Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research
Abstract
1. Introduction
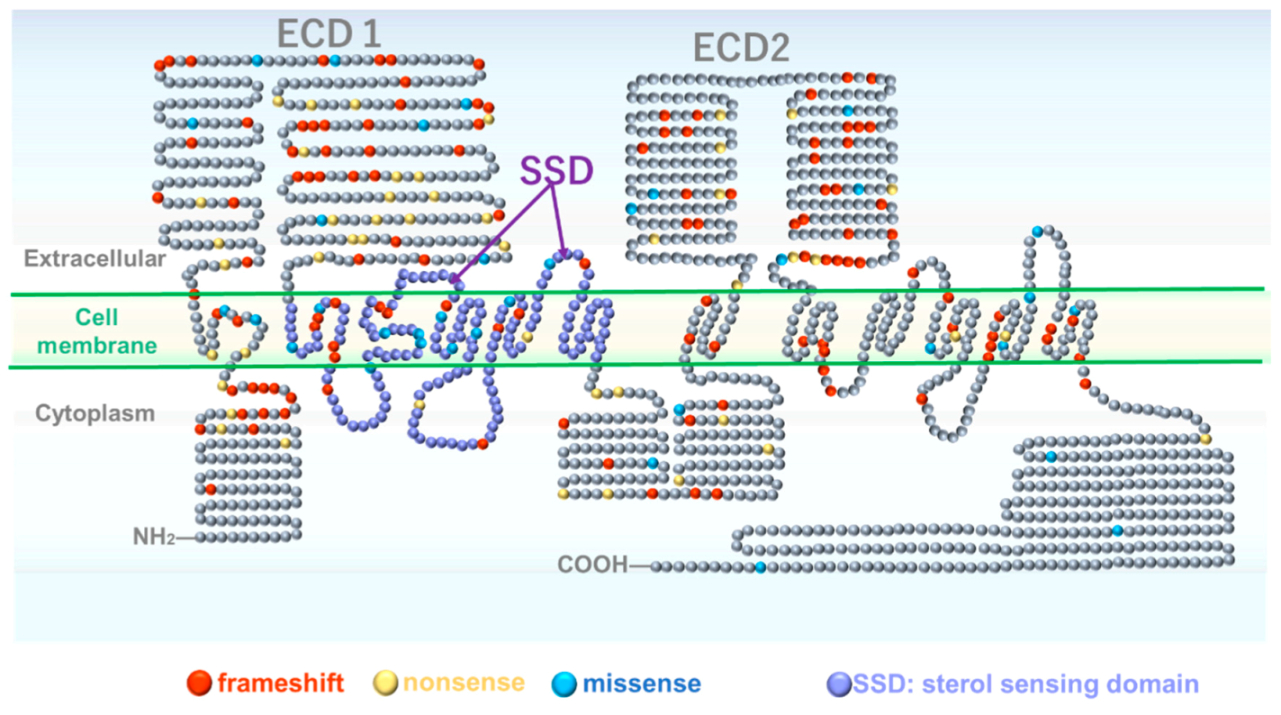
2. Genetic and Molecular Structural Aspects of Gorlin Syndrome
3. Genomic Instability
4. Bone Metabolism, Hedgehog Over-Activation and Pathological Mechanism
5. Tumors
5.1. Skin Cancer
5.2. Medulloblastoma
5.3. Keratocystic Odontogenic Tumor
5.4. Other Tumors
6. Cancer Development and Hh Pathway Activation
7. Disease-Specific Induced Pluripotent Stem Cells
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GS | Gorlin syndrome |
| BCC | Basal cell carcinomas |
| Hh | Hedgehog |
| hiPSCs | Human induced pluripotent stem cells |
| iPSC | Induced pluripotent stem cells |
| KCOT | Keratocystic odontogenic tumors |
| OKC | Odontogenic keratocysts |
References
- Gorlin, R.J.; Goltz, R.W. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N. Engl. J. Med. 1960, 262, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Gorlin Syndrome—Genetics Home Reference—NIH. Available online: https://ghr.nlm.nih.gov/condition/gorlin-syndrome (accessed on 13 August 2020).
