Abstract
CC chemokines (or β-chemokines) are 28 chemotactic cytokines with an N-terminal CC domain that play an important role in immune system cells, such as CD4+ and CD8+ lymphocytes, dendritic cells, eosinophils, macrophages, monocytes, and NK cells, as well in neoplasia. In this review, we discuss human CC motif chemokine ligands: CCL1, CCL3, CCL4, CCL5, CCL18, CCL19, CCL20, CCL21, CCL25, CCL27, and CCL28 (CC motif chemokine receptor CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands). We present their functioning in human physiology and in neoplasia, including their role in the proliferation, apoptosis resistance, drug resistance, migration, and invasion of cancer cells. We discuss the significance of chemokine receptors in organ-specific metastasis, as well as the influence of each chemokine on the recruitment of various cells to the tumor niche, such as cancer-associated fibroblasts (CAF), Kupffer cells, myeloid-derived suppressor cells (MDSC), osteoclasts, tumor-associated macrophages (TAM), tumor-infiltrating lymphocytes (TIL), and regulatory T cells (Treg). Finally, we show how the effect of the chemokines on vascular endothelial cells and lymphatic endothelial cells leads to angiogenesis and lymphangiogenesis.
1. Introduction: The Dual Properties of Chemokines Are Key for Understanding of the Tumor Microenvironment during Therapy
The CC (β) subfamily of chemokines is a group of chemotactic cytokines known as CC motif chemokine ligands (CCL)1–28. Their shared characteristic is the N-terminal CC domain and digits in their symbols depend on the order of discovery [1,2]. The actual number of CC chemokines is 27, as CCL9 and CCL10 denote the same chemokine. All these chemokines are ligands for 10 receptors—CC motif chemokine receptors (CCR)1–10. Just like the rest of chemokines, CC chemokines are crucial for the functioning of the immune system cells [2]. However, apart from their anti-cancer properties, they also show some pro-cancer characteristics and thus play an important role in neoplasia.
Below, we have listed the properties of selected chemokines in the tumor. These properties can be divided into anti- and pro-cancer. The former are associated mainly with the recruitment of anti-cancer tumor-infiltrating lymphocytes (TIL), which destroy cancer cells [3]. In turn, the pro-cancer properties of chemokines are related to recruiting cells supporting tumor development [4], as well as increasing or causing the proliferation [5], migration, and invasion of cancer cells [6]. Very often, if not always, a given chemokine shows both pro- and anti-cancer properties, something we wished to emphasize in this paper. Therefore, the increased expression of a single chemokine is not always a clear indicator in establishing a patient prognosis in all cancers. The table below shows where the increased expression of a given chemokine improves the prognosis for one type of cancer or worsens the prognosis for patients with other cancers [7,8] (Table 1 and Table 2). There is no single CC chemokine that would worsen or improve the prognosis in all types of cancers.

Table 1.
Influence of increased expression of individual CC chemokines discussed in this review on the prognosis of patients with various cancers according to “The Human Protein Atlas”. (https://www.proteinatlas.org/) [7,8].

Table 2.
Effects of increased expression of individual CC chemokine receptors discussed in this review on the prognosis of patients with various cancers according to “The Human Protein Atlas” (https://www.proteinatlas.org/) [7,8].
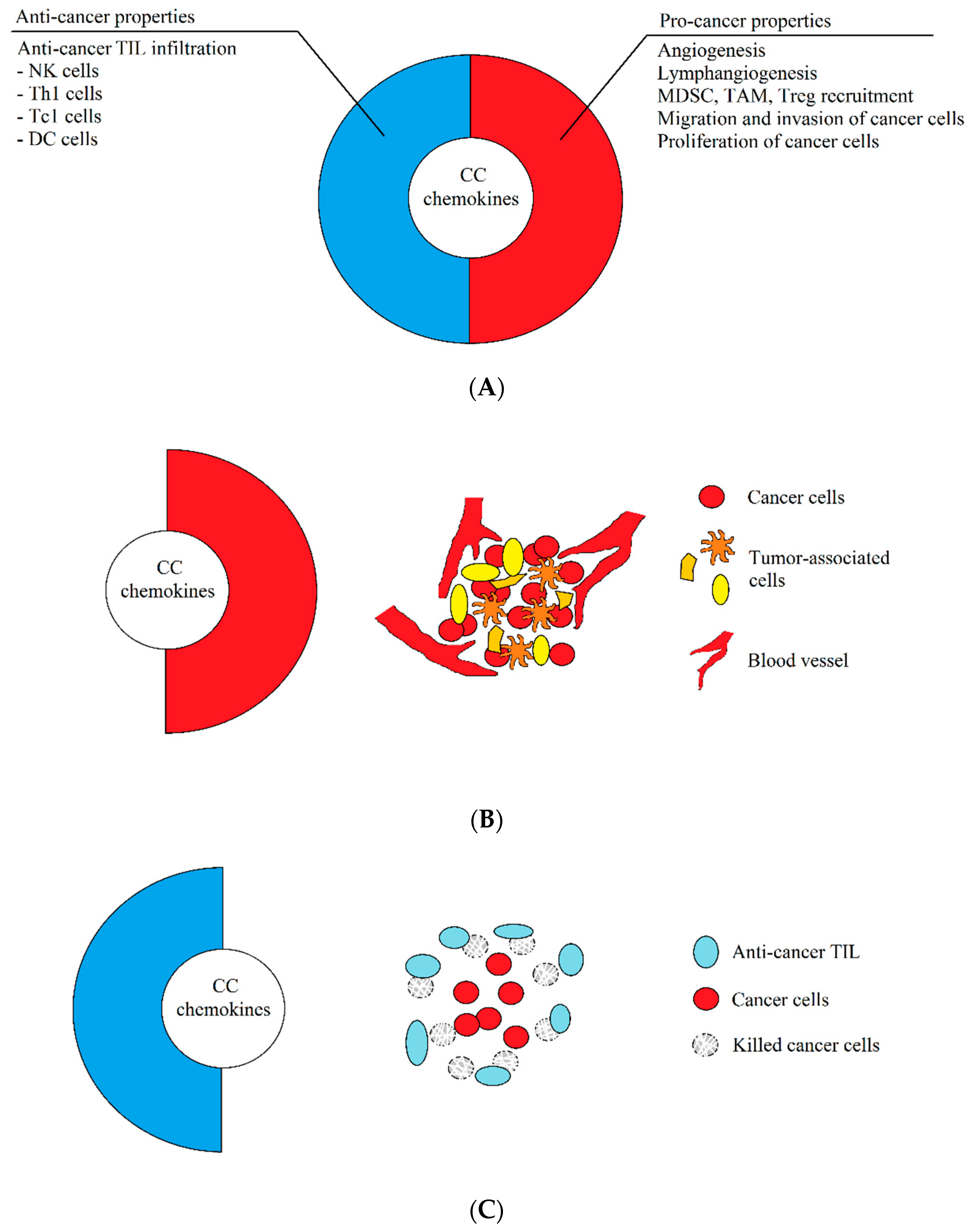
Another important premise of this review is the intratumor heterogeneity. A tumor is not a homogenous environment and consists of areas with different properties. The most significant is the area affected by chronic hypoxia [9], characterized by the accumulation of tumor-associated macrophages (TAM) [10,11,12,13], regulatory T cells (Treg) [14,15,16], and myeloid-derived suppressor cells (MDSC) [17,18]. The functions of these recruited pro-cancer cells in this microenvironment are enhanced by chronic hypoxia [11,19,20,21] and cancer acidification [22], which increases the resistance of cancer cells to anticancer therapy and the action of the immune system [20,22,23,24]. In such hypoxic areas, chemokines show only pro-cancer properties, despite their aforementioned dual nature. However, during the effective anti-cancer response of the immune system, the same chemokines will exhibit anti-cancer properties [3] (Figure 1).
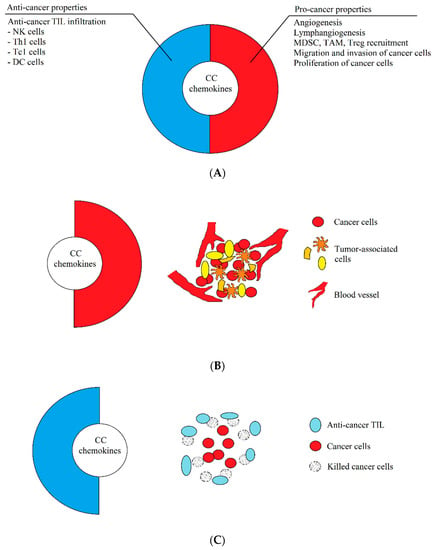
Figure 1.
The dual properties of CC chemokines. (A) Most, if not all, the chemokines described in this paper have both pro- and anti-cancer properties. The anti-cancer properties consist of the recruitment of anti-cancer tumor-infiltrating lymphocytes (TIL), which infiltrates the tumor and destroys tumor cells. The pro-cancer properties of chemokines, on the other hand, consist in causing angiogenesis and lymphangiogenesis, recruitment of pro-cancer cells supporting the development of the tumor, and the stimulation of proliferation, the induction of migration, and the invasion of cancer cells. (B) In a growing tumor, CC chemokines have enhanced pro-cancer properties, while anti-cancer properties are suppressed. As a result, these chemokines participate in the development of a tumor by causing angiogenesis, migration of tumor cells, and recruitment of cells supporting the development of a tumor, which results in the progress of cancer. (C) During immunotherapy or an effective anticancer response of the immune system, the same CC chemokines show enhanced anti-cancer properties, which result in the infiltration of a tumor by anti-cancer TIL, which destroy tumor cells. The immune system fights with the tumor, which often leads to recovery.
Knowledge of the anti-cancer and pro-cancer properties of individual chemokines allows a prediction of the consequences to then improve the effectiveness of anti-cancer therapies. One example is radiotherapy, which leads to an increased expression of certain chemokines, e.g., CCL2 and CCL5, resulting in the recruitment of TAM and Treg [25,26,27]. This has a pro-cancer effect and nullifies the therapeutic benefits of radiotherapy. On the other hand, the same chemokines have anti-cancer properties, because they infiltrate the tumor with anti-cancer TILs [3,28]. For this reason, radiotherapy may be more effective if used prior to immunotherapy [29]. Another application of knowledge presented in this paper is the use of gene therapy to enhance the expression of a given chemokine followed by immunotherapy [30,31,32,33] or chemotherapy [34]. As in the previous case, the increased expression of a chemokine may enhance the effectiveness of immunotherapy.
The role of chemokines in cancer has been the subject matter of a considerable number of papers. PubMed (https://pubmed.ncbi.nlm.nih.gov/) includes almost 22 thousand articles containing the words “chemokine” and “cancer” (title + abstract + keywords). However, the sheer number of papers makes it difficult to write a comprehensive and meaningful review. One solution is to focus on literature reviews that deal with individual chemokines, although one problem is that due to the fast advances in science they quickly become out-of-date. Another problem is the low attention paid in literature to less important chemokines and the emphasis on the best known chemokines. That is why, in this review, we address the significance of all human chemokines in cancer. Due to the considerable amount of data, we decided to discuss in this paper only CC chemokines that have been assigned to receptors: CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 (CCL1, CCL3, CCL4, CCL5, CCL18, CCL19, CCL20, CCL21, CCL25, CCL27, and CCL28) (Table 3 and Table 4).

Table 3.
Nomenclature for CC chemokines discussed in this review, including cells recruited into the tumor niche.

Table 4.
Receptors for CC chemokines described in this review and their respective ligands and functions in a tumor.
2. CCR5 Ligands
2.1. CCL5
Chemokine CCL5 (also known as regulated on activation, normally T cell expressed and secreted (RANTES)) is a ligand for receptors CCR5 [35,36], CCR3 [37,38,39], and CCR1 [1,2,40]. After secretion of this chemokine outside the cell, two amino acids are truncated from the N-terminus by CD26/dipeptidyl peptidase IV (DPPIV), resulting in an increased affinity to CCR5 and reduced affinity to CCR1 and CCR3 [41]. CCL5 is also a ligand for G-protein-coupled receptor 75 (GPR75), whose expression occurs on brain nerve cells [42,43]. The effect of CCL5 is mitigated by the atypical chemokine receptor 2 (ACKR2)/D6, which reduces the level of this chemokine [44,45,46].
Pro-inflammatory cytokines elevate CCL5 expression, which triggers the accumulation of various immune cells at inflammatory sites [47], e.g., contributing to asthma by CCR3–mediated chemotaxis of eosinophils [47]. The CCL5→CCR5 axis also induces the accumulation of and increases the cytotoxic properties of anti-cancer TIL in a tumor [28,30,48,49,50,51,52,53,54]. CCL5 is responsible for the infiltration of a tumor by NK cells [48,49,52,53], conventional type 1 dendritic cells [54], T helper cell type 1 (Th1) [28], and type 1 cytotoxic cells (Tc1) [22]. For this reason, some postulate that cancer therapies should combine immunotherapy with increasing the expression of CCL5 in the tumor to enhance the infiltration of the tumor by various immune cells [30,49,50]. On the other hand, the genome of Kaposi’s sarcoma-associated herpesvirus 8 contains viral macrophage-inflammatory protein-II (vMIP-II) [55,56,57]. Cells infected by this virus secrete vMIP-II, which acts as a CCR5 antagonist and therefore reduces the infiltration of the tumor by anti-cancer TIL. vMIP-II also induces the recruitment of Th2-type cells via CCR3 and CCR8, which decreases the activity of cytotoxic lymphocytes [58,59].
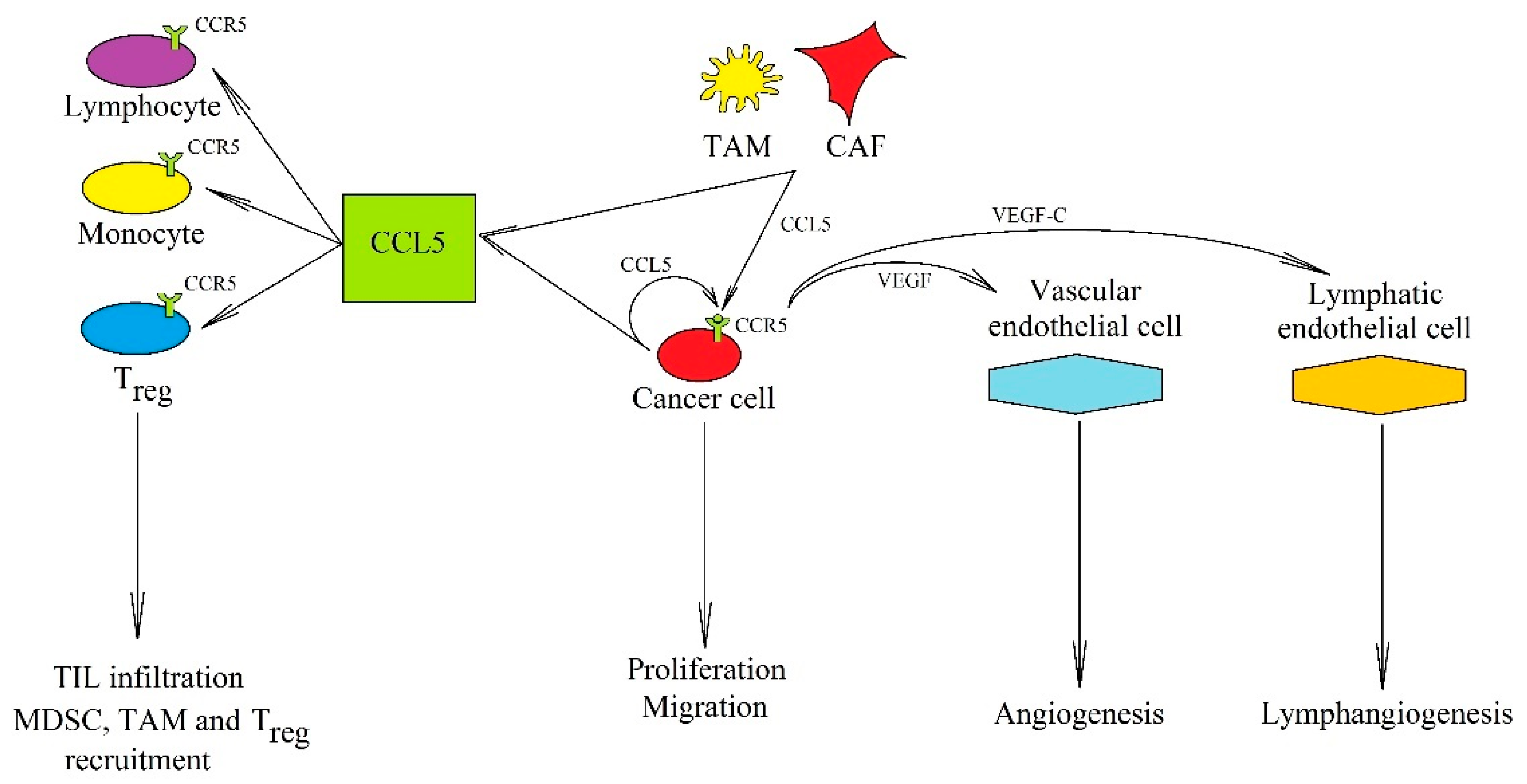
On the other hand, CCL5 has also several pro-cancer properties (Figure 2). Its expression is elevated in breast cancer [60], glioblastoma multiforme [61], and hepatocellular carcinoma [62]. In a tumor, CCL5 expression is found in cancer cells [63,64], cancer-associated fibroblasts (CAF) [65], mesenchymal stem cells (MSC) [66], MDSC [67], TAM [68,69,70,71], and TIL [72]. CCL5 expression also occurs in lymphatic endothelial cells, which is crucial for the formation of the metastatic niche [73].
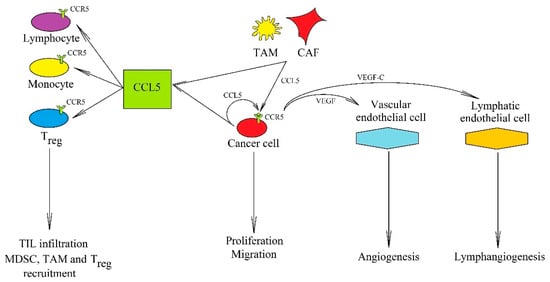
Figure 2.
The significance of the CCL5→CCR5 axis in cancer processes. In a tumor, CCL5 is secreted by tumor cells and also by TAM and CAF. Its anti-cancer properties consist of inducing cytotoxic TIL infiltration that increases the anticancer response of the immune system. Its pro-cancer effect is associated with (i) recruitment of cells participating in cancer immune evasion: MDSC, TAM, and Treg, (ii) induction of proliferation, migration, and invasion of cancer cells, and (iii) causing an increased production of VEGF, which leads to angiogenesis and lymphangiogenesis.
