Diabetes Mellitus-Related Dysfunction of the Motor System
Abstract
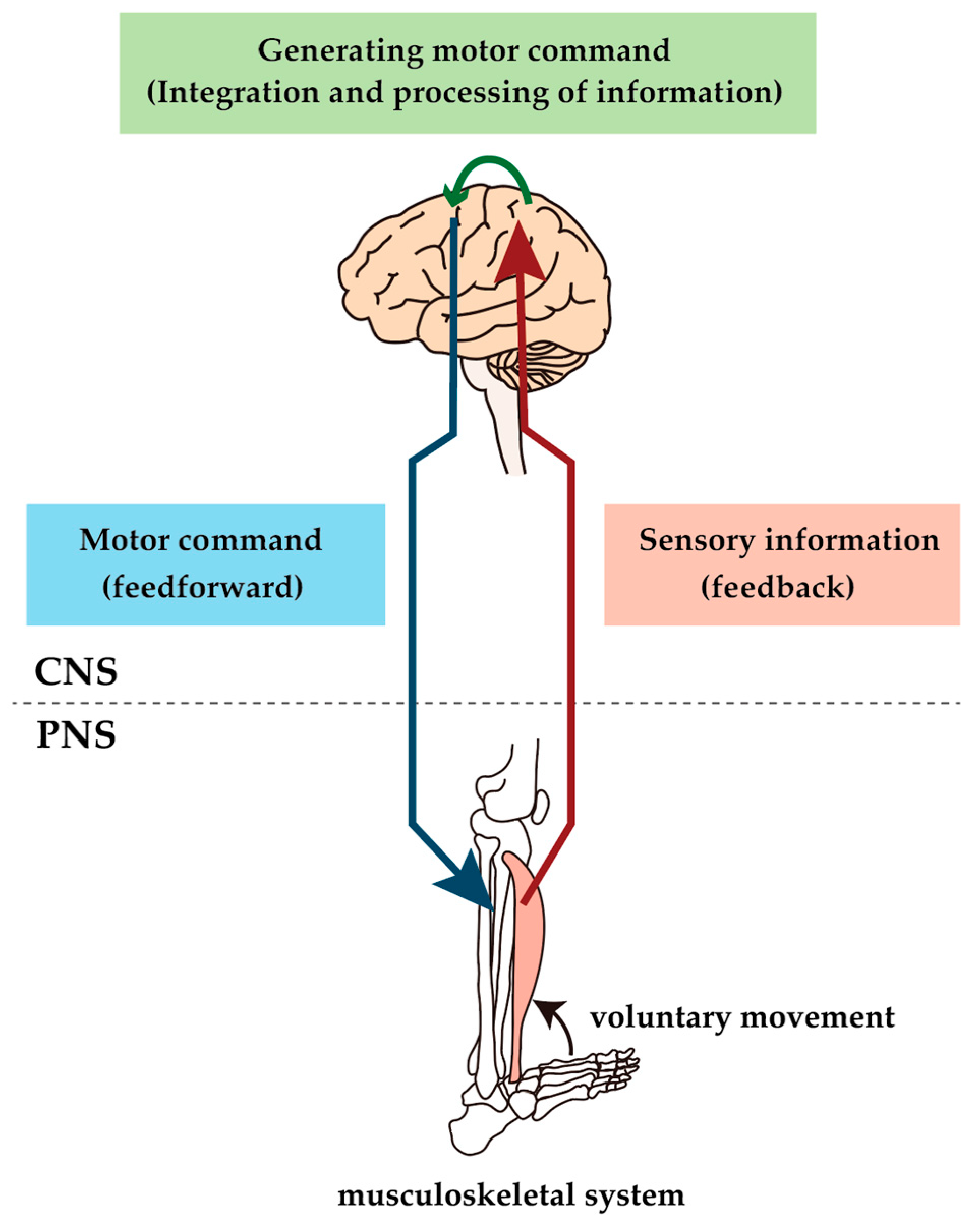
1. Introduction
2. Alteration of the Neuromuscular Pathway
2.1. Cell Bodies of MNs
2.2. Motor Axon
2.3. NMJ and Muscle Fibers

2.4. Differences in Vulnerability to Diabetes between MNs
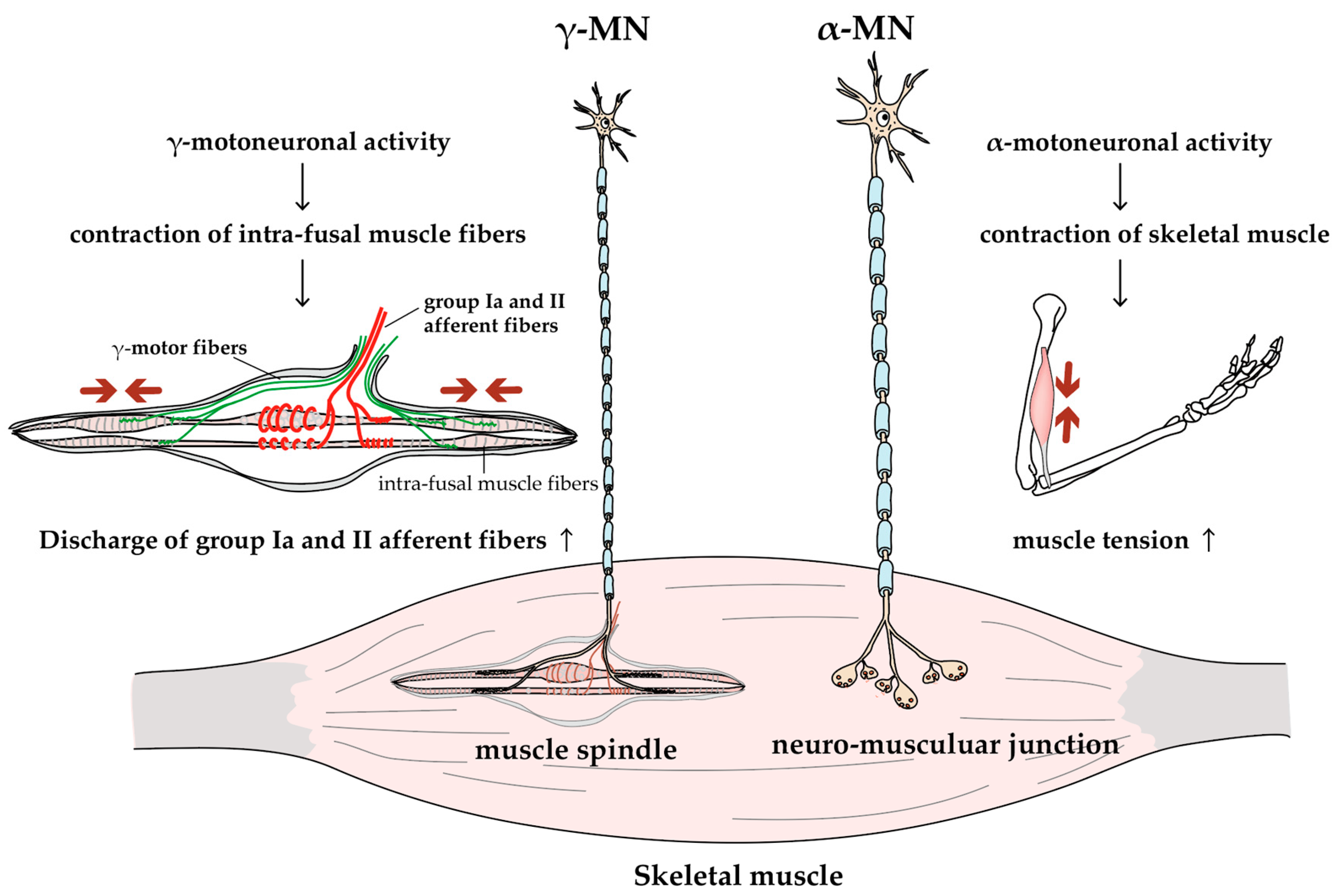
2.5. γ-MNs
3. Alteration of Corticomotoneuronal Pathway
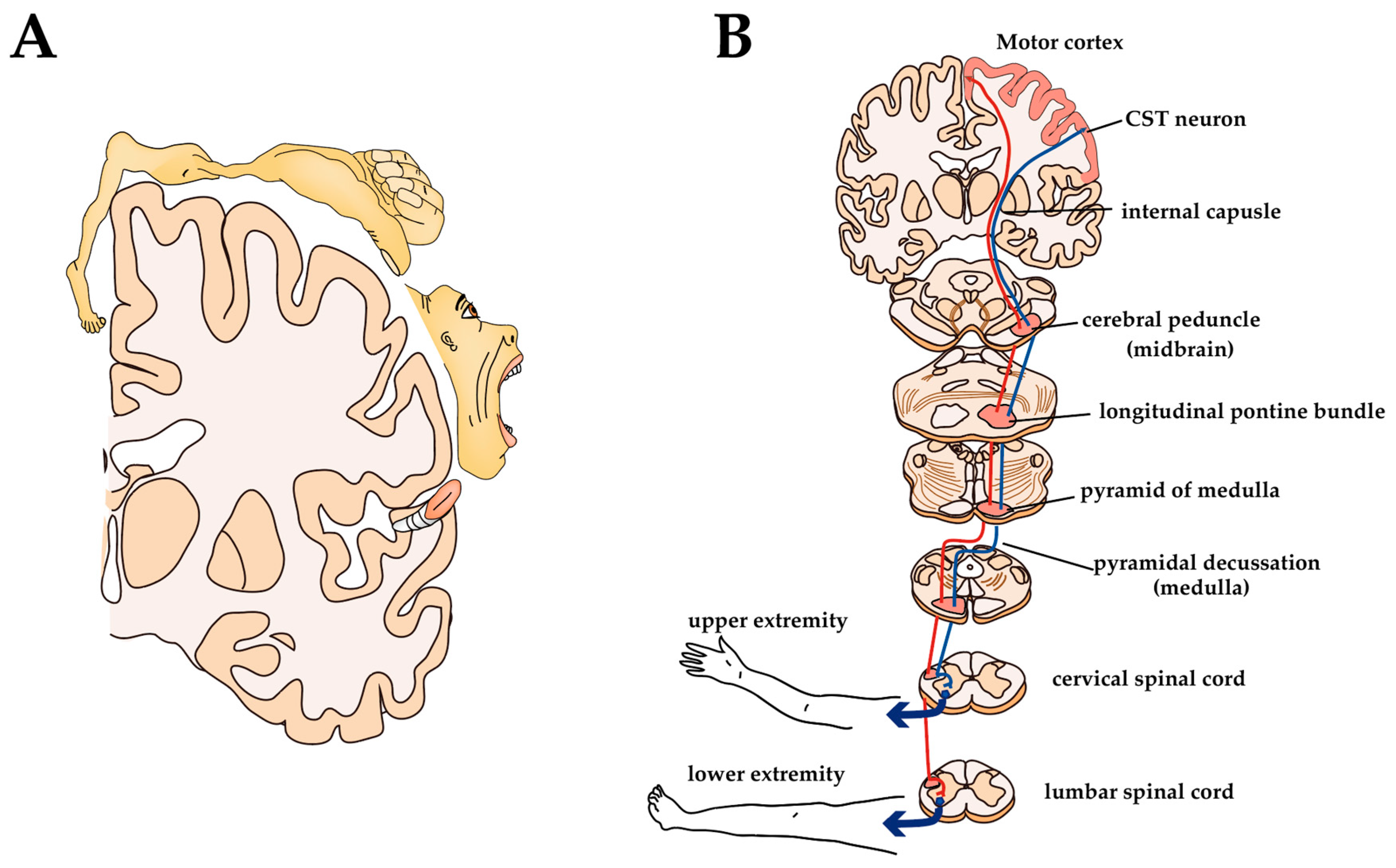
3.1. Primary Motor Cortex and CST
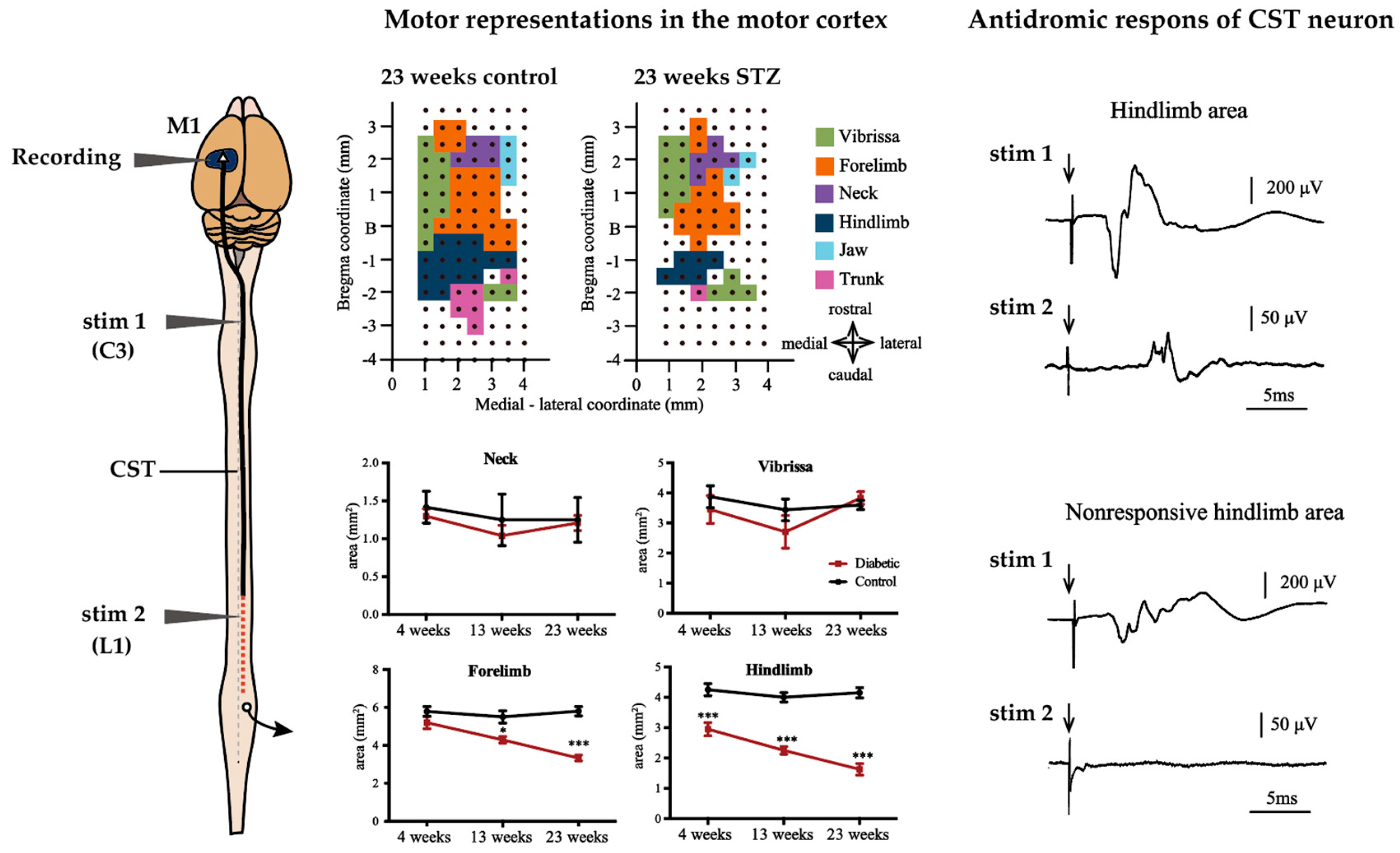
3.2. Alterations of M1
3.3. Alterations of CST
3.4. Possible Movement Disorders Caused by Diabetes-Induced Lesions of M1 and CST
3.5. Possible Therapies on Corticomotoneuronal Pathway Lesion in Diabetes
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DN | diabetic neuropathy |
| PNS | peripheral nervous system |
| DRG | dorsal root ganglion |
| BBB | blood-brain barrier |
| STZ | streptozotocin |
| BB | Bio-Breeding |
| MNCV | motor nerve conduction velocity |
| NMJ | neuromuscular junction |
| M1 | primary motor cortex |
| CST | corticospinal tract |
| MN | motoneuron |
| ACh | acetylcholine |
| ROS | reactive oxygen species |
| AGEs | advanced glycation end-products |
| RAGE | receptor for AGEs |
References
- Said, G. Diabetic neuropathy—A review. Nat. Rev. Neurol. 2007, 3, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, X.; Zhang, Q.; Zou, R. Diabetes mellitus and risk of falls in older adults: A systematic review and meta-analysis. Age Ageing 2016, 45, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Lee, S.; Cuneo, M.L. Gait characteristics in patients with type 2 diabetes; improvement after administration of rosiglitazone. Med. Sci. Monit. 2005, 11, 43–51. [Google Scholar]
- Uccioli, L.; Giacomini, P.G.; Monticone, G.; Magrini, A.; Durola, L.; Bruno, E.; Parisi, L.; Di Girolamo, S.; Menzinger, G. Body sway in diabetic neuropathy. Diabetes Care 1995, 18, 339–344. [Google Scholar] [CrossRef]
- Volpato, S.; Leveille, S.G.; Blaum, C.; Fried, L.P.; Guralnik, J.M. Risk factors for falls in older disabled women with diabetes: The women’s health and aging study. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1539–1545. [Google Scholar] [CrossRef]
- Wong, E.; Backholer, K.; Gearon, E.; Harding, J.; Freak-Poli, R.; Stevenson, C.; Peeters, A. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013, 1, 106–114. [Google Scholar] [CrossRef]
- Zochodne, D.W.; Ramji, N.; Toth, C. Neuronal Targeting in Diabetes Mellitus: A Story of Sensory Neurons and Motor Neurons. Neuroscientist 2008, 14, 311–318. [Google Scholar] [CrossRef]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817. [Google Scholar] [CrossRef]
- Zochodne, D.W.; Verge, V.M.K.; Cheng, C.; Sun, H.; Johnston, J. Does diabetes target ganglion neurones? Brain 2001, 124, 2319–2334. [Google Scholar] [CrossRef]
- Ramji, N.; Toth, C.; Kennedy, J.; Zochodne, D.W. Does diabetes mellitus target motor neurons? Neurobiol. Dis. 2007, 26, 301–311. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.-A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Sweetnam, D.; Holmes, A.; Tennant, K.A.; Zamani, A.; Walle, M.; Jones, P.; Wong, C.; Brown, C.E. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J. Neurosci. 2012, 32, 5132–5143. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D. Stroke and diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 167–174. [Google Scholar] [PubMed]
- Huynh, W.; Kwai, N.; Arnold, R.; Krishnan, A.V.; Lin, C.S.Y.; Vucic, S.; Kiernan, M.C. The Effect of Diabetes on Cortical Function in Stroke: Implications for Poststroke Plasticity. Diabetes 2017, 66, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Poulsen, P.L.; Mogensen, C.E.; Jakobsen, J. Isokinetic Muscle Strength in Long-Term IDDM Patients in Relation to Diabetic Complications. Diabetes 1996, 45, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Nielsen, S.; Mogensen, C.E.; Jakobsen, J. Muscle Strength in Type 2 Diabetes. Diabetes 2004, 53, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.N.; Tuttle, L.J.; Bohnert, K.L.; Mueller, M.J.; Sinacore, D.R. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals with Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association with Performance and Function. Phys. Ther. 2008, 88, 1336–1344. [Google Scholar] [CrossRef]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’s Disease. Front. Endocrinol. 2014, 5, 102. [Google Scholar] [CrossRef]
- Malone, J.I. Diabetic Central Neuropathy: CNS Damage Related to Hyperglycemia. Diabetes 2016, 65, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fang, P.; An, J.; Lin, H.; Liang, Y.; Shen, W.; Leng, X.; Zhang, C.; Zheng, Y.; Qiu, S. Micro-structural white matter abnormalities in type 2 diabetic patients: A DTI study using TBSS analysis. Neuroradiology 2016, 58, 1209–1216. [Google Scholar] [CrossRef]
- Eaton, S.E.; Harris, N.D.; Rajbhandari, S.M.; Greenwood, P.; Wilkinson, I.D.; Ward, J.D.; Griffiths, P.D.; Tesfaye, S. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet 2001, 358, 35–36. [Google Scholar] [CrossRef]
