A Brief History of Adherons: The Discovery of Brain Exosomes
Abstract
1. Introduction
2. Cell Adhesion Is Dependent Upon High Molecular Weight Protein Complexes
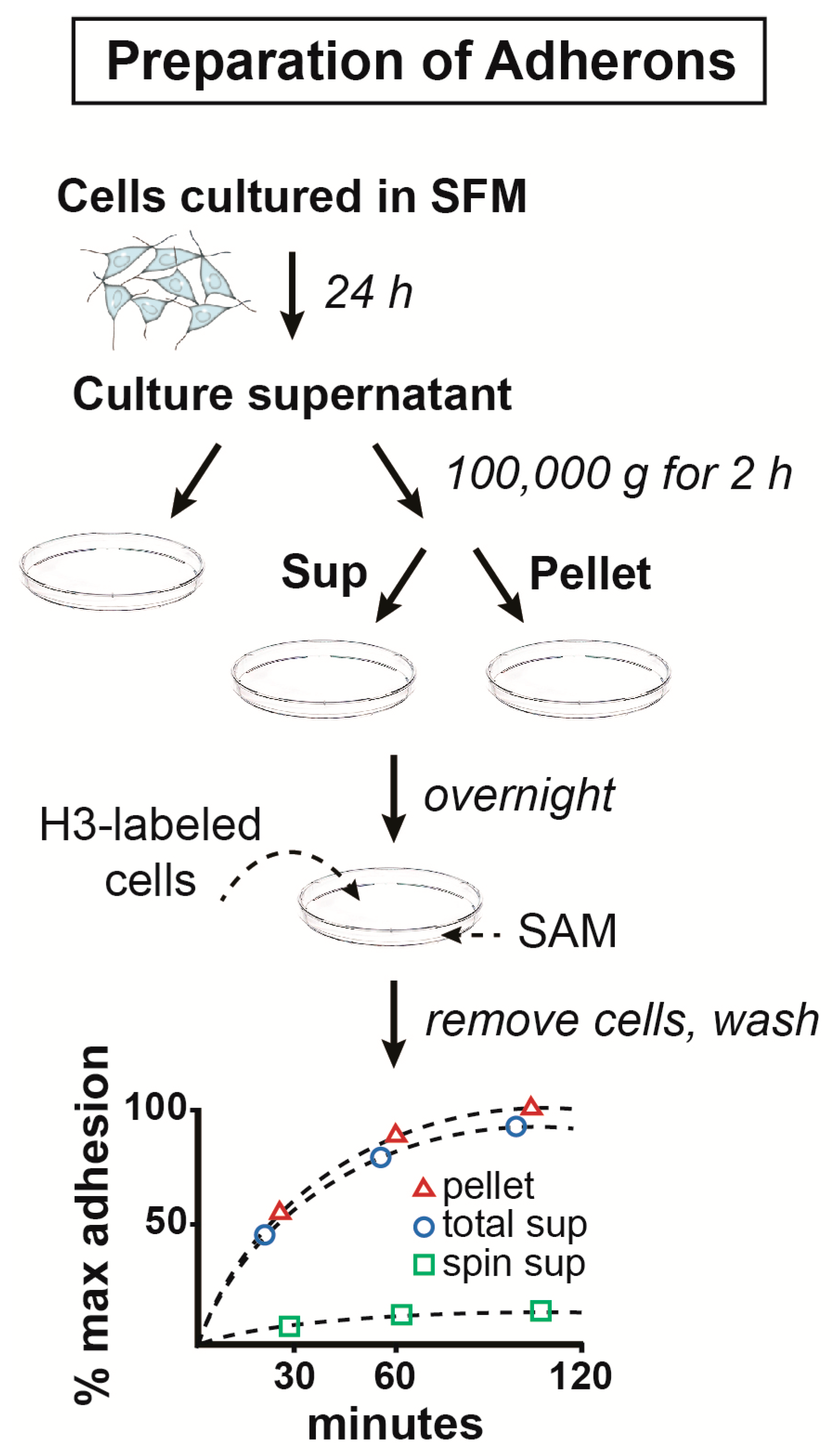
2.1. The Structure and Composition of Adherons
2.2. Isolation and Cloning of a Neurotrophic Factor from Adherons
2.3. Adherons vs. Exosomes
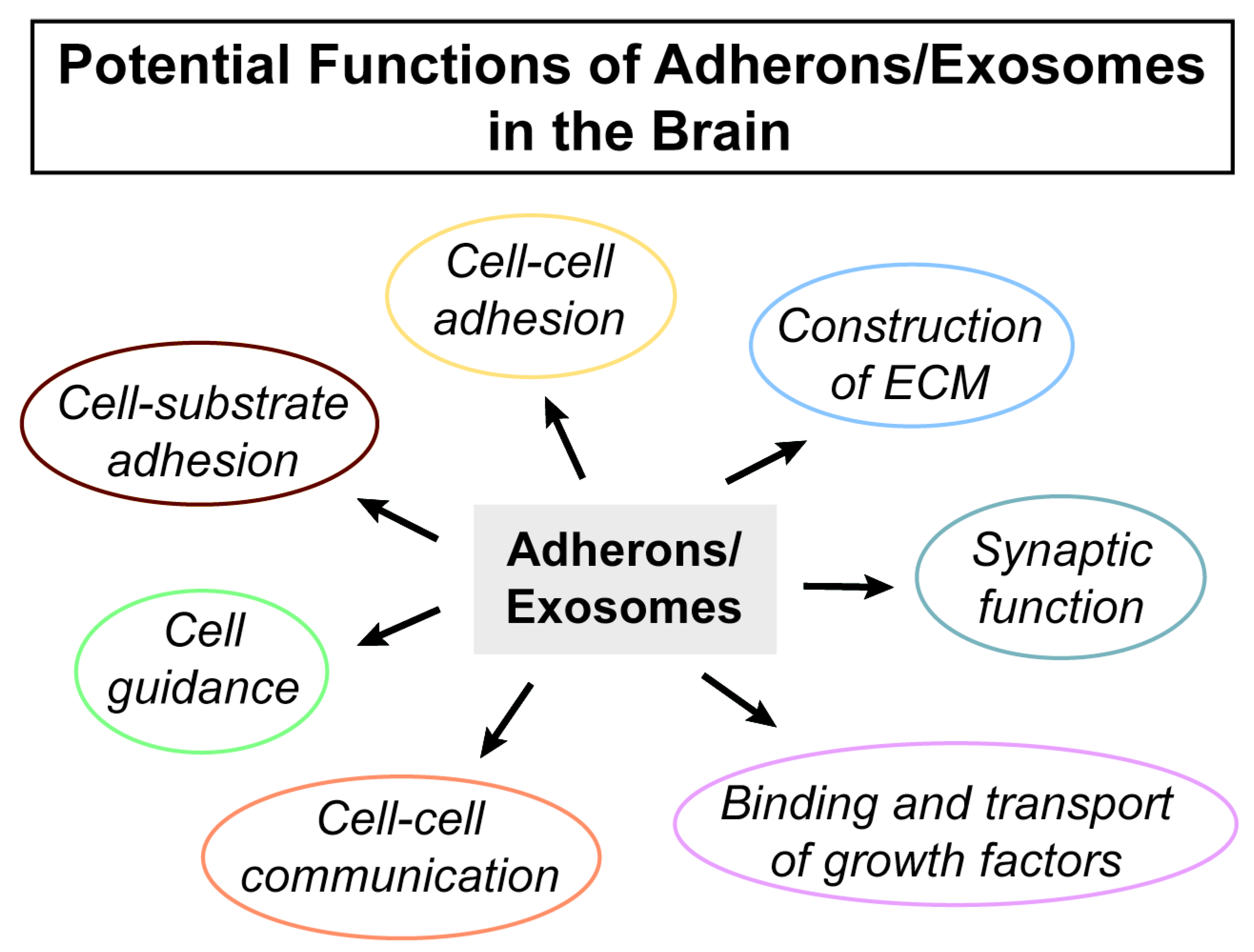
2.4. Exosomes, Adhesion, and the Brain ECM
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| ECM | Extracellular matrix |
| FGF | Fibroblast growth factor |
| GAG | Glycosaminoglycan |
| GDGF | Glial-derived growth factor |
| HSPG | Heparin sulfate proteoglycan |
| NGF | Nerve growth factor |
| PNN | Perineuronal net |
| SAM | Substrate-attached material |
| TR | Transferrin receptor |
References
- Bargmann, C.I.; Hung, M.C.; Weinberg, R.A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986, 319, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Engele, J.; Schubert, D.; Bohn, M.C. Conditioned media derived from glial cell lines promote survival and differentiation of dopaminergic neurons in vitro: Role of mesencephalic glia. J. Neurosci. Res. 1991, 30, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Klar, A.; Park, M.; Dargusch, R.; Fischer, W.H. F-spondin promotes nerve precursor differentiation. J. Neurochem. 2006, 96, 444–453. [Google Scholar] [CrossRef]
- Kidokoro, Y.; Heinemann, S.; Schubert, D.; Brandt, B.L.; Klier, F.G. Synapse formation and neurotrophic effects on muscle cell lines. Cold Spring Harb. Symp. Quant. Biol. 1976, 40, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Heinemann, S.; Carlisle, W.; Tarikas, H.; Kimes, B.; Patrick, J.; Steinbach, J.H.; Culp, W.; Brandt, B.L. Clonal cell lines from the rat central nervous system. Nature 1974, 249, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Bergmann, R.; Beyer, G.J.; Manfrass, P.; Steinbach, J.; Kretzschmar, M.; Enghardt, W.; Hoyer, D.; Gunther, K.; Schubert, H.; et al. Investigations of cerebral glucose utilization into the newborn brain: A [18F]-FDG positron emission tomography study using a high resolution multiwire proportional chamber detector device. Exp. Pathol. 1991, 42, 229–233. [Google Scholar] [CrossRef]
- Schubert, D. The substrate attached material synthesized by clonal cell lines of nerve, glia, and muscle. Brain Res. 1977, 132, 337–346. [Google Scholar] [CrossRef]
- Schubert, D. NGF-induced alterations in protein secretion and substrate-attached material of a clonal nerve cell line. Brain Res. 1978, 155, 196–200. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M. Role of a 16S glycoprotein complex in cellular adhesion. Proc. Natl. Acad. Sci. USA 1980, 77, 4137–4141. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M. A role of seceted glycosaminoglycans in cell-substratum adhesion. J. Biol. Chem. 1980, 255, 11564–11569. [Google Scholar]