CCL5 acts on cancer cells by increasing their proliferation [62,64,74,75]. By activating the Wnt/β-catenin→signal transducer and activator of transcription 3 (STAT3) signaling pathways, it induces the self-renewal of prostate cancer stem cells [70]. Similar to other chemokines, CCL5 causes migration, invasion, and epithelial-to-mesenchymal transition (EMT) of cancer cells via its receptor [62,64,76,77], which is related to the activation of hedgehog [78], Wnt/β-catenin [70,79], and the Akt/PKB→nuclear factor κB (NF-κB) pathways [80,81,82]. The induction of migration by CCL5 is associated with increased secretion of matrix metalloproteinases (MMPs) by cancer cells [80,83,84]. In addition, CCL5 increases apoptosis resistance and drug resistance through the activation of Akt/PKB→NF-κB and STAT3 pathways [72,85,86,87]. It also causes an increase in programmed death-ligand 1 (PD-L1) expression on cancer cells, which protects them against cytotoxic lymphocytes [71].
CCL5 not only affects cancer cells but also tumor-associated cells. It recruits MDSC [88,89], MSC [90], TAM [77,91,92], and T-helper cells 17 (Th17) [93] into the tumor niche. Via CCR5, it participates in recruiting Treg and elevating its activity in the tumor niche [67,94,95,96]. CCL5 is secreted by diffuse large B cell lymphoma, which causes the recruitment of monocytes that increase the proliferation of those cancer cells [97]. In human papillomavirus (HPV) 16, E7 protein increases the expression of CCL5 [98]. This chemokine is responsible for recruiting mast cells and through these cell enhances the growth of a tumor.
CCL5 participates in angiogenesis, which is associated with an increase in vascular endothelial growth factor (VEGF) expression in cancer cells and vascular endothelial cells via the activation of CCR1 and CCR5 [99,100,101,102]. CCL5 may cause differentiation of cancer stem cells into endothelial cells as observed in the ovarian cancer model [103]. This chemokine is also induced by the Epstein–Barr virus (EBV) (human herpesvirus 4) in nasopharyngeal carcinoma cells, which leads to angiogenesis [104]. It may also indirectly cause lymphangiogenesis by increasing VEGF-C expression, as observed in chondrosarcoma cells [105].
2.2. CCL3 and CCL4
CCL3 (also known as macrophage inflammatory protein-1α, i.e., MIP-1α) and CCL4 (also known as macrophage inflammatory protein 1β, i.e., MIP-1β) are pro-inflammatory chemokines. In humans, there are also additional copy number variations (CNV) of CCL3 and CCL4 genes, known as CCL3L and CCL4L, which are produced by duplicating the genes of the corresponding chemokines [106,107]. The products of these additional genes have the same amino acid sequence as CCL3 and CCL4 and the same properties.
CCL3 is a ligand for receptors CCR1 and CCR5 [35,36], while CCL4 interacts with CCR5 [35,36], and with low affinity with CCR1 [40]. CCL3 and CCL4 are not ligands for CCR3 [38,39,108], but Combadiere et al. show that CCL3 and CCL4 are potent agonists for CCR3 [37]. Due to their anti-cancer properties, we decided to include them in the section on CCR5 ligands. Both these CC chemokines are important in the onset of the immune response [109]. They induce the recruitment of dendritic cells, neutrophils, monocytes, macrophages, NK cells, and T cells to inflammatory sites [109]. CCL3 is responsible for the correct function of CD8+ T cells and CCL4 acts on CD4+ T cells [110,111,112]. The increase in the number of immune cells, induced by CCL3 and CCL4, makes these chemokines a potentially important element of cancer immunotherapy [113,114,115].
In a tumor, an active immune response results in the production of CCL3 and CCL4 by B cells [116] and basophils [117]. The produced chemokines act as chemoattractants for anti-cancer TIL with CCR1 and CCR5 [115,118,119,120,121]. However, in a tumor both CCL3 and CCL4 may be cleaved by cathepsin D [122] and chemokine decoy receptor ACKR2/D6 [2,123], which suppresses the anti-cancer effect of these two CC chemokines.
On the other hand, CCL3 and CCL4 also have pro-cancer effects. Their expression has been found in tumor-associated and cancer cells. In a tumor, they are produced by TAM [69,124,125,126,127], MDSC [68], and MSC [66], which promotes tumor growth. The expression of CCL4 may also occur in tumor-associated neutrophils (TAN) [128]. CCL3 may be expressed in a cancer cell [127]. The immune response reduces the expression of CCL3 in cancer cells via the activation of β-catenin [129]. Both CCL3 and CCL4 are also expressed in chronic myeloid leukemia and multiple myeloma [130,131,132,133]. CCL3 and CCL4 lead to the inhibition of normal osteoblast function and an increase in osteoclast activity and thus to bone destruction [130,134,135,136]. In addition, the production of CCL3 by acute myeloid leukemia cells causes severe anemia by inhibiting erythropoiesis [137]. In bone marrow, hypoxia induces an increase in CCR1 expression on multiple myeloma cells [138]. The activation of this receptor by CCL3 affects the function of CXC motif chemokine receptor 4 (CXCR4), which lead to the egress of those cells to the blood. The elevated level of CCL3 in serum is associated with a worse prognosis for patients with diffuse large B cell lymphoma [139], extranodal NK/T-cell lymphoma [140], and multiple myeloma [141].
Both chemokines can cause Treg recruitment via CCR1 and CCR5. CCL3 causes the recruitment of Treg to the leukemic hematopoietic microenvironment, which promotes the development of acute myeloid leukemia [142]. CCL4 also induces the recruitment of Treg to the tumor niche, as shown in melanoma [67]. Both CCL3 and CCL4 cause the recruitment of MDSC into the tumor niche via CCR5 [143,144].
There are no reports on the recruitment of TAM by CCL3 or CCL4; they support the anticancer functions of monocytes [145], so they will not recruit TAM but will be more responsible for the infiltration of anti-cancer M1 macrophages. In a hepatocellular carcinoma model, CCL3—via CCR1—causes the recruitment of Kupffer cells, resident macrophages in the liver [146]. In addition, CCL3 [147] and CCL4 [148] cause the recruitment of CAF via CCR5, which is of great importance for the functioning of a tumor and the initial stages of metastatic niche formation in bones.
Although CCL3 and CCL4 have an anti-cancer effect by causing cytotoxic TIL infiltration to the tumor, they can support the development of the tumor if they act directly on a cancer cell. For example, CCL3 increases cancer cell proliferation [144]. In multiple myeloma, this chemokine causes drug resistance by activating the PI3K→Akt/PKB→mTOR and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathways [133]. In acute lymphoblastic leukemia, anticancer drugs increase the expression of CCL3 and CCL4, which leads to drug resistance of these cells [149].
CCL3 causes migration and invasion of cancer cells via CCR5 [124,127,144]. This is related, among other things, to the activation of PI3K→Akt/PKB and ERK MAPK pathways [127]. Both CCL3 and CCL4 may also participate in metastasis. CCL3 is secreted by the tumor, which causes an increase in CCL2, CCL7, and CCL8 expression in the lungs and brain [150], which facilitates the metastasis to these organs. After getting to the blood, a cancer cell is encased in platelets, which protects it against NK cells [151]. Platelets also secrete many factors, including CCL3, which support the cancer cell [152]. After reaching another organ, CCL3 participates in the formation of the metastatic niche. The cancer cell recruits macrophages through the CCL2→CCR2 axis [153]; these macrophages then secrete CCL3, which causes the retention of these cells in the metastatic niche in an autocrine manner via CCR1. In bones, on the other hand, there is an increase in the expression of CCL3 in bone-marrow-derived monocytes caused by the secretion of epidermal growth factor (EGF) by cancer cells. This causes the differentiation of these monocytes into osteoclasts [154], which leads to bone remodeling around the forming metastases.
CCL3 and CCL4 also indirectly cause angiogenesis. CCL3 [127,155] and CCL4 [156] cause increased expression of VEGF in a cancer cell, a growth factor causing angiogenesis. CCL4 also increases the expression of VEGF-C in a cancer cell, which leads to lymphangiogenesis and lymph node metastasis [157].
3. CCR6 and CCL20
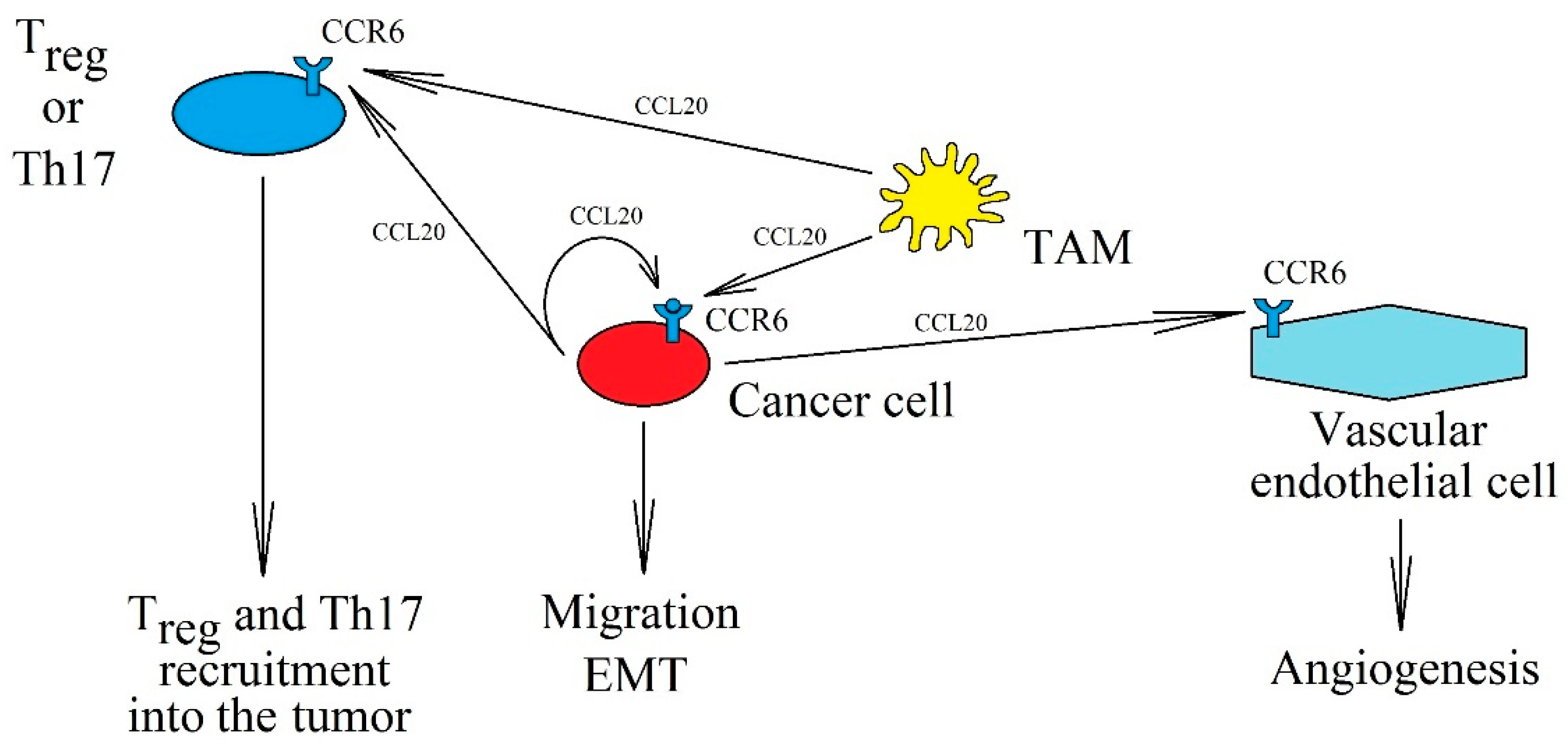
CCL20 (also known as liver activation regulated chemokine (LARC) or macrophage inflammatory protein-3α (MIP-3α) or Exodus-1) is a proinflammatory chemokine; a ligand for CCR6 [1,2,158,159]. This makes it significant for the correct function of dendritic cells, T cells, and B cells—cells with expression of this receptor [159]. CCL20 is produced by Th17 cells and is thus responsible for the function of these lymphocytes [160]. In addition, CCL20 also plays an important role in neoplastic processes (Figure 3).
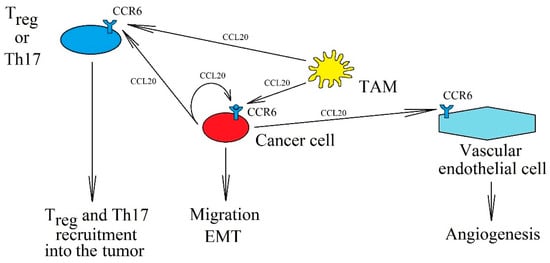
Figure 3.
The significance of the CCL20→CCR6 axis in cancer processes. CCL20 is expressed in tumor cells and TAM, which can activate CCR6 on cancer cells in an autocrine manner, causing their migration and EMT. In addition, increased concentration of CCL20 in the cancer microenvironment recruits Treg and Th17 into the tumor niche and causes angiogenesis via CCR6.
CCL20 expression is elevated in many cancers, such as breast cancer [60], hepatocellular carcinoma [161], and pancreatic cancer [162,163]. While its expression occurs in cancer cells [63,162], TAM are also a significant source of this chemokine in the tumor [162,164,165]. CCR6 expression has also been shown on tumor cells. Thus, in some cancers, the cells can stimulate their own proliferation and migration in an autocrine manner via CCL20→CCR6 [63,166,167,168]. At the same time, CCL20 directly causes angiogenesis by activating CCR6 on vascular endothelial cells [169]. CCR6 activation may also increase the expression of VEGF in cancer cells, which contributes to angiogenesis [170].
The main primary function of CCL20 in a tumor is to recruit Treg and Th17 for the tumor niche [171,172,173,174]. This leads to tumor immune evasion. The CCL20 also recruits TAMs [175] through CCR6. This chemokine is also responsible for recruiting dendritic cells, which increases the anticancer response of the immune system [176]. However, it seems that the impact of cancer immune evasion is stronger than this process.
In addition to recruiting cells to a tumor niche, CCL20 increases cancer cell proliferation [166,177], causes cancer cell migration and invasion [167,177,178,179], and induces EMT of cancer cells [165,180,181]. It also participates in organ-specific patterns of metastasis—high expression of CCL20 in the liver results in the metastasis of tumor cells with high expression of CCR6 to this organ [182,183,184]. CCR6 is also important in lung metastasis in breast cancer patients [185] and adrenal metastasis in lung cancer patients [186]. However, in the adrenal gland and lungs, CCL20 expression is low [187]. This indicates that this chemokine can only participate in the induction of cell migration into the bloodstream. The expression of CCR6 on B-cell non-Hodgkin’s lymphomas leads to the localization of those cells at mucosal sites [188]. The CCL20→CCR6 axis is also significant in osteolytic bone lesions caused by multiple myeloma [189].
4. CCR7, CCL19, and CCL21
The most important physiological functions of CCL19 (also known as EBI1 ligand chemokine (ELC) or macrophage inflammatory protein-3 (MIP-3) or Exodus-3) and CCL21 (also known as secondary lymphoid tissue chemokine (SLC) or 6Ckine or Exodus-2) include the homing of T cells to a lymph node [190,191,192,193,194]. This process is dependent on the receptor of these chemokines: CCR7 [2,190]. Therefore, the increased expression of these chemokines in a tumor has an anti-cancer effect via cytotoxic TIL [195,196,197,198].
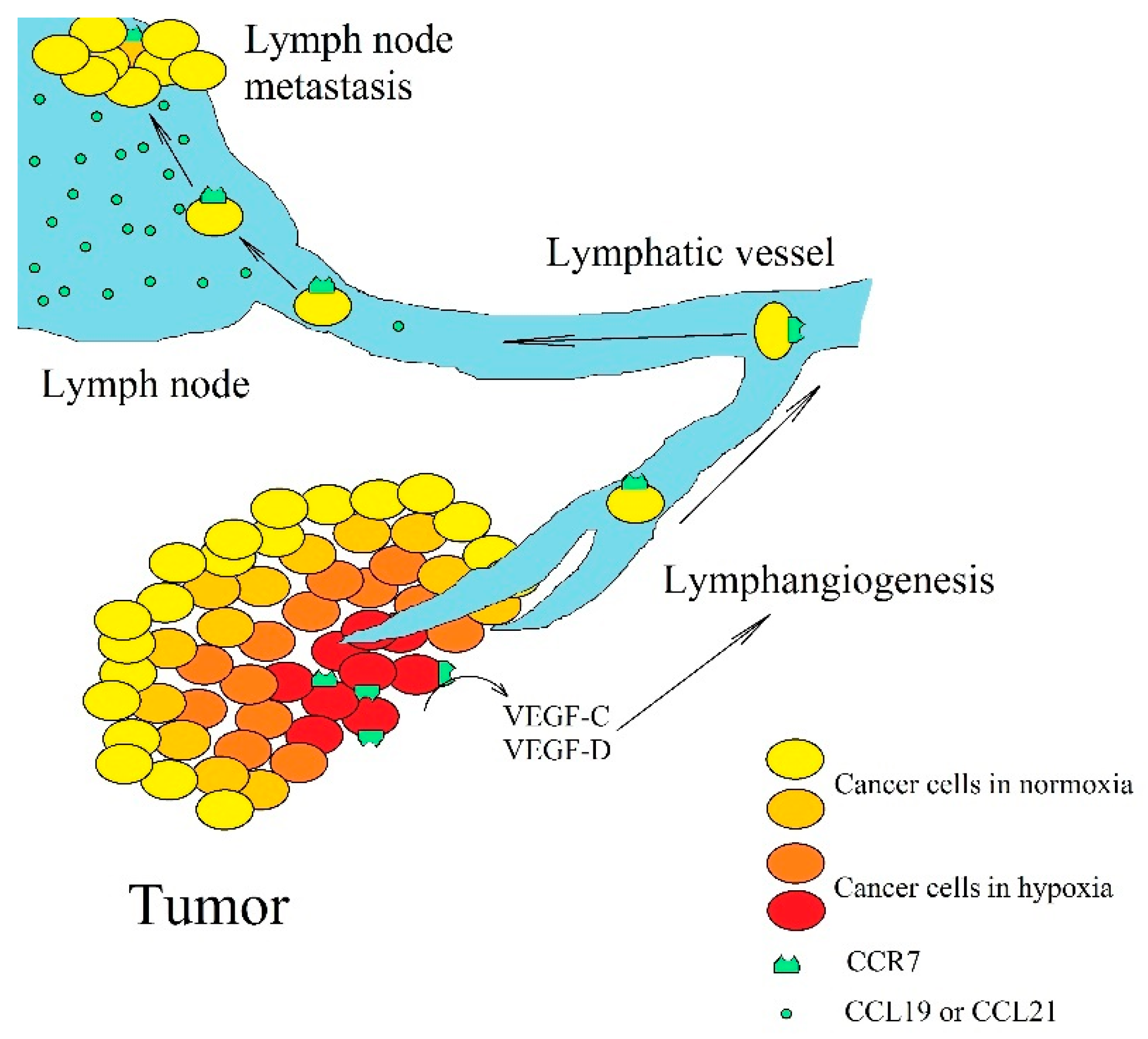
However, this axis may also have pro-cancer properties if CCR7 is located on a cancer cell. Hypoxia [199,200,201] and prostaglandin E2 (PGE2) [202,203] increases the expression of CCR7 on a cancer cell (Figure 4). CCR7 enhances proliferation [204,205,206] and stemness of cancer cells [207,208,209]. The activation of CCR7 increases angiogenesis by activating NF-κB and thus increases VEGF-A expression in esophageal squamous carcinoma cells [210]. In contrast, in colorectal cancer cells, CCL19 inhibits angiogenesis by increasing the expression of miR-206, which inhibits the ERK MAPK→HIF-1→VEGF-A pathway [211]. Activation of CCR7 also increases the expression of VEGF-C and VEGF-D in non-small-cell lung cancer cells [210], head and neck cancer cells [212], and esophageal squamous carcinoma cells [213]. These are growth factors responsible for lymphangiogenesis. The activation of CCR7 on a cancer cell causes EMT and migration of cancer cells [199,200,204,205,206,209]. In blood and lymphatic vessels CCR7 on cancer cells prevents anoikis [205,214]. In lymphangiogenesis, cancer cells penetrate lymphatic vessels where a cancer cell with CCR7 expression is kept in a lymph node [215,216,217,218,219,220,221], which is related to the high expression of a ligands for this receptor [190,191,192,194] in these peripheral lymphoid organs. In addition, some leukemias, such as B cell chronic lymphocytic leukemia but not multiple myeloma cells, show CCR7 expression [222]. This leads to the homing of those cells to secondary lymphoid tissues. CCR7 is also significant in the dissemination of non-Hodgkin’s lymphoma [223]. In leukemias, the expression of CCR7 is a significant prognostic factor. Higher expression is associated with worse prognosis for patients with diffuse large B-cell lymphoma [224]. Metastasis to lymph nodes is not only associated with the described mechanism but also with the high expression of CCL1 in a lymph node combined with the expression of CCR8 on a cancer cell [225]. This process is crucial for the metastasis of malignant melanoma to a lymph node. CCR7 expression has also been associated with skin metastasis in patients with breast cancer [185].
