Alanine Scanning Mutagenesis of the DRYxxI Motif and Intracellular Loop 2 of Human Melanocortin-4 Receptor
Abstract
1. Introduction
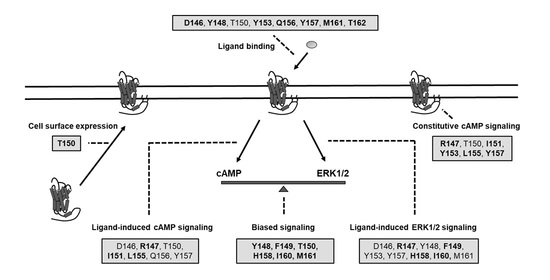
2. Results
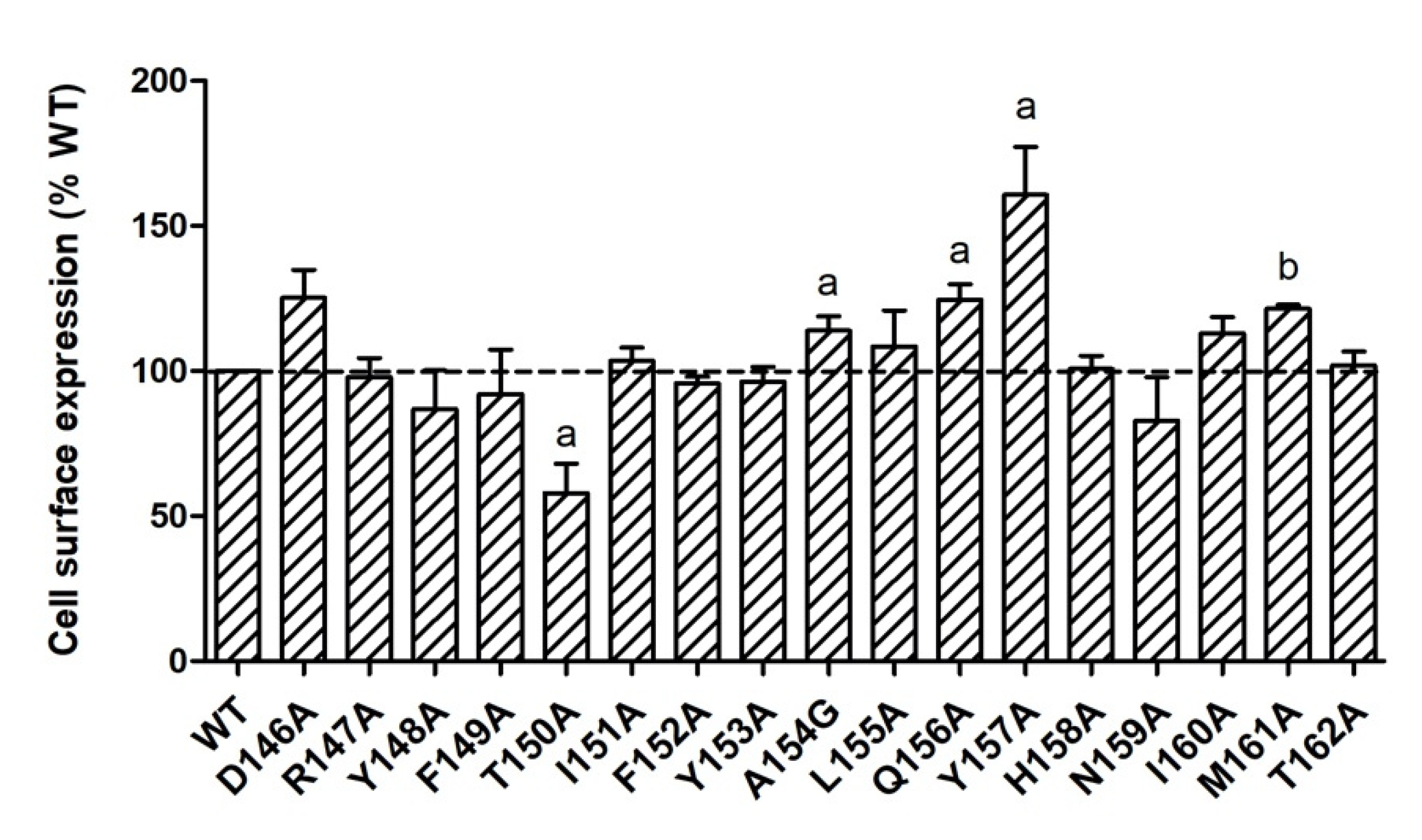
2.1. Cell Surface Expression of the Mutant MC4Rs
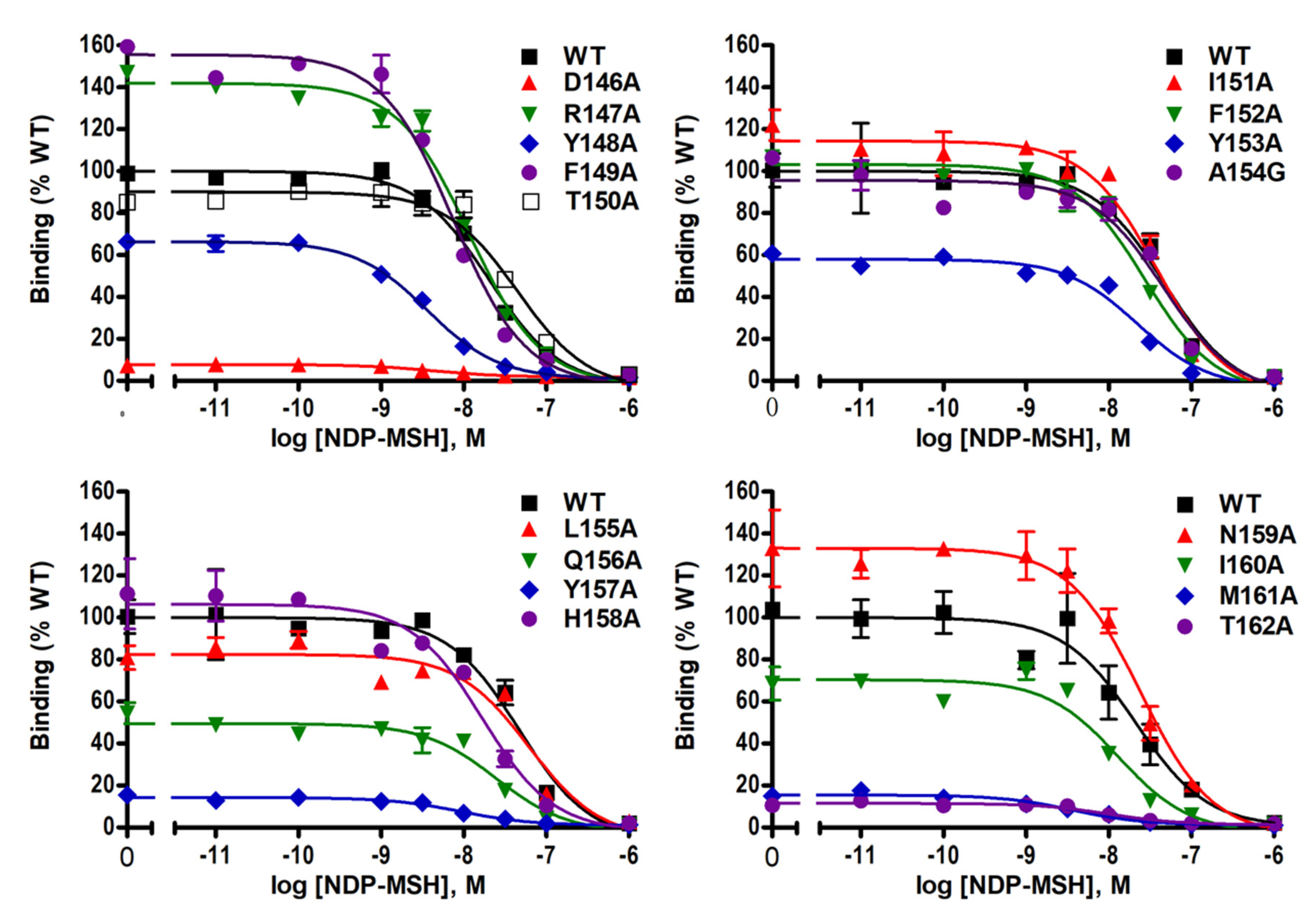
2.2. Ligand-Binding Properties of the Mutant MC4Rs
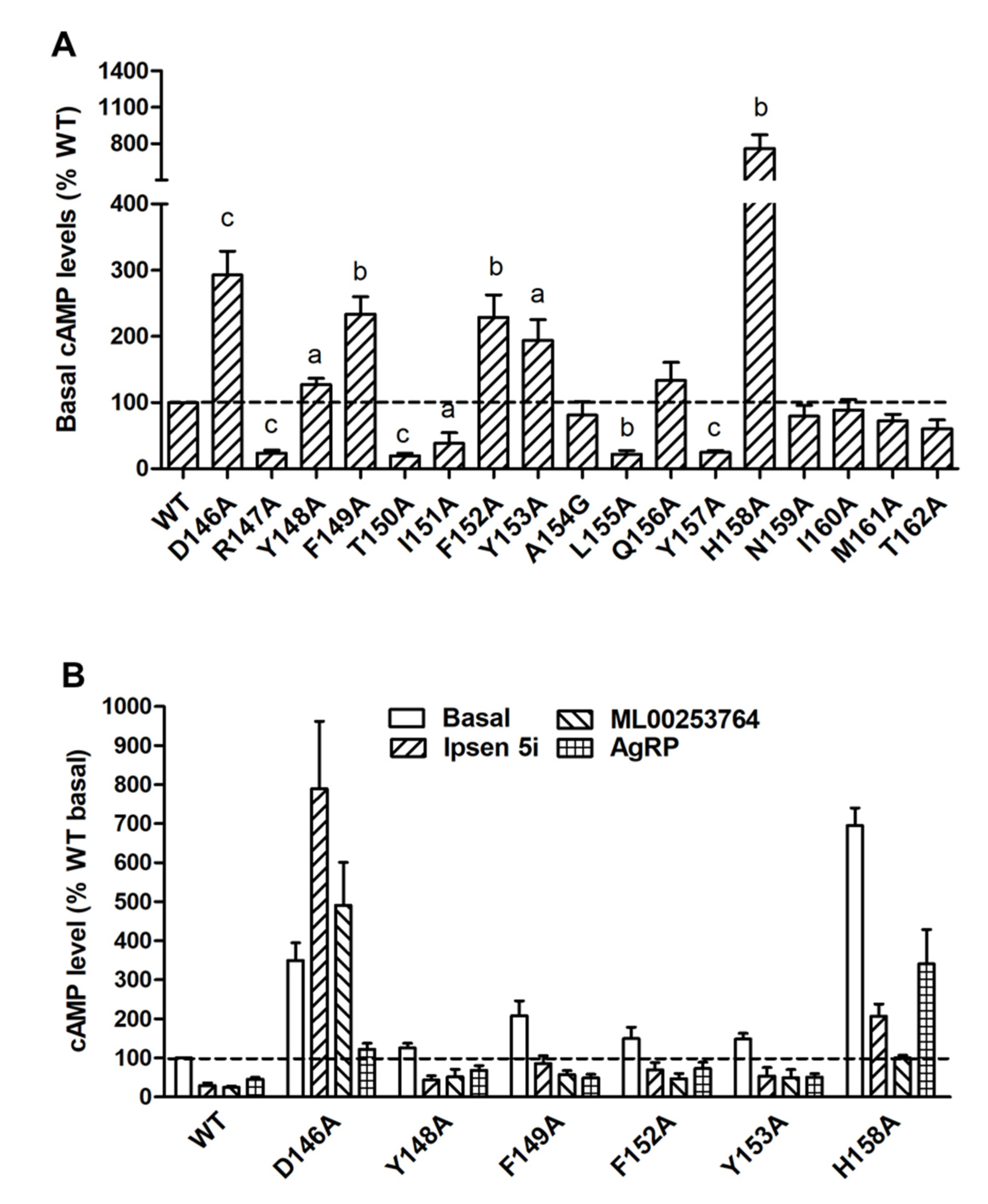
2.3. Signaling Properties of the Mutant MC4Rs in the cAMP Pathway
2.4. Signaling Properties of the Mutant MC4Rs in the ERK1/2 Pathway
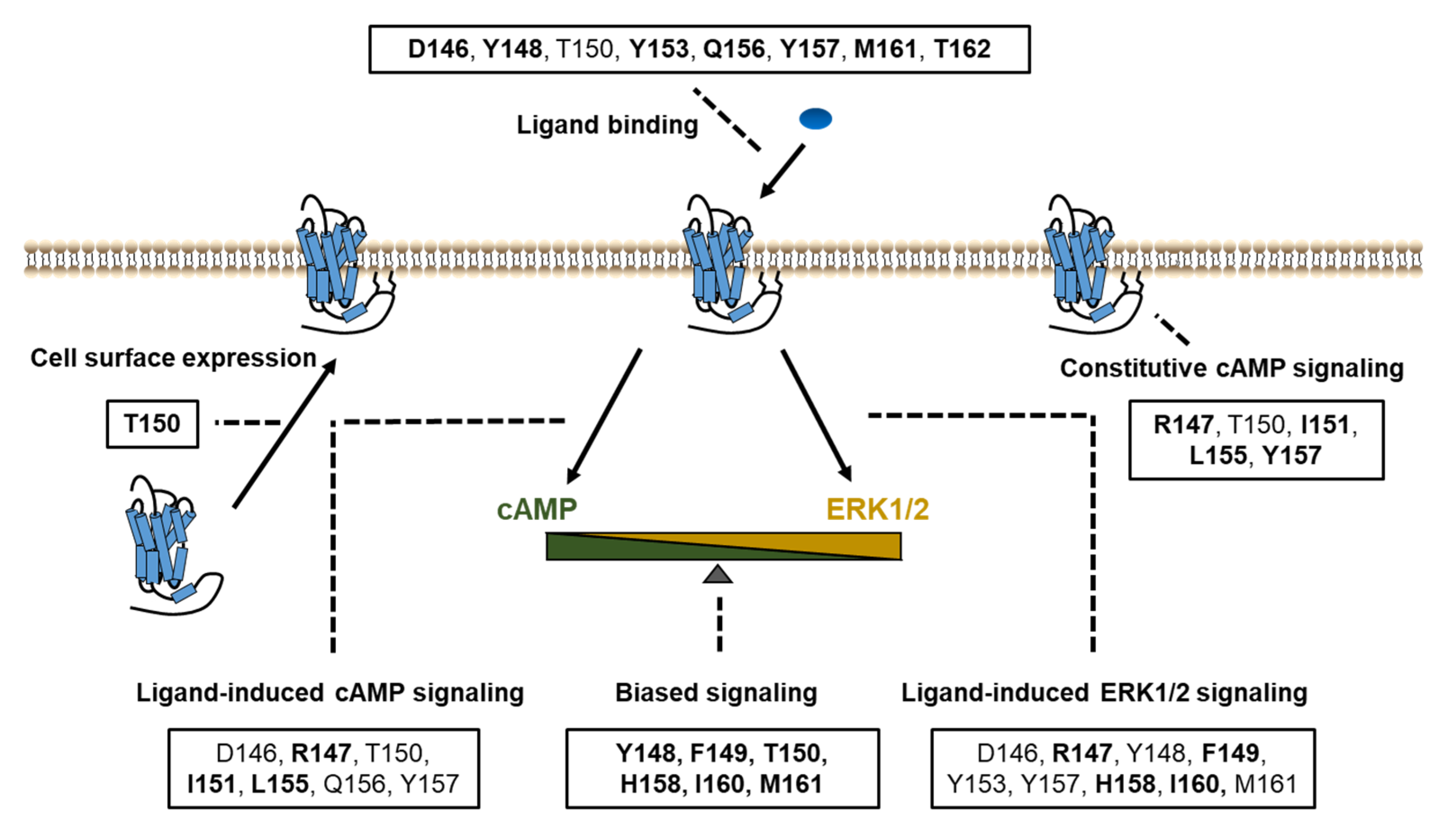
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Site-Directed Mutagenesis
4.3. Cell Culture and Transfection
4.4. Quantification of Receptor Cell Surface Expression by Flow Cytometry
4.5. Competitive Ligand Binding Assay
4.6. cAMP Assay
4.7. Protein Preparation and Western Blot
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Adenylyl cyclase |
| AgRP | Agouti-related peptide |
| CNS | Central nervous system |
| EC50 | Half maximal effective concentration |
| ECL | Extracellular loop |
| ERK1/2 | Extracellular signal-regulated protein kinases 1 and 2 |
| GPCR | G protein-coupled receptor |
| Gs | Stimulatory G protein |
| HEK293T | Human embryonic kidney 293T |
| IC50 | Half maximal inhibitory concentration |
| ICL | Intracellular loop |
| MAPK | Mitogen-activated protein kinase |
| MC3R | Melanocortin-3 receptor |
| MC4R | Melanocortin-4 receptor |
| MSH | Melanocyte-stimulating hormone |
| NDP-MSH | [Nle4, D-Phe7]-α-melanocyte-stimulating hormone |
| pERK1/2 | Phosphorylated ERK1/2 |
| PKA | Protein kinase A |
| TMD | Transmembrane domain |
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, S.; Farooqi, I.S.; Yeo, G.S.; Challis, B.G. Minireview: Human obesity-lessons from monogenic disorders. Endocrinology 2003, 144, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef]
- Tao, Y.X. The melanocortin-4 receptor: Physiology, pharmacology, and pathophysiology. Endocr. Rev. 2010, 31, 506–543. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.; Patten, C.S.; Roth, J.D.; Yee, D.K.; Fluharty, S.J. Melanocortin receptor signaling through mitogen-activated protein kinase in vitro and in rat hypothalamus. Brain Res. 2003, 986, 1–11. [Google Scholar] [CrossRef]
- Vongs, A.; Lynn, N.M.; Rosenblum, C.I. Activation of MAP kinase by MC4-R through PI3 kinase. Regul. Pept. 2004, 120, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tao, Y.X. Pleiotropic functions of the transmembrane domain 6 of human melanocortin-4 receptor. J. Mol. Endocrinol. 2012, 49, 237–248. [Google Scholar] [CrossRef]
