Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains
Abstract
1. Introduction
2. Results
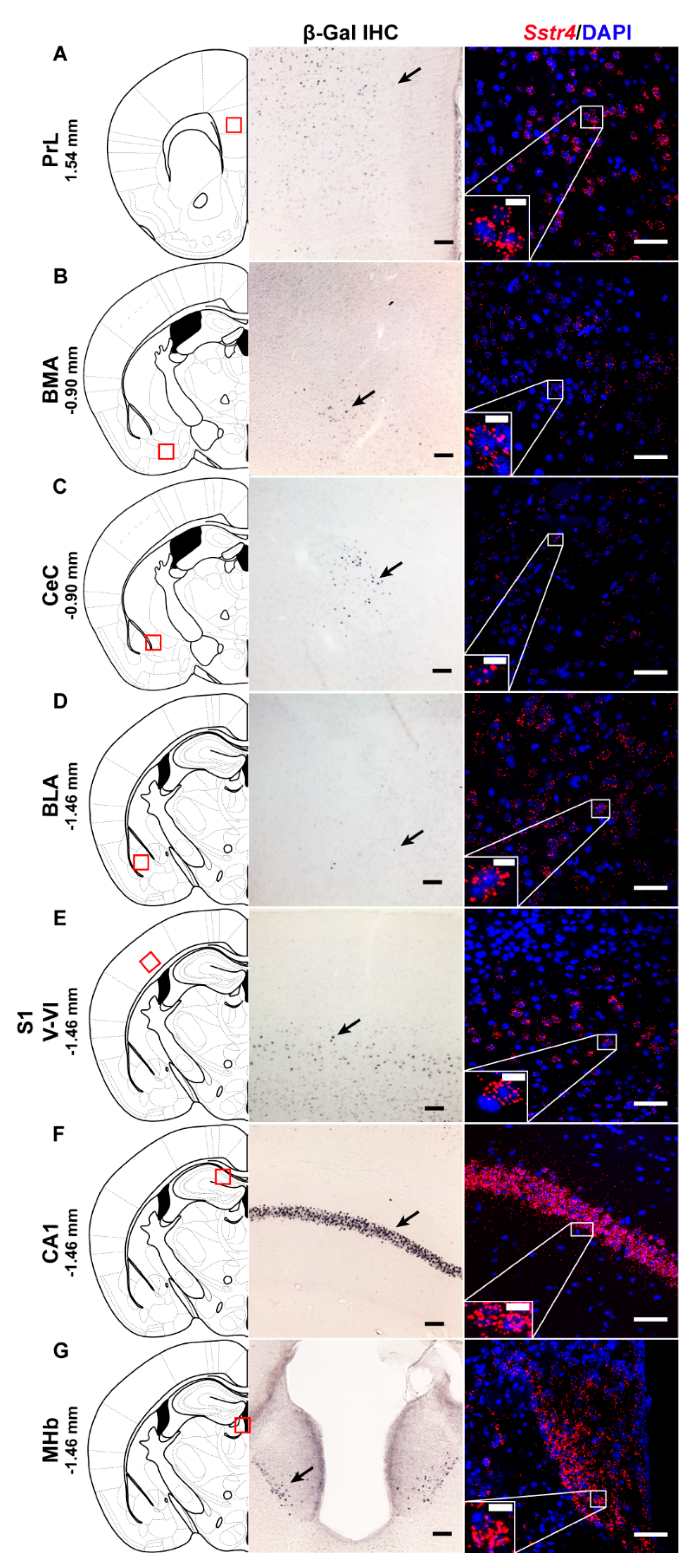
2.1. Sstr4 Expression in Several Pain- and Mood Regulation-Related Brain Regions
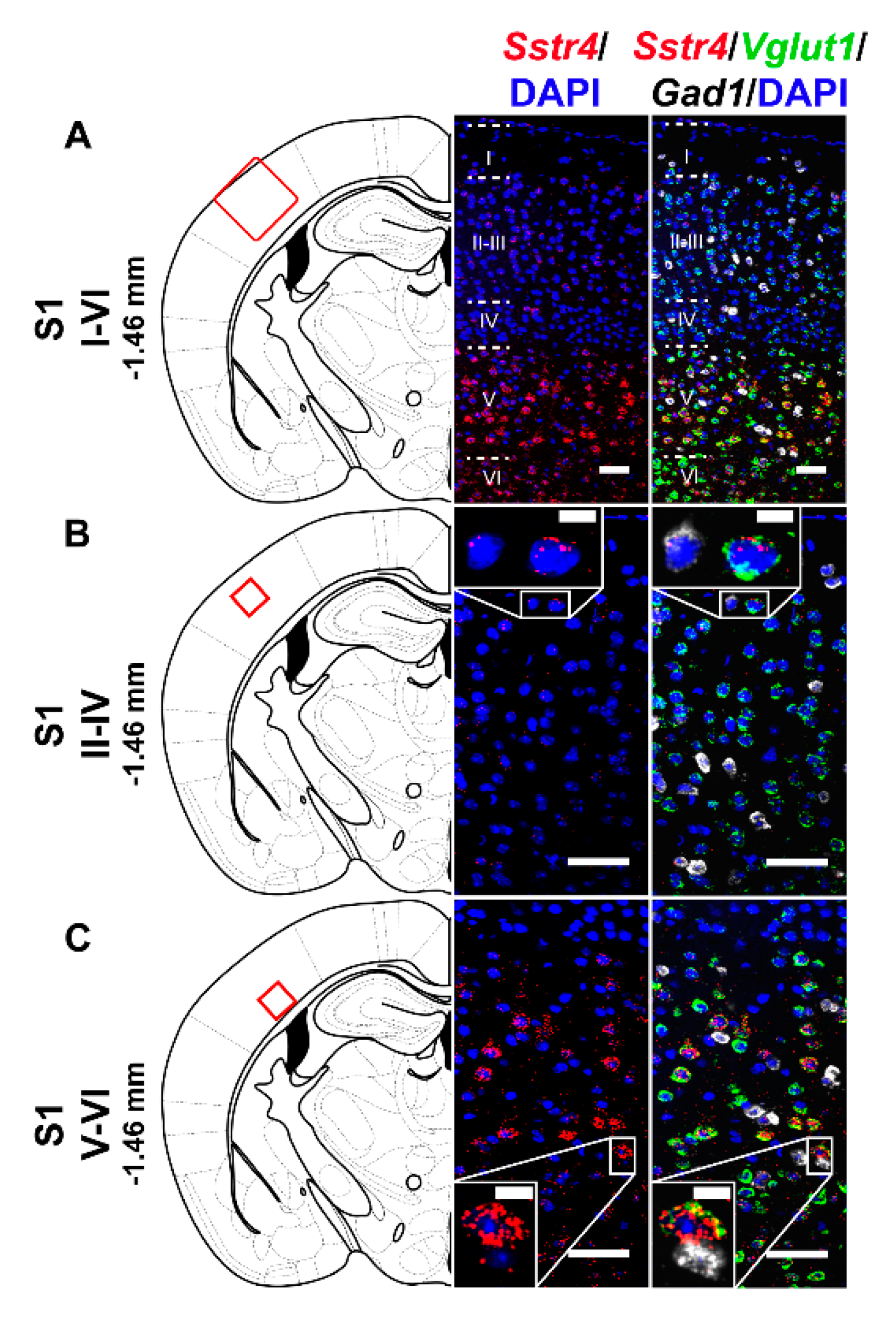
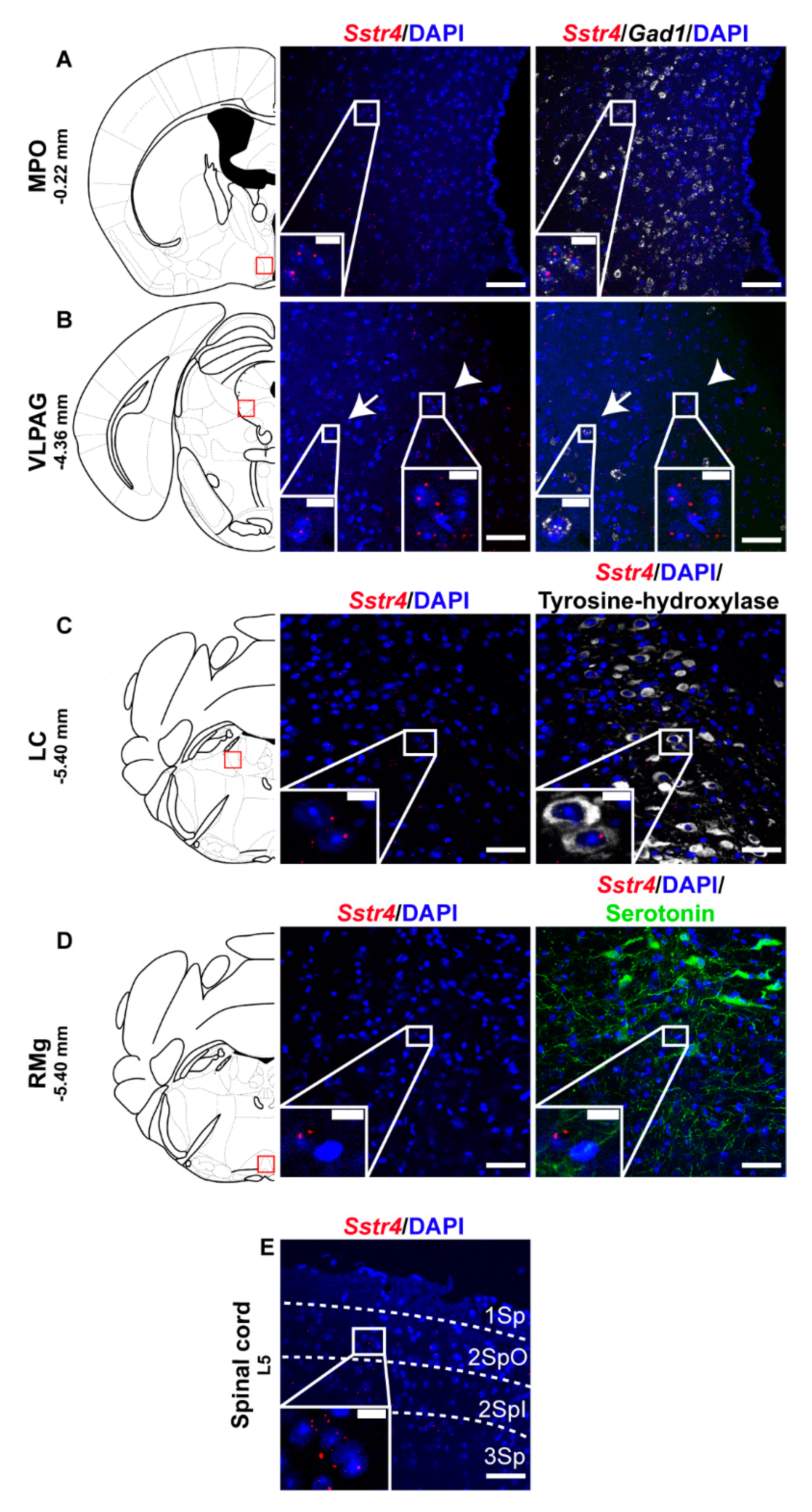
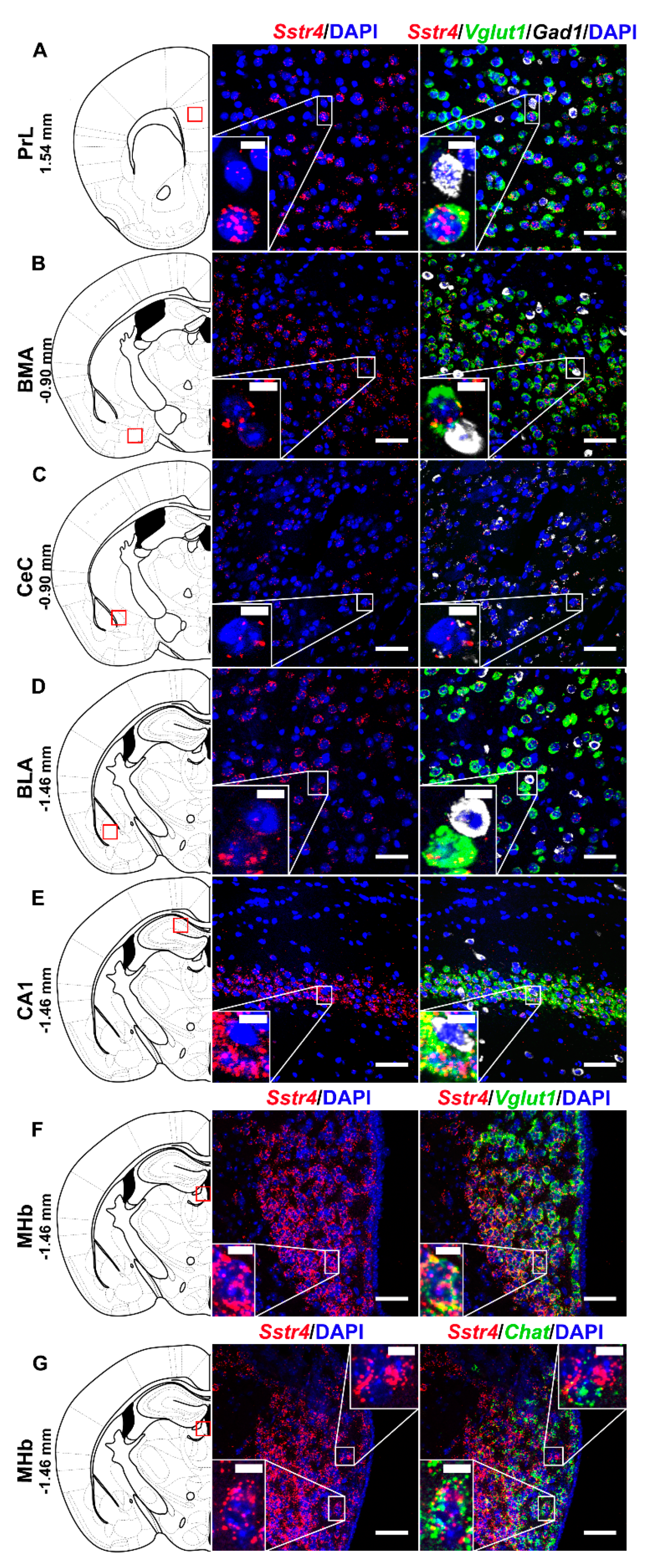
2.2. Sstr4 Is Detectable Both in Nociceptive and Anti-Nociceptive Centers as Well as in Glutamatergic Excitatory Neurons of Brain Regions Involved in Mood Regulation
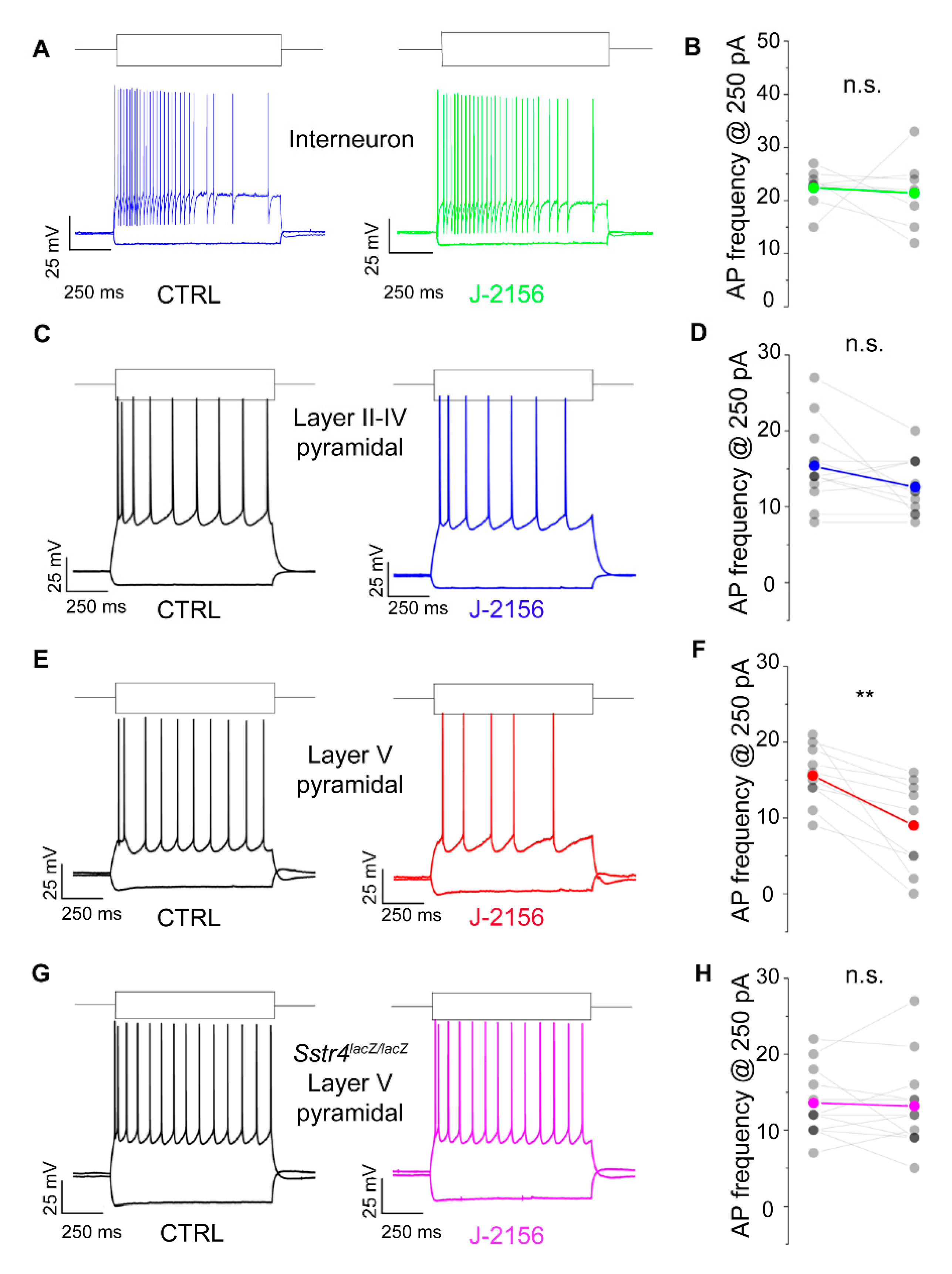
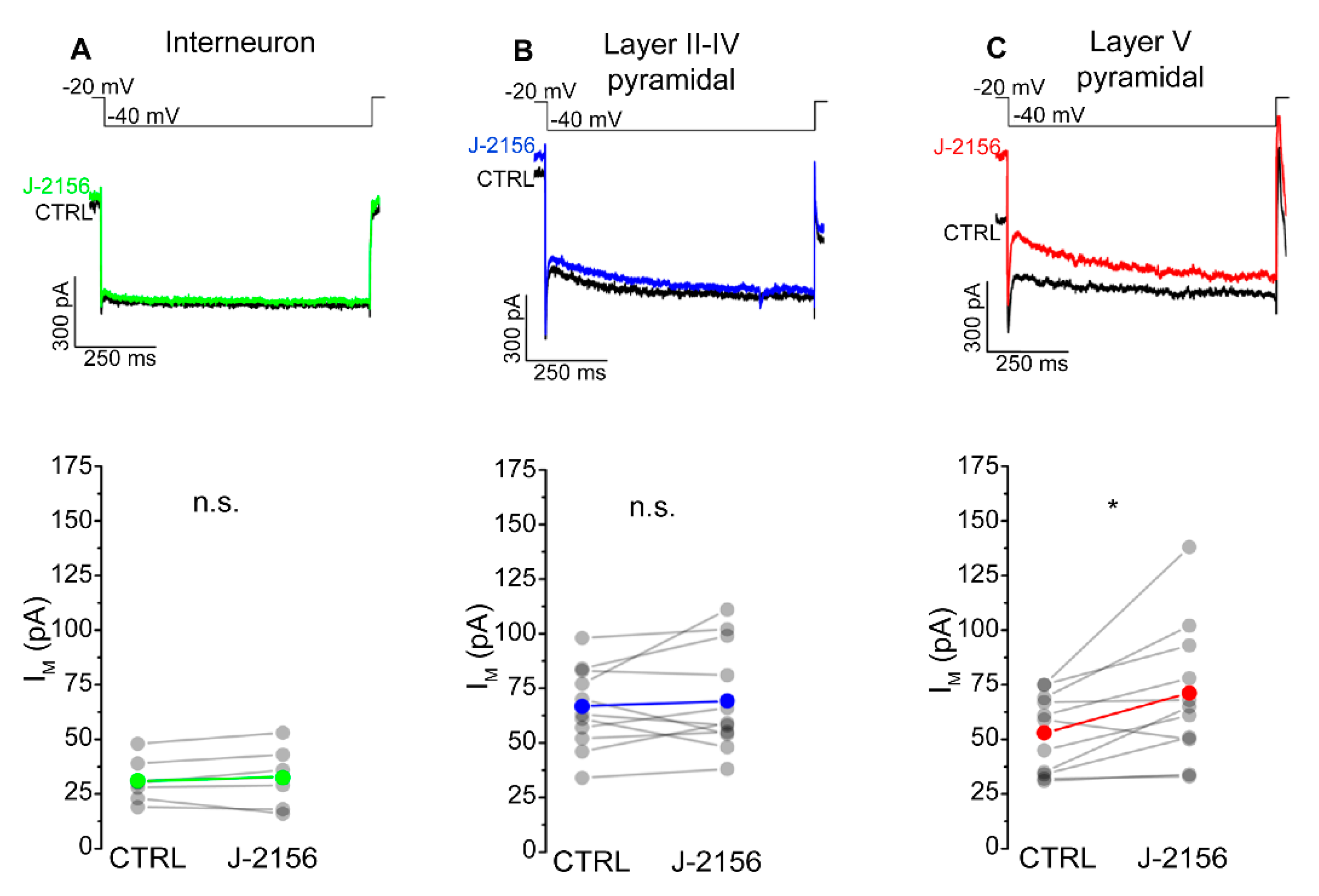
2.3. Activation of the SST4 Receptor Decreases the Excitability of Layer V Pyramidal Neurons
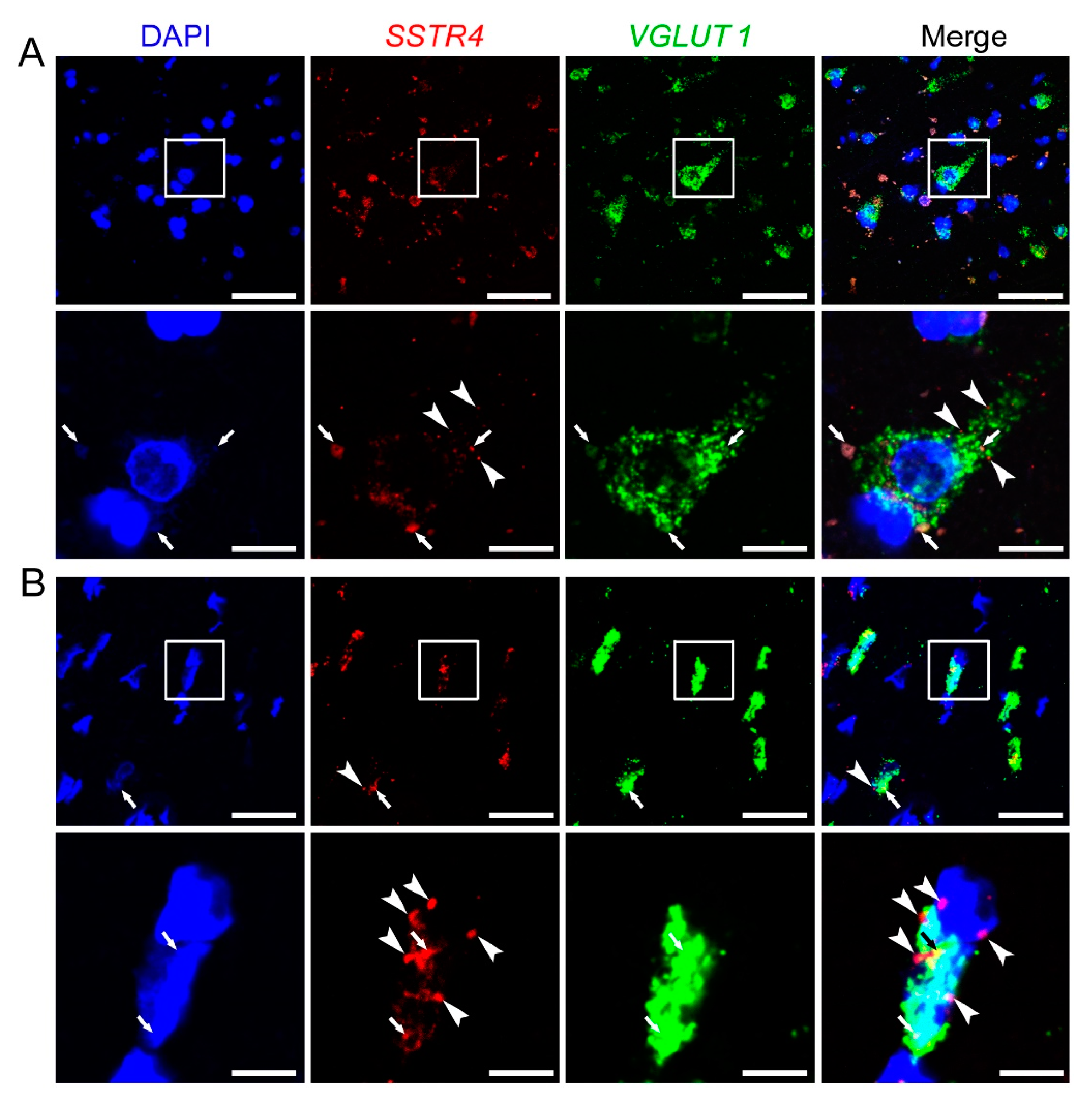
2.4. SSTR4 mRNA Co-Localizes on VGLUT1-Expressing Pyramidal Neurons in Human Neurosurgical Cortical Tissue Samples
3. Discussion
4. Materials and Methods
4.1. G-Protein Activation Assay
4.2. Samples
4.2.1. Mice
4.2.2. Human Post-Mortem and Neurosurgical Cortical Tissues
4.3. Sample Preparation and Investigational Techniques
4.3.1. Mouse
Real-Time Quantitative RT-qPCR
Perfusion and Tissue Processing for Histological Studies