- Xiong, Y.; Sui, Y.; Xu, Z.; Zhang, Q.; Karaman, M.M.; Cai, K.; Anderson, T.M.; Zhu, W.; Wang, J.; Zhou, X.J. A Diffusion Tensor Imaging Study on White Matter Abnormalities in Patients with Type 2 Diabetes Using Tract-Based Spatial Statistics. Am. J. Neuroradiol. 2016, 37, 1462–1469. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, X.; Wang, X.; Xu, X.; Li, H.; Cao, B.; Yang, Y.; Lu, J.; Chen, Z. Abnormal subcortical nuclei shapes in patients with type 2 diabetes mellitus. Eur. Radiol. 2017, 27, 4247–4256. [Google Scholar] [CrossRef]
- Emerick, A.J.; Richards, M.P.; Kartje, G.L.; Neafsey, E.J.; Stubbs, E.B. Experimental diabetes attenuates cerebral cortical-evoked forelimb motor responses. Diabetes 2005, 54, 2764–2771. [Google Scholar] [CrossRef]
- Muramatsu, K.; Ikutomo, M.; Tamaki, T.; Shimo, S.; Niwa, M. Effect of streptozotocin-induced diabetes on motor representations in the motor cortex and corticospinal tract in rats. Brain Res. 2018, 1680, 115–126. [Google Scholar] [CrossRef]
- Svoboda, K.; Li, N. Neural mechanisms of movement planning: Motor cortex and beyond. Curr. Opin. Neurol. 2018, 49, 33–41. [Google Scholar] [CrossRef]
- Lemon, R.N. Neural control of dexterity: What has been achieved? Exp. Brain Res. 1999, 128, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2020, 69, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Isa, T.; Ohki, Y.; Alstermark, B.; Pettersson, L.-G.; Sasaki, S. Direct and Indirect Cortico-Motoneuronal Pathways and Control of Hand/Arm Movements. Physiology 2007, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.; Zytnicki, D. Alpha, beta and gamma motoneurons: Functional diversity in the motor system’s final pathway. J. Integr. Neurosci. 2012, 10, 243–276. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation–contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Sherrington, C.S. The Integrative Action of the Nervous System; Archibald Constable and Co., Ltd.: London, UK, 1906; pp. 36–69. [Google Scholar]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef]
- Eldred, E.; Granit, R.; Merton, P.A. Supraspinal control of the muscle spindles and its significance. J. Physiol. 1953, 122, 498–523. [Google Scholar] [CrossRef]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes. Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.M.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Reske-Nielsen, E.; Lundbaek, K. Pathological changes in the central and peripheral nervous system of young long-term diabetics. II. The spinal cord and peripheral nerves. Diabetologia 1968, 4, 34–43. [Google Scholar] [CrossRef] [PubMed]
- MacDonell, C.W.; Chopek, J.W.; Gardiner, K.R.; Gardiner, P.F. α-Motoneurons maintain biophysical heterogeneity in obesity and diabetes in Zucker rats. J. Neurophysiol. 2017, 118, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Sidenius, P.; Jakobsen, J. Reduced Perikaryal Volume of Lower Motor and Primary Sensory Neurons in Early Experimental Diabetes. Diabetes 1980, 29, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, V.B.; Vega, M.C.; Coirini, H. Reduction of the spinal nucleus of the bulbocavernosous volume by experimental diabetes. Brain Res. 2004, 1019, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.J.; Martinez, J.A.; Liu, W.Q.; Zochodne, D.W.; Hanson, L.R.; Frey, W.H.; Toth, C. Motor end plate innervation loss in diabetes and the role of insulin. J. Neuropathol. Exp. Neurol. 2011, 70, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulos-Stournaras, S.; Iles, J.F. Motor neuron columns in the lumbar spinal cord of the rat. J. Comp. Neurol. 1983, 217, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, K.; Niwa, M.; Nagai, M.; Kamimura, T.; Sasaki, S.-I.; Ishiguro, T. The size of motoneurons of the gastrocnemius muscle in rats with diabetes. Neurosci. Lett. 2012, 531, 109–113. [Google Scholar] [CrossRef]
- Muramatsu, K.; Niwa, M.; Tamaki, T.; Ikutomo, M.; Masu, Y.; Hasegawa, T.; Shimo, S.; Sasaki, S.-I. Effect of streptozotocin-induced diabetes on motoneurons and muscle spindles in rats. Neurosci. Res. 2017, 115, 21–28. [Google Scholar] [CrossRef]
- Tamaki, T.; Muramatsu, K.; Ikutomo, M.; Oshiro, N.; Hayashi, H.; Niwa, M. Effects of streptozotocin-induced diabetes on leg muscle contractile properties and motor neuron morphology in rats. Anat. Sci. Int. 2018, 93, 502–513. [Google Scholar] [CrossRef]
- Wilson, N.M.; Wright, D.E. Experimental motor neuropathy in diabetes. Handb. Clin. Neurol. 2014, 126, 461–467. [Google Scholar]
- Sima, A.A.F.; Zhang, W.-X.; Greene, D.A. Diabetic and hypoglycemic neuropathy—A comparison in the BB rat. Diabetes Res. Clin. Pract. 1989, 6, 279–296. [Google Scholar] [CrossRef]