- Mo, X.L.; Yang, R.; Tao, Y.X. Functions of transmembrane domain 3 of human melanocortin-4 receptor. J. Mol. Endocrinol. 2012, 49, 221–235. [Google Scholar] [CrossRef]
- Sutton, G.M.; Duos, B.; Patterson, L.M.; Berthoud, H.R. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 2005, 146, 3739–3747. [Google Scholar] [CrossRef]
- Damm, E.; Buech, T.R.; Gudermann, T.; Breit, A. Melanocortin-induced PKA activation inhibits AMPK activity via ERK-1/2 and LKB-1 in hypothalamic GT1-7 cells. Mol. Endocrinol. 2012, 26, 643–654. [Google Scholar] [CrossRef]
- He, S.; Tao, Y.X. Defect in MAPK signaling as a cause for monogenic obesity caused by inactivating mutations in the melanocortin-4 receptor gene. Int. J. Biol. Sci. 2014, 10, 1128–1137. [Google Scholar] [CrossRef]
- Srinivasan, S.; Lubrano-Berthelier, C.; Govaerts, C.; Picard, F.; Santiago, P.; Conklin, B.R.; Vaisse, C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J. Clin. Investig. 2004, 114, 1158–1164. [Google Scholar] [CrossRef]
- Tao, Y.X. Constitutive activity in melanocortin-4 receptor: Biased signaling of inverse agonists. Adv. Pharmacol. 2014, 70, 135–154. [Google Scholar]
- Agosti, F.; Cordisco Gonzalez, S.; Martinez Damonte, V.; Tolosa, M.J.; Di Siervi, N.; Schioth, H.B.; Davio, C.; Perello, M.; Raingo, J. Melanocortin 4 receptor constitutive activity inhibits L-type voltage-gated calcium channels in neurons. Neuroscience 2017, 346, 102–112. [Google Scholar] [CrossRef]
- Vogel, R.; Mahalingam, M.; Lüdeke, S.; Huber, T.; Siebert, F.; Sakmar, T.P. Functional role of the “ionic lock”—An interhelical hydrogen-bond network in family A heptahelical receptors. J. Mol. Biol. 2008, 380, 648–655. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Parnot, C.; Deupi, X.; Ratnala, V.R.P.; Swaminath, G.; Farrens, D.; Kobilka, B. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol. 2006, 2, 417. [Google Scholar] [CrossRef]
- Raman, D.; Osawa, S.; Weiss, E.R. Binding of arrestin to cytoplasmic loop mutants of bovine rhodopsin. Biochemistry 1999, 38, 5117–5123. [Google Scholar] [CrossRef]
- Huang, H.; Tao, Y.X. Functions of the DRY motif and intracellular loop 2 of human melanocortin 3 receptor. J. Mol. Endocrinol. 2014, 53, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-phenylalanine-α-melanocyte-stimulating hormone: A highly potent α-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. USA 1980, 77, 5754–5758. [Google Scholar] [CrossRef]