- Schubert, D.; LaCorbiere, M.; Klier, F.G.; Birdwell, C. The structure and function of myoblast adherons. Cold Spring Harb. Symp. Quant. Biol. 1983, 48, 539–549. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Pan, B.; Johnstone, R. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M.; Klier, F.G.; Birdwell, C. A role for adherons in neural retina cell adhesion. J. Cell Biol. 1983, 96, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Bandari, S.K.; Liu, J.; Mobley, J.A.; Brown, E.E.; Sanderson, R.D. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J. Biol. Chem. 2016, 291, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; LaCorbiere, M. The specificity of extracellular glycoprotein complexes in mediating cellular adhesion. J. Neurosci. 1982, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lilien, J.; Moscona, A.A. Cell aggregation: Its enhancement by a supernatant from culutres of homologous cells. Science 1967, 157, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.J.; Schubert, D.; Glaser, L. Cell-substratum adhesion in chick neural retina depends upon protein-heparan sulfate interactions. J. Cell Biol. 1985, 100, 1192–1199. [Google Scholar] [CrossRef]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef]
- Cole, G.; Glaser, L. A heparin-binding domain from N-CAM is involved in neural cell-substratum adhesion. J. Cell Biol. 1986, 102, 403–412. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M. Isolation of an adhesion-mediating protein from chick neural retina adherons. J. Cell Biol. 1985, 101, 1071–1077. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M.; Esch, F. A chick neural retina adhesion and survival molecule is a retinal-binding protein. J. Cell Biol. 1986, 102, 2295–2301. [Google Scholar] [CrossRef]
- Berman, P.; Gray, P.; Chen, E.; Keyser, K.; Ehrlich, D.; Karten, H.; LaCorbiere, M.; Esch, F.; Schubert, D. Sequence analysis, cellular localization and expression of a neuroretina adhesion and cell survival molecule. Cell 1987, 51, 134–142. [Google Scholar] [CrossRef]
- Matsukawa, T.; Sugitani, K.; Mawatari, K.; Koriyama, Y.; Liu, Z.; Tanaka, M.; Kato, S. Role of purpurin as a retinol-binding protein in goldfish retina during the early stage of optic nerve regeneration: Its priming action on neurite outgrowth. J. Neurosci. 2004, 24, 8346–8353. [Google Scholar] [CrossRef]