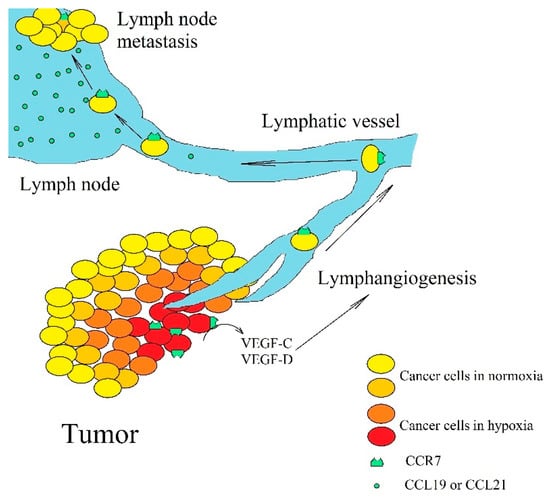
Figure 4.
The significance of CCR7 expression in lymph node metastasis. Hypoxia increases CCR7 expression on cancer cells, which leads to an increase in VEGF-C and VEGF-D expression, which in turn, leads to lymphangiogenesis. Then, cancer cells enter lymphatic vessels where they migrate to lymph nodes. As lymph nodes have a high expression of CCL19 and CCL21, cancer cells with CCR7 expression become trapped and form metastasis there.
In addition to the effect on a cancer cell, the described axis CCL19/CCL21→CCR7 also affects the cellular composition of a tumor. Increased expression of CCL19 and CCL21 in a tumor results in anti-cancer TIL infiltration and an improved prognosis for many tumor patients [195,196,197,198]. Cancer immune evasion mechanisms reduce the expression of these chemokines in a cancer cell [129]. Nevertheless, if the tumor microenvironment has favorable conditions for tumor development, then CCL19 and CCL21 may participate in Treg recruitment to the tumor niche [226,227]. Treg are cells involved in cancer immune evasion and so their recruitment to a tumor niche inhibits the correct anti-cancer response of the immune system.
5. CCR8
5.1. CCL1
Chemokine CCL1(also known as I-309) was first identified as a cytokine secreted by activated human T lymphocytes [228]. CCL1 is a ligand for just one receptor, CCR8 [1,2,229], and is considered a Th2-related cytokine due to the expression of the CCR8 receptor on Th2 cells, but not on Th1 cells [230]. Therefore, this chemokine plays an important role in the pathogenesis of asthma [231]. The CCL1→CCR8 axis also plays an important role in the homing of lymphocytes to the healthy skin and therefore plays an important role in the physiology of this tissue [232,233].
Elevated expression of CCL1 in a cancer cell occurs in leukemia caused by viruses, e.g., human T cell leukemia virus type 1 (HTLV-1) [234,235]. Properties similar to this chemokine are also shown by viral macrophage inflammatory protein-I (vMIP-I) (homolog to CCL1)—a viral protein expressed in tumor cells transformed by the herpes virus [236]. Another example is Kaposi sarcoma-related human herpes virus-8 [237,238]. In addition, encoded in by the genome of that virus, vMIP-II leads to the recruitment of Th2-type cells via CCR3 and CCR8, which reduces the activity of cytotoxic lymphocytes [58]. In breast cancer, however, the expression of CCL1 is no different than in healthy tissue [60]. In hepatoma cells, it is absent or very low [239]. In gliomas, on the other hand, it is even lower than in the brain tissue [61]. In hepatocellular carcinomas, the expression of CCL1 is elevated in tumor stroma and peritumoral tissue [240]. In solid tumors, CCL1 is produced by CAF [241,242], TAM [243], CCR8-CD11b+ myeloid cells [244], and Treg [245]. The expression of this chemokine has also been demonstrated in breast cancer stem cells [246], bladder cancer tumors [244,247], and renal cell carcinomas [244].
In cancer, CCL1 has an antiapoptotic activity and induces chemoresistance to anticancer drugs due to the activation of the ERK MAPK cascade via CCR8 (Figure 5) [234,236,242,248,249]. This has a special significance in apoptosis resistance adult T-cell leukemia [234] and murine T cell lymphomas [248]. CCL1 also stimulates proliferation [247] and causes the migration of bladder cancer cells [241]. At the same time, due to the expression of CCL1 in lymph nodes, this chemokine participates in metastasis to these peripheral lymphoid organ through the CCR8 receptor on cancer cells that have entered lymphatic vessels [225]. This process is important in metastasis to lymph nodes in malignant melanoma, which often leads to increased CCR8 expression in the cancer cells of this tumor [225]. However, the CCL1→CCR8 axis is not the only molecular route of metastasis to lymph nodes. In this process, CCL19 and CCL21 acting on CCR7 are also significant [215,216,217,218,219,220,221].
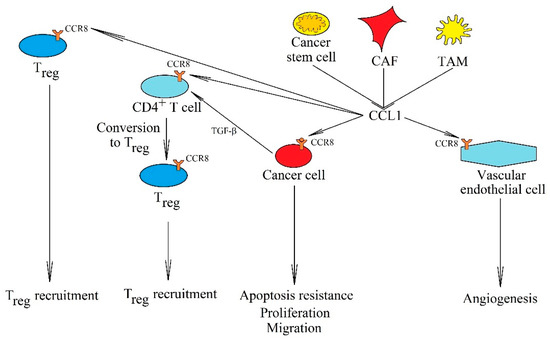
Figure 5.
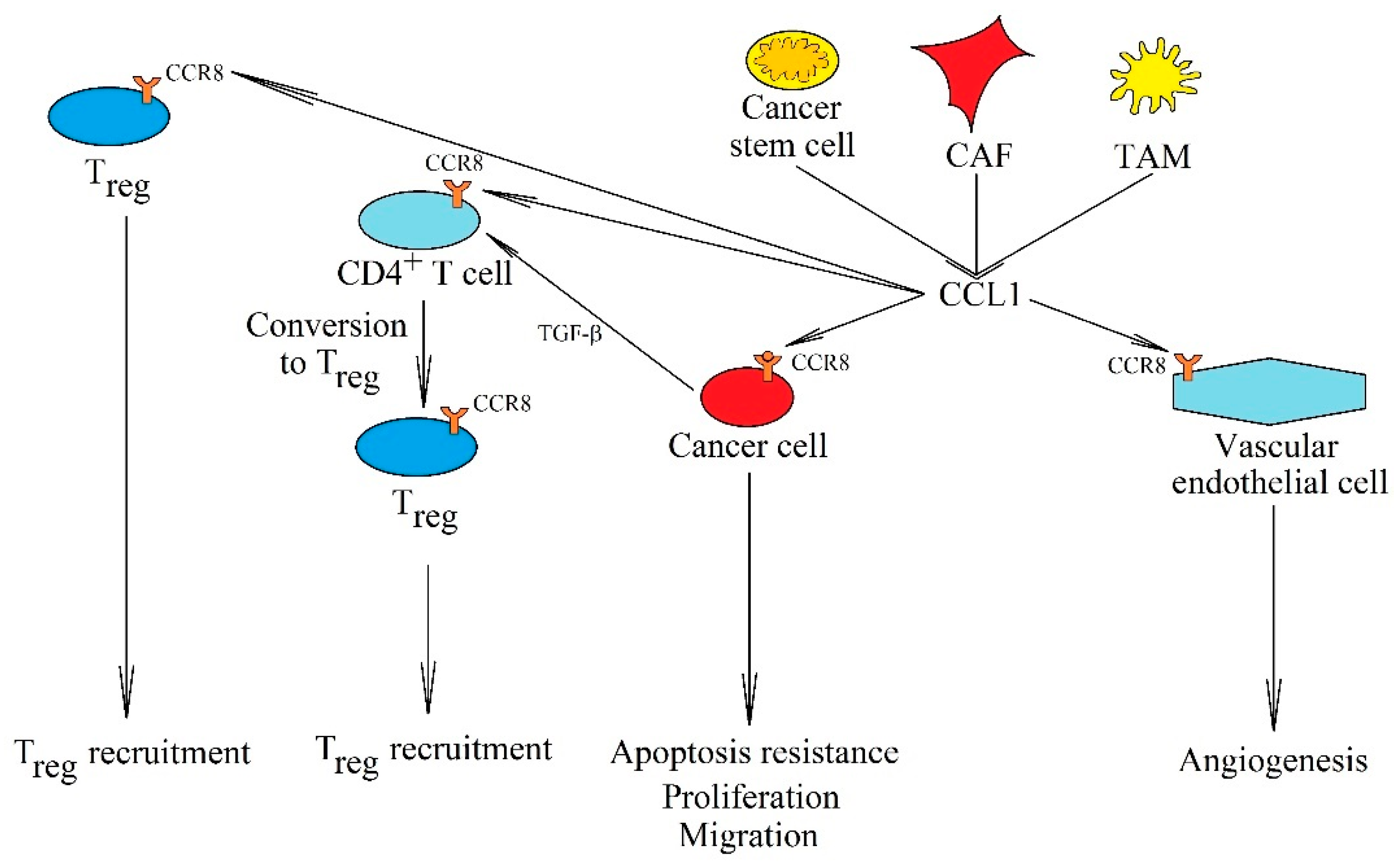
The significance of the CCL1→CCR8 axis in cancer processes. CCL1 is secreted into the cancer microenvironment by cancer stem cells, CAF and TAM. This chemokine has many primary tumor functions. By activating the CCR8 receptor on cancer cells, it causes their proliferation, apoptosis resistance, and migration. It also causes angiogenesis by activating its CCR8 receptor on endothelial cells. Another important function is the recruitment of Treg into the tumor niche and causing the conversion of CD4+ T cells into Treg.
In addition to the effect on a cancer cell, CCL1 causes angiogenesis on vascular endothelial cells via CCR8 [238,250]. It is involved in the recruitment of CCR8-CD11b+ myeloid cells [244] and Treg [246,251,252] to a tumor niche. However, CCL1 can also participate in the conversion of CD4+ T cells into Treg [245], in a process dependent on transforming growth factor-β (TGF-β), which increases the expression of CCL1 in CD4+ T cells. This is followed by an autocrine conversion of this cell to Treg, which also involves CCL1. In addition, CCL1 supports the immunosuppressive function of Treg in a tumor niche [245], which is crucial for the interaction of cancer stem cells and CAF with Treg [241,242,246]. Finally, CCL1 increases the expression of interleukin 6 (IL-6) in MDSC, which acts as a proinflammatory in the cancer microenvironment [244].
5.2. CCL18
CCL18 (also known as pulmonary and activation regulated chemokine (PARC), alternative macrophage activation-associated C-C chemokine-1 (AMAC-1), dendritic cell-derived C-C chemokine 1 (DC-CK1), and macrophage inflammatory protein 4 (MIP-4)) is produced by dendritic cells, especially in germinal centers of regional lymph nodes. This chemokine then causes the chemoattraction of naïve T cells to these cells [253,254,255]. This leads to the initiation of an immunological response. CCL18 also acts as an anti-inflammatory chemokine, a marker for the macrophage M2 subset [256].
In a tumor, CCL18 is mainly produced by TAM (Figure 6) [69,257,258]. This chemokine causes cancer cell migration and EMT by activating its receptors, phosphatidylinositol transfer protein 3 (PITPNM3) and CCR8 [2,259,260,261,262,263]. CCL18 also increases the expression of cancer stem cell markers [264], is involved in angiogenesis by acting on vascular endothelial cells via PITPNM3 [265], and affects non-cancer cells in the tumor niche. Acting as an immunosuppressive cytokine, it causes polarization of macrophages to phenotype M2 [256] and recruits naïve CD4+ T cells into the tumor niche via its receptor PITPNM3 [266], which then differentiate into Treg cells responsible for tumor immune evasion. CCL18 recruits immature dendritic cells to the tumor niche [267]. In addition, this chemokine participates in the differentiation of immature dendritic cells into tumor-associated dendritic cells (TADC) [268,269]. Other functions of CCL18 in the tumor include participation in the intercellular communication dependent on extracellular vesicles [270]. This chemokine binds to glycosaminoglycans on extracellular vesicles, which allows them to be retained on cells with an expression of CCR8, a receptor for CCL18. CCL18 also increases the proliferation of cancer cells, but this effect depends on the type of tumor. For example, CCL18 decreases the proliferation of acute lymphocytic leukemia B cells [271] and cutaneous T-cell lymphoma (CTCL) [272] in a process dependent on GPR30 that affects the activity of CXCR4 [271]. Increased expression of CCL18 in lesional skin and serum of patients with CTCL [273] and in patients with diffuse large B cell lymphoma [274] is associated with a worse prognosis. In addition, CCL18 decreases the proliferation of non-small-cell lung cancer cells [67] and increases the proliferation of glioma cells [275] and oral squamous cell carcinoma cells [276].
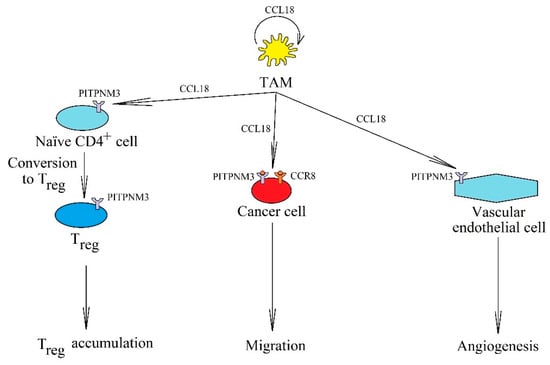
Figure 6.
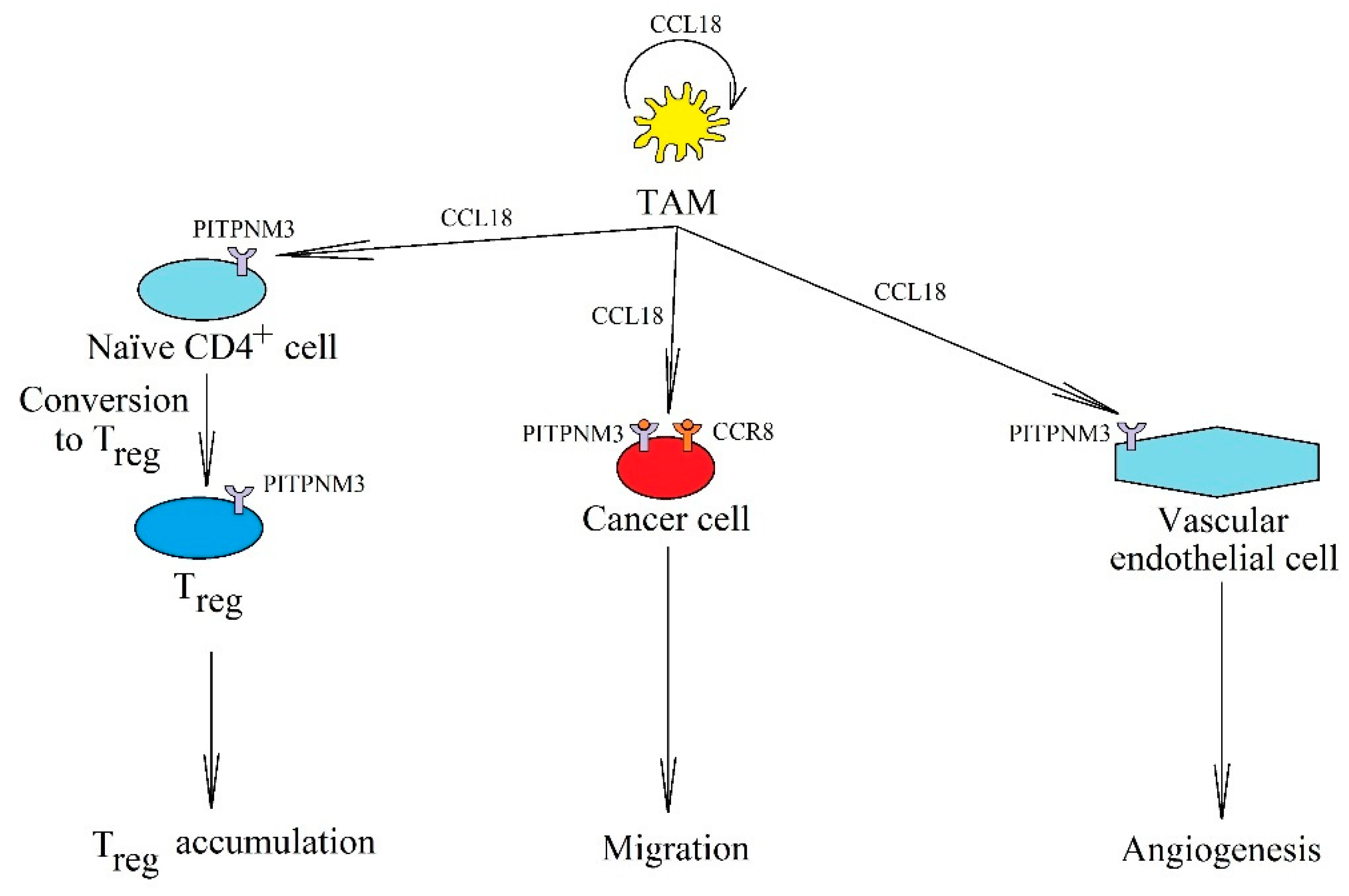
The significance of CCL18 in cancer processes. In a tumor, it is produced and secreted by TAM, where it acts on these cells in an autocrine manner. It also causes cancer cell migration and invasion via receptors PITPNM3 and CCR8. CCL18 is important in angiogenesis by acting on PITPNM3 on endothelial cells. It also causes the recruitment of naïve CD4+ T cells into the tumor niche.