β-Galactosidase-Specific Immunohistochemistry (β-Gal IHC)
SST4-Specific Immunohistochemistry
RNAscope In Situ Hybridization (ISH) on Mouse Samples
Acute Brain Slice Preparation
In Vitro Electrophysiological Recordings
4.3.2. Human
RT-qPCR on Post-Mortem Human Cortical Tissues
RNAscope ISH on Neurosurgical Cortical Tissue Samples
4.4. Experimental Design and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SS | Somatostatin |
| SST1-5 | somatostatin receptor subtype 1-5 |
| SSTR1-5/Sstr1-5 | gene of somatostatin receptor subtype 1-5 in human/mouse |
| Sstr4lacZ/lacZ mice | Sstr4-deficient, lacZ knock-in reporter mice |
| GIRK | G protein-coupled inward-rectifying potassium channels |
| ISH | In situ hybridization |
| β-Gal IHC | β-galactosidase immunostaining |
| DRG | Dorsal root ganglia |
| TG | Trigeminal ganglia |
| PrL | Prelimbic cortex |
| MPO | Medial preoptic area of the hypothalamus |
| BMA | Basomedial amygdala |
| CeC | Core of central amygdala |
| BLA | Basolateral amygdala |
| S1 | Primary somatosensory cortex |
| CA1 | Cornu Ammonis 1 region of hippocampus |
| MHb | Medial nucleus of habenula |