- Pastorino, L.; Cusano, R.; Nasti, S.; Faravelli, F.; Forzano, F.; Baldo, C.; Barile, M.; Gliori, S.; Muggianu, M.; Ghigliotti, G.; et al. Molecular characterization of Italian nevoid basal cell carcinoma syndrome patients. Hum. Mutat. 2005, 25, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Savino, M.; d’Apolito, M.; Formica, V.; Baorda, F.; Mari, F.; Renieri, A.; Carabba, E.; Tarantino, E.; Andreucci, E.; Belli, S.; et al. Spectrum of PTCH mutations in Italian nevoid basal cell-carcinoma syndrome patients: Identification of thirteen novel alleles. Hum. Mutat. 2004, 24, 441. [Google Scholar] [CrossRef] [PubMed]
- Shanley, S.; Ratcliffe, J.; Hockey, A.; Haan, E.; Oley, C.; Ravine, D.; Martin, N.; Wicking, C.; Chenevix-Trench, G. Nevoid basal cell carcinoma syndrome: Review of 118 affected individuals. Am. J. Med. Genet. 1994, 50, 282–290. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Ladusans, E.J.; Rimmer, S.; Burnell, L.D.; Thakker, N.; Farndon, P.A. Complications of the naevoid basal cell carcinoma syndrome: Results of a population based study. J. Med. Genet. 1993, 30, 460–464. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. Part A 2010, 152, 327–332. [Google Scholar] [CrossRef]
- Endo, M.; Fujii, K.; Sugita, K.; Saito, K.; Kohno, Y.; Miyashita, T. Nationwide survey of nevoid basal cell carcinoma syndrome in Japan revealing the low frequency of basal cell carcinoma. Am. J. Med. Genet. A 2012, 158, 351–357. [Google Scholar] [CrossRef]
- Rahbari, H.; Mehregan, A.H. Basal cell epithelioma (carcinoma) in children and teenagers. Cancer 1982, 49, 350–353. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Kamihara, J.; Evans, D.G.R.; Brugières, L.; Bourdeaut, F.; Molenaar, J.J.; Walsh, M.F.; Brodeur, G.M.; Diller, L. Cancer surveillance in Gorlin syndrome and rhabdoid tumor predisposition syndrome. Clin. Cancer Res. 2017, 23, e62–e67. [Google Scholar] [CrossRef]
- Smith, M.J.; Beetz, C.; Williams, S.G.; Bhaskar, S.S.; O’Sullivan, J.; Anderson, B.; Daly, S.B.; Urquhart, J.E.; Bholah, Z.; Oudit, D.; et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J. Clin. Oncol. 2015, 32, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahawan, M.G.; Trevino, S.; Jacob, R.; Murray, J.C.; Al-Rahawan, M.M. Medulloblastoma in a toddler with Gorlin syndrome. Baylor Univ. Med. Cent. Proc. 2018, 31, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, S.; Robinson, G.; Ellison, D.W.; Wu, G.; He, X.; Lu, Q.R.; Mclendon, R.; Grant, G.; Driscoll, T.; Neuberg, R. Gorlin syndrome and desmoplastic medulloblastoma: Report of 3 cases with unfavorable clinical course and novel mutations. Pediatr. Blood Cancer 2015, 62, 1855–1858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo Muzio, L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J. Rare Dis. 2008, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Li, C.; Kim, A.L.; Spiegelman, V.S.; Bickers, D.R. Sonic hedgehog signaling in Basal cell nevus syndrome. Cancer Res. 2014, 74, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Chang, L.; Nguyen, A.; James, A.W. A review of hedgehog signaling in cranial bone development. Front. Physiol. 2013, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Bresler, S.C.; Padwa, B.L.; Granter, S.R. Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome). Head Neck Pathol. 2016, 10, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wilding, A.; Ingham, S.L.; Lalloo, F.; Clancy, T.; Huson, S.M.; Moran, A.; Evans, D.G. Life expectancy in hereditary cancer predisposing diseases: An observational study. J. Med. Genet. 2012, 49, 264–269. [Google Scholar] [CrossRef]
- Kimonis, V.E.; Goldstein, A.M.; Pastakia, B.; Yang, M.L.; Kase, R.; DiGiovanna, J.J.; Bale, A.E.; Bale, S.J. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997, 69, 299–308. [Google Scholar] [CrossRef]