6. CCR9 and CCL25
CCL25 (also known as thymus-expressed chemokine (TECK)) is a ligand for just one receptor: CCR9 [1,2,277,278]. This chemokine is important for the correct function of the thymus [279,280]. Due to the expression of CCL25 in the gastrointestinal tract, lymphocyte homing to the tissues of this system is one of the main functions of the CCL25→CCR9 axis [281,282,283]. This is of great importance in the immunological functions of the intestinal and gastric mucosa. However, this function of CCL25 results in intestinal metastasis of cancer cells with CCR9 expression [284,285]. The CCL25→CCR9 axis is also significant in the homing of diffuse large B-cell lymphoma and follicular lymphoma to the gastrointestinal tract [286].
In a tumor, CCL25 is produced by cancer cells, such as breast cancer cells [287] and pancreatic cancer cells [288], and also by cancer-related cells, e.g., pancreatic stellate cells in pancreatic cancer [289]. CCL25 participates in the migration and invasion of cancer cells by causing an increase in metalloproteinase expression [290,291,292]. It also causes EMT of cancer cells [287]. However, in colon cancer, CCR9 activation inhibits cancer cell migration [293]. The activation of CCR9 is also associated with apoptosis resistance and drug resistance, which is related to Akt/PKB→PI3K pathway activation [294,295,296]. Additionally, the CCL25→CCR9 axis stimulates tumor cell proliferation [288].
There are no data on the influence of CCL25 on the recruitment of cells cooperating in the development of a tumor or its participation in angiogenesis [297]. However, this chemokine may cause infiltration of the tumor by cytotoxic TIL exhibiting CCR9 expression, which has an anticancer effect [298]. There are also indications that CCL25 causes lymphangiogenesis because activation of the CCL25→CCR9 axis increases the expression of VEGF-C and VEGF-D on the non-small-cell lung cancer cells [292]. CCL25 can also recruit MDSC into a tumor niche, as indicated in a study by Sun et al. on endometriosis [299].
7. CCR10, CCL28, and CCL27
CCL28 (also known as mucosae-associated epithelial chemokine (MEC)) is a chemokine essential for normal mucosal immune function [300,301]. It activates two receptors: CCR3 [302] and CCR10 [303]. Another chemokine that activates CCR10 is CCL27 (also known as ESkine) [2,304,305], and for this reason, its effects are similar to those of CCL28. CCL27 causes CD3+ and CD4+ lymphocyte migration [306]. CCL28 and CCL27 cause B cell and T cell migration via CCR10, especially IgA plasma blasts [300,301,307]. CCL27 is produced by keratinocytes and therefore acts mainly in the skin [301], whereas CCL28 is produced in mucosal tissues [301]. For this reason, these chemokines are important in the homing of immune system cells to mucosal and epithelial tissues and thus participate in immunological reactions against microorganisms. CCL28 also causes eosinophil migration via CCR3 [302], which is associated with the development of allergies.
CCL27 also has an intracellular function. The CCL27 gene, through alternative splicing, creates—in addition to CCL27—PESKY protein [306,308,309], a nuclear protein with expression in the eye, brain, and testes. This protein alters the expression of genes associated with the actin cytoskeleton, which leads to cell migration and changes in cell morphology [308,310]. CCL27 also contains a nuclear location sequence—after internalization of the CCR10 receptor with this chemokine, CCL27 is transported to the cell nucleus where it has a similar function to PESKY [308].
Expression of CCL28 is down-regulated in breast cancer [311], colon cancer [312], and multiforme glioblastoma [61]. In endometrial cancer [313], basal cell carcinoma [314], and squamous cell carcinoma [314], the expression of CCL27 is reduced. This suggests that these chemokines have an anticancer effect, at least in the early stages of tumor development. This is confirmed by studies on the survival of patients with different types of breast cancer [315]. In patients with luminal-like breast cancer, elevated concentrations of CCL28 in the tumor improve prognosis, in contrast to triple-negative breast cancer [315]. In turn, elevated CCL27 expression improves the prognosis in patients with cutaneous malignant melanoma [316]. CCL28 and CCL27 participate in the anticancer response of the immune system, causing an infiltration of the tumor by anticancer NK cells, which leads to improved prognosis with an increased expression of these chemokines in the tumor [313,317,318,319]. For this reason, gene therapies that increase the expression of these two chemokines have an anticancer effect [317,318,320].
However, numerous in vitro studies show that CCL28 and CCL27 support tumor development (Figure 7). For this reason, some researchers suggest that CCL28 can act locally, especially in the hypoxic regions of the tumor [14,16,17,321,322]. CCL28 and CCL27 stimulate proliferation and have anti-apoptotic effects on cancer cells [323,324,325]. In vitro experiments also show that CCL28 reduces migration and EMT in oral squamous cell carcinoma [326], but the effect on migration varies among other types of cancer. In hepatocellular carcinoma, CCL28 increases the migration of these cells [327]. The same activation of CCR10 by both the ligands causes the migration of breast cancer cells [325,328] and glioblastoma multiforme cells [324]. After entering the bloodstream, a cancer cell is trapped in organs and tissues, which have a high expression of chemokines for the receptors on the cancer cell. Due to the production of CCL28 and CCL27 in the skin, the expression of CCR10 on a cancer cell increases the probability of skin metastasis [329].
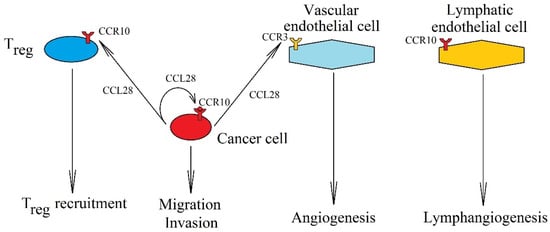
Figure 7.
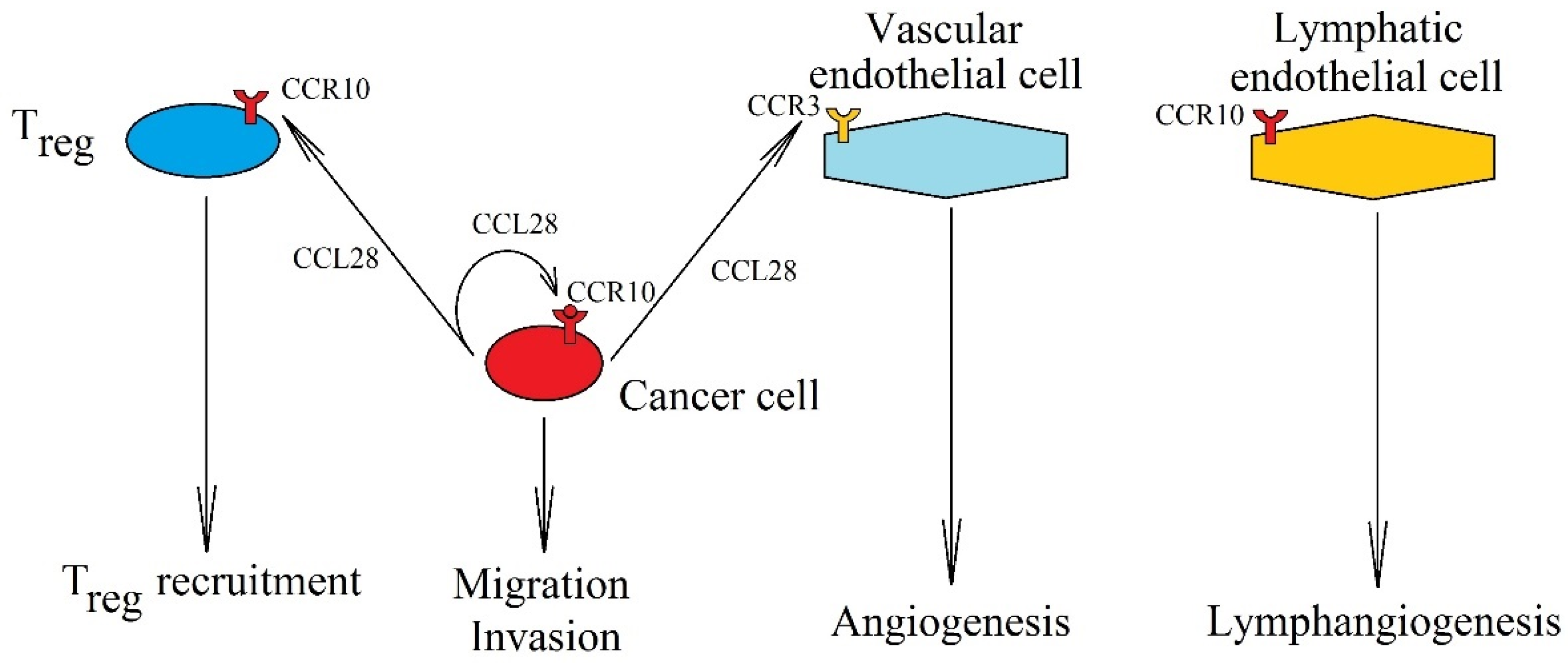
Significance of CCL28 expression increase in cancer processes. Expression of CCL28 in a cancer cell causes the migration and invasion of cancer cells via CCR10. CCL28 is also involved in Treg recruitment into the tumor niche by interacting with receptor CCR10 on these cells. It also causes vascularization of the tumor, causing angiogenesis by activating CCR3 on endothelial cells and lymphangiogenesis by activating CCR10 on lymphatic endothelial cells.
In addition to its effect on cancer cells, CCL28 also acts on non-cancer cells in a tumor niche. In particular, it participates in the recruitment of Treg [14,16], and in pancreatic ductal adenocarcinoma, in the recruitment of cancer-associated stellate cells [330] caused by the expression of CCR10 on these cells. The CCL27→CCR10 axis has also been shown to be involved in the recruitment of Th22 to malignant ascites; even though no effect of an increased number of these cells on patient prognosis has been demonstrated [331], Th22 may have a pro-cancer effect, as shown in a study on colorectal cancer [332]. In turn, CCL28 causes angiogenesis by activating CCR3 on vascular endothelial cells [322]. Both chemokines, CCL28 and CCL27, cause lymphangiogenesis by activating CCR10 on lymphatic endothelial cells [333].
8. Further Direction in the Research on the Role of CC Chemokines in Cancer Processes
The role of individual chemokines in neoplastic processes is very well known. They cause the recruitment of various cells into the cancer niche and cause cancer cell migration and invasion. However, little is known about the interactions between cells in a tumor, particularly the direct or indirect influence of cancer cells on non-cancer cells and the interactions between non-cancer cells. Especially interesting are cancer-cell-induced changes in the expression of chemokines produced by cancer-associated cells, e.g., by TAM [68,69,70,71,257,258], MDSC [67], and CAF [65]. In a tumor, cancer cells are not isolated but interact with cancer-associated cells, and that is why these cells (e.g., TAM, TIL, MDSC, CAF) should be investigated more often to discover new mechanisms in a tumor. Unfortunately, there are few research tools that can be used to show the interactions between cancer-associated cells and the actual cancer cells. The most notable are the co-culture of cancer cells and cancer-associated cells [334] and the use of a conditioned medium from cancer cells to culture non-cancer cells [335,336]. The understanding of those interactions could help foster new therapeutic approaches if the discovered mechanisms are universal in one type of tumor or even common for all neoplastic diseases.
Author Contributions
J.K.: writing—original draft preparation; S.G.: investigation; I.G.: investigation; K.B.: investigation; I.B.-B.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry Pomeranian Medical University in Szczecin, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CAF | Cancer-associated fibroblasts |
| CCL | CC motif chemokine ligand |
| CCR | CC motif chemokine receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| MDSC | Myeloid-derived suppressor cells |
| NF-κB | Nuclear factor κB |
| NK | Natural killers |
| TAM | Tumor-associated macrophages |
| Th17 | T-helper cells 17 |
| TIL | Tumor-infiltrating lymphocytes |
| Treg | Regulatory T cells |
| VEGF | Vascular endothelial growth factor |
References
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Lança, T.; Costa, M.F.; Gonçalves-Sousa, N.; Rei, M.; Grosso, A.R.; Penido, C.; Silva-Santos, B. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J. Immunol. 2013, 190, 6673–6680. [Google Scholar] [CrossRef] [PubMed]
- Low-Marchelli, J.M.; Ardi, V.C.; Vizcarra, E.A.; van Rooijen, N.; Quigley, J.P.; Yang, J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013, 73, 662–671. [Google Scholar] [CrossRef]
- Mou, T.; Xie, F.; Zhong, P.; Hua, H.; Lai, L.; Yang, Q.; Wang, J. MiR-345-5p functions as a tumor suppressor in pancreatic cancer by directly targeting CCL8. Biomed. Pharmacother. 2019, 111, 891–900. [Google Scholar] [CrossRef]
- Dagouassat, M.; Suffee, N.; Hlawaty, H.; Haddad, O.; Charni, F.; Laguillier, C.; Vassy, R.; Martin, L.; Schischmanoff, P.O.; Gattegno, L.; et al. Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int. J. Cancer 2010, 126, 1095–1108. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Daniel, S.K.; Sullivan, K.M.; Labadie, K.P.; Pillarisetty, V.G. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin. Transl. Med. 2019, 8, 10. [Google Scholar] [CrossRef]
- Wang, S.C.; Hong, J.H.; Hsueh, C.; Chiang, C.S. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab. Investig. 2012, 92, 151–162. [Google Scholar] [CrossRef]
- Tripathi, C.; Tewari, B.N.; Kanchan, R.K.; Baghel, K.S.; Nautiyal, N.; Shrivastava, R.; Kaur, H.; Bhatt, M.L.; Bhadauria, S. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget 2014, 5, 5350–5368. [Google Scholar] [CrossRef]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef]
- Saha, J.; Sarkar, D.; Pramanik, A.; Mahanti, K.; Adhikary, A.; Bhattacharyya, S. PGE2-HIF1α reciprocal induction regulates migration, phenotypic alteration and immunosuppressive capacity of macrophages in tumor microenvironment. Life Sci. 2020, 253, 117731. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jene, N.; Byrne, D.; Millar, E.K.; O’Toole, S.A.; McNeil, C.M.; Bates, G.J.; Harris, A.L.; Banham, A.H.; Sutherland, R.L.; et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011, 13, R47. [Google Scholar] [CrossRef]
- Ren, L.; Yu, Y.; Wang, L.; Zhu, Z.; Lu, R.; Yao, Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget 2016, 7, 75763–75773. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.K.; Xu, I.M.; Lai, R.K.; Tse, A.P.; Wei, L.L.; Koh, H.Y.; Li, L.L.; Lee, D.; Lo, R.C.; Wong, C.M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016, 64, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.K.; Tse, A.P.; Xu, I.M.; Di Cui, J.; Lai, R.K.; Li, L.L.; Koh, H.Y.; Tsang, F.H.; Wei, L.L.; Wong, C.M.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, H.Z.; Williams, E.P.; Hickey, M.M.; Patel, S.A.; Durham, A.C.; Yuan, L.J.; Hammond, R.; Gimotty, P.A.; Keith, B.; Simon, M.C. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Investig. 2010, 120, 2699–2714. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Deng, J.; Li, J.; Sarde, A.; Lines, J.L.; Lee, Y.C.; Qian, D.C.; Pechenick, D.A.; Manivanh, R.; Le Mercier, I.; Lowrey, C.H.; et al. Hypoxia-Induced VISTA Promotes the Suppressive Function of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 1079–1090. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Hang, J.; Zhang, J.; Zhang, T.; Huo, Y.; Liu, J.; Lai, S.; Luo, D.; Wang, L.; et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol. Res. 2020. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, Q.; Shao, N.; Shan, Z.; Cheang, T.; Zhang, Z.; Su, Q.; Wang, S.; Lin, Y. Respiratory hyperoxia reverses immunosuppression by regulating myeloid-derived suppressor cells and PD-L1 expression in a triple-negative breast cancer mouse model. Am. J. Cancer Res. 2019, 9, 529–545. [Google Scholar]
- Connolly, K.A.; Belt, B.A.; Figueroa, N.M.; Murthy, A.; Patel, A.; Kim, M.; Lord, E.M.; Linehan, D.C.; Gerber, S.A. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget 2016, 7, 86522–86535. [Google Scholar] [CrossRef]
- Bravatà, V.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Russo, G.; Di Maggio, F.M.; Augello, G.; Lio, D.; Gilardi, M.C. Cytokine profile of breast cell lines after different radiation doses. Int. J. Radiat. Biol. 2017, 93, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Mondini, M.; Loyher, P.L.; Hamon, P.; Gerbé de Thoré, M.; Laviron, M.; Berthelot, K.; Clémenson, C.; Salomon, B.L.; Combadière, C.; Deutsch, E.; et al. CCR2-Dependent Recruitment of Tregs and Monocytes Following Radiotherapy Is Associated with TNFα-Mediated Resistance. Cancer Immunol. Res. 2019, 7, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.S.; Bardhan, K.; Chen, M.R.; Paschall, A.V.; Lu, C.; Bollag, R.J.; Kong, F.C.; Jin, J.; Kong, F.M.; Waller, J.L.; et al. NF-κB functions as a molecular link between tumor cells and Th1/Tc1 T cells in the tumor microenvironment to exert radiation-mediated tumor suppression. Oncotarget 2016, 7, 23395–23415. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Z.; Zhu, Y.Y.; Ruan, M.; Chen, L.; Zhang, Q.Y. Local Irradiation Sensitized Tumors to Adoptive T Cell Therapy via Enhancing the Cross-Priming, Homing, and Cytotoxicity of Antigen-Specific CD8 T Cells. Front. Immunol. 2019, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, N.; Aldrich, M.; Weksberg, D.; Rollins, L.; Goltsova, T.; Chen, S.Y.; Huang, X.F. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 2009, 32, 145–156. [Google Scholar] [CrossRef]
- Igoucheva, O.; Grazzini, M.; Pidich, A.; Kemp, D.M.; Larijani, M.; Farber, M.; Lorton, J.; Rodeck, U.; Alexeev, V. Immunotargeting and eradication of orthotopic melanoma using a chemokine-enhanced DNA vaccine. Gene Ther. 2013, 20, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Li, L.; Wu, H.; Wang, C.; Yan, Z.; Wang, Y.; Su, C.; Jin, H.; Zhou, F.; et al. The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma. Oncol. Rep. 2013, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Nakamoto, Y.; Sakai, Y.; Marukawa, Y.; Kitahara, M.; Mukaida, N.; Kaneko, S. Prolonged, NK cell-mediated antitumor effects of suicide gene therapy combined with monocyte chemoattractant protein-1 against hepatocellular carcinoma. J. Immunol. 2007, 178, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Raport, C.J.; Gosling, J.; Schweickart, V.L.; Gray, P.W.; Charo, I.F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1 beta, and MIP-1 alpha. J. Biol. Chem. 1996, 271, 17161–17166. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, C.; Ahuja, S.K.; Tiffany, H.L.; Murphy, P.M. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J. Leukoc. Biol. 1996, 60, 147–152. [Google Scholar] [CrossRef]
- Combadiere, C.; Ahuja, S.K.; Murphy, P.M. Cloning and functional expression of a human eosinophil CC chemokine receptor. J. Biol. Chem. 1995, 270, 16491–16494. [Google Scholar] [CrossRef]
- Daugherty, B.L.; Siciliano, S.J.; DeMartino, J.A.; Malkowitz, L.; Sirotina, A.; Springer, M.S. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J. Exp. Med. 1996, 183, 2349–2354. [Google Scholar] [CrossRef]
- Ponath, P.D.; Qin, S.; Post, T.W.; Wang, J.; Wu, L.; Gerard, N.P.; Newman, W.; Gerard, C.; Mackay, C.R. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J. Exp. Med. 1996, 183, 2437–2448. [Google Scholar] [CrossRef]
- Neote, K.; DiGregorio, D.; Mak, J.Y.; Horuk, R.; Schall, T.J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 1993, 72, 415–425. [Google Scholar] [CrossRef]