| VPL | Ventral posterolateral thalamic nucleus |
| VLPAG | Ventrolateral periaqueductal gray matter |
| RMg | Raphe magnus nucleus |
| LC | Locus coeruleus |
| Vglut1 | Vesicular glutamate transporter 1 |
| Gad1 | Glutamate decarboxylase 1 |
| Chat | Choline acetyltransferase |
| Rbfox3 | RNA Binding Fox-1 Homolog 3, NeuN |
| TH | Tyrosine-hydroxylase |
| HPA | Hypothalamus-pituitary-adrenal axis |
| DAB | 3,3′-diaminobenzidine |
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Viollet, C.; Lepousez, G.; Loudes, C.; Videau, C.; Simon, A.; Epelbaum, J. Somatostatinergic systems in brain: Networks and functions. Mol. Cell. Endocrinol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Rivier, J.; Tache, Y. Central Actions of Somatostatin-28 and Oligosomatostatin Agonists to Prevent Components of the Endocrine, Autonomic and Visceral Responses to Stress Through Interaction with Different Somatostatin Receptor Subtypes. Curr. Pharm. Des. 2012, 19, 98–105. [Google Scholar] [CrossRef][Green Version]
- Pintér, E.; Helyes, Z.; Szolcsányi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef]
- Hoyer, D.; Bell, G.I.; Berelowitz, M.; Epelbaum, J.; Feniuk, W.; Humphrey, P.P.A.; O’Carroll, A.M.; Patel, Y.C.; Schonbrunn, A.; Taylor, J.E.; et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol. Sci. 1995, 16, 86–88. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Németh, J.; Sándor, K.; Elekes, K.; Szabó, Á.; Pozsgai, G.; Keszthelyi, D.; Kereskai, L.; Engström, M.; et al. Effects of the somatostatin receptor subtype 4 selective agonist J-2156 on sensory neuropeptide release and inflammatory reactions in rodents. Br. J. Pharmacol. 2006. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Sándor, K.; Elekes, K.; Bánvölgyi, Á.; Keszthelyi, D.; Szoke, É.; Tóth, D.M.; Sándor, Z.; Kereskai, L.; et al. Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc. Natl. Acad. Sci. USA 2009, 106, 13088–13093. [Google Scholar] [CrossRef]
- Markovics, A.; Szőke, É.; Sándor, K.; Börzsei, R.; Bagoly, T.; Kemény, Á.; Elekes, K.; Pintér, E.; Szolcsányi, J.; Helyes, Z. Comparison of the anti-inflammatory and anti-nociceptive effects of cortistatin-14 and somatostatin-14 in distinct in vitro and in vivo model systems. J. Mol. Neurosci. 2012, 46, 40–50. [Google Scholar] [CrossRef]