- Tao, Y.X.; Conn, P.M. Chaperoning G protein-coupled receptors: From cell biology to therapeutics. Endocr. Rev. 2014, 35, 602–647. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Litherland, S.A.; Sorensen, N.B.; Proneth, B.; Wood, M.S.; Shaw, A.M.; Millard, W.J.; Haskell-Luevano, C. Pharmacological characterization of 40 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists and the agouti-related protein (AGRP) antagonist. Biochemistry 2006, 45, 7277–7288. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Mutations in melanocortin-4 receptor and human obesity. Prog. Mol. Biol. Transl. Sci. 2009, 88, 173–204. [Google Scholar] [PubMed]
- Yu, J.; Gimenez, L.E.; Hernandez, C.C.; Wu, Y.; Wein, A.H.; Han, G.W.; McClary, K.; Mittal, S.R.; Burdsall, K.; Stauch, B.; et al. Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science 2020, 368, 428–433. [Google Scholar]
- Kleinau, G.; Heyder, N.A.; Tao, Y.X.; Scheerer, P. Structural complexity and plasticity of signaling regulation at the melanocortin-4 receptor. Int. J. Mol. Sci. 2020, 21, 5728. [Google Scholar] [CrossRef]
- Erlenbach, I.; Kostenis, E.; Schmidt, C.; Serradeil-Le Gal, C.; Raufaste, D.; Dumont, M.E.; Pausch, M.H.; Wess, J. Single amino acid substitutions and deletions that alter the G protein coupling properties of the V2 vasopressin receptor identified in yeast by receptor random mutagenesis. J. Biol. Chem. 2001, 276, 29382–29392. [Google Scholar] [CrossRef]
- Lu, Z.L.; Gallagher, R.; Sellar, R.; Coetsee, M.; Millar, R.P. Mutations remote from the human gonadotropin-releasing hormone (GnRH) receptor-binding sites specifically increase binding affinity for GnRH II but not GnRH I: Evidence for ligand-selective, receptor-active conformations. J. Biol. Chem. 2005, 280, 29796–29803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Z.L.; Tao, Y.X. Functions of DPLIY motif and helix 8 of human melanocortin-3 receptor. J. Mol. Endocrinol. 2015, 55, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: Cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002, 366, 381–416. [Google Scholar] [CrossRef]