- Mortier, A.; Van Damme, J.; Proost, P. Regulation of chemokine activity by posttranslational modification. Pharmacol. Ther. 2008, 120, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Pease, J.E. Tails of the unexpected—An atypical receptor for the chemokine RANTES/CCL5 expressed in brain. Br. J. Pharmacol. 2006, 149, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Dedoni, S.; Campbell, L.A.; Harvey, B.K.; Avdoshina, V.; Mocchetti, I. The orphan G-protein-coupled receptor 75 signaling is activated by the chemokine CCL5. J. Neurochem. 2018, 146, 526–539. [Google Scholar] [CrossRef]
- Wu, F.Y.; Ou, Z.L.; Feng, L.Y.; Luo, J.M.; Wang, L.P.; Shen, Z.Z.; Shao, Z.M. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol. Cancer Res. 2008, 6, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Langenes, V.; Svensson, H.; Börjesson, L.; Gustavsson, B.; Bemark, M.; Sjöling, Å.; Quiding-Järbrink, M. Expression of the chemokine decoy receptor D6 is decreased in colon adenocarcinomas. Cancer Immunol. Immunother. 2013, 62, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Massara, M.; Bonavita, O.; Mantovani, A.; Locati, M.; Bonecchi, R. Atypical chemokine receptors in cancer: Friends or foes? J. Leukoc. Biol. 2016, 99, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Sayers, T.J.; Carter, C.R.; Ortaldo, J.R. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 1995, 155, 3877–3888. [Google Scholar]
- Lavergne, E.; Combadière, C.; Iga, M.; Boissonnas, A.; Bonduelle, O.; Maho, M.; Debré, P.; Combadiere, B. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J. Immunol. 2004, 173, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, N.; Huang, X.F. CCL5 as an adjuvant for cancer immunotherapy. Expert Opin. Biol. Ther. 2010, 10, 725–733. [Google Scholar] [CrossRef]
- Chew, V.; Chen, J.; Lee, D.; Loh, E.; Lee, J.; Lim, K.H.; Weber, A.; Slankamenac, K.; Poon, R.T.; Yang, H.; et al. Chemokine-driven lymphocyte infiltration: An early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012, 61, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mgrditchian, T.; Arakelian, T.; Paggetti, J.; Noman, M.Z.; Viry, E.; Moussay, E.; Van Moer, K.; Kreis, S.; Guerin, C.; Buart, S.; et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, E9271–E9279. [Google Scholar] [CrossRef]
- Araujo, J.M.; Gomez, A.C.; Aguilar, A.; Salgado, R.; Balko, J.M.; Bravo, L.; Doimi, F.; Bretel, D.; Morante, Z.; Flores, C.; et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci. Rep. 2018, 8, 4899. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis, E.; et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Lindow, M.; Nansen, A.; Bartholdy, C.; Stryhn, A.; Hansen, N.J.; Boesen, T.P.; Wells, T.N.; Schwartz, T.W.; Thomsen, A.R. The virus-encoded chemokine vMIP-II inhibits virus-induced Tc1-driven inflammation. J. Virol. 2003, 77, 7393–7400. [Google Scholar] [CrossRef]
- Rubant, S.; Ludwig, R.J.; Pfeffer, J.; Schulze-Johann, P.; Kaufmann, R.; Pfeilschifter, J.M.; Boehncke, W.H.; Radeke, H.H. Eukaryotic expression of the broad-spectrum chemokine receptor antagonist vMIP-II and its effects on T-cell function in vitro and in vivo. Exp. Dermatol. 2006, 15, 634–642. [Google Scholar] [CrossRef]
- Yamin, R.; Kaynan, N.S.; Glasner, A.; Vitenshtein, A.; Tsukerman, P.; Bauman, Y.; Ophir, Y.; Elias, S.; Bar-On, Y.; Gur, C.; et al. The viral KSHV chemokine vMIP-II inhibits the migration of Naive and activated human NK cells by antagonizing two distinct chemokine receptors. PLoS Pathog. 2013, 9, e1003568. [Google Scholar] [CrossRef]
- Sozzani, S.; Luini, W.; Bianchi, G.; Allavena, P.; Wells, T.N.; Napolitano, M.; Bernardini, G.; Vecchi, A.; D’Ambrosio, D.; Mazzeo, D.; et al. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood 1998, 92, 4036–4039. [Google Scholar] [CrossRef]
- Weber, K.S.; Gröne, H.J.; Röcken, M.; Klier, C.; Gu, S.; Wank, R.; Proudfoot, A.E.; Nelson, P.J.; Weber, C. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 2001, 31, 2458–2466. [Google Scholar] [CrossRef]
- Thomas, J.K.; Mir, H.; Kapur, N.; Bae, S.; Singh, S. CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Sci. Rep. 2019, 9, 4014. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Singh, A.; Sharma, K.C.; Saxena, S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017, 18, 1307–1313. [Google Scholar] [PubMed]
- Singh, S.K.; Mishra, M.K.; Rivers, B.M.; Gordetsky, J.B.; Bae, S.; Singh, R. Biological and Clinical Significance of the CCR5/CCL5 Axis in Hepatocellular Carcinoma. Cancers 2020, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.A.; Rolke, D.; Rave-Fränk, M.; Schirmer, M.; Eicheler, W.; Doerfler, A.; Hille, A.; Hess, C.F.; Matthias, C.; Rödel, R.M.; et al. Analysis of chemokine and chemokine receptor expression in squamous cell carcinoma of the head and neck (SCCHN) cell lines. Radiat. Environ. Biophys. 2011, 50, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Shen, Y.C.; Chang, J.W.; Hsieh, J.J.; Chu, Y.; Wang, C.H. Autocrine CCL5 promotes tumor progression in esophageal squamous cell carcinoma in vitro. Cytokine 2018, 110, 94–103. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.; Yang, X.; Zhang, X.; Zhang, Z.; Sun, Y.; Xu, B.; Hua, J.; He, Z.; Song, Z. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol. Ther. 2017, 18, 806–815. [Google Scholar] [CrossRef]
- Nishikawa, G.; Kawada, K.; Nakagawa, J.; Toda, K.; Ogawa, R.; Inamoto, S.; Mizuno, R.; Itatani, Y.; Sakai, Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019, 10, 264. [Google Scholar] [CrossRef]
- Schlecker, E.; Stojanovic, A.; Eisen, C.; Quack, C.; Falk, C.S.; Umansky, V.; Cerwenka, A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol. 2012, 189, 5602–5611. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, L.; Dai, S.; Li, L.; Wang, F.; Shan, B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed. Pharmacother. 2016, 77, 142–149. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 2016, 1, e85841. [Google Scholar] [CrossRef]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Z.; Wang, J.; Zhang, W.; Yang, Y.; Zhang, Y.; Qu, X.; Zhu, Y.; Zou, J.; Peng, S.; et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020, 27, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Jin, S.; Yang, Y.; Sheng, J.; He, Q.; Song, Y.; Yu, W.; Hu, S.; Jin, J. Infiltrating CD4+ T cells attenuate chemotherapy sensitivity in prostate cancer via CCL5 signaling. Prostate 2019, 79, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Fertig, E.J.; Jin, K.; Sukumar, S.; Pandey, N.B.; Popel, A.S. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 2014, 5, 4715. [Google Scholar] [CrossRef] [PubMed]
- Murooka, T.T.; Rahbar, R.; Fish, E.N. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem. Biophys. Res. Commun. 2009, 387, 381–386. [Google Scholar] [CrossRef]
- Gao, D.; Rahbar, R.; Fish, E.N. CCL5 activation of CCR5 regulates cell metabolism to enhance proliferation of breast cancer cells. Open Biol. 2016, 6, 160122. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef]
- An, G.; Wu, F.; Huang, S.; Feng, L.; Bai, J.; Gu, S.; Zhao, X. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol. Rep. 2019, 42, 2499–2511. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Wang, W.; Ning, B.F.; Chen, F.; Shen, W.; Ding, J.; Chen, W.; Xie, W.F.; Zhang, X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016, 379, 49–59. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Tsang, L.L.; Ye, Q.; Chan, H.C.; Sun, Y.; Jiang, X. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/β-catenin/Slug pathway. Cell Death Dis. 2017, 8, e2819. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Yang, W.H.; Chen, H.T.; Huang, C.Y.; Tan, T.W.; Lin, Y.T.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J. Cell. Physiol. 2009, 220, 418–426. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fong, Y.C.; Lee, C.Y.; Chen, M.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem. Pharmacol. 2009, 77, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xiang, T.; Qi, W.; Huang, J.; Chen, J.; He, L.; Liang, Z.; Guo, B.; Li, Y.; Xie, R.; et al. CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition. Oncotarget 2015, 6, 5846–5859. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Fujita, Y.; Nakane, K.; Mizutani, K.; Terazawa, R.; Ehara, H.; Kanimoto, Y.; Kojima, T.; Nozawa, Y.; Deguchi, T.; et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine 2013, 64, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dréau, D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhes. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef]
- Yi, E.H.; Lee, C.S.; Lee, J.K.; Lee, Y.J.; Shin, M.K.; Cho, C.H.; Kang, K.W.; Lee, J.W.; Han, W.; Noh, D.Y.; et al. STAT3-RANTES autocrine signaling is essential for tamoxifen resistance in human breast cancer cells. Mol. Cancer Res. 2013, 11, 31–42. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, C.; Li, N.; Shan, W.; Lu, H.; Guo, L.; Guo, E.; Xia, M.; Weng, D.; Meng, L.; et al. Cisplatin-induced CCL5 secretion from CAFs promotes cisplatin-resistance in ovarian cancer via regulation of the STAT3 and PI3K/Akt signaling pathways. Int. J. Oncol. 2016, 48, 2087–2097. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Park, S.J.; Jo, E.; Hwang, I.H.; Lee, K.B.; Kim, S.W.; Kim, D.J.; Joo, J.C.; Hong, S.H.; Lee, M.G.; et al. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-κB signaling pathway. Cell Death Discov. 2018, 4, 62. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Qin, J.; Zhou, H.; Majumdar, A.P.N.; Peng, F. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol. Immunotoxicol. 2018, 40, 91–97. [Google Scholar] [CrossRef]
- Ban, Y.; Mai, J.; Li, X.; Mitchell-Flack, M.; Zhang, T.; Zhang, L.; Chouchane, L.; Ferrari, M.; Shen, H.; Ma, X. Targeting Autocrine CCL5-CCR5 Axis Reprograms Immunosuppressive Myeloid Cells and Reinvigorates Antitumor Immunity. Cancer Res. 2017, 77, 2857–2868. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Gilkes, D.M.; Takano, N.; Semenza, G.L. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc. Natl. Acad. Sci. USA 2014, 111, E2120–E2129. [Google Scholar] [CrossRef]
- Datar, I.; Qiu, X.; Ma, H.Z.; Yeung, M.; Aras, S.; de la Serna, I.; Al-Mulla, F.; Thiery, J.P.; Trumbly, R.; Fan, X.; et al. RKIP regulates CCL5 expression to inhibit breast cancer invasion and metastasis by controlling macrophage infiltration. Oncotarget 2015, 6, 39050–39061. [Google Scholar] [CrossRef] [PubMed]
- Brauß, T.F.; Winslow, S.; Lampe, S.; Scholz, A.; Weigert, A.; Dehne, N.; von Stedingk, K.; Schmid, T.; Brüne, B. The RNA-binding protein HuR inhibits expression of CCL5 and limits recruitment of macrophages into tumors. Mol. Carcinog 2017, 56, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ye, J.; Hsueh, E.C.; Zhang, Y.; Hoft, D.F.; Peng, G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010, 184, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Goedegebuure, P.S.; Belt, B.A.; Flaherty, B.; Sankpal, N.; Gillanders, W.E.; Eberlein, T.J.; Hsieh, C.S.; Linehan, D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009, 182, 1746–1755. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, Y.C.; Mahalingam, J.; Huang, C.T.; Chen, T.W.; Kang, C.W.; Peng, H.M.; Chu, Y.Y.; Chiang, J.M.; Dutta, A.; et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012, 72, 1092–1102. [Google Scholar] [CrossRef]
- Wang, X.; Lang, M.; Zhao, T.; Feng, X.; Zheng, C.; Huang, C.; Hao, J.; Dong, J.; Luo, L.; Li, X.; et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3+Treg cells in pancreatic ductal adenocarcinoma. Oncogene 2017, 36, 3048–3058. [Google Scholar] [CrossRef]
- Mueller, C.G.; Boix, C.; Kwan, W.H.; Daussy, C.; Fournier, E.; Fridman, W.H.; Molina, T.J. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J. Leukoc. Biol. 2007, 82, 567–575. [Google Scholar] [CrossRef]
- Bergot, A.S.; Ford, N.; Leggatt, G.R.; Wells, J.W.; Frazer, I.H.; Grimbaldeston, M.A. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5- mediated recruitment of mast cells. PLoS Pathog. 2014, 10, e1004466. [Google Scholar] [CrossRef]
- Suffee, N.; Hlawaty, H.; Meddahi-Pelle, A.; Maillard, L.; Louedec, L.; Haddad, O.; Martin, L.; Laguillier, C.; Richard, B.; Oudar, O.; et al. RANTES/CCL5-induced pro-angiogenic effects depend on CCR1, CCR5 and glycosaminoglycans. Angiogenesis 2012, 15, 727–744. [Google Scholar] [CrossRef]
- Liu, G.T.; Chen, H.T.; Tsou, H.K.; Tan, T.W.; Fong, Y.C.; Chen, P.C.; Yang, W.H.; Wang, S.W.; Chen, J.C.; Tang, C.H. CCL5 promotes VEGF-dependent angiogenesis by down-regulating miR-200b through PI3K/Akt signaling pathway in human chondrosarcoma cells. Oncotarget 2014, 5, 10718–10731. [Google Scholar] [CrossRef]
- Wang, S.W.; Liu, S.C.; Sun, H.L.; Huang, T.Y.; Chan, C.H.; Yang, C.Y.; Yeh, H.I.; Huang, Y.L.; Chou, W.Y.; Lin, Y.M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sax, M.J.; Gasch, C.; Athota, V.R.; Freeman, R.; Rasighaemi, P.; Westcott, D.E.; Day, C.J.; Nikolic, I.; Elsworth, B.; Wei, M.; et al. Cancer cell CCL5 mediates bone marrow independent angiogenesis in breast cancer. Oncotarget 2016, 7, 85437–85449. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xiang, T.; Huang, S.; Zhou, J.; Wang, Z.; Xie, R.; Long, H.; Zhu, B. Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumor angiogenesis through autocrine CCL5 signaling. Cancer Lett. 2016, 376, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Feng, L.; Zhang, S.; Zhang, H.; Zhang, X.; Qi, X.; Zhang, Y.; Feng, Q.; Xiang, T.; Zeng, Y.X. Induction of chemokine (C-C motif) ligand 5 by Epstein-Barr virus infection enhances tumor angiogenesis in nasopharyngeal carcinoma. Cancer Sci. 2018, 109, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Lin, C.Y.; Liu, S.C.; Liu, G.T.; Chen, Y.L.; Chen, J.J.; Chan, C.H.; Lin, T.Y.; Chen, C.K.; Xu, G.H.; et al. CCL5 promotes VEGF-C production and induces lymphangiogenesis by suppressing miR-507 in human chondrosarcoma cells. Oncotarget 2016, 7, 36896–36908. [Google Scholar] [CrossRef]
- Modi, W.S. CCL3L1 and CCL4L1 chemokine genes are located in a segmental duplication at chromosome 17q12. Genomics 2004, 83, 735–738. [Google Scholar] [CrossRef][Green Version]
- Colobran, R.; Pedrosa, E.; Carretero-Iglesia, L.; Juan, M. Copy number variation in chemokine superfamily: The complex scene of CCL3L-CCL4L genes in health and disease. Clin. Exp. Immunol. 2010, 162, 41–52. [Google Scholar] [CrossRef]
- Pease, J.E.; Wang, J.; Ponath, P.D.; Murphy, P.M. The N-terminal extracellular segments of the chemokine receptors CCR1 and CCR3 are determinants for MIP-1alpha and eotaxin binding, respectively, but a second domain is essential for efficient receptor activation. J. Biol. Chem. 1998, 273, 19972–19976. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef]
- Taub, D.D.; Conlon, K.; Lloyd, A.R.; Oppenheim, J.J.; Kelvin, D.J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science 1993, 260, 355–358. [Google Scholar] [CrossRef]
- Schall, T.J.; Bacon, K.; Camp, R.D.; Kaspari, J.W.; Goeddel, D.V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 1993, 177, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.; Huang, A.Y.; Altan-Bonnet, G.; Stoll, S.; Scheinecker, C.; Germain, R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 2006, 440, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Y.; Liang, A.; Xie, Y.; Liu, S.; Guo, J.; Wang, W.; Qi, R.; An, H.; Zhang, M.; et al. Intratumoral expression of MIP-1beta induces antitumor responses in a pre-established tumor model through chemoattracting T cells and NK cells. Cell. Mol. Immunol. 2004, 1, 199–204. [Google Scholar] [PubMed]
- Schaller, T.H.; Batich, K.A.; Suryadevara, C.M.; Desai, R.; Sampson, J.H. Chemokines as adjuvants for immunotherapy: Implications for immune activation with CCL3. Expert Rev. Clin. Immunol. 2017, 13, 1049–1060. [Google Scholar] [CrossRef]
- Williford, J.M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.M.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 2019, 5, eaay1357. [Google Scholar] [CrossRef]
- Griss, J.; Bauer, W.; Wagner, C.; Simon, M.; Chen, M.; Grabmeier-Pfistershammer, K.; Maurer-Granofszky, M.; Roka, F.; Penz, T.; Bock, C.; et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019, 10, 4186. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tüting, T.; Rudensky, A.Y.; Hämmerling, G.J. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef]
- Iida, N.; Nakamoto, Y.; Baba, T.; Kakinoki, K.; Li, Y.Y.; Wu, Y.; Matsushima, K.; Kaneko, S.; Mukaida, N. Tumor cell apoptosis induces tumor-specific immunity in a CC chemokine receptor 1- and 5-dependent manner in mice. J. Leukoc. Biol. 2008, 84, 1001–1010. [Google Scholar] [CrossRef]
- González-Martín, A.; Gómez, L.; Lustgarten, J.; Mira, E.; Mañes, S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4(+) and CD8(+) T cells. Cancer Res. 2011, 71, 5455–5466. [Google Scholar] [CrossRef]