- Bree, A.F.; Shah, M.R. Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am. J. Med. Genet. A 2011, 155, 2091–2097. [Google Scholar] [CrossRef]
- Tanioka, M.; Takahashi, K.; Kawabata, T.; Kosugi, S.; Murakami, K.I.; Miyachi, Y.; Nishigori, C.; Iizuka, T. Germline mutations of the PTCH gene in Japanese patients with nevoid basal cell carcinoma syndrome. Arch. Dermatol. Res. 2005, 296, 303–308. [Google Scholar] [CrossRef]
- Yu, F.Y.; Hong, Y.Y.; Qu, J.F.; Chen, F.; Li, T.J. The large intracellular loop of ptch1 mediates the non-canonical Hedgehog pathway through cyclin B1 in nevoid basal cell carcinoma syndrome. Int. J. Mol. Med. 2014, 34, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Di Minin, G.; Vercellino, I.; Wutz, A.; Korkhov, V.M. Structural basis of sterol recognition by human hedgehog receptor PTCH1. Sci. Adv. 2019, 5, eaaw6490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bulkley, D.P.; Xin, Y.; Roberts, K.J.; Asarnow, D.E.; Sharma, A.; Myers, B.R.; Cho, W.; Cheng, Y.; Beachy, P.A. Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched. Cell 2018, 175, 1352–1364.e14. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Qian, H.; Cao, P.; Zhao, X.; Zhou, Q.; Lei, J.; Yan, N. Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science (80-.) 2018, 361, eaas8935. [Google Scholar] [CrossRef]
- Fleet, A.J.; Hamel, P.A. The protein-specific activities of the transmembrane modules of Ptch1 and Ptch2 are determined by their adjacent protein domains. J. Biol. Chem. 2019, 293, 16583–16595. [Google Scholar] [CrossRef]
- Fujii, K.; Miyashita, T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): Update and literature review. Pediatr. Int. 2014, 56, 667–674. [Google Scholar] [CrossRef]
- Evans, D.G.; Oudit, D.; Smith, M.J.; Rutkowski, D.; Allan, E.; Newman, W.G.; Lear, J.T. First evidence of genotype-phenotype correlations in Gorlin syndrome. J. Med. Genet. 2017, 54, 530–536. [Google Scholar] [CrossRef]
- Stojanov, I.J.; Schaefer, I.M.; Menon, R.S.; Wasman, J.; Gokozan, H.N.; Garcia, E.P.; Baur, D.A.; Woo, S.; Sholl, L.M. Biallelic PTCH1 Inactivation Is a Dominant Genomic Change in Sporadic Keratocystic Odontogenic Tumors. Am. J. Surg. Pathol. 2020, 44, 553–560. [Google Scholar] [CrossRef]
- Torrelo, A.; Hernández-Martín, A.; Bueno, E.; Colmenero, I.; Rivera, I.; Requena, L.; Happle, R.; González-Sarmiento, R. Molecular evidence of type 2 mosaicism in Gorlin syndrome. Br. J. Dermatol. 2013, 169, 1342–1345. [Google Scholar] [CrossRef]
- Ikemoto, Y.; Takayama, Y.; Fujii, K.; Masuda, M.; Kato, C.; Hatsuse, H.; Fujitani, K.; Nagao, K.; Kameyama, K.; Ikehara, H.; et al. Somatic mosaicism containing double mutations in PTCH1 revealed by generation of induced pluripotent stem cells from nevoid basal cell carcinoma syndrome. J. Med. Genet. 2017, 54, 579–584. [Google Scholar] [CrossRef]
- Barakat, M.T.; Humke, E.W.; Scott, M.P. Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol. Med. 2010, 16, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Solomon, J.A. Hedgehog Pathway Inhibition for the Treatment of Basal Cell Carcinoma. Target. Oncol. 2019, 14, 253–267. [Google Scholar] [CrossRef]
- Onodera, S.; Saito, A.; Hasegawa, D.; Morita, N.; Watanabe, K.; Nomura, T.; Shibahara, T.; Ohba, S.; Yamaguchi, A.; Azuma, T. Multi-layered mutation in hedgehog-related genes in Gorlin syndrome may affect the phenotype. PLoS ONE 2017, 12, e0184702. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.I.; Naruto, T.; Tanimoto, K.; Yasukawa, C.; Oikawa, Y.; Masuda, K.; Imoto, I.; Inazawa, J.; Omura, K.; Harada, H. Simultaneous detection of both single nucleotide variations and copy number alterations by next-generation sequencing in gorlin syndrome. PLoS ONE 2015, 10, e140480. [Google Scholar] [CrossRef]