- Bond, R.A.; IJzerman, A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 2006, 27, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Constitutive activation of G protein-coupled receptors and diseases: Insights into mechanism of activation and therapeutics. Pharmacol. Ther. 2008, 120, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Segaloff, D.L. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 5632–5638. [Google Scholar] [CrossRef]
- Fan, Z.C.; Tao, Y.X. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J. Cell. Mol. Med. 2009, 13, 3268–3282. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tao, Y.X. Functional studies on twenty novel naturally occurring melanocortin-4 receptor mutations. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1190–1199. [Google Scholar] [CrossRef]
- Vaisse, C.; Clement, K.; Durand, E.; Hercberg, S.; Guy-Grand, B.; Froguel, P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J. Clin. Investig. 2000, 106, 253–262. [Google Scholar] [CrossRef]
- Hinney, A.; Hohmann, S.; Geller, F.; Vogel, C.; Hess, C.; Wermter, A.K.; Brokamp, B.; Goldschmidt, H.; Siegfried, W.; Remschmidt, H.; et al. Melanocortin-4 receptor gene: Case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J. Clin. Endocrinol. Metab. 2003, 88, 4258–4267. [Google Scholar] [CrossRef]
- Valli-Jaakola, K.; Lipsanen-Nyman, M.; Oksanen, L.; Hollenberg, A.N.; Kontula, K.; Bjorbaek, C.; Schalin-Jantti, C. Identification and characterization of melanocortin-4 receptor gene mutations in morbidly obese Finnish children and adults. J. Clin. Endocrinol. Metab. 2004, 89, 940–945. [Google Scholar] [CrossRef]
- Mo, X.L.; Tao, Y.X. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1939–1948. [Google Scholar] [CrossRef]
- Yi, T.L.; Yang, L.K.; Ruan, G.L.; Yang, D.Q.; Tao, Y.X. Melanocortin-4 receptor in swamp eel (Monopterus albus): Cloning, tissue distribution, and pharmacology. Gene 2018, 678, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, X.F.; Li, G.L.; Tao, Y.X. Biased signaling in fish melanocortin-4 receptors (MC4Rs): Divergent pharmacology of four ligands on spotted scat (Scatophagus argus) and grass carp (Ctenopharyngodon idella) MC4Rs. Mol. Cell. Endocrinol. 2020, 515, 110929. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, M.; Lai, Y.; Gantz, I.; Georgeson, K.E.; Harmon, C.M. Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J. Biol. Chem. 2002, 277, 20328–20335. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, J.A.; Jensen, A.D.; Liapakis, G.; Rasmussen, S.G.; Shi, L.; Gether, U.; Javitch, J.A. Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 2001, 276, 29171–29177. [Google Scholar] [CrossRef] [PubMed]