- Mohseni, S. Hypoglycaemic neuropathy in diabetic BB/Wor rats treated with insulin implants affects ventral root axons but not dorsal root axons. Acta Neuropathol. 2000, 100, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.G.; Locke, S. Motor Nerve Conduction Velocity in Diabetes. Arch. Neurol. 1961, 5, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, G. Variations in motor conduction velocity produced by acute changes of the metabolic state in diabetic patients. Diabetologia 1968, 4, 273–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrington, A.L.; Shaw, J.E.; Van Schie, C.H.M.; Abbott, C.A.; Vileikyte, L.; Boulton, A.J.M. Can Motor Nerve Conduction Velocity Predict Foot Problems in Diabetic Subjects Over a 6-Year Outcome Period? Diabetes Care 2002, 25, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Yamada, T.; Nelson, P.S. Distal slowing of motor nerve conduction velocity in diabetic polyneuropathy. J. Neurol. Sci. 1979, 42, 291–302. [Google Scholar] [CrossRef]
- Coppey, L.J.; Davidson, E.P.; Dunlap, J.A.; Lund, D.D.; Yorek, M.A. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int. J. Exp. Diabetes Res. 2000, 1, 131–143. [Google Scholar] [CrossRef]
- Brismar, T. Nodal function of pathological nerve fibers. Cell. Mol. Life Sci. 1983, 39, 946–953. [Google Scholar] [CrossRef]
- Sima, A.A.F.; Brismar, T. Reversible diabetic nerve dysfunction: Structural correlates to electrophysiological abnormalities. Ann. Neurol. 1985, 18, 21–29. [Google Scholar] [CrossRef]
- VargheseCherian, P.; Kamijo, M.; Angelides, K.J.; Sima, A.A.F. Nodal Na+-channel displacement is associated with nerve-conduction slowing in the chronically diabetic BB/W rat: Prevention by aldose reductase inhibition. J. Diabetes. Complicat. 1996, 10, 192–200. [Google Scholar]
- Sima, A.A.; Nathaniel, V.; Bril, V.; McEwen, T.A.; Greene, D.A. Histopathological heterogeneity of neuropathy in insulin-dependent and non-insulin-dependent diabetes, and demonstration of axo-glial dysjunction in human diabetic neuropathy. J. Clin. Investig. 1988, 81, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.A.; Lattimer, S.A.; Yagihashi, S.; Greene, D.A. Axo-glial dysjunction. A novel structural lesion that accounts for poorly reversible slowing of nerve conduction in the spontaneously diabetic bio-breeding rat. J. Clin. Investig. 1986, 77, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.A.; Zhang, W.; Xu, G.; Sugimoto, K.; Guberski, D.; Yorek, M.A. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia 2000, 43, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cheng, C.; Sun, H.; Zochodne, D.W. Near nerve local insulin prevents conduction slowing in experimental diabetes. Brain Res. 1997, 763, 209–214. [Google Scholar] [CrossRef]
- Sima, A.A.F.; Zhang, W.; Li, Z.-G.; Murakawa, Y.; Pierson, C.R. Molecular alterations underlie nodal and paranodal degeneration in type 1 diabetic neuropathy and are prevented by C-peptide. Diabetes 2004, 53, 1556–1563. [Google Scholar] [CrossRef]
- Wada, R.; Yagihashi, S. Role of Advanced Glycation End Products and Their Receptors in Development of Diabetic Neuropathy. Ann. N. Y. Acad. Sci. 2005, 1043, 598–604. [Google Scholar] [CrossRef]
- Schlaepfer, W.W.; Gerritsen, G.C.; Dulin, W.E. Segmental demyelination in the distal peripheral nerves of chronically diabetic chinese hamsters. Diabetologia 1974, 10, 541–548. [Google Scholar] [CrossRef][Green Version]
- Almeida, S.; Riddell, M.C.; Cafarelli, E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 2008, 37, 231–240. [Google Scholar] [CrossRef]
- De Vos, K.J.; Hafezparast, M. Neurobiology of axonal transport defects in motor neuron diseases: Opportunities for translational research? Neurobiol. Dis. 2017, 105, 283–299. [Google Scholar] [CrossRef]
- Cragg, B.G. What is the signal for chromatolysis? Brain Res. 1970, 23, 1–21. [Google Scholar] [CrossRef]
- Fink, D.J.; Purkiss, D.; Mata, M. Alterations in retrograde axonal transport in streptozocin-induced diabetic rats. Diabetes 1987, 36, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Li, C.; Hu, J.; Zhang, D.L.; Wu, Z.Y.; Ding, T.; Qu, J.; Li, H.; Li, Y.Q. Alterations in the neural circuits from peripheral afferents to the spinal cord: Possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front. Neural Circuits 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.G.; Hohman, T.C.; Cai, F.; Regalia, J.; Helke, C.J. Streptozotocin-Induced Diabetes Causes Metabolic Changes and Alterations in Neurotrophin Content and Retrograde Transport in the Cervical Vagus Nerve. Exp. Neurol. 2001, 170, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Kablar, B.; Belliveau, A.C. Presence of neurotrophic factors in skeletal muscle correlates with survival of spinal cord motor neurons. Dev. Dyn. 2005, 234, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Sidenius, P. Decreased axonal flux of retrogradely transported glycoproteins in early experimental diabetes. J. Neurochem. 1979, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Meiri, K.F.; McLean, W.G. Axonal transport of protein in motor fibres of experimentally diabetic rats—Fast anterograde transport. Brain Res. 1982, 238, 77–88. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Modert, C.W.; Yip, H.K.; Johnson, E.M. Retrograde axonal transport of intravenously administered 125I-nerve growth factor in rats with streptozotocin-induced diabetes. Diabetes 1983, 32, 654–663. [Google Scholar] [CrossRef]