- Shiohama, T.; Fujii, K.; Miyashita, T.; Takatani, T.; Ikehara, H.; Uchikawa, H.; Motojima, T.; Uchida, T.; Shimojo, N. MicroRNAs profiling in fibroblasts derived from patients with Gorlin syndrome. J. Hum. Genet. 2019, 64, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; García-Muse, T. Causes of genome instability. Annu. Rev. Genet. 2013, 47, 1–32. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Reiter, J.F. Misactivation of Hedgehog signaling causes inherited and sporadic cancers. J. Clin. Investig. 2019, 129, 465–475. [Google Scholar] [CrossRef]
- Goodrich, L.V.; Milenković, L.; Higgins, K.M.; Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (80-.) 1997, 277, 1109–1113. [Google Scholar] [CrossRef]
- Palle, K.; Mani, C.; Tripathi, K.; Athar, M. Aberrant GLI1 activation in DNA damage response, carcinogenesis and chemoresistance. Cancers (Basel) 2015, 7, 2330–2352. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.K.; Chen, M.H.; Day, T.F.; Chuang, P.T.; Yang, Y. Wnt/β-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 2006, 133, 3695–3707. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; Giardullo, P.; Leonardi, S.; Tanno, B.; Meschini, R.; Cordelli, E.; Benassi, B.; Longobardi, M.G.; Izzotti, A.; Pulliero, A.; et al. Transgenerational inheritance of enhanced susceptibility to radiation-induced medulloblastoma in newborn Ptch1+/− mice after paternal irradiation. Oncotarget 2015, 6, 36098–36112. [Google Scholar] [CrossRef][Green Version]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Kawaguchi, H.; Kugimiya, F.; Ogasawara, T.; Kawamura, N.; Saito, T.; Ikeda, T.; Fujii, K.; Miyajima, T.; Kuramochi, A.; et al. Patched1 haploinsufficiency increases adult bone mass and modulates Gli3 repressor activity. Dev. Cell 2008, 14, 689–699. [Google Scholar] [CrossRef]
- Ohta, S.; Wang, B.; Mansour, S.L.; Schoenwolf, G.C. SHH ventralizes the otocyst by maintaining basal PKA activity and regulating GLI3 signaling. Dev. Biol. 2016, 420, 100–109. [Google Scholar] [CrossRef]
- Onodera, S.; Saito, A.; Hojo, H.; Nakamura, T.; Zujur, D.; Watanabe, K.; Morita, N.; Hasegawa, D.; Masaki, H.; Nakauchi, H.; et al. Hedgehog Activation Regulates Human Osteoblastogenesis. Stem Cell Rep. 2020, 15, 125–139. [Google Scholar] [CrossRef]
- Mak, K.K.; Bi, Y.; Wan, C.; Chuang, P.-T.; Clemens, T.; Young, M.; Yang, Y. Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev. Cell 2008, 14, 674–688. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, J.; Zhang, H.; Li, X.; Qu, J.; Zhai, J.; Zhang, L.; Chen, F.; Li, T. Heterozygous PTCH1 Mutations Impact the Bone Metabolism in Patients with Nevoid Basal Cell Carcinoma Syndrome Likely by Regulating SPARC Expression. J. Bone Miner. Res. 2016, 31, 1413–1428. [Google Scholar] [CrossRef]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef]
- Pellegrini, C.; Maturo, M.G.; Di Nardo, L.; Ciciarelli, V.; Gutiérrez García-Rodrigo, C.; Fargnoli, M.C. Understanding the molecular genetics of basal cell carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Gailani, M.R.; Stahle-Backdahl, M.; Leffell, D.J.; Glynn, M.; Zaphiropoulos, P.G.; Pressman, C.; Unden, A.B.; Dean, M.; Brash, D.E.; Bale, A.E.; et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat. Genet. 1996, 14, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Stacey, S.N.; Sulem, P.; Masson, G.; Gudjonsson, S.A.; Thorleifsson, G.; Jakobsdottir, M.; Sigurdsson, A.; Gudbjartsson, D.F.; Sigurgeirsson, B.; Benediktsdottir, K.R.; et al. New common variants affecting susceptibility to basal cell carcinoma. Nat. Genet. 2009, 41, 909–914. [Google Scholar] [CrossRef]
- Wong, S.Y.; Seol, A.D.; So, P.-L.; Ermilov, A.N.; Bichakjian, C.K.; Epstein, E.H.; Dlugosz, A.A.; Reiter, J.F. Primary cilia can both mediate and suppress Hedgehog pathway–dependent tumorigenesis. Nat. Med. 2009, 15, 1055–1061. [Google Scholar] [CrossRef]
- Basal Cell Carcinoma: Pathogenesis, Epidemiology, Clinical Features, Diagnosis, Histopathology, and Management—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26029015/ (accessed on 11 August 2020).