- Qiu, C.; Zeyda, T.; Johnson, B.; Hochgeschwender, U.; De Lecea, L.; Tallent, M.K. Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. J. Neurosci. 2008, 106, 13088–13093. [Google Scholar] [CrossRef]
- Engin, E.; Treit, D. Anxiolytic and antidepressant actions of somatostatin: The role of sst2 and sst3 receptors. Psychopharmacology 2009, 206, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Scheich, B.; Gaszner, B.; Kormos, V.; László, K.; Ádori, C.; Borbély, É.; Hajna, Z.; Tékus, V.; Bölcskei, K.; Ábrahám, I.; et al. Somatostatin receptor subtype 4 activation is involved in anxiety and depression-like behavior in mouse models. Neuropharmacology 2016, 101, 204–215. [Google Scholar] [CrossRef]
- Scheich, B.; Csekő, K.; Borbély, É.; Ábrahám, I.; Csernus, V.; Gaszner, B.; Helyes, Z. Higher susceptibility of somatostatin 4 receptor gene-deleted mice to chronic stress-induced behavioral and neuroendocrine alterations. Neuroscience 2017. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Dutar, P.; Epelbaum, J.; Viollet, C. Somatostatinergic systems: An update on brain functions in normal and pathological aging. Front. Endocrinol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.V.; Lamp, T.; Tallent, M.K. Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. J. Neurophysiol. 2002. [Google Scholar] [CrossRef]