- Allen, F.; Rauhe, P.; Askew, D.; Tong, A.A.; Nthale, J.; Eid, S.; Myers, J.T.; Tong, C.; Huang, A.Y. CCL3 Enhances Antitumor Immune Priming in the Lymph Node via IFNγ with Dependency on Natural Killer Cells. Front. Immunol. 2017, 8, 1390. [Google Scholar] [CrossRef]
- Zhao, N.; Dang, H.; Ma, L.; Martin, S.P.; Forgues, M.; Ylaya, K.; Hewitt, S.M.; Wang, X.W. Intratumoral γδ T-cell infiltrates, CCL4/5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Clark-Lewis, I.; Buri, C.; Langen, H.; Lis, M.; Mazzucchelli, L. Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-1 alpha, macrophage inflammatory protein-1 beta, and SLC that are expressed in human breast cancer. Am. J. Pathol. 2003, 162, 1183–1190. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Wylie, S.M.; Pragnell, I.B.; Graham, G.J. Cloning and characterization of a novel murine beta chemokine receptor, D6. Comparison to three other related macrophage inflammatory protein-1alpha receptors, CCR-1, CCR-3, and CCR-5. J. Biol. Chem. 1997, 272, 12495–12504. [Google Scholar] [CrossRef] [PubMed]
- Baghel, K.S.; Tewari, B.N.; Shrivastava, R.; Malik, S.A.; Lone, M.U.; Jain, N.K.; Tripathi, C.; Kanchan, R.K.; Dixit, S.; Singh, K.; et al. Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1β dependent upregulation of MYO3A gene in breast cancer cells. Oncoimmunology 2016, 5, e1196299. [Google Scholar] [CrossRef]
- Iriki, T.; Ohnishi, K.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Pan, C.; Ikeda, K.; Mori, T.; Suzuki, M.; Ichiyasu, H.; et al. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer 2017, 106, 22–32. [Google Scholar] [CrossRef]
- Zeiner, P.S.; Preusse, C.; Golebiewska, A.; Zinke, J.; Iriondo, A.; Muller, A.; Kaoma, T.; Filipski, K.; Müller-Eschner, M.; Bernatz, S.; et al. Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas. Brain Pathol. 2019, 29, 513–529. [Google Scholar] [CrossRef]
- Kodama, T.; Koma, Y.I.; Arai, N.; Kido, A.; Urakawa, N.; Nishio, M.; Shigeoka, M.; Yokozaki, H. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab. Investig. 2020, 100, 1140–1157. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Fechner, M.K.; Hoffmann, T.K.; Lang, S.; Brandau, S. A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J. Leukoc. Biol. 2012, 91, 591–598. [Google Scholar] [CrossRef]
- Ren, R.; Yu, J.; Zhang, Y.; Wang, S.F.; Guo, X.; Shen, M.; Xu, M.D.; Jiang, M.; Zhi, Q.; Chen, K.; et al. Inflammation Promotes Progression of Pancreatic Cancer Through WNT/β-Catenin Pathway-Dependent Manner. Pancreas 2019, 48, 1003–1014. [Google Scholar] [CrossRef]
- Abe, M.; Hiura, K.; Wilde, J.; Moriyama, K.; Hashimoto, T.; Ozaki, S.; Wakatsuki, S.; Kosaka, M.; Kido, S.; Inoue, D.; et al. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood 2002, 100, 2195–2202. [Google Scholar] [CrossRef]
- Dairaghi, D.J.; Oyajobi, B.O.; Gupta, A.; McCluskey, B.; Miao, S.; Powers, J.P.; Seitz, L.C.; Wang, Y.; Zeng, Y.; Zhang, P.; et al. CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood 2012, 120, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Naka, K.; Morishita, S.; Komatsu, N.; Hirao, A.; Mukaida, N. MIP-1α/CCL3-mediated maintenance of leukemia-initiating cells in the initiation process of chronic myeloid leukemia. J. Exp. Med. 2013, 210, 2661–2673. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Takeda, T.; Tomonari, Y.; Mashimo, K.; Koumoto, Y.I.; Hoshida, S.; Itoh, T.; Imano, M.; Satou, T.; Sakaguchi, K.; et al. The MIP-1α autocrine loop contributes to decreased sensitivity to anticancer drugs. J. Cell. Physiol. 2018, 233, 4258–4271. [Google Scholar] [CrossRef]
- Choi, S.J.; Oba, Y.; Gazitt, Y.; Alsina, M.; Cruz, J.; Anderson, J.; Roodman, G.D. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J. Clin. Investig. 2001, 108, 1833–1841. [Google Scholar] [CrossRef]
- Oba, Y.; Lee, J.W.; Ehrlich, L.A.; Chung, H.Y.; Jelinek, D.F.; Callander, N.S.; Horuk, R.; Choi, S.J.; Roodman, G.D. MIP-1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp. Hematol. 2005, 33, 272–278. [Google Scholar] [CrossRef]
- Vallet, S.; Pozzi, S.; Patel, K.; Vaghela, N.; Fulciniti, M.T.; Veiby, P.; Hideshima, T.; Santo, L.; Cirstea, D.; Scadden, D.T.; et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia 2011, 25, 1174–1181. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, A.; Zhao, H.; Lu, P.; Cheng, H.; Dong, F.; Gong, Y.; Ma, S.; Zheng, Y.; Zhang, H.; et al. Leukemia cell infiltration causes defective erythropoiesis partially through MIP-1α/CCL3. Leukemia 2016, 30, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Vandyke, K.; Zeissig, M.N.; Hewett, D.R.; Martin, S.K.; Mrozik, K.M.; Cheong, C.M.; Diamond, P.; To, L.B.; Gronthos, S.; Peet, D.J.; et al. HIF-2α Promotes Dissemination of Plasma Cells in Multiple Myeloma by Regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017, 77, 5452–5463. [Google Scholar] [CrossRef]
- Takahashi, K.; Sivina, M.; Hoellenriegel, J.; Oki, Y.; Hagemeister, F.B.; Fayad, L.; Romaguera, J.E.; Fowler, N.; Fanale, M.A.; Kwak, L.W.; et al. CCL3 and CCL4 are biomarkers for B cell receptor pathway activation and prognostic serum markers in diffuse large B cell lymphoma. Br. J. Haematol. 2015, 171, 726–735. [Google Scholar] [CrossRef]
- Kim, H.S.; Ryu, K.J.; Ko, Y.H.; Kim, H.J.; Kim, S.H.; Kim, W.S.; Kim, S.J. Macrophage inflammatory protein 1 alpha (MIP-1α) may be associated with poor outcome in patients with extranodal NK/T-cell lymphoma. Hematol. Oncol. 2017, 35, 310–316. [Google Scholar] [CrossRef]
- Botta, C.; Di Martino, M.T.; Ciliberto, D.; Cucè, M.; Correale, P.; Rossi, M.; Tagliaferri, P.; Tassone, P. A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J. 2016, 6, e511. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Feng, W.; Wang, H.; Wang, L.; Yang, X.; Yang, F.; Zhang, Y.; Liu, X.; Zhang, D.; Ren, Q.; et al. Blocking migration of regulatory T cells to leukemic hematopoietic microenvironment delays disease progression in mouse leukemia model. Cancer Lett. 2020, 469, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Blattner, C.; Fleming, V.; Weber, R.; Himmelhan, B.; Altevogt, P.; Gebhardt, C.; Schulze, T.J.; Razon, H.; Hawila, E.; Wildbaum, G.; et al. CCR5+ Myeloid-Derived Suppressor Cells Are Enriched and Activated in Melanoma Lesions. Cancer Res. 2018, 78, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Meng, M.; Wang, G.; Han, R.; Zhang, Y.; Jing, X.; Zhao, L.; Gu, S.; Zhao, X. Myeloid-Derived Suppressor Cells Recruited by Chemokine (C-C Motif) Ligand 3 Promote the Progression of Breast Cancer via Phosphoinositide 3-Kinase-Protein Kinase B-Mammalian Target of Rapamycin Signaling. J. Breast Cancer 2020, 23, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Chattopadhya, S.; Chattopadhyay, U.; Sharma, N.K. Macrophage inflammatory protein (MIP)1alpha and MIP1beta differentially regulate release of inflammatory cytokines and generation of tumoricidal monocytes in malignancy. Cancer Immunol. Immunother. 2006, 55, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, P.; Fujii, C.; Nakamoto, Y.; Gao, J.L.; Kaneko, S.; Murphy, P.M.; Mukaida, N. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int. J. Cancer 2006, 118, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Shinagawa, K.; Matsushima, K.; Mukaida, N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int. J. Cancer 2014, 135, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Nishimura, T.; Hayakawa, Y.; Hashimoto, S.; Gotoh, N.; Mukaida, N. Essential roles of the interaction between cancer cell-derived chemokine, CCL4, and intra-bone CCR5-expressing fibroblasts in breast cancer bone metastasis. Cancer Lett. 2016, 378, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tang, C.; Zhang, M.Y.; Huang, W.L.; Xu, Y.; Sun, H.Y.; Yang, F.; Song, L.L.; Wang, H.; Mu, L.L.; et al. Blocking ATM-dependent NF-κB pathway overcomes niche protection and improves chemotherapy response in acute lymphoblastic leukemia. Leukemia 2019, 33, 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, E.; Kaza, V.; Papavassiliou, A.G.; Chatzistamou, I.; Kiaris, H. Induction of the MCP chemokine cluster cascade in the periphery by cancer cell-derived Ccl3. Cancer Lett. 2017, 389, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chu, H.Y.; Zhong, Z.M.; Qi, X.; Cheng, R.; Qin, R.J.; Liang, J.; Zhu, X.F.; Zeng, M.S.; Sun, C.Z. Platelet-secreted CCL3 and its receptor CCR5 promote invasive and migratory abilities of anaplastic thyroid carcinoma cells via MMP-1. Cell. Signal. 2019, 63, 109363. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.C.; Qian, J.; Zhang, Y.N.; Wang, W.; Kang, X.; Xu, W.; Wu, J.; Zheng, W. Colorectal cancer cells promote osteoclastogenesis and bone destruction through regulating EGF/ERK/CCL3 pathway. Biosci. Rep. 2020, 40, BSR20201175. [Google Scholar]
- Liao, Y.Y.; Tsai, H.C.; Chou, P.Y.; Wang, S.W.; Chen, H.T.; Lin, Y.M.; Chiang, I.P.; Chang, T.M.; Hsu, S.K.; Chou, M.C.; et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget 2016, 7, 4310–4325. [Google Scholar] [CrossRef]
- Hua, F.; Tian, Y. CCL4 promotes the cell proliferation, invasion and migration of endometrial carcinoma by targeting the VEGF-A signal pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 11288–11299. [Google Scholar]
- Lien, M.Y.; Tsai, H.C.; Chang, A.C.; Tsai, M.H.; Hua, C.H.; Wang, S.W.; Tang, C.H. Chemokine CCL4 Induces Vascular Endothelial Growth Factor C Expression and Lymphangiogenesis by miR-195-3p in Oral Squamous Cell Carcinoma. Front. Immunol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 1997, 272, 14893–14898. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Louten, J.; Boniface, K.; de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 2009, 123, 1004–1011. [Google Scholar] [CrossRef]
- Rubie, C.; Frick, V.O.; Wagner, M.; Rau, B.; Weber, C.; Kruse, B.; Kempf, K.; Tilton, B.; König, J.; Schilling, M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand. J. Immunol. 2006, 63, 468–477. [Google Scholar] [CrossRef]
- Kleeff, J.; Kusama, T.; Rossi, D.L.; Ishiwata, T.; Maruyama, H.; Friess, H.; Büchler, M.W.; Zlotnik, A.; Korc, M. Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int. J. Cancer 1999, 81, 650–657. [Google Scholar] [CrossRef]
- Rubie, C.; Frick, V.O.; Ghadjar, P.; Wagner, M.; Grimm, H.; Vicinus, B.; Justinger, C.; Graeber, S.; Schilling, M.K. CCL20/CCR6 expression profile in pancreatic cancer. J. Transl. Med. 2010, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, R.; Gutiérrez-González, A.; Gutiérrez-Seijo, A.; Sánchez-Gregorio, S.; García-Giménez, J.; Mercader, E.; Márquez-Rodas, I.; Avilés, J.A.; Relloso, M.; Sánchez-Mateos, P. CCL20 Expression by Tumor-Associated Macrophages Predicts Progression of Human Primary Cutaneous Melanoma. Cancer Immunol. Res. 2018, 6, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 Axis. Cancers 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Itoh, Y.; Yamaguchi, K.; Yamauchi, N.; Harano, Y.; Nakajima, T.; Minami, M.; Okanoue, T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem. Biophys. Res. Commun. 2004, 322, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Kimsey, T.F.; Campbell, A.S.; Albo, D.; Wilson, M.; Wang, T.N. Co-localization of macrophage inflammatory protein-3alpha (Mip-3alpha) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer J. 2004, 10, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, L.; Sun, X.; Wang, L.; Wang, X.; Chen, C. Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J. Cell. Mol. Med. 2016, 20, 920–929. [Google Scholar] [CrossRef]
- Benkheil, M.; Van Haele, M.; Roskams, T.; Laporte, M.; Noppen, S.; Abbasi, K.; Delang, L.; Neyts, J.; Liekens, S. CCL20, a direct-acting pro-angiogenic chemokine induced by hepatitis C virus (HCV): Potential role in HCV-related liver cancer. Exp. Cell Res. 2018, 372, 168–177. [Google Scholar] [CrossRef]
- Zhu, C.C.; Chen, C.; Xu, Z.Q.; Zhao, J.K.; Ou, B.C.; Sun, J.; Zheng, M.H.; Zong, Y.P.; Lu, A.G. CCR6 promotes tumor angiogenesis via the AKT/NF-κB/VEGF pathway in colorectal cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 387–397. [Google Scholar] [CrossRef]
- Chen, K.J.; Lin, S.Z.; Zhou, L.; Xie, H.Y.; Zhou, W.H.; Taki-Eldin, A.; Zheng, S.S. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 2011, 6, e24671. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, R.; Mao, C.; Shi, L.; Wang, S.; Yu, L.; Hu, Q.; Dai, D.; Xu, H. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum. Immunol. 2012, 73, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lou, X.M.; He, Y. Preferential recruitment of Th17 cells to cervical cancer via CCR6-CCL20 pathway. PLoS ONE 2015, 10, e0120855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Qi, Y.; Li, X.N.; Yang, Y.; Liu, D.L.; Zhao, J.; Zhu, D.Y.; Wu, K.; Zhou, X.D.; Zhao, S. The role of CCL20/CCR6 axis in recruiting Treg cells to tumor sites of NSCLC patients. Biomed. Pharmacother. 2015, 69, 242–248. [Google Scholar] [CrossRef]
- Nandi, B.; Shapiro, M.; Samur, M.K.; Pai, C.; Frank, N.Y.; Yoon, C.; Prabhala, R.H.; Munshi, N.C.; Gold, J.S. Stromal CCR6 drives tumor growth in a murine transplantable colon cancer through recruitment of tumor-promoting macrophages. Oncoimmunology 2016, 5, e1189052. [Google Scholar] [CrossRef]
- Bonnotte, B.; Crittenden, M.; Larmonier, N.; Gough, M.; Vile, R.G. MIP-3alpha transfection into a rodent tumor cell line increases intratumoral dendritic cell infiltration but enhances (facilitates) tumor growth and decreases immunogenicity. J. Immunol. 2004, 173, 4929–4935. [Google Scholar] [CrossRef]
- Brand, S.; Olszak, T.; Beigel, F.; Diebold, J.; Otte, J.M.; Eichhorst, S.T.; Göke, B.; Dambacher, J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J. Cell Biochem. 2006, 97, 709–723. [Google Scholar] [CrossRef]
- Campbell, A.S.; Albo, D.; Kimsey, T.F.; White, S.L.; Wang, T.N. Macrophage inflammatory protein-3alpha promotes pancreatic cancer cell invasion. J. Surg. Res. 2005, 123, 96–101. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Marsigliante, S. CCL20 promotes migration and invasiveness of human cancerous breast epithelial cells in primary culture. Mol. Carcinog. 2017, 56, 2461–2473. [Google Scholar] [CrossRef]
- Hou, K.Z.; Fu, Z.Q.; Gong, H. Chemokine ligand 20 enhances progression of hepatocellular carcinoma via epithelial-mesenchymal transition. World J. Gastroenterol. 2015, 21, 475–483. [Google Scholar] [CrossRef]
- Marsigliante, S.; Vetrugno, C.; Muscella, A. Paracrine CCL20 loop induces epithelial-mesenchymal transition in breast epithelial cells. Mol. Carcinog. 2016, 55, 1175–1186. [Google Scholar] [CrossRef]
- Dellacasagrande, J.; Schreurs, O.J.; Hofgaard, P.O.; Omholt, H.; Steinsvoll, S.; Schenck, K.; Bogen, B.; Dembic, Z. Liver metastasis of cancer facilitated by chemokine receptor CCR6. Scand. J. Immunol. 2003, 57, 534–544. [Google Scholar] [CrossRef]
- Ghadjar, P.; Coupland, S.E.; Na, I.K.; Noutsias, M.; Letsch, A.; Stroux, A.; Bauer, S.; Buhr, H.J.; Thiel, E.; Scheibenbogen, C.; et al. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J. Clin. Oncol. 2006, 24, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Rubie, C.; Oliveira, V.; Kempf, K.; Wagner, M.; Tilton, B.; Rau, B.; Kruse, B.; Konig, J.; Schilling, M. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumour Biol. 2006, 27, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Cabioglu, N.; Assi, H.; Sabourin, J.C.; Delaloge, S.; Sahin, A.; Broglio, K.; Spano, J.P.; Combadiere, C.; Bucana, C.; et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann. Oncol. 2006, 17, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, C.M.; Mercier, O.; Dartevelle, P.; Commo, F.; Olaussen, K.A.; de Montpreville, V.; André, F.; Sabatier, L.; Soria, J.C. Expression of chemokine receptor CCR6 as a molecular determinant of adrenal metastatic relapse in patients with primary lung cancer. Clin. Lung Cancer 2010, 11, 187–191. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Rodig, S.J.; Jones, D.; Shahsafaei, A.; Dorfman, D.M. CCR6 is a functional chemokine receptor that serves to identify select B-cell non-Hodgkin’s lymphomas. Hum. Pathol. 2002, 33, 1227–1233. [Google Scholar] [CrossRef]
- Giuliani, N.; Lisignoli, G.; Colla, S.; Lazzaretti, M.; Storti, P.; Mancini, C.; Bonomini, S.; Manferdini, C.; Codeluppi, K.; Facchini, A.; et al. CC-chemokine ligand 20/macrophage inflammatory protein-3α and CC-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res. 2008, 68, 6840–6850. [Google Scholar] [CrossRef]
- Gunn, M.D.; Tangemann, K.; Tam, C.; Cyster, J.G.; Rosen, S.D.; Williams, L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 258–263. [Google Scholar] [CrossRef]