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4567. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science (80-.) 2013, 340, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Radtke, F.; Clevers, H. Self-renewal and cancer of the gut: Two sides of a coin. Science (80-.) 2005, 307, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical hedgehog signaling pathway in cancer: Activation of GLI transcription factors beyond smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.E.; Stahl, D.; Kreiborg, S.; Rosenberg, T.; Kalscheuer, V.; Larsen, L.A.; Tommerup, N.; Brøndum-Nielsen, K.; Tümer, Z. Delineation of an interstitial 9q22 deletion in basal cell nevus syndrome. Am. J. Med. Genet. A 2005, 132, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Adebali, O.; Adar, S.; Sancar, A. Dynamic maps of UV damage formation and repair for the human genome. Proc. Natl. Acad. Sci. USA 2017, 114, 6758–6763. [Google Scholar] [CrossRef]
- Young, L.C.; Listgarten, J.; Trotter, M.J.; Andrew, S.E.; Tron, V.A. Evidence that dysregulated DNA mismatch repair characterizes human nonmelanoma skin cancer. Br. J. Dermatol. 2008, 158, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Šitum, M.; Buljan, M.; Bulat, V.; Mihic, L.L.; Bolanèa, Z.; Simic, D. The role of UV radiation in the development of Basal cell carcinoma. Coll. Antropol. 2008, 32, 167–170. [Google Scholar]
- Nacev, B.A.; Feng, L.; Bagert, J.D.; Lemiesz, A.E.; Gao, J.J.; Soshnev, A.A.; Kundra, R.; Schultz, N.; Muir, T.W.; Allis, C.D. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature 2019, 567, 473–478. [Google Scholar] [CrossRef]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef]
- Wilson, C.B. Brain Tumors. N. Engl. J. Med. 1979, 300, 1469–1471. [Google Scholar] [CrossRef]
- Millard, N.E.; De Braganca, K.C. Medulloblastoma. J. Child Neurol. 2016, 31, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Huse, J.T. 2016 World Health Organization Classification of Central Nervous System Tumors. Contin. Lifelong Learn. Neurol. 2017, 23, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Farndon, P.A. Nevoid Basal Cell Carcinoma Syndrome. 2002 Jun 20 [Updated 2018 Mar 29]; Adam, M., Ardinger, H., Pagon, R., Wallace, S., Bean, L., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Schroeder, K.; Gururangan, S. Molecular variants and mutations in medulloblastoma. Pharmgenom. Pers. Med. 2014, 7, 43–51. [Google Scholar] [PubMed]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Prim. 2019, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pak, E.; Segal, R.A. Hedgehog Signal Transduction: Key Players, Oncogenic Drivers, and Cancer Therapy. Dev. Cell 2016, 38, 333–344. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Lackey, E.P.; Heck, D.H. Recent advances in understanding the mechanisms of cerebellar granule cell development and function and their contribution to behavior. F1000 Res. 2018, 7. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Nör, C.; Taylor, M.D. p53 and meduloblastoma. Cold Spring Harb. Perspect. Med. 2016, 6, a026278. [Google Scholar] [CrossRef] [PubMed]