- Jakobsen, J.; Sidenius, P. Decreased axonal transport of structural proteins in streptozotocin diabetic rats. J. Clin. Investig. 1980, 66, 292–297. [Google Scholar] [CrossRef]
- Mayer, J.H.; Tomlinson, D.R.; McLean, W.G. Slow Orthograde Axonal Transport of Radiolabelled Protein in Sciatic Motoneurones of Rats with Short-Term Experimental Diabetes: Effects of Treatment with an Aldose Reductase Inhibitor or myo-Inositol. J. Neurochem. 1984, 43, 1265–1270. [Google Scholar] [CrossRef]
- Medori, R.; Autilio-Gambetti, L.; Jenich, H.; Gambetti, P. Changes in axon size and slow axonal transport are related in experimental diabetic neuropathy. Neurology 1988, 38, 597. [Google Scholar] [CrossRef]
- Tomlinson, D.R.; Filliatreau, G.; Figliomeni, B.; Hassig, R.; Di Giamberardino, L.; Willars, G.B. Proteins of slow axonal transport in sciatic motoneurones of rats with streptozotocin-induced diabetes or galactosaemia. Diabetes Res. Clin. Pract. 1990, 9, 15–21. [Google Scholar] [CrossRef]
- Medori, R.; Autilio-Gambetti, L.; Monaco, S.; Gambetti, P. Experimental diabetic neuropathy: Impairment of slow transport with changes in axon cross-sectional area. Proc. Natl. Acad. Sci. USA 1985, 82, 7716–7720. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Mizukami, H.; Horie, H.; Yagihashi, S. Impaired Axonal Regeneration in Diabetes. Perspective on the Underlying Mechanism from In Vivo and In Vitro Experimental Studies. Front. Endocrinol. 2017, 8, 668. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Larsen, E.; Holzbaur, E.L.F. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Mclean, W.G. The Role of the Axonal Cytoskeleton in Diabetic Neuropathy. Neurochem. Res. 1997, 22, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Buras, E.; Terashima, T.; Serrano, F.; Massaad, C.A.; Hu, L.; Bitner, B.; Inoue, T.; Chan, L.; Pautler, R.G. Hyperglycemia Induces Oxidative Stress and Impairs Axonal Transport Rates in Mice. PLoS ONE 2010, 5, e13463. [Google Scholar] [CrossRef]
- Juranek, J.K.; Geddis, M.S.; Rosario, R.; Schmidt, A.M. Impaired slow axonal transport in diabetic peripheral nerve is independent of RAGE. Eur. J. Neurosci. 2013, 38, 3159–3168. [Google Scholar] [CrossRef]
- Tomlinson, D.R.; Moriarty, R.J.; Mayer, J.H. Prevention and Reversal of Defective Axonal Transport and Motor Nerve Conduction Velocity in Rats with Experimental Diabetes by Treatment with the Aldose Reductase Inhibitor Sorbinil. Diabetes 1984, 33, 470–476. [Google Scholar] [CrossRef]
- Picconi, F.; Mataluni, G.; Ziccardi, L.; Parravano, M.; Di Renzo, A.; Ylli, D.; Pasqualetti, P.; Studer, V.; Chioma, L.; Marfia, G.A.; et al. Association between Early Neuroretinal Dysfunction and Peripheral Motor Unit Loss in Patients with Type 1 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 9763507. [Google Scholar] [CrossRef]
- Reske-Nielsen, E.; Gregersen, G.; Harmsen, A.; Lundbaek, K. Morphological abnormalities of the terminal neuromuscular apparatus in recent juvenile diabetes. Diabetologia 1970, 6, 104–109. [Google Scholar] [CrossRef][Green Version]
- Fahim, M.A.; El-Sabban, F.; Davidson, N. Muscle contractility decrement and correlated morphology during the pathogenesis of streptozotocin-diabetic mice. Anat. Rec. 1998, 251, 240–244. [Google Scholar] [CrossRef]
- Fahim, M.A.; Hasan, M.Y.; Alshuaib, W.B. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J. Appl. Physiol. 2000, 89, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Okazaki, M.; Kimura, M. Streptozocin-Diabetes Modifies Acetylcholine Release from Mouse Phrenic Nerve Terminal and Presynaptic Sensitivity to Succinylcholine. Jpn. J. Pharmacol. 1993, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kawae, T.; Kataoka, H.; Ikeda, Y. Loss of lower extremity muscle strength based on diabetic polyneuropathy in older patients with type 2 diabetes: Multicenter Survey of the Isometric Lower Extremity Strength in Type 2 Diabetes: Phase 2 study. J. Diabetes. Investig. 2020, 2019, 170. [Google Scholar] [CrossRef]
- Ijzerman, T.H.; Schaper, N.C.; Melai, T.; Meijer, K.; Willems, P.J.B.; Savelberg, H.H.C.M. Lower extremity muscle strength is reduced in people with type 2 diabetes, with and without polyneuropathy, and is associated with impaired mobility and reduced quality of life. Diabetes Res. Clin. Pract. 2012, 95, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Kimpinski, K.; Doherty, T.J.; Rice, C.L. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin. Neurophysiol. 2014, 125, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Choi, I.H.; Kimpinski, K.; Doherty, T.J.; Rice, C.L. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve 2013, 48, 298–300. [Google Scholar] [CrossRef]
- Andersen, H.; Stålberg, E.; Gjerstad, M.D.; Jakobsen, J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve 1998, 21, 1647–1654. [Google Scholar] [CrossRef]
- Andersen, H.; Gadeberg, P.C.; Brock, B.; Jakobsen, J. Muscular atrophy in diabetic neuropathy: A stereological magnetic resonance imaging study. Diabetologia 1997, 40, 1062–1069. [Google Scholar] [CrossRef]