- Nagashima, M.; Sakurai, H.; Mawatari, K.; Koriyama, Y.; Matsukawa, T.; Kato, S. Involvement of retinoic acid signaling in goldfish optic nerve regeneration. Neurochem. Int. 2009, 54, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Murayama, D.; Nagashima, M.; Higashi, T.; Mawatari, K.; Matsukawa, T.; Kato, S. Purpurin expression in the zebrafish retina during early development and after optic nerve lesion in adults. Brain Res. 2007, 1153, 34–42. [Google Scholar] [CrossRef]
- Nagashima, M.; Saito, J.; Mawatari, K.; Mori, Y.; Matsukawa, T.; Koriyama, Y.; Kato, S. A hypoplastic retinal lamination in the purpurin knock down embryo in zebrafish. Adv. Exp. Med. Biol. 2010, 664, 517–524. [Google Scholar]
- Kato, S.; Matsukawa, T.; Koriyama, Y.; Sugitani, K.; Ogai, K. A molecular mechanism of optic nerve regeneration in fish: The retinoid signaling pathway. Prog. Retin. Eye Res. 2013, 37, 13–30. [Google Scholar] [CrossRef]
- Park, H.; Shim, J.S.; Kim, B.S.; Jung, H.J.; Huh, T.L.; Kwon, H.J. Purpurin inhibits adipocyte-derived leucine aminopeptidase and angiogenesis in a zebrafish model. Biochem. Biophys. Res. Commun. 2014, 450, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.B.; Gao, H.W.; Peng, C. Quinones as preventive agents in Alzheimer’s diseases: Focus on NLRP3 inflammasomes. J. Pharm. Pharmacol. 2020, 72, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Boing, A.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–708. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V.; Nagaeva, O.; Dehlin, E. Isolation and Characterization of Exosomes from Cultures of Tissue Explants and Cell Lines. Curr. Protoc. Immunol. 2016, 115, 14.42.1–14.42.21. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Benbenishty, A.; Blinder, P.; Sagi, I. Demystifying the extracellular matrix and its proteolytic remodeling in the brain: Structural and functional insights. Cell. Mol. Life Sci. 2019, 76, 3229–3248. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Balzac, C.A.; Lazaro-Pena, M.; Tecle, E.; Gomez, N.; Bulow, H.E. Complex cooperative functions of heparan sulfate proteoglycans shape nervous system development in Caenorhabditis elegans. G3 Genes Genomes Genet. 2014, 4, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Nakamura, F.; Fukunaga, S. Diverse function of perlecan in central nervous system cells in vitro. Anim. Sci. J. 2015, 86, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I.; Toth, E.A.; Sodar, B.W.; Szabo-Taylor, K.E. Molecular interactions at the surface of extracellular vesicles. Sem. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D. The possible role of adhesion in synaptic modification. Trends Neurosci. 1991, 14, 127–130. [Google Scholar] [CrossRef]