- Vanner, R.J.; Remke, M.; Gallo, M.; Selvadurai, H.J.; Coutinho, F.; Lee, L.; Kushida, M.; Head, R.; Morrissy, S.; Zhu, X.; et al. Quiescent Sox2+ Cells Drive Hierarchical Growth and Relapse in Sonic Hedgehog Subgroup Medulloblastoma. Cancer Cell 2014, 26, 33–47. [Google Scholar] [CrossRef]
- Golitz, L.E. Nevoid basal cell carcinoma syndrome. Multiple basal cell carcinomas of the palms after radiation therapy. Arch. Dermatol. 1980, 116, 1159–1163. [Google Scholar] [CrossRef]
- Strong, L.C. Genetic and environmental interactions. Cancer 1977, 40, 1861–1866. [Google Scholar] [CrossRef]
- Khaliq, M.I.U.; Shah, A.A.; Ahmad, I.; Hasan, S.; Jangam, S.S. Keratocystic odontogenic tumors related to Gorlin-Goltz syndrome: A clinicopathological study. J. Oral Biol. Cranio-fac. Res. 2016, 6, 93–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashmi, A.A.; Edhi, M.M.; Faridi, N.; Hosein, M.; Khan, M. Mutiple keratocystic odontogenic tumors (KCOT) in a patient with Gorlin syndrome: A case report with late presentation and absence of skin manifestations. BMC Res. Notes 2016, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Fidele, N.B.; Zheng, Y.; Zhao, Y.; Wu, T.; Liu, J.; Sun, Y.; Liu, B. Recurrence of odontogenic keratocysts and possible prognostic factors: Review of 455 patients. Med. Oral Patol. Oral y Cir. Bucal 2019, 24, e491–e501. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Saghravanian, N.; Habibi, M.; Mellati, E.; Habibi, M. Keratocystic odontogenic tumor: A 10-year retrospective study of 83 cases in an Iranian population. J. Oral Sci. 2007, 49, 229–235. [Google Scholar] [CrossRef]
- Stoelinga, P.J.W. Long-term follow-up on keratocysts treated according to a defined protocol. Int. J. Oral Maxillofac. Surg. 2001, 30, 14–25. [Google Scholar] [CrossRef]
- International Histological Classification of Tumours No. 5: Histological Typing of Odontogenic Tumours. Jaw Cysts, and Allied Lesions. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC477552/ (accessed on 11 August 2020).
- Stoelinga, P.J.W. Excision of the overlying, attached mucosa, in conjunction with cyst enucleation and treatment of the bony defect with carnoy solution. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 407–414. [Google Scholar] [CrossRef]
- Stoelinga, P.J.W.; Peters, J.H. A note on the origin of keratocysts of the jaws. Int. J. Oral Surg. 1973, 2, 37–44. [Google Scholar] [CrossRef]
- Kramer, I.R.H.; Pindborg, J.J.; Shear, M. The WHO Histological typing of odontogenic tumours. A commentary on the second edition. Cancer 1992, 70, 2988–2994. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhang, J.Y.; Li, X.F.; Luo, H.Y.; Chen, F.; Li, T.J. PTCH1 Gene Mutations in Keratocystic Odontogenic Tumors: A Study of 43 Chinese Patients and a Systematic Review. PLoS ONE 2013, 8, e77305. [Google Scholar] [CrossRef]
- Gorlin, R.J. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet. Med. 2004, 6, 530–539. [Google Scholar] [CrossRef]
- Kerbrat, A.; Beaufrere, A.; Neiva-Vaz, C.; Galmiche, L.; Belhous, K.; Orbach, D.; Gauthier-Villars, M.; Picard, A.; Kadlub, N. Rhabdomyosarcoma and rhabdomyoma associated with nevoid basal cell carcinoma syndrome: Local treatment strategy. Pediatr. Dermatol. 2018, 35, e245–e247. [Google Scholar] [CrossRef] [PubMed]