- Andersen, H.; Gjerstad, M.D.; Jakobsen, J. Atrophy of foot muscles: A measure of diabetic neuropathy. Diabetes Care 2004, 27, 2382–2385. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Murakawa, Y.; Sima, A.A.F. Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J. Peripher. Nerv. Syst. 2002, 7, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.W.; Wright, D.E. A Role for Insulin in Diabetic Neuropathy. Front. Neurosci. 2016, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H. Motor function in diabetic neuropathy. Acta Neurol. Scand. 1999, 100, 211–220. [Google Scholar] [CrossRef]
- Andersen, H. Motor dysfunction in diabetes. Diabetes Metab. Res. Rev. 2012, 28, 89–92. [Google Scholar] [CrossRef]
- Andersen, H. Motor neuropathy. Handb. Clin. Neurol. 2014, 126, 81–95. [Google Scholar]
- Perry, B.D.; Caldow, M.K.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; Asrar Ul Haq, M.; Hare, D.L.; et al. Muscle atrophy in patients with Type 2 Diabetes Mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2016, 22, 94–109. [Google Scholar]
- Monaco, C.M.F.; Gingrich, M.A.; Hawke, T.J. Considering Type 1 Diabetes as a Form of Accelerated Muscle Aging. Exerc. Sport Sci. Rev. 2019, 47, 98–107. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]
- Gutierrez, E.M.; Helber, M.D.; Dealva, D.; Ashton-Miller, J.A.; Richardson, J.K. Mild diabetic neuropathy affects ankle motor function. Clin. Biomech. 2001, 16, 522–528. [Google Scholar] [CrossRef]
- Sacchetti, M.; Scotto Sacchetti, M.; Balducci, S.; Bazzucchi, I.; Carlucci, F.; Scotto di Palumbo, A.; Di Palumbo, A.S.; Haxhi, J.; Conti, F.; Di Biase, N.; et al. Neuromuscular dysfunction in diabetes: Role of nerve impairment and training status. Med. Sci. Sports Exerc. 2013, 45, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated Loss of Skeletal Muscle Strength in Older Adults with Type 2 Diabetes. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Miller, T.A.; Armstrong, R.B. Mechanisms of force loss in diabetic mouse skeletal muscle. Muscle Nerve 2003, 28, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Eshima, H.; Tamura, Y.; Kakehi, S.; Nakamura, K.; Kurebayashi, N.; Murayama, T.; Kakigi, R.; Sakurai, T.; Kawamori, R.; Watada, H. Dysfunction of muscle contraction with impaired intracellular Ca2+ handling in skeletal muscle and the effect of exercise training in male db/dbmice. J. Appl. Physiol. 2019, 126, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.B.; Gollnick, P.D.; Ianuzzo, C.D. Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res. 1975, 162, 387–393. [Google Scholar] [CrossRef] [PubMed]
- MÅrin, P.; Andersson, B.; Krotkiewski, M.; Björntorp, P. Muscle Fiber Composition and Capillary Density in Women and Men with NIDDM. Diabetes Care 1994, 17, 382–386. [Google Scholar] [CrossRef]
- Greene, D.A.; Lewis, R.A.; Lattimer, S.A.; Brown, M.J. Selective effects of myo-inositol administration on sciatic and tibial motor nerve conduction parameters in the streptozocin-diabetic rat. Diabetes 1982, 31, 573–578. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A.; Harrison, J. Effect of diabetes on motor conduction velocity in different branches of the rat sciatic nerve. Exp. Neurol. 1986, 92, 757–761. [Google Scholar] [CrossRef]
- Fritzsch, B. Fast axonal diffusion of 3000 molecular weight dextran amines. J. Neurosci. Methods 1993, 50, 95–103. [Google Scholar] [CrossRef]
- Ishihara, A.; Naitoh, H.; Araki, H.; Nishihira, Y. Soma size and oxidative enzyme activity of motoneurones supplying the fast twitch and slow twitch muscles in the rat. Brain Res. 1988, 446, 195–198. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A.; Robertson, S. Changes in skeletal muscle contractile properties in streptozocin-induced diabetic rats and role of polyol pathway and hypoinsulinemia. Diabetes 1990, 39, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Major, B.; Kimpinski, K.; Doherty, T.J.; Rice, C.L. Skeletal muscle morphology and contractile function in relation to muscle denervation in diabetic neuropathy. J. Appl. Physiol. 2014, 116, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, Y.; Shinohara, M.; Kouzaki, M.; Fukunaga, T. Fluctuations in plantar flexion force are reduced after prolonged tendon vibration. J. Appl. Physiol. 2004, 97, 2090–2097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Deursen, R.W.; Sanchez, M.M.; Ulbrecht, J.S.; Cavanagh, P.R. The role of muscle spindles in ankle movement perception in human subjects with diabetic neuropathy. Exp. Brain Res. 1998, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Swash, M.; Fox, K.P. The pathology of the human muscle spindle: Effect of denervation. J. Neurol. Sci. 1974, 22, 1–24. [Google Scholar] [CrossRef]