- Angelova, K.; Fanelli, F.; Puett, D. A model for constitutive lutropin receptor activation based on molecular simulation and engineered mutations in transmembrane helices 6 and 7. J. Biol. Chem. 2002, 277, 32202–32213. [Google Scholar] [CrossRef] [PubMed]
- Greasley, P.J.; Fanelli, F.; Rossier, O.; Abuin, L.; Cotecchia, S. Mutagenesis and modelling of the a1b-adrenergic receptor highlight the role of the helix 3/helix 6 interface in receptor activation. Mol. Pharmacol. 2002, 61, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mizrachi, D.; Fanelli, F.; Segaloff, D.L. The formation of a salt bridge between helices 3 and 6 is responsible for the constitutive activity and lack of hormone responsiveness of the naturally occurring L457R mutation of the human lutropin receptor. J. Biol. Chem. 2005, 280, 26169–26176. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef]
- Rovati, G.E.; Capra, V.; Neubig, R.R. The highly conserved DRY motif of class A G protein-coupled receptors: Beyond the ground state. Mol. Pharmacol. 2007, 71, 959–964. [Google Scholar] [CrossRef]
- Scheerer, P.; Park, J.H.; Hildebrand, P.W.; Kim, Y.J.; Krauss, N.; Choe, H.W.; Hofmann, K.P.; Ernst, O.P. Crystal structure of opsin in its G-protein-interacting conformation. Nature 2008, 455, 497–502. [Google Scholar] [CrossRef]
- Rasmussen, S.G.; Choi, H.J.; Fung, J.J.; Pardon, E.; Casarosa, P.; Chae, P.S.; Devree, B.T.; Rosenbaum, D.M.; Thian, F.S.; Kobilka, T.S.; et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 2011, 469, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hinney, A.; Bettecken, T.; Tarnow, P.; Brumm, H.; Reichwald, K.; Lichtner, P.; Scherag, A.; Nguyen, T.T.; Schlumberger, P.; Rief, W.; et al. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J. Clin. Endocrinol. Metab. 2006, 91, 1761–1769. [Google Scholar] [CrossRef]
- Timossi, C.; Maldonado, D.; Vizcaíno, A.; Lindau-Shepard, B.; Conn, P.M.; Ulloa-Aguirre, A. Structural determinants in the second intracellular loop of the human follicle-stimulating hormone receptor are involved in Gs protein activation. Mol. Cell. Endocrinol. 2002, 189, 157–168. [Google Scholar] [CrossRef]
- Miura, S.I.; Zhang, J.; Karnik, S.S. Angiotensin II type 1 receptor-function affected by mutations in cytoplasmic loop CD. FEBS Lett. 2000, 470, 331–335. [Google Scholar] [CrossRef]
- Tao, Y.X.; Segaloff, D.L. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J. Clin. Endocrinol. Metab. 2004, 89, 3936–3942. [Google Scholar] [CrossRef][Green Version]