- Jiang, N.; Furue, H.; Katafuchi, T.; Yoshimura, M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci. Res. 2003. [Google Scholar] [CrossRef]
- Moore, S.D.; Madamba, S.G.; Joëls, M.; Siggins, G.R. Somatostatin augments the M-current in hippocampal neurons. Science 1988, 239, 278–280. [Google Scholar] [CrossRef]
- Shenoy, P.A.; Kuo, A.; Khan, N.; Gorham, L.; Nicholson, J.R.; Corradini, L.; Vetter, I.; Smith, M.T. The somatostatin receptor-4 agonist J-2156 alleviates mechanical hypersensitivity in a rat model of breast cancer induced bone pain. Front. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Schreff, M.; Schulz, S.; Händel, M.; Keilhoff, G.; Braun, H.; Pereira, G.; Klutzny, M.; Schmidt, H.; Wolf, G.; Höllt, V. Distribution, targeting, and internalization of the sst4 somatostatin receptor in rat brain. J. Neurosci. 2000, 20, 3785–3797. [Google Scholar] [CrossRef]
- Hannon, J.P.; Petrucci, C.; Fehlmann, D.; Viollet, C.; Epelbaum, J.; Hoyer, D. Somatostatin sst2 receptor knock-out mice: Localisation of sst1-5 receptor mRNA and binding in mouse brain by semi-quantitative RT-PCR, in situ hybridisation histochemistry and receptor autoradiography. Neuropharmacology 2002. [Google Scholar] [CrossRef]
- Wang, F.; Flanagan, J.; Su, N.; Wang, L.C.; Bui, S.; Nielson, A.; Wu, X.; Vo, H.T.; Ma, X.J.; Luo, Y. RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Botz, B.; Bölcskei, K.; Helyes, Z. Challenges to develop novel anti-inflammatory and analgesic drugs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Pozsgai, G.; Hajna, Z.; Helyes, Z.; Szolcsányi, J. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br. J. Clin. Pharmacol. 2014, 77, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kántás, B.; Börzsei, R.; Szőke, É.; Bánhegyi, P.; Horváth, Á.; Hunyady, Á.; Borbély, É.; Hetényi, C.; Pintér, E.; Helyes, Z. Novel Drug-Like Somatostatin Receptor 4 Agonists are Potential Analgesics for Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 6245. [Google Scholar] [CrossRef]
- Available online: https://www.lilly.com/discovery/clinical-development-pipeline#/ (accessed on 10 September 2020).