- Weis, J.; Schr der, J.M.; Dimpfel, W. Nerve conduction changes and fine structural alterations of extra- and intrafusal muscle and nerve fibers in streptozotocin diabetic rats. Muscle Nerve 1995, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.F.; Andersen, H.; Sinkjaer, T. Decreased stiffness at the ankle joint in patients with long-term Type 1 diabetes. Diabet. Med. 2004, 21, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.A.; Ryals, J.M.; Feldman, E.L.; Wright, D.E. Abnormal muscle spindle innervation and large-fiber neuropathy in diabetic mice. Diabetes 2008, 57, 1693–1701. [Google Scholar] [CrossRef]
- Friese, A.; Friese, A.; Kaltschmidt, J.A.; Kaltschmidt, J.A.; Ladle, D.R.; Ladle, D.R.; Sigrist, M.; Sigrist, M.; Jessell, T.M.; Jessell, T.M.; et al. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc. Natl. Acad. Sci. USA 2009, 106, 13588–13593. [Google Scholar] [CrossRef]
- Kucera, J. Splitting of the nuclear bag fiber in the course of muscle spindle denervation and reinnervation. J. Histochem. Cytochem. 1977, 25, 1102–1104. [Google Scholar] [CrossRef]
- Sveen, K.A.; Karimé, B.; Jørum, E.; Mellgren, S.I.; Fagerland, M.W.; Monnier, V.M.; Dahl-Jørgensen, K.; Hanseen, K.F. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes: Associations with glycemic control and advanced protein glycation: The Oslo Study. Diabetes Care 2013, 36, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Riddle, C.N.; Baker, S.N. Convergence of Pyramidal and Medial Brain Stem Descending Pathways onto Macaque Cervical Spinal Interneurons. J. Neurophysiol. 2010, 103, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Adaptive plasticity in motor cortex: Implications for rehabilitation after brain injury. J. Rehabil. Med. 2003, 41, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Lewallen, K.A.; Pfaff, S.L. Spatial organization of cortical and spinal neurons controlling motor behavior. Curr. Opin. Neurol. 2012, 22, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Welniarz, Q.; Dusart, I.; Roze, E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 2017, 77, 810–829. [Google Scholar] [CrossRef]
- Seki, K.; Perlmutter, S.I.; Fetz, E.E. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat. Neurosci. 2003, 6, 1309–1316. [Google Scholar] [CrossRef]
- Ueno, M.; Nakamura, Y.; Li, J.; Gu, Z.; Niehaus, J.; Maezawa, M.; Crone, S.A.; Goulding, M.; Baccei, M.L.; Yoshida, Y. Corticospinal Circuits from the Sensory and Motor Cortices Differentially Regulate Skilled Movements through Distinct Spinal Interneurons. Cell Rep. 2018, 23, 1286–1300.e7. [Google Scholar] [CrossRef]
- Lawrence, D.G.; Kuypers, H.G.J.M. The functional organization of the motor system in the monkey. Brain 1968, 91, 1–14. [Google Scholar] [CrossRef]
- Sasaki, S.; Isa, T.; Pettersson, L.-G.; Alstermark, B.; Naito, K.; Yoshimura, K.; Seki, K.; Ohki, Y. Dexterous Finger Movements in Primate Without Monosynaptic Corticomotoneuronal Excitation. J. Neurophysiol. 2004, 92, 3142–3147. [Google Scholar] [CrossRef]
- Brands, A.M.A.; Biessels, G.-J.; de Haan, E.H.F.; Kappelle, L.J.; Roy, P.C. Kessels The Effects of Type 1 Diabetes on Cognitive Performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Biessels, G.-J.; Gispen, W.H.; Ramakers, G.M.J. Synaptic transmission changes in the pyramidal cells of the hippocampus in streptozotocin-induced diabetes mellitus in rats. Brain Res. 2006, 1073–1074, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Reijmer, Y.D.; van den Berg, E.; de Bresser, J.; Kessels, R.P.C.; Kappelle, L.J.; Algra, A.; Biessels, G.-J. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes. Metab. Res. Rev. 2011, 27, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.N.; Younan, S.M.; Youssef, M.F.; Rashed, L.A.; Mohamady, I. A histological and functional study on hippocampal formation of normal and diabetic rats. F1000Res. 2013, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, L.; He, D.; Ling, S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. Biomed. Res. Int. 2013, 2013, 924327. [Google Scholar] [CrossRef]
- Marks, J.L.; Porte, D., Jr.; Stahl, W.L.; Baskin, D.G. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 1990, 127, 3234–3236. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Parihar, M.S.; Chaudhary, M.; Shetty, R.; Hemnani, T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: Prevention by extracts of Withania somnifera and Aloe vera. J. Clin. Neurosci. 2004, 11, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Srivastava, S.; Kakkar, P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ. Toxicol. Pharmacol. 2009, 27, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Restivo, F.; Vercellinatto, I.; Danni, O.; Brignardello, E.; Aragno, M.; Boccuzzi, G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J. Endocrinol. 2005, 187, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Aragno, M.; Brignardello, E.; Tamagno, E.; Gatto, V.; Danni, O.; Boccuzzi, G. Dehydroepiandrosterone administration prevents the oxidative damage induced by acute hyperglycemia in rats. J. Endocrinol. 1997, 155, 233–240. [Google Scholar] [CrossRef]
- Andersen, H.; Nielsen, S.; Nielsen, J.F. Motor cortical excitability remains unaffected of short-term hyperglycemia in Type 1 diabetic patients. J. Diabetes Complicat. 2006, 20, 51–55. [Google Scholar] [CrossRef]