- Wacker, J.L.; Feller, D.B.; Tang, X.B.; Defino, M.C.; Namkung, Y.; Lyssand, J.S.; Mhyre, A.J.; Tan, X.; Jensen, J.B.; Hague, C. Disease-causing mutation in GPR54 reveals the importance of the second intracellular loop for class A G-protein-coupled receptor function. J. Biol. Chem. 2008, 283, 31068–31078. [Google Scholar] [CrossRef]
- Strickland, S.; Loeb, J.N. Obligatory separation of hormone binding and biological response curves in systems dependent upon secondary mediators of hormone action. Proc. Natl. Acad. Sci. USA 1981, 78, 1366–1370. [Google Scholar] [CrossRef]
- Tao, Y.X.; Segaloff, D.L. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology 2003, 144, 4544–4551. [Google Scholar] [CrossRef]
- Nickolls, S.A.; Fleck, B.; Hoare, S.R.; Maki, R.A. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: Evidence for ligand-specific conformational states. J. Pharmacol. Exp. Ther. 2005, 313, 1281–1288. [Google Scholar] [CrossRef]
- Büch, T.R.; Heling, D.; Damm, E.; Gudermann, T.; Breit, A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J. Biol. Chem. 2009, 284, 26411–26420. [Google Scholar] [CrossRef]
- Ghamari-Langroudi, M.; Digby, G.J.; Sebag, J.A.; Millhauser, G.L.; Palomino, R.; Matthews, R.; Gillyard, T.; Panaro, B.L.; Tough, I.R.; Cox, H.M.; et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 2015, 520, 94–98. [Google Scholar] [CrossRef]
- Yang, Z.; Tao, Y.X. Biased signaling initiated by agouti-related peptide through human melanocortin-3 and -4 receptors. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 1485–1494. [Google Scholar] [CrossRef]
- Yang, L.K.; Tao, Y.X. Biased signaling at neural melanocortin receptors in regulation of energy homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.K.; Hou, Z.S.; Tao, Y.X. Biased signaling in naturally occurring mutations of G protein-coupled receptors associated with diverse human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1867, 165973. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; IJzerman, A.P. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, M.; Kim, K.; Lee, D.; Jung, Y.; Oh, D.; Ko, Y.J.; Cho, A.E.; Cho, H.S.; Lee, W. Solution structure of the transmembrane 2 domain of the human melanocortin-4 receptor in sodium dodecyl sulfate (SDS) micelles and the functional implication of the D90N mutant. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Link, R.; Veiksina, S.; Tahk, M.J.; Laasfeld, T.; Paiste, P.; Kopanchuk, S.; Rinken, A. The constitutive activity of melanocortin-4 receptors in cAMP pathway is allosterically modulated by zinc and copper ions. J. Neurochem. 2020, 153, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Huang, H. Ipsen 5i is a novel potent pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Front. Endocrinol. 2014, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.L.; Kipnis, D.M.; Utiger, R.; Parker, C. Radioimmunoassay for the measurement of adenosine 3’,5’-cyclic phosphate. Proc. Natl. Acad. Sci. USA 1969, 64, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987, 7, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Fan, Z.C.; Tao, Y.X. Functions of acidic transmembrane residues in human melanocortin-3 receptor binding and activation. Biochem. Pharmacol. 2008, 76, 520–530. [Google Scholar] [CrossRef] [PubMed]

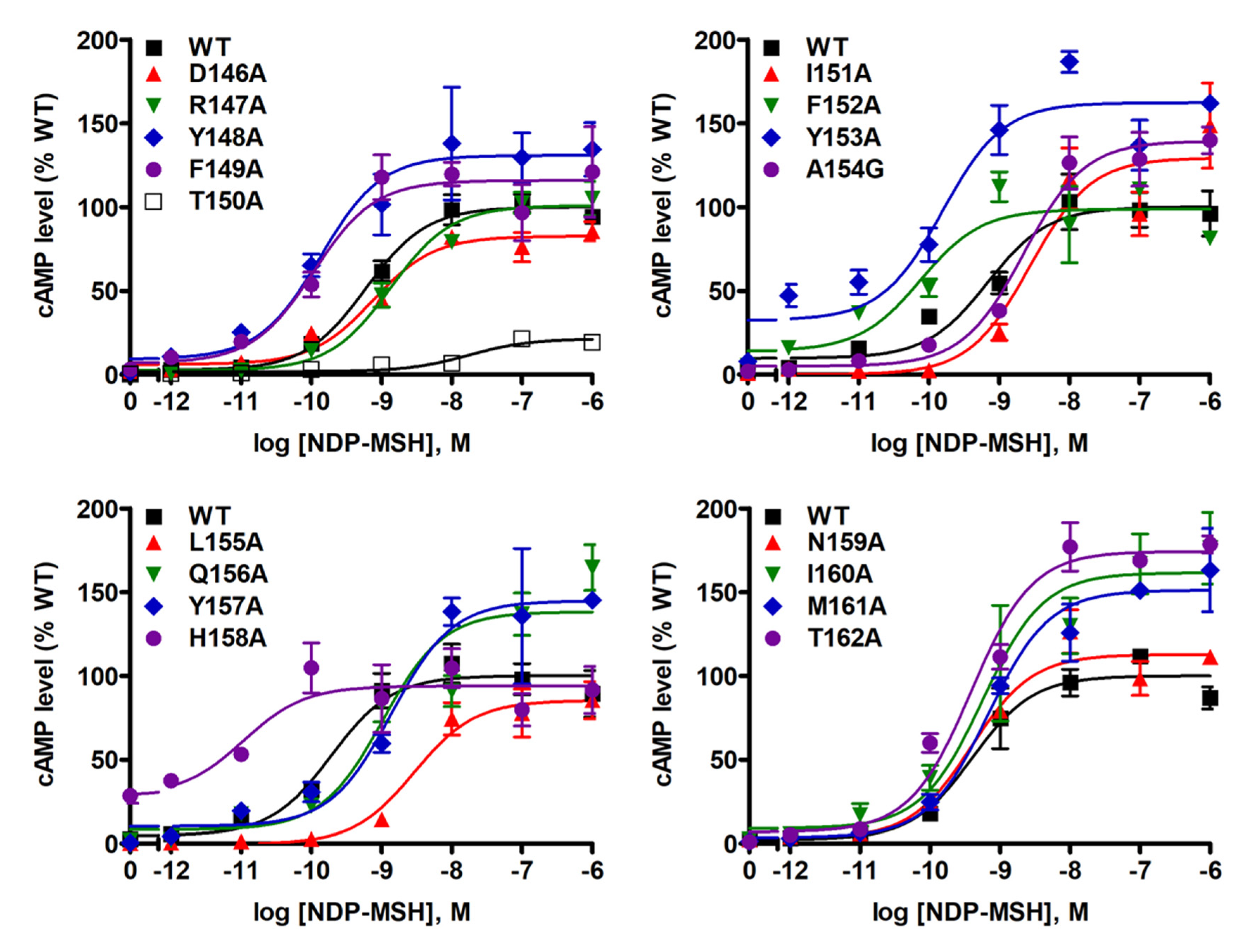
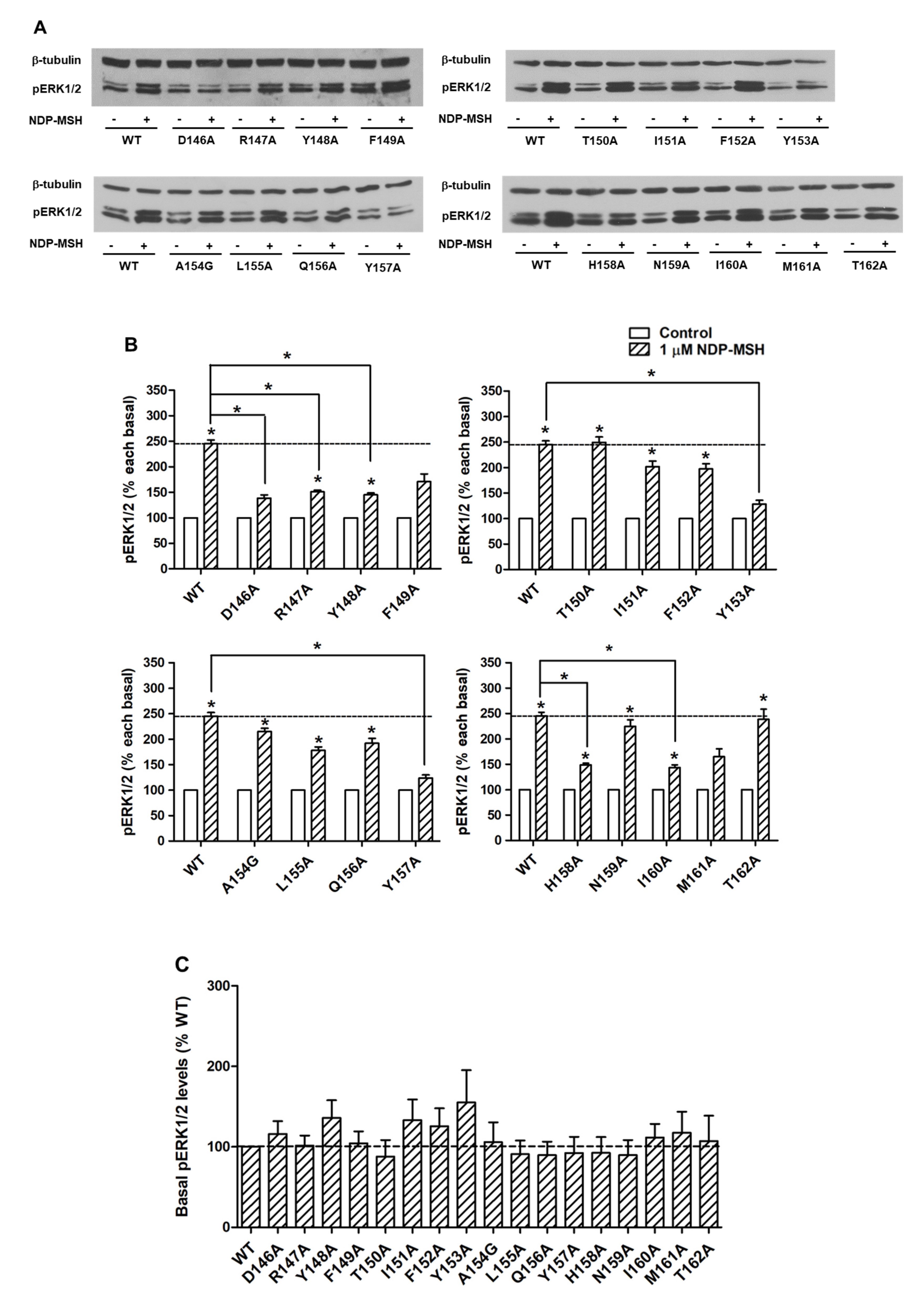
| NDP-MSH Binding | Basal Activity (% WT) | NDP-MSH-Stimulated cAMP | n | NDP-MSH-Stimulated ERK1/2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | IC50 (nM) | Bmax (% WT) | n | Basal cAMP levels | n | EC50 (nM) | Rmax (% WT) | |||
| WT | 14 | 19.43 ± 2.10 | 100 ± 0 | 31 | 100 ± 0 | 25 | 0.38 ± 0.07 | 100 ± 0 | 16 | + |
| D146A | 3 | 3.24 ± 0.70 a | 6.47 ± 0.97 b | 9 | 292.67 ± 35.94 b | 4 | 1.07 ± 0.18 a | 108.52 ± 12.25 | 6 | – |
| R147A | 3 | 11.86 ± 0.25 | 176.71 ± 38.93 | 4 | 23.62 ± 4.31 b | 4 | 1.73 ± 0.44 a | 121.75 ± 14.65 | 6 | – |
| Y148A | 3 | 4.31 ± 1.78 b | 60.38 ± 10.62 a | 9 | 126.90 ± 9.86 a | 3 | 0.21 ± 0.09 | 136.92 ± 8.17 a | 6 | – |
| F149A | 3 | 6.30 ± 0.83 | 156.37 ± 13.73 a | 8 | 233.51 ± 26.15 a | 3 | 0.24 ± 0.08 | 154.21 ± 31.84 | 5 | – |
| T150A | 3 | 40.25 ± 6.11 a | 95.30 ± 3.43 | 4 | 19.34 ± 4.05 b | 4 | 11.55 ± 2.38 b | 17.62 ± 3.60 b | 6 | + |
| I151A | 4 | 17.78 ± 6.96 | 124.47 ± 28.35 | 3 | 38.42 ± 15.77 a | 3 | 3.29 ± 0.27 b | 93.52 ± 24.82 | 5 | + |
| F152A | 3 | 17.29 ± 5.63 | 76.21 ± 14.64 | 11 | 228.74 ± 33.95 b | 5 | 0.06 ± 0.02 a | 115.47 ± 14.57 | 5 | + |
| Y153A | 3 | 13.02 ± 5.03 | 56.84 ± 4.52 b | 9 | 194.08 ± 31.05 b | 3 | 0.26 ± 0.10 | 152.24 ± 28.47 | 5 | – |
| A154G | 3 | 28.72 ± 9.72 | 84.83 ± 6.18 | 3 | 80.70 ± 21.17 | 3 | 0.87 ± 0.10 | 110.06 ± 10.78 | 8 | + |
| L155A | 3 | 50.80 ± 14.05 | 79.61 ± 8.63 | 4 | 21.77 ± 5.64 b | 4 | 6.03 ± 1.42 b | 100.44 ± 5.25 | 7 | + |