- Willimann, K.; Legler, D.F.; Loetscher, M.; Roos, R.S.; Delgado, M.B.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 1998, 28, 2025–2034. [Google Scholar] [CrossRef]
- Yoshida, R.; Nagira, M.; Imai, T.; Baba, M.; Takagi, S.; Tabira, Y.; Akagi, J.; Nomiyama, H.; Yoshie, O. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: Activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 1998, 10, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Nomura, T.; Kohno, M.; Tateishi, N.; Suzuki, Y.; Maeda, N.; Fujisawa, R.; Yoshie, O.; Fujita, S. Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood 2000, 95, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Baekkevold, E.S.; Yamanaka, T.; Palframan, R.T.; Carlsen, H.S.; Reinholt, F.P.; von Andrian, U.H.; Brandtzaeg, P.; Haraldsen, G. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 2001, 193, 1105–1112. [Google Scholar] [CrossRef]
- Sharma, S.; Stolina, M.; Luo, J.; Strieter, R.M.; Burdick, M.; Zhu, L.X.; Batra, R.K.; Dubinett, S.M. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J. Immunol. 2000, 164, 4558–4563. [Google Scholar] [CrossRef]
- Liang, C.M.; Zhong, C.P.; Sun, R.X.; Liu, B.B.; Huang, C.; Qin, J.; Zhou, S.; Shan, J.; Liu, Y.K.; Ye, S.L. Local expression of secondary lymphoid tissue chemokine delivered by adeno-associated virus within the tumor bed stimulates strong anti-liver tumor immunity. J. Virol. 2007, 81, 9502–9511. [Google Scholar] [CrossRef]
- Wu, S.; Lu, X.; Zhang, Z.L.; Lei, P.; Hu, P.; Wang, M.; Huang, B.; Xing, W.; Jiang, X.T.; Liu, H.J.; et al. CC chemokine ligand 21 enhances the immunogenicity of the breast cancer cell line MCF-7 upon assistance of TLR2. Carcinogenesis 2011, 32, 296–304. [Google Scholar] [CrossRef]
- Correale, P.; Rotundo, M.S.; Botta, C.; Del Vecchio, M.T.; Ginanneschi, C.; Licchetta, A.; Conca, R.; Apollinari, S.; De Luca, F.; Tassone, P.; et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin. Cancer Res. 2012, 18, 850–857. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, X.; Zhang, S.; Zhang, Q.; Wang, E. Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha correlates with migration and invasion in lung cancer cells. Cancer Biol. Ther. 2009, 8, 322–330. [Google Scholar] [CrossRef]
- Cheng, S.; Han, L.; Guo, J.; Yang, Q.; Zhou, J.; Yang, X. The essential roles of CCR7 in epithelial-to-mesenchymal transition induced by hypoxia in epithelial ovarian carcinomas. Tumour Biol. 2014, 35, 12293–12298. [Google Scholar] [CrossRef]
- Basheer, H.A.; Pakanavicius, E.; Cooper, P.A.; Shnyder, S.D.; Martin, L.; Hunter, K.D.; Vinader, V.; Afarinkia, K. Hypoxia modulates CCR7 expression in head and neck cancers. Oral Oncol. 2018, 80, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.R.; Hou, M.F.; Chang, H.C.; Hung, W.C. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008, 283, 11155–11163. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.W.; Pan, M.R.; Hou, M.F.; Hung, W.C. Cyclooxygenase-2 up-regulates CCR7 expression via AKT-mediated phosphorylation and activation of Sp1 in breast cancer cells. J. Cell. Physiol. 2013, 228, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Vieira, J.M.; Casalou, C.; Mesquita, M.; Pereira, T.; Cavaco, B.M.; Dias, S.; Leite, V. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. J. Endocrinol. 2006, 191, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Su, M.L.; Chang, T.M.; Chiang, C.H.; Chang, H.C.; Hou, M.F.; Li, W.S.; Hung, W.C. Inhibition of chemokine (C-C motif) receptor 7 sialylation suppresses CCL19-stimulated proliferation, invasion and anti-anoikis. PLoS ONE 2014, 9, e98823. [Google Scholar] [CrossRef]
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.F.; Chen, Z.; Zu, X.B. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE 2015, 10, e0119506. [Google Scholar] [CrossRef]
- Boyle, S.T.; Ingman, W.V.; Poltavets, V.; Faulkner, J.W.; Whitfield, R.J.; McColl, S.R.; Kochetkova, M. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene 2016, 35, 105–115. [Google Scholar] [CrossRef]
- Boyle, S.T.; Gieniec, K.A.; Gregor, C.E.; Faulkner, J.W.; McColl, S.R.; Kochetkova, M. Interplay between CCR7 and Notch1 axes promotes stemness in MMTV-PyMT mammary cancer cells. Mol. Cancer 2017, 16, 19. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Z.; Jiang, E.; Zhou, X.; Wang, L.; Wang, H.; Luo, X.; Chen, Q.; Liu, K.; Shang, Z. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J. Cell. Physiol. 2020, 235, 5995–6009. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Liang, G.Y.; Zheng, Y.F.; Tan, Q.Y.; Wang, R.W.; Li, K. CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells in vitro via activation of the NF-κB/VEGF signaling pathway. Am. J. Transl. Res. 2017, 9, 3282–3292. [Google Scholar]
- Xu, Z.; Zhu, C.; Chen, C.; Zong, Y.; Feng, H.; Liu, D.; Feng, W.; Zhao, J.; Lu, A. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Q.; Li, Y.; Tang, N.; Qiu, X. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 15729–15738. [Google Scholar] [PubMed]
- Yu, J.; Tao, S.; Hu, P.; Wang, R.; Fang, C.; Xu, Y.; Qi, D.; Wei, Z.; Zhang, J.; Tan, Q. CCR7 promote lymph node metastasis via regulating VEGF-C/D-R3 pathway in lung adenocarcinoma. J. Cancer 2017, 8, 2060–2068. [Google Scholar] [CrossRef]
- Kochetkova, M.; Kumar, S.; McColl, S.R. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009, 16, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.T.; Hwang, S.T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shimada, Y.; Maeda, M.; Kawabe, A.; Kaganoi, J.; Komoto, I.; Hashimoto, Y.; Miyake, M.; Hashida, H.; Imamura, M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 2003, 9, 3406–3412. [Google Scholar] [PubMed]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weissbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef]
- Wilson, J.L.; Burchell, J.; Grimshaw, M.J. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006, 66, 11802–11807. [Google Scholar] [CrossRef]
- Cunningham, H.D.; Shannon, L.A.; Calloway, P.A.; Fassold, B.C.; Dunwiddie, I.; Vielhauer, G.; Zhang, M.; Vines, C.M. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl. Oncol. 2010, 3, 354–361. [Google Scholar] [CrossRef]
- Irino, T.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T.; et al. CC-Chemokine receptor CCR7: A key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 291. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Li, N.; Gao, J.; Yu, J.; Zhao, J.; Li, M.; Zhao, Z. High Expression of CCR7 Predicts Lymph Node Metastasis and Good Prognosis in Triple Negative Breast Cancer. Cell. Physiol. Biochem. 2017, 43, 531–539. [Google Scholar] [CrossRef] [PubMed]
- López-Giral, S.; Quintana, N.E.; Cabrerizo, M.; Alfonso-Pérez, M.; Sala-Valdés, M.; De Soria, V.G.; Fernández-Rañada, J.M.; Fernández-Ruiz, E.; Muñoz, C. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J. Leukoc. Biol. 2004, 76, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Zhao, G.; Sun, B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: Clinical and experimental study. J. Exp. Clin. Cancer Res. 2011, 30, 51. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhang, L.; Li, G.; Liu, W.; Tang, W.; Zhang, H.; Luan, J.; Gao, L.; Wang, X. CXCR4 and CCR7 Expression in Primary Nodal Diffuse Large B-Cell Lymphoma-A Clinical and Immunohistochemical Study. Am. J. Med. Sci. 2019, 357, 302–310. [Google Scholar] [CrossRef]
- Das, S.; Sarrou, E.; Podgrabinska, S.; Cassella, M.; Mungamuri, S.K.; Feirt, N.; Gordon, R.; Nagi, C.S.; Wang, Y.; Entenberg, D.; et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 2013, 210, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, D.; Zhou, J.; Li, Q.; Zhou, L.; Li, S.M.; Zhu, L.; Chou, K.Y.; Zhou, L.; Tao, L.; et al. High CCR6/CCR7 expression and Foxp3+ Treg cell number are positively related to the progression of laryngeal squamous cell carcinoma. Oncol Rep 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sharma, S.C.; N. Das, S. Dynamics of regulatory T cells (Tregs) in patients with oral squamous cell carcinoma. J. Surg. Oncol. 2017, 116, 1103–1113. [Google Scholar] [CrossRef]
- Miller, M.D.; Wilson, S.D.; Dorf, M.E.; Seuanez, H.N.; O’Brien, S.J.; Krangel, M.S. Sequence and chromosomal location of the I-309 gene. Relationship to genes encoding a family of inflammatory cytokines. J. Immunol. 1990, 145, 2737–2744. [Google Scholar]
- Goya, I.; Gutiérrez, J.; Varona, R.; Kremer, L.; Zaballos, A.; Márquez, G. Identification of CCR8 as the specific receptor for the human beta-chemokine I-309: Cloning and molecular characterization of murine CCR8 as the receptor for TCA-3. J. Immunol. 1998, 160, 1975–1981. [Google Scholar]
- Zingoni, A.; Soto, H.; Hedrick, J.A.; Stoppacciaro, A.; Storlazzi, C.T.; Sinigaglia, F.; D’Ambrosio, D.; O’Garra, A.; Robinson, D.; Rocchi, M.; et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J. Immunol. 1998, 161, 547–551. [Google Scholar]
- Mutalithas, K.; Guillen, C.; Raport, C.; Kolbeck, R.; Soler, D.; Brightling, C.E.; Pavord, I.D.; Wardlaw, A.J. Expression of CCR8 is increased in asthma. Clin. Exp. Allergy 2010, 40, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Schaerli, P.; Ebert, L.; Willimann, K.; Blaser, A.; Roos, R.S.; Loetscher, P.; Moser, B. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 2004, 199, 1265–1275. [Google Scholar] [CrossRef]
- Ebert, L.M.; Meuter, S.; Moser, B. Homing and function of human skin gammadelta T cells and NK cells: Relevance for tumor surveillance. J. Immunol. 2006, 176, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Ruckes, T.; Saul, D.; Van Snick, J.; Hermine, O.; Grassmann, R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 2001, 98, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Sejima, H.; Naito, T.; Ushirogawa, H.; Matsuzaki, T.; Matsuura, E.; Tanaka, Y.; Nakamura, T.; Takashima, H. The CC chemokine ligand (CCL) 1, upregulated by the viral transactivator Tax, can be downregulated by minocycline: Possible implications for long-term treatment of HTLV-1-associated myelopathy/tropical spastic paraparesis. Virol. J. 2017, 14, 234. [Google Scholar] [CrossRef]
- Louahed, J.; Struyf, S.; Demoulin, J.B.; Parmentier, M.; Van Snick, J.; Van Damme, J.; Renauld, J.C. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur. J. Immunol. 2003, 33, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.J.; Garlisi, C.G.; Xiao, H.; Shan, L.; Hedrick, J.A. The Kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 1999, 189, 1993–1998. [Google Scholar] [CrossRef]
- Haque, N.S.; Fallon, J.T.; Taubman, M.B.; Harpel, P.C. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 2001, 97, 39–45. [Google Scholar] [CrossRef]
- Shin, E.C.; Choi, Y.H.; Kim, J.S.; Kim, S.J.; Park, J.H. Expression patterns of cytokines and chemokines genes in human hepatoma cells. Yonsei Med. J. 2002, 43, 657–664. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Röhrle, N.; Makeschin, M.C.; Fesseler, J.; Endres, S.; Mayr, D.; Anz, D. Peritumoural CCL1 and CCL22 expressing cells in hepatocellular carcinomas shape the tumour immune infiltrate. Pathology 2019, 51, 586–592. [Google Scholar] [CrossRef]
- Yeh, C.R.; Hsu, I.; Song, W.; Chang, H.; Miyamoto, H.; Xiao, G.Q.; Li, L.; Yeh, S. Fibroblast ERα promotes bladder cancer invasion via increasing the CCL1 and IL-6 signals in the tumor microenvironment. Am. J. Cancer Res. 2015, 5, 1146–1157. [Google Scholar]
- Li, Z.; Chan, K.; Qi, Y.; Lu, L.; Ning, F.; Wu, M.; Wang, H.; Wang, Y.; Cai, S.; Du, J. Participation of CCL1 in Snail-Positive Fibroblasts in Colorectal Cancer Contribute to 5-Fluorouracil/Paclitaxel Chemoresistance. Cancer Res. Treat. 2018, 50, 894–907. [Google Scholar] [CrossRef]
- Sun, D.; Luo, T.; Dong, P.; Zhang, N.; Chen, J.; Zhang, S.; Dong, L.; Janssen, H.L.A.; Zhang, S. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J. Cell. Biochem. 2020, 121, 2828–2838. [Google Scholar] [CrossRef]
- Eruslanov, E.; Stoffs, T.; Kim, W.J.; Daurkin, I.; Gilbert, S.M.; Su, L.M.; Vieweg, J.; Daaka, Y.; Kusmartsev, S. Expansion of CCR8(+) inflammatory myeloid cells in cancer patients with urothelial and renal carcinomas. Clin. Cancer Res. 2013, 19, 1670–1680. [Google Scholar] [CrossRef]
- Hoelzinger, D.B.; Smith, S.E.; Mirza, N.; Dominguez, A.L.; Manrique, S.Z.; Lustgarten, J. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J. Immunol. 2010, 184, 6833–6842. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, X.; Qi, P.; Ye, Y.; Shen, W.; Leng, L.; Wang, L.; Li, X.; Luo, X.; Chen, Y.; et al. Sox2 Communicates with Tregs Through CCL1 to Promote the Stemness Property of Breast Cancer Cells. Stem Cells 2017, 35, 2351–2365. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, N.; Qi, J.; Gu, Z.; Shen, H. Long non-coding RNA-GAS5 acts as a tumor suppressor in bladder transitional cell carcinoma via regulation of chemokine (C-C motif) ligand 1 expression. Mol. Med. Rep. 2016, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, J.; Houssiau, F.; Proost, P.; Van Damme, J.; Renauld, J.C. I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J. Immunol. 1996, 157, 2570–2576. [Google Scholar] [PubMed]
- Spinetti, G.; Bernardini, G.; Camarda, G.; Mangoni, A.; Santoni, A.; Capogrossi, M.C.; Napolitano, M. The chemokine receptor CCR8 mediates rescue from dexamethasone-induced apoptosis via an ERK-dependent pathway. J. Leukoc. Biol. 2003, 73, 201–207. [Google Scholar] [CrossRef]
- Bernardini, G.; Spinetti, G.; Ribatti, D.; Camarda, G.; Morbidelli, L.; Ziche, M.; Santoni, A.; Capogrossi, M.C.; Napolitano, M. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 2000, 96, 4039–4045. [Google Scholar] [CrossRef]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001, 194, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kuehnemuth, B.; Piseddu, I.; Wiedemann, G.M.; Lauseker, M.; Kuhn, C.; Hofmann, S.; Schmoeckel, E.; Endres, S.; Mayr, D.; Jeschke, U.; et al. CCL1 is a major regulatory T cell attracting factor in human breast cancer. BMC Cancer 2018, 18, 1278. [Google Scholar] [CrossRef] [PubMed]
- Adema, G.J.; Hartgers, F.; Verstraten, R.; de Vries, E.; Marland, G.; Menon, S.; Foster, J.; Xu, Y.; Nooyen, P.; McClanahan, T.; et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997, 387, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Hieshima, K.; Imai, T.; Baba, M.; Shoudai, K.; Ishizuka, K.; Nakagawa, T.; Tsuruta, J.; Takeya, M.; Sakaki, Y.; Takatsuki, K.; et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J. Immunol. 1997, 159, 1140–1149. [Google Scholar]
- Lindhout, E.; Vissers, J.L.; Hartgers, F.C.; Huijbens, R.J.; Scharenborg, N.M.; Figdor, C.G.; Adema, G.J. The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J. Immunol. 2001, 166, 3284–3289. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Proost, P.; Opdenakker, G.; Laureys, G.; Verhasselt, B.; Peperstraete, L.; Van de Putte, I.; Saccani, A.; Allavena, P.; et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J. Biol. Chem. 2002, 277, 24584–24593. [Google Scholar] [CrossRef]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef]
- Hong, R.; Shen, M.H.; Xie, X.H.; Ruan, S.M. Inhibition of breast cancer metastasis via PITPNM3 by pachymic acid. Asian Pac. J. Cancer Prev. 2012, 13, 1877–1880. [Google Scholar] [CrossRef]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of human CCR8 as a CCL18 receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef]
- Ploenes, T.; Scholtes, B.; Krohn, A.; Burger, M.; Passlick, B.; Müller-Quernheim, J.; Zissel, G. CC-chemokine ligand 18 induces epithelial to mesenchymal transition in lung cancer A549 cells and elevates the invasive potential. PLoS ONE 2013, 8, e53068. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W.; Zhang, H.; Wu, W.; Peng, Y.; Zeng, Y.; Wan, Y.; Wang, J.; Ouyang, N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-κB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016, 37, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, X.; Deng, W.; Huang, M.; Wu, Y.; Zhou, Z.; Zhu, K.; Wang, Y.; Cheng, X.; Zhou, X.; et al. CCL18 enhances migration, invasion and EMT by binding CCR8 in bladder cancer cells. Mol. Med. Rep. 2019, 19, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, X.; Li, M.; Tao, X.; Tai, S.; Fan, Z.; Wang, Z.; Cheng, B.; Xia, J. Chemokine (CC motif) ligand 18 upregulates Slug expression to promote stem-cell like features by activating the mammalian target of rapamycin pathway in oral squamous cell carcinoma. Cancer Sci. 2017, 108, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Y.S.; Yao, Y.D.; Chen, J.Q.; Chen, J.N.; Huang, S.Y.; Zeng, Y.J.; Yao, H.R.; Zeng, S.H.; Fu, Y.S.; et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 2015, 6, 34758–34773. [Google Scholar] [CrossRef]