- Engström, M.; Savola, J.M.; Wurster, S. Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J. Pharmacol. Exp. Ther. 2006, 316, 1262–1268. [Google Scholar] [CrossRef]
- Gebhart, G.F. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004, 27, 729–737. [Google Scholar] [CrossRef]
- Harris, J.A. Descending antinociceptive mechanisms in the brainstem: Their role in the animal’s defensive system. J. Physiol. Paris 1996. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019. [Google Scholar] [CrossRef]
- Prager, E.M.; Bergstrom, H.C.; Wynn, G.H.; Braga, M.F.M. The basolateral amygdala γ-aminobutyric acidergic system in health and disease. J. Neurosci. Res. 2016, 94, 548–567. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015. [Google Scholar] [CrossRef]
- Duvarci, S.; Pare, D. Amygdala microcircuits controlling learned fear. Neuron 2014, 82, 966–980. [Google Scholar] [CrossRef]
- Kalin, N.H.; Shelton, S.E.; Davidson, R.J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004, 24, 5506–5515. [Google Scholar] [CrossRef]
- Beaulieu, S.; Di Paolo, T.; Barden, N. Control of ACTH secretion by the central nucleus of the amygdala: Implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 1986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, L.; Ren, Y.; Liang, J.; Lin, R.; Feng, Q.; Zhou, J.; Hu, F.; Ren, J.; Wei, C.; et al. Presynaptic Excitation via GABAB Receptors in Habenula Cholinergic Neurons Regulates Fear Memory Expression. Cell 2016. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-W.A.; Morton, G.; Guy, E.G.; Wang, S.D.; Turner, E.E. Dorsal Medial Habenula Regulation of Mood-Related Behaviors and Primary Reinforcement by Tachykinin-Expressing Habenula Neurons. Eneuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Yang, S.H.; Kim, J.Y.; Kim, H. The role of the medial habenula cholinergic system in addiction and emotion-associated behaviors. Front. Psychiatry 2019. [Google Scholar] [CrossRef]
- Han, S.; Yang, S.H.; Kim, J.Y.; Mo, S.; Yang, E.; Song, K.M.; Ham, B.J.; Mechawar, N.; Turecki, G.; Lee, H.W.; et al. Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Jacobson, L.; Sapolsky, R. The Role of the Hippocampus in Feedback Regulation of the Hypothalamic-Pituitary-Adrenocortical Axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef]
- Prévôt, T.D.; Gastambide, F.; Viollet, C.; Henkous, N.; Martel, G.; Epelbaum, J.; Béracochéa, D.; Guillou, J.L. Roles of Hippocampal Somatostatin Receptor Subtypes in Stress Response and Emotionality. Neuropsychopharmacology 2017, 42, 1647–1656. [Google Scholar] [CrossRef]
- Yavorska, I.; Wehr, M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front. Neural Circuits 2016. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Timme, K.A.; Wood, J.R. Using Single Molecule mRNA Fluorescent in Situ Hybridization (RNA-FISH) to Quantify mRNAs in Individual Murine Oocytes and Embryos. Sci. Rep. 2018, 8, 7930. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, F.; Haipt, A.; Rosenbaum, D.; Rohe, T.; Fallgatter, A.J.; Hautzinger, M.; Ehlis, A.-C. The Positive Brain—Resting State Functional Connectivity in Highly Vital and Flourishing Individuals. Front. Hum. Neurosci. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://algonist.com/services/ (accessed on 10 October 2020).
- Palkovits, M.; Graf, L.; Hermann, I.; Borvendeg, J.; Acs, Z.S.; Lang, T. Regional Distribution of Enkephalins, Endorphins and ACTH in the Central Nervous System of Rats Determined by Radioimmunoassay; Academic Press: Budapest, Hungary, 1978; pp. 187–195. [Google Scholar]
- Paxinos, G.; Franklin, K.B.J. Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2001; ISBN 0125476361. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29. [Google Scholar] [CrossRef]
- Helyes, Z.; Tékus, V.; Szentes, N.; Pohóczky, K.; Botz, B.; Kiss, T.; Kemény, Á.; Környei, Z.; Tóth, K.; Lénárt, N.; et al. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1–induced mechanisms. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Léger, L.; Charnay, Y.; Burlet, S.; Gay, N.; Schaad, N.; Bouras, C.; Cespuglio, R. Comparative distribution of nitric oxide synthase- and serotonin- containing neurons in the raphe nuclei of four mammalian species. Histochem. Cell Biol. 1998. [Google Scholar] [CrossRef]
- Kormos, V.; Gáspár, L.; Kovács, L.; Farkas, J.; Gaszner, T.; Csernus, V.; Balogh, A.; Hashimoto, H.; Reglodi, D.; Helyes, Z.; et al. Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 2016, 330, 335–358. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kecskés, A.; Pohóczky, K.; Kecskés, M.; Varga, Z.V.; Kormos, V.; Szőke, É.; Henn-Mike, N.; Fehér, M.; Kun, J.; Gyenesei, A.; et al. Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains. Int. J. Mol. Sci. 2020, 21, 7788. https://doi.org/10.3390/ijms21207788
Kecskés A, Pohóczky K, Kecskés M, Varga ZV, Kormos V, Szőke É, Henn-Mike N, Fehér M, Kun J, Gyenesei A, et al. Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains. International Journal of Molecular Sciences. 2020; 21(20):7788. https://doi.org/10.3390/ijms21207788
Chicago/Turabian StyleKecskés, Angéla, Krisztina Pohóczky, Miklós Kecskés, Zoltán V. Varga, Viktória Kormos, Éva Szőke, Nóra Henn-Mike, Máté Fehér, József Kun, Attila Gyenesei, and et al. 2020. "Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains" International Journal of Molecular Sciences 21, no. 20: 7788. https://doi.org/10.3390/ijms21207788
APA StyleKecskés, A., Pohóczky, K., Kecskés, M., Varga, Z. V., Kormos, V., Szőke, É., Henn-Mike, N., Fehér, M., Kun, J., Gyenesei, A., Renner, É., Palkovits, M., Ferdinandy, P., Ábrahám, I. M., Gaszner, B., & Helyes, Z. (2020). Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains. International Journal of Molecular Sciences, 21(20), 7788. https://doi.org/10.3390/ijms21207788