- Gavet, O.; Pines, J. Progressive Activation of CyclinB1-Cdk1 Coordinates Entry to Mitosis. Dev. Cell 2010, 18, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.A.; Kong, M.; Ollendorff, V.; Donoghue, D.J. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001, 20, 2214–2223. [Google Scholar] [CrossRef] [PubMed]
- Kenney, A.M.; Rowitch, D.H. Sonic hedgehog Promotes G1 Cyclin Expression and Sustained Cell Cycle Progression in Mammalian Neuronal Precursors. Mol. Cell. Biol. 2000, 20, 9055–9067. [Google Scholar] [CrossRef] [PubMed]
- Matus, D.Q.; Magie, C.R.; Pang, K.; Martindale, M.Q.; Thomsen, G.H. The Hedgehog gene family of the cnidarian, Nematostella vectensis, and implications for understanding metazoan Hedgehog pathway evolution. Dev. Biol. 2008, 313, 501–518. [Google Scholar] [CrossRef]
- Perler, F.B. Protein splicing of inteins and hedgehog autoproteolysis: Structure, function, and evolution. Cell 1998, 92, 1–4. [Google Scholar] [CrossRef]
- Kopinke, D.; Roberson, E.C.; Reiter, J.F. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell 2017, 170, 340–351.e12. [Google Scholar] [CrossRef]
- Elliott, K.H.; Brugmann, S.A. Sending mixed signals: Cilia-dependent signaling during development and disease. Dev. Biol. 2019, 447, 28–41. [Google Scholar] [CrossRef]
- Chaudhry, P.; Singh, M.; Triche, T.J.; Guzman, M.; Merchant, A.A. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood 2017, 129, 3465–3475. [Google Scholar] [CrossRef]
- Chung, J.H.; Bunz, F. A loss-of-function mutation in PTCH1 suggests a role for autocrine hedgehog signaling in colorectal tumorigenesis. Oncotarget 2013, 4, 2208–2211. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Harth, M.; Sader, R. Vismodegib hedgehog-signaling inhibition and treatment of basal cell carcinomas as well as keratocystic odontogenic tumors in Gorlin syndrome. Ann. Maxillofac. Surg. 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Di Prete, M.; Lozzi, F.; Lanna, C.; Spallone, G.; Mazzeo, M.; Cosio, T.; Rapanotti, C.; Dika, E.; Gaziano, R.; et al. High-risk recurrence basal cell carcinoma: Focus on hedgehog pathway inhibitors and review of the literature. Chemotherapy 2020, 65, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Scarfì, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Berrocal, A. Management of high-risk and advanced basal cell carcinoma. Clin. Transl. Oncol. 2015, 17, 497–503. [Google Scholar] [CrossRef]
- Kesireddy, M.; Mendiola, V.L.; Jana, B.; Patel, S. Long-term Response to Vismodegib in a Patient with Gorlin-Goltz Syndrome: A Case Report and Review of Pathological Mechanisms Involved. Cureus 2019, 11, e5383. [Google Scholar] [CrossRef]
- Dréno, B.; Kunstfeld, R.; Hauschild, A.; Fosko, S.; Zloty, D.; Labeille, B.; Grob, J.J.; Puig, S.; Gilberg, F.; Bergström, D.; et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017, 18, 404–412. [Google Scholar] [CrossRef]