- Orlandi, C.; Omori, Y.; Wang, Y.; Cao, Y.; Ueno, A.; Roux, M.J.; Condomitti, G.; de Wit, J.; Kanagawa, M.; Furukawa, T.; et al. Transsynaptic binding of orphan receptor GPR179 to dystroglycan-pikachurin complex is essential for the synaptic organization of photoreceptors. Cell Rep. 2018, 25, 130–145. [Google Scholar] [CrossRef]
- Schubert, D.; Schroeder, R.; LaCorbiere, M.; Saitoh, T.; Cole, G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science 1988, 241, 223–226. [Google Scholar] [CrossRef]
- Schubert, D.; LaCorbiere, M.; Saitoh, T.; Cole, G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc. Natl. Acad. Sci. USA 1989, 86, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Jin, L.-W.; Saitoh, T.; Cole, G. The regulation of amyloid beta protein precursor secretion and its modulatory role in cell adhesion. Neuron 1989, 3, 689–694. [Google Scholar] [CrossRef]
- Klier, F.G.; Cole, G.; Stallcup, W.; Schubert, D. Amyloid beta protein precursor is associated with extracellular matrix. Brain Res. 1990, 515, 336–342. [Google Scholar] [CrossRef]
- Miranda, A.M.; Lasiecka, Z.M.; Xu, Y.; Neufeld, J.; Shahriar, S.; Simoes, S.; Chan, R.B.; Oliveira, T.G.; Small, S.A.; Di Paolo, G. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 2018, 9, 291. [Google Scholar] [CrossRef]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhaes, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin and artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef]
- Saengsawang, W.; Kongoun, S.; Chanda, M. Exercise increases brain-derived neurotrophic factor in serum exosomes. FASEB J. 2017, 31, 839–845. [Google Scholar]
- Goetzi, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J. 2019, 33, 231–238. [Google Scholar] [CrossRef]
- Hormung, S.; Dutta, S.; Bitan, G. CNS-derived blood exosomes as a promising source of biomarkers: Opportunities and challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, D. A Brief History of Adherons: The Discovery of Brain Exosomes. Int. J. Mol. Sci. 2020, 21, 7673. https://doi.org/10.3390/ijms21207673
Schubert D. A Brief History of Adherons: The Discovery of Brain Exosomes. International Journal of Molecular Sciences. 2020; 21(20):7673. https://doi.org/10.3390/ijms21207673
Chicago/Turabian StyleSchubert, David. 2020. "A Brief History of Adherons: The Discovery of Brain Exosomes" International Journal of Molecular Sciences 21, no. 20: 7673. https://doi.org/10.3390/ijms21207673
APA StyleSchubert, D. (2020). A Brief History of Adherons: The Discovery of Brain Exosomes. International Journal of Molecular Sciences, 21(20), 7673. https://doi.org/10.3390/ijms21207673