- Su, S.; Liao, J.; Liu, J.; Huang, D.; He, C.; Chen, F.; Yang, L.; Wu, W.; Chen, J.; Lin, L.; et al. Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell Res. 2017, 27, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Vulcano, M.; Struyf, S.; Scapini, P.; Cassatella, M.; Bernasconi, S.; Bonecchi, R.; Calleri, A.; Penna, G.; Adorini, L.; Luini, W.; et al. Unique regulation of CCL18 production by maturing dendritic cells. J. Immunol. 2003, 170, 3843–3849. [Google Scholar] [CrossRef]
- Azzaoui, I.; Yahia, S.A.; Chang, Y.; Vorng, H.; Morales, O.; Fan, Y.; Delhem, N.; Ple, C.; Tonnel, A.B.; Wallaert, B.; et al. CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood 2011, 118, 3549–3558. [Google Scholar] [CrossRef]
- Cheng, D.E.; Tsai, Y.M.; Hsu, Y.L.; Hou, M.F.; Tsai, E.M.; Wang, J.Y.; Kan, J.Y.; Kuo, P.L. Cluster of differentiation 45 activation is crucial in interleukin-10-dependent tumor-associated dendritic cell differentiation. Oncol. Lett. 2014, 8, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Lagerweij, T.; Zhao, X.W.; Dusoswa, S.; van der Stoop, P.; Westerman, B.; de Gooijer, M.C.; Zoetemelk, M.; Zomer, A.; Crommentuijn, M.H.W.; et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J. Extracell. Vesicles 2018, 7, 1446660. [Google Scholar] [CrossRef]
- Catusse, J.; Wollner, S.; Leick, M.; Schröttner, P.; Schraufstätter, I.; Burger, M. Attenuation of CXCR4 responses by CCL18 in acute lymphocytic leukemia B cells. J. Cell. Physiol. 2010, 225, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Zimmermann, N.; Berndt, N.; Grosser, M.; Stein, A.; Koch, A.; Meurer, M. Up-regulation of the chemokine CCL18 by macrophages is a potential immunomodulatory pathway in cutaneous T-cell lymphoma. Am. J. Pathol. 2011, 179, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Increased CCL18 expression in patients with cutaneous T-cell lymphoma: Association with disease severity and prognosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, L.; Gu, Y.; Lu, H.; Feng, Z. The expression of CCL18 in diffuse large B cell lymphoma and its mechanism research. Cancer Biomark. 2018, 21, 925–934. [Google Scholar] [CrossRef]
- Ma, L.; Wang, H.; Li, Z.; Geng, X.; Li, M. Chemokine (C-C motif) ligand 18 is highly expressed in glioma tissues and promotes invasion of glioblastoma cells. J. Cancer Res. Ther. 2019, 15, 358–364. [Google Scholar]
- Jiang, X.; Liu, J.; Li, S.; Jia, B.; Huang, Z.; Shen, J.; Luo, H.; Zhao, J. CCL18-induced LINC00319 promotes proliferation and metastasis in oral squamous cell carcinoma via the miR-199a-5p/FZD4 axis. Cell Death Dis. 2020, 11, 777. [Google Scholar] [CrossRef]
- Zaballos, A.; Gutiérrez, J.; Varona, R.; Ardavín, C.; Márquez, G. Cutting edge: Identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J. Immunol. 1999, 162, 5671–5675. [Google Scholar] [PubMed]
- Wurbel, M.A.; Philippe, J.M.; Nguyen, C.; Victorero, G.; Freeman, T.; Wooding, P.; Miazek, A.; Mattei, M.G.; Malissen, M.; Jordan, B.R.; et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 2000, 30, 262–271. [Google Scholar] [CrossRef]
- Carramolino, L.; Zaballos, A.; Kremer, L.; Villares, R.; Martín, P.; Ardavín, C.; Martínez-A, C.; Márquez, G. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood 2001, 97, 850–857. [Google Scholar] [CrossRef]
- Uehara, S.; Song, K.; Farber, J.M.; Love, P.E. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J. Immunol. 2002, 168, 134–142. [Google Scholar] [CrossRef]
- Hosoe, N.; Miura, S.; Watanabe, C.; Tsuzuki, Y.; Hokari, R.; Oyama, T.; Fujiyama, Y.; Nagata, H.; Ishii, H. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G458–G466. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Jaimes, M.C.; Lazarus, N.H.; Monak, D.; Zhang, C.; Butcher, E.C.; Greenberg, H.B. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J. Immunol. 2006, 176, 5749–5759. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Jangani, M.; Parmar, A.; Wang, G.; Coe, D.; Spear, S.; Sandrock, I.; Capasso, M.; Coles, M.; Cornish, G.; et al. A Subset of CCL25-Induced Gut-Homing T Cells Affects Intestinal Immunity to Infection and Cancer. Front. Immunol. 2019, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Letsch, A.; Keilholz, U.; Schadendorf, D.; Assfalg, G.; Asemissen, A.M.; Thiel, E.; Scheibenbogen, C. Functional CCR9 expression is associated with small intestinal metastasis. J. Investig. Dermatol. 2004, 122, 685–690. [Google Scholar] [CrossRef]
- Amersi, F.F.; Terando, A.M.; Goto, Y.; Scolyer, R.A.; Thompson, J.F.; Tran, A.N.; Faries, M.B.; Morton, D.L.; Hoon, D.S. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res. 2008, 14, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Doan, N.; Said, J.; Karunasiri, D.; Pullarkat, S.T. Strong expression of chemokine receptor CCR9 in diffuse large B-cell lymphoma and follicular lymphoma strongly correlates with gastrointestinal involvement. Hum. Pathol. 2014, 45, 1451–1458. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Shen, Y.; Qi, L.; Zhang, Z.; Tian, H.; Zou, Z. Thymus-expressed chemokine secreted by breast cancer cells promotes metastasis and inhibits apoptosis. Oncol. Rep. 2020, 43, 1875–1884. [Google Scholar] [CrossRef]
- Shen, X.; Mailey, B.; Ellenhorn, J.D.; Chu, P.G.; Lowy, A.M.; Kim, J. CC chemokine receptor 9 enhances proliferation in pancreatic intraepithelial neoplasia and pancreatic cancer cells. J. Gastrointest. Surg. 2009, 13, 1955–1962. [Google Scholar] [CrossRef]
- Heinrich, E.L.; Arrington, A.K.; Ko, M.E.; Luu, C.; Lee, W.; Lu, J.; Kim, J. Paracrine Activation of Chemokine Receptor CCR9 Enhances The Invasiveness of Pancreatic Cancer Cells. Cancer Microenviron. 2013, 6, 241–245. [Google Scholar] [CrossRef][Green Version]
- Singh, S.; Singh, U.P.; Stiles, J.K.; Grizzle, W.E.; Lillard, J.W., Jr. Expression and functional role of CCR9 in prostate cancer cell migration and invasion. Clin. Cancer Res. 2004, 10, 8743–8750. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, P.K.; Mir, H.; Singh, R.; Singh, N.; Kloecker, G.H.; Lillard, J.W., Jr.; Singh, S. CCR9/CCL25 expression in non-small cell lung cancer correlates with aggressive disease and mediates key steps of metastasis. Oncotarget 2014, 5, 10170–10179. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Tang, D.; Fan, L.; Gao, W.; Lin, H. CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner. Exp. Ther. Med. 2020, 19, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Edwards, R.; Tucci, S.; Bu, P.; Milsom, J.; Lee, S.; Edelmann, W.; Gümüs, Z.H.; Shen, X.; Lipkin, S. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J. Clin. Investig. 2012, 122, 3184–3196. [Google Scholar] [CrossRef]
- Johnson, E.L.; Singh, R.; Johnson-Holiday, C.M.; Grizzle, W.E.; Partridge, E.E.; Lillard, J.W., Jr.; Singh, S. CCR9 interactions support ovarian cancer cell survival and resistance to cisplatin-induced apoptosis in a PI3K-dependent and FAK-independent fashion. J. Ovarian Res. 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Holiday, C.; Singh, R.; Johnson, E.L.; Grizzle, W.E.; Lillard, J.W., Jr.; Singh, S. CCR9-CCL25 interactions promote cisplatin resistance in breast cancer cell through Akt activation in a PI3K-dependent and FAK-independent fashion. World J. Surg. Oncol. 2011, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.; Zhong, Y.; Lan, J.; Li, X.; Lin, H. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway. Med. Oncol. 2015, 32, 66. [Google Scholar] [CrossRef]
- Xu, B.; Deng, C.; Wu, X.; Ji, T.; Zhao, L.; Han, Y.; Yang, W.; Qi, Y.; Wang, Z.; Yang, Z.; et al. CCR9 and CCL25: A review of their roles in tumor promotion. J. Cell. Physiol. 2020, 235, 9121–9132. [Google Scholar] [CrossRef]
- Chen, H.; Cong, X.; Wu, C.; Wu, X.; Wang, J.; Mao, K.; Li, J.; Zhu, G.; Liu, F.; Meng, X.; et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9+ T cells. Sci. Adv. 2020, 6, eaax4690. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, J.; Jiang, F.; Wang, Y.; Yan, Q.; Yu, N.; Zhang, J.; Zhang, J.; Li, M.; He, Y. CD33+ CD14+ CD11b+ HLA-DR- monocytic myeloid-derived suppressor cells recruited and activated by CCR9/CCL25 are crucial for the pathogenic progression of endometriosis. Am. J. Reprod. Immunol. 2019, 81, e13067. [Google Scholar] [CrossRef]
- Lazarus, N.H.; Kunkel, E.J.; Johnston, B.; Wilson, E.; Youngman, K.R.; Butcher, E.C. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 2003, 170, 3799–3805. [Google Scholar] [CrossRef]
- Xiong, N.; Fu, Y.; Hu, S.; Xia, M.; Yang, J. CCR10 and its ligands in regulation of epithelial immunity and diseases. Protein Cell 2012, 3, 571–580. [Google Scholar] [CrossRef]
- John, A.E.; Thomas, M.S.; Berlin, A.A.; Lukacs, N.W. Temporal production of CCL28 corresponds to eosinophil accumulation and airway hyperreactivity in allergic airway inflammation. Am. J. Pathol. 2005, 166, 345–353. [Google Scholar] [CrossRef]
- Wang, W.; Soto, H.; Oldham, E.R.; Buchanan, M.E.; Homey, B.; Catron, D.; Jenkins, N.; Copeland, N.G.; Gilbert, D.J.; Nguyen, N.; et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). J. Biol. Chem. 2000, 275, 22313–22323. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Wang, W.; Soto, H.; Buchanan, M.E.; Wiesenborn, A.; Catron, D.; Müller, A.; McClanahan, T.K.; Dieu-Nosjean, M.C.; Orozco, R.; et al. Cutting edge: The orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J. Immunol. 2000, 164, 3465–3470. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Alenius, H.; Müller, A.; Soto, H.; Bowman, E.P.; Yuan, W.; McEvoy, L.; Lauerma, A.I.; Assmann, T.; Bünemann, E.; et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 2002, 8, 157–165. [Google Scholar] [CrossRef]
- Baird, J.W.; Nibbs, R.J.; Komai-Koma, M.; Connolly, J.A.; Ottersbach, K.; Clark-Lewis, I.; Liew, F.Y.; Graham, G.J. ESkine, a novel beta-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J. Biol. Chem. 1999, 274, 33496–33503. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Deng, L.; Wang, B.Z. CCL28 chemokine: An anchoring point bridging innate and adaptive immunity. Int. Immunopharmacol. 2017, 51, 165–170. [Google Scholar] [CrossRef]
- Gortz, A.; Nibbs, R.J.; McLean, P.; Jarmin, D.; Lambie, W.; Baird, J.W.; Graham, G.J. The chemokine ESkine/CCL27 displays novel modes of intracrine and paracrine function. J. Immunol. 2002, 169, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Ledee, D.R.; Chen, J.; Tonelli, L.H.; Takase, H.; Gery, I.; Zelenka, P.S. Differential expression of splice variants of chemokine CCL27 mRNA in lens, cornea, and retina of the normal mouse eye. Mol. Vis. 2004, 10, 663–667. [Google Scholar]
- Nibbs, R.J.; Graham, G.J. CCL27/PESKY: A novel paradigm for chemokine function. Expert Opin. Biol. Ther. 2003, 3, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mickanin, C.S.; Bhatia, U.; Labow, M. Identification of a novel beta-chemokine, MEC, down-regulated in primary breast tumors. Int. J. Oncol. 2001, 18, 939–944. [Google Scholar] [PubMed]
- Dimberg, J.; Hugander, A.; Wågsäter, D. Protein expression of the chemokine, CCL28, in human colorectal cancer. Int. J. Oncol. 2006, 28, 315–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Degos, C.; Heinemann, M.; Barrou, J.; Boucherit, N.; Lambaudie, E.; Savina, A.; Gorvel, L.; Olive, D. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsi, A.; Müller, A.; Hippe, A.; Rieker, J.; van Lierop, A.; Steinhoff, M.; Seeliger, S.; Kubitza, R.; Pippirs, U.; Meller, S.; et al. Tumor immune escape by the loss of homeostatic chemokine expression. Proc. Natl. Acad. Sci. USA 2007, 104, 19055–19060. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.H.; Chen, Y.Y.; Ma, D.; Chen, H.Y.; Ding, K.F.; Yu, K.D. Complicated prognostic values of CCL28 in breast cancer by subtype. J. Thorac. Dis. 2019, 11, 777–787. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.; Thompson, A.K.; Monteagudo, C. High CCL27 immunoreactivity in ‘supratumoral’ epidermis correlates with better prognosis in patients with cutaneous malignant melanoma. J. Clin. Pathol. 2017, 70, 15–19. [Google Scholar] [CrossRef]
- Gao, J.Q.; Tsuda, Y.; Katayama, K.; Nakayama, T.; Hatanaka, Y.; Tani, Y.; Mizuguchi, H.; Hayakawa, T.; Yoshie, O.; Tsutsumi, Y.; et al. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res. 2003, 63, 4420–4425. [Google Scholar]
- Gao, J.Q.; Tsuda, Y.; Han, M.; Xu, D.H.; Kanagawa, N.; Hatanaka, Y.; Tani, Y.; Mizuguchi, H.; Tsutsumi, Y.; Mayumi, T.; et al. NK cells are migrated and indispensable in the anti-tumor activity induced by CCL27 gene therapy. Cancer Immunol. Immunother. 2009, 58, 291–299. [Google Scholar] [CrossRef]
- Maghazachi, A.A.; Sand, K.L.; Al-Jaderi, Z. Glatiramer Acetate, Dimethyl Fumarate, and Monomethyl Fumarate Upregulate the Expression of CCR10 on the Surface of Natural Killer Cells and Enhance Their Chemotaxis and Cytotoxicity. Front. Immunol. 2016, 7, 437. [Google Scholar] [CrossRef]
- Okada, N.; Sasaki, A.; Niwa, M.; Okada, Y.; Hatanaka, Y.; Tani, Y.; Mizuguchi, H.; Nakagawa, S.; Fujita, T.; Yamamoto, A. Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther. 2006, 13, 393–405. [Google Scholar] [CrossRef][Green Version]
- Xie, F.; Liu, L.B.; Shang, W.Q.; Chang, K.K.; Meng, Y.H.; Mei, J.; Yu, J.J.; Li, D.J.; Li, M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef]
- Huang, G.; Tao, L.; Shen, S.; Chen, L. Hypoxia induced CCL28 promotes angiogenesis in lung adenocarcinoma by targeting CCR3 on endothelial cells. Sci. Rep. 2016, 6, 27152. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Cardones, A.R.; Finkelstein, S.E.; Restifo, N.P.; Klaunberg, B.A.; Nestle, F.O.; Castillo, S.S.; Dennis, P.A.; Hwang, S.T. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J. Exp. Med. 2003, 198, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Zhang, H.Y.; Du, W.; Qin, Z.; Yao, Y.; Mao, Y.; Zhou, L. Upregulation of chemokine receptor CCR10 is essential for glioma proliferation, invasion and patient survival. Oncotarget 2014, 5, 6576–6583. [Google Scholar] [CrossRef][Green Version]
- Yang, X.L.; Liu, K.Y.; Lin, F.J.; Shi, H.M.; Ou, Z.L. CCL28 promotes breast cancer growth and metastasis through MAPK-mediated cellular anti-apoptosis and pro-metastasis. Oncol. Rep. 2017, 38, 1393–1401. [Google Scholar] [CrossRef]
- Park, J.; Zhang, X.; Lee, S.K.; Song, N.Y.; Son, S.H.; Kim, K.R.; Shim, J.H.; Park, K.K.; Chung, W.Y. CCL28-induced RARβ expression inhibits oral squamous cell carcinoma bone invasion. J. Clin. Investig. 2019, 129, 5381–5399. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, B.H.; Yin, X.; Ren, Z.G. Role of chemokine CCL28 in hypoxia-induced migration of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2013, 21, 524–527. [Google Scholar] [PubMed]
- Lin, H.Y.; Sun, S.M.; Lu, X.F.; Chen, P.Y.; Chen, C.F.; Liang, W.Q.; Peng, C.Y. CCR10 activation stimulates the invasion and migration of breast cancer cells through the ERK1/2/MMP-7 signaling pathway. Int. Immunopharmacol. 2017, 51, 124–130. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Roy, I.; Boyle, K.A.; Vonderhaar, E.P.; Zimmerman, N.P.; Gorse, E.; Mackinnon, A.C.; Hwang, R.F.; Franco-Barraza, J.; Cukierman, E.; Tsai, S.; et al. Cancer cell chemokines direct chemotaxis of activated stellate cells in pancreatic ductal adenocarcinoma. Lab. Investig. 2017, 97, 302–317. [Google Scholar] [CrossRef]
- Yang, X.W.; Jiang, H.X.; Lei, R.; Lu, W.S.; Tan, S.H.; Qin, S.Y. Recruitment and significance of Th22 cells and Th17 cells in malignant ascites. Oncol. Lett. 2018, 16, 5389–5397. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Cao, Y.F.; Jiang, Z.Y.; Zhang, S.; Gao, F. Th22 cell accumulation is associated with colorectal cancer development. World J. Gastroenterol. 2015, 21, 4216–4224. [Google Scholar] [CrossRef]
- Karnezis, T.; Farnsworth, R.H.; Harris, N.C.; Williams, S.P.; Caesar, C.; Byrne, D.J.; Herle, P.; Macheda, M.L.; Shayan, R.; Zhang, Y.F.; et al. CCL27/CCL28-CCR10 Chemokine Signaling Mediates Migration of Lymphatic Endothelial Cells. Cancer Res. 2019, 79, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Heneweer, M.; Muusse, M.; Dingemans, M.; de Jong, P.C.; van den Berg, M.; Sanderson, J.T. Co-culture of primary human mammary fibroblasts and MCF-7 cells as an in vitro breast cancer model. Toxicol. Sci. 2005, 83, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.J.; O’Sullivan, J.N.; Ryan, E.J. Tumor conditioned media from colorectal cancer patients inhibits dendritic cell maturation. Oncoimmunology 2012, 1, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wang, R.; Boulbes, D.R.; Zhang, H.; Watowich, S.S.; Xia, L.; Ye, X.; Bhattacharya, R.; Ellis, L.M. Macrophage conditioned medium promotes colorectal cancer stem cell phenotype via the hedgehog signaling pathway. PLoS ONE 2018, 13, e0190070. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).