- Andersen, H.; Nielsen, J.F.; Poulsen, P.L.; Mogensen, C.E.; Jakobsen, J. Motor pathway function in normoalbuminuric IDDM patients. Diabetologia 1995, 38, 1191–1196. [Google Scholar] [CrossRef]
- Liu, D.; Duan, S.; Zhang, J.; Zhou, C.; Liang, M.; Yin, X.; Wei, P.; Wang, J. Aberrant Brain Regional Homogeneity and Functional Connectivity in Middle-Aged T2DM Patients: A Resting-State Functional MRI Study. Front. Hum. Neurosci. 2016, 10, 539. [Google Scholar] [CrossRef]
- Hughes, T.M.; Ryan, C.M.; Aizenstein, H.J.; Nunley, K.; Gianaros, P.J.; Miller, R.; Costacou, T.; Strotmeyer, E.S.; Orchard, T.J.; Rosano, C. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J. Diabetes Complicat. 2013, 27, 558–564. [Google Scholar] [CrossRef]
- Peng, B.; Chen, Z.; Ma, L.; Dai, Y. Cerebral alterations of type 2 diabetes mellitus on MRI: A pilot study. Neurosci. Lett. 2015, 606, 100–105. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Sun, J.; Ma, L. Mapping the brain in type II diabetes: Voxel-based morphometry using DARTEL. Eur. J. Radiol. 2012, 81, 1870–1876. [Google Scholar] [CrossRef]
- Yoon, S.; Cho, H.; Kim, J.; Lee, D.-W.; Kim, G.H.; Hong, Y.S.; Moon, S.; Park, S.; Lee, S.; Lee, S.; et al. Brain changes in overweight/obese and normal-weight adults with type 2 diabetes mellitus. Diabetologia 2017, 60, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- van Duinkerken, E.; Schoonheim, M.M.; IJzerman, R.G.; Klein, M.; Ryan, C.M.; Moll, A.C.; Snoek, F.J.; Barkhof, F.; Diamant, M.; Pouwels, P.J.W. Diffusion tensor imaging in type 1 diabetes: Decreased white matter integrity relates to cognitive functions. Diabetologia 2012, 55, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, L.; IJzerman, R.G.; Jennifer, S.; Barkhof, F.; Diamant, M.; Veltman, D.J.; van Duinkerken, E. Alterations in white matter volume and integrity in obesity and type 2 diabetes. Metab. Brain Dis. 2016, 31, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fonseca, J.P.; Rincón, J. Structural and ultrastructural analysis of cerebral cortex, cerebellum, and hypothalamus from diabetic rats. Exp. Diabetes Res. 2009, 2009, 329632. [Google Scholar] [CrossRef]
- Martínez-Tellez, R.; Gómez-Villalobos, M.d.J.; Flores, G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005, 1048, 108–115. [Google Scholar] [CrossRef]
- Mukai, N.; Hori, S.; Pomeroy, M. Cerebral lesions in rats with streptozotocin-induced diabetes. Acta Neuropathol. 1980, 51, 79–84. [Google Scholar] [CrossRef]
- Jakobsen, J.; Sidenius, P.; Gundersen, H.J.G.; Østerby, R. Quantitative Changes of Cerebral Neocortical Structure in Insulin-Treated Long-Term Streptozocin-lnduced Diabetes in Rats. Diabetes 1987, 36, 597–601. [Google Scholar] [CrossRef]
- Reske-Nielsen, E.; Lundbaek, K.; Rafaelsen, O.J. Pathological changes in the central and peripheral nervous system of young long-term diabetics: I. Diabetic encephalopathy. Diabetologia 1966, 1, 233–241. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Osmanovic-Barilar, J.; Brückner, M.K.; Hoyer, S.; Arendt, T.; Riederer, P. Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: A long-term follow up study. J. Neural. Transm. 2011, 118, 765–772. [Google Scholar] [CrossRef]
- Larsson, M.; Lietzau, G.; Nathanson, D.; Östenson, C.-G.; Mallard, C.; Johansson, M.E.; Nyström, T.; Patrone, C.; Darsalia, V. Diabetes negatively affects cortical and striatal GABAergic neurons: An effect that is partially counteracted by exendin-4. Biosci. Rep. 2016, 36, e00421. [Google Scholar] [CrossRef]
- Aye, T.; Barnea-Goraly, N.; Ambler, C.; Hoang, S.; Schleifer, K.; Park, Y.; Drobny, J.; Wilson, D.M.; Reiss, A.L.; Buckingham, B.A. White Matter Structural Differences in Young Children with Type 1 Diabetes: A Diffusion Tensor Imaging Study. Diabetes Care 2012, 35, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; WANG, J.; Zhou, X.; Shu, N.; Wang, Y.; Zhang, Z. White Matter Integrity Disruptions Associated with Cognitive Impairments in Type 2 Diabetic Patients. Diabetes 2014, 63, 3596–3605. [Google Scholar] [CrossRef] [PubMed]
- Perantie, D.C.; Wu, J.; Koller, J.M.; Lim, A.; Warren, S.L.; Black, K.J.; Sadler, M.; White, N.H.; Hershey, T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 2007, 30, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Callaghan, M.F.; Heni, M.; Weiskopf, N.; Scheffler, K.; Häring, H.-U.; Fritsche, A.; Veit, R.; Preissl, H. Specific white matter tissue microstructure changes associated with obesity. Neuroimage 2016, 125, 36–44. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Schenone, A.; Scramuzza, G.; Caponnetto, C.; Gasparetto, B.; Adezati, L.; Abbruzzese, M.; Viviani, G.L. Impairment of central motor conduction in diabetic patients. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 335–340. [Google Scholar] [CrossRef]
- Dolu, H.; Ulas, U.H.; Bolu, E.; Ozkardes, A.; Odabasi, Z.; Ozata, M.; Vural, O. Evaluation of central neuropathy in type II diabetes mellitus by multimodal evoked potentials. Acta Neurol. Belg. 2003, 103, 206–211. [Google Scholar]