- Di Magno, L.; Basile, A.; Coni, S.; Manni, S.; Sdruscia, G.; D’Amico, D.; Antonucci, L.; Infante, P.; De Smaele, E.; Cucchi, D.; et al. The energy sensor AMPK regulates Hedgehog signaling in human cells through a unique Gli1 metabolic checkpoint. Oncotarget 2016, 7, 9538–9549. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Basset-Seguin, N.; Garbe, C.; Gesierich, A.; Lao, C.D.; Miller, C.; Mortier, L.; Murrell, D.F.; Hamid, O.; et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017, 17. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yang, W. iPSC reprogramming from human peripheral blood using Sendai Virus mediated gene transfer. StemBook 2014. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Spitzer, E.D. Promoting appropriate urine culture management to improve health care outcomes and the accuracy of catheter-associated urinary tract infections. Am. J. Infect. Control 2017, 45, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, H.; Fujii, K.; Miyashita, T.; Ikemoto, Y.; Nagamine, M.; Shimojo, N.; Umezawa, A. Establishment of a Gorlin syndrome model from induced neural progenitor cells exhibiting constitutive GLI1 expression and high sensitivity to inhibition by smoothened (SMO). Lab. Investig. 2020, 100, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, Y.; Miyashita, T.; Nasu, M.; Hatsuse, H.; Kajiwara, K.; Fujii, K.; Motojima, T.; Kokido, I.; Toyoda, M.; Umezawa, A. Gorlin syndrome-induced pluripotent stem cells form medulloblastoma with loss of heterozygosity in PTCH1. Aging (Albany. N.Y.) 2020, 12, 9935–9947. [Google Scholar] [CrossRef]
- Hasegawa, D.; Ochiai-Shino, H.; Onodera, S.; Nakamura, T.; Saito, A.; Onda, T.; Watanabe, K.; Nishimura, K.; Ohtaka, M.; Nakanishi, M.; et al. Gorlin syndrome-derived induced pluripotent stem cells are hypersensitive to hedgehog-mediated osteogenic induction. PLoS ONE 2017, 12, e0186879. [Google Scholar] [CrossRef]
- Huang, M.; Tailor, J.; Zhen, Q.; Gillmor, A.H.; Miller, M.L.; Weishaupt, H.; Chen, J.; Zheng, T.; Nash, E.K.; McHenry, L.K.; et al. Engineering Genetic Predisposition in Human Neuroepithelial Stem Cells Recapitulates Medulloblastoma Tumorigenesis. Cell Stem Cell 2019, 25, 433–446.e7. [Google Scholar] [CrossRef]
- Susanto, E.; Navarro, A.M.; Zhou, L.; Sundström, A.; van Bree, N.; Stantic, M.; Moslem, M.; Tailor, J.; Rietdijk, J.; Zubillaga, V.; et al. Modeling SHH-driven medulloblastoma with patient iPS cell-derived neural stem cells. Proc. Natl. Acad. Sci. USA 2020, 117, 20127–20138. [Google Scholar] [CrossRef]
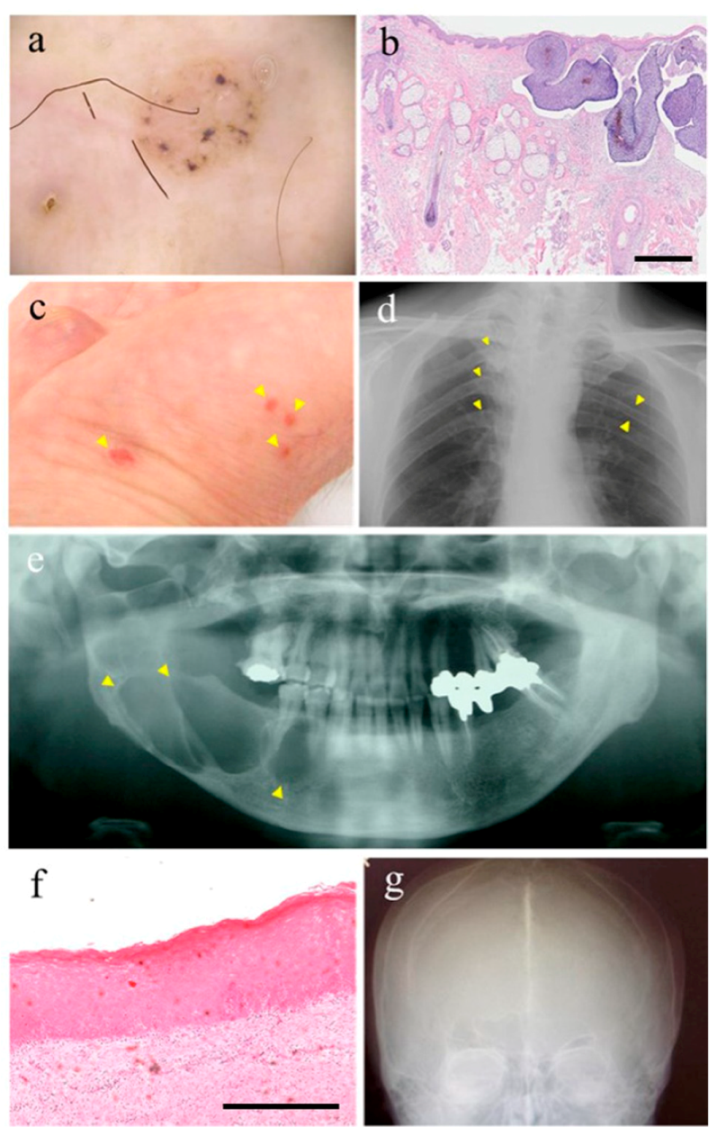
| Major Criteria (Diagnostic Criteria for GS—from Kimonis, V.E. et al. [19]) |
|---|
|
| Minor Criteria (Any One of the Following Features) |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, S.; Nakamura, Y.; Azuma, T. Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research. Int. J. Mol. Sci. 2020, 21, 7559. https://doi.org/10.3390/ijms21207559
Onodera S, Nakamura Y, Azuma T. Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research. International Journal of Molecular Sciences. 2020; 21(20):7559. https://doi.org/10.3390/ijms21207559
Chicago/Turabian StyleOnodera, Shoko, Yuriko Nakamura, and Toshifumi Azuma. 2020. "Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research" International Journal of Molecular Sciences 21, no. 20: 7559. https://doi.org/10.3390/ijms21207559
APA StyleOnodera, S., Nakamura, Y., & Azuma, T. (2020). Gorlin Syndrome: Recent Advances in Genetic Testing and Molecular and Cellular Biological Research. International Journal of Molecular Sciences, 21(20), 7559. https://doi.org/10.3390/ijms21207559