| Q156A | 3 | 15.41 ± 5.45 | 61.26 ± 6.10 b | 4 | 133.59 ± 27.19 | 4 | 1.76 ± 0.43 a | 129.25 ± 23.87 | 8 | + |
| Y157A | 3 | 7.43 ± 2.02 | 14.88 ± 2.18 b | 5 | 24.72 ± 2.89 b | 4 | 2.85 ± 0.61 b | 131.64 ± 18.30 | 7 | – |
| H158A | 3 | 10.36 ± 3.15 | 138.97 ± 25.34 | 8 | 761.86 ± 111.54 b | 3 | 0.03 ± 0.02 a | 109.75 ± 26.84 | 7 | – |
| N159A | 3 | 15.19 ± 5.31 | 119.92 ± 6.62 a | 4 | 79.16 ± 16.55 | 4 | 0.30 ± 0.07 | 92.29 ± 16.32 | 7 | + |
| I160A | 3 | 6.77 ± 2.07 a | 95.24 ± 16.43 | 3 | 88.84 ± 15.64 | 3 | 0.20 ± 0.18 | 109.91 ± 28.73 | 7 | – |
| M161A | 4 | 3.47 ± 1.19 b | 13.18 ± 2.67 b | 4 | 72.38 ± 9.95 | 4 | 0.41 ± 0.16 | 137.22 ± 11.70 a | 7 | – |
| T162A | 3 | 5.79 ± 2.35 | 13.73 ± 1.17 b | 4 | 60.37 ± 13.41 | 4 | 0.46 ± 0.10 | 145.81 ± 13.95 a | 8 | + |
| Constructs | Forward Primer Sequences (5′→3′) |
|---|---|
| D146A | CAATTGCAGTGGCCAGGTACTTTAC |
| R147A | CAATTGCAGTGGACGCGTACTTTACT |
| Y148A | GCAGTGGACAGGGCCTTTACTATCTT |
| F149A | CAGTGGACAGGTACGCTACTATCTTC |
| T150A | GACAGGTACTTTGCTATCTTCTATG |
| I151A | CAGGTACTTTACTGCCTTCTATGCTCT |
| F152A | GTACTTTACTATCGCCTATGCTCTCCA |
| Y153A | CTTTACTATCTTCGCTGCTCTCCAGTA |
| A154G | CTATCTTCTATGGTCTCCAGTACC |
| L155A | CTATCTTCTATGCTGCCCAGTACCATA |
| Q156A | CTATGCTCTCGCGTACCATAAC |
| Y157A | CTATGCTCTCCAGGCCCATAACATTAT |
| H158A | GCTCTCCAGTACGCTAACATTATGAC |
| N159A | CTCCAGTACCATGCCATTATGACAG |
| I160A | CCAGTACCATAACGCTATGACAGTTA |
| M161A | GTACCATAACATTGCGACAGTTAAGC |
| T162A | CATAACATTATGGCAGTTAAGCGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-K.; Tao, Y.-X. Alanine Scanning Mutagenesis of the DRYxxI Motif and Intracellular Loop 2 of Human Melanocortin-4 Receptor. Int. J. Mol. Sci. 2020, 21, 7611. https://doi.org/10.3390/ijms21207611
Yang L-K, Tao Y-X. Alanine Scanning Mutagenesis of the DRYxxI Motif and Intracellular Loop 2 of Human Melanocortin-4 Receptor. International Journal of Molecular Sciences. 2020; 21(20):7611. https://doi.org/10.3390/ijms21207611
Chicago/Turabian StyleYang, Li-Kun, and Ya-Xiong Tao. 2020. "Alanine Scanning Mutagenesis of the DRYxxI Motif and Intracellular Loop 2 of Human Melanocortin-4 Receptor" International Journal of Molecular Sciences 21, no. 20: 7611. https://doi.org/10.3390/ijms21207611
APA StyleYang, L.-K., & Tao, Y.-X. (2020). Alanine Scanning Mutagenesis of the DRYxxI Motif and Intracellular Loop 2 of Human Melanocortin-4 Receptor. International Journal of Molecular Sciences, 21(20), 7611. https://doi.org/10.3390/ijms21207611