- Goldenberg, Z.; Kucera, P.; Brezinova, M.; Kurca, E.; Barak, L.; Traubner, P. Clinically unapparent central motor pathways lesion in patients with type I diabetes mellitus. A transcranial magnetic stimulation study. Bratisl. Lek. Listy. 2004, 105, 400–403. [Google Scholar]
- El Bardawil, M.M.; El Hamid, M.; El Sawy, N.; Megallaa, M.; El Emary, W. Postural control and central motor pathway involvement in type 2 diabetes mellitus: Dynamic posturographic and electrophysiologic studies. Alex. Med. J. 2013, 49, 299–307. [Google Scholar] [CrossRef]
- Uccioli, L.; Giacomini, P.G.; Pasqualetti, P.; Di Girolamo, S.; Ferrigno, P.; Monticone, G.; Bruno, E.; Boccasena, P.; Magrini, A.; Parisi, L.; et al. Contribution of central neuropathy to postural instability in IDDM patients with peripheral neuropathy. Diabetes Care 1997, 20, 929–934. [Google Scholar] [CrossRef]
- Biessels, G.J.; Cristino, N.A.; Rutten, G.J.; Hamers, F.P.; Erkelens, D.W.; Gispen, W.H. Neurophysiological changes in the central and peripheral nervous system of streptozotocin-diabetic rats. Course of development and effects of insulin treatment. Brain 1999, 122 Pt 4, 757–768. [Google Scholar] [CrossRef]
- Madsen, J.G.; Østergaard, J.A.; Andersen, H.; Pedersen, M. Attenuation of Cortically Evoked Motor-Neuron Potential in Streptozotocin-Induced Diabetic Rats: A Study about the Effect of Diabetes upon Cortical-Initiated Movement. Biomed. Res. Int. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pekiner, C.; McLean, W.G. Neurofilament Protein Phosphorylation in Spinal Cord of Experimentally Diabetic Rats. J. Neurochem. 1991, 56, 1362–1367. [Google Scholar] [CrossRef]
- Pekiner, C.; Cullum, N.A.; Hughes, J.N.; Hargreaves, A.J.; Mahon, J.; Casson, I.F.; McLean, W.G. Glycation of Brain Actin in Experimental Diabetes. J. Neurochem. 1993, 61, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Hnaway, J.; Young, R.R. Localization of the pyramidal tract in the internal capsule of man. J. Neurol. Sci. 1977, 34, 63–70. [Google Scholar] [CrossRef]
- Passingham, R.E.; Perry, V.H.; Wilkinson, F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain 1983, 106, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Roy, R.R.; Raven, J.; Hodgson, J.; Brain, H.M. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 2005, 128, 2338–2358. [Google Scholar] [CrossRef]
- Liddell, E.G.T.; Phillips, C.G. Pyramidal section in the cat. Brain 1944, 67, 1–9. [Google Scholar] [CrossRef]
- Benitez, S.U.; Carneiro, E.M.; de Oliveira, A.L.R. Synaptic input changes to spinal cord motoneurons correlate with motor control impairments in a type 1 diabetes mellitus model. Brain Behav. 2015, 5, e00372. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, B. White matter inhibitors in CNS axon regeneration failure. Exp. Neurol. 2008, 209, 302–312. [Google Scholar] [CrossRef]
- Xu, X.M.; Guénard, V.; Kleitman, N.; Aebischer, P.; Bunge, M.B. A Combination of BDNF and NT-3 Promotes Supraspinal Axonal Regeneration into Schwann Cell Grafts in Adult Rat Thoracic Spinal Cord. Exp. Neurol. 1995, 134, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Blits, B.; Dijkhuizen, P.A.; Boer, G.J.; Verhaagen, J. Intercostal Nerve Implants Transduced with an Adenoviral Vector Encoding Neurotrophin-3 Promote Regrowth of Injured Rat Corticospinal Tract Fibers and Improve Hindlimb Function. Exp. Neurol. 2000, 164, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Guénard, V.; Kleitman, N.; Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 2004, 351, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Vavrek, R.; Pearse, D.D.; Fouad, K. Neuronal Populations Capable of Regeneration following a Combined Treatment in Rats with Spinal Cord Transection. J. Neurotrauma 2007, 24, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Zaaimi, B.; Fisher, K.M.; Edgley, S.A.; Soteropoulos, D.S. Pathways mediating functional recovery. Prog. Brain Res. 2015, 218, 389–412. [Google Scholar]
- Zaaimi, B.; Edgley, S.A.; Soteropoulos, D.S.; Brain, S.B. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 2012, 135, 2277–2289. [Google Scholar] [CrossRef]
- Umeda, T.; Takahashi, M.; Isa, K.; Isa, T. Formation of Descending Pathways Mediating Cortical Command to Forelimb Motoneurons in Neonatally Hemidecorticated Rats. J. Neurophysiol. 2010, 104, 1707–1716. [Google Scholar] [CrossRef]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef]
- Tohyama, T.; Kinoshita, M.; Kobayashi, K.; Isa, K.; Watanabe, D.; Kobayashi, K.; Liu, M.; Isa, T. Contribution of propriospinal neurons to recovery of hand dexterity after corticospinal tract lesions in monkeys. Proc. Natl. Acad. Sci. USA 2017, 114, 604–609. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hayashi, T.; Murata, Y.; Ose, T.; Higo, N. Premotor Cortical-Cerebellar Reorganization in a Macaque Model of Primary Motor Cortical Lesion and Recovery. J. Neurosci. 2019, 39, 8484–8496. [Google Scholar] [CrossRef]
- Jaillard, A.; Martin, C.D.; Garambois, K.; Brain, J.L. Vicarious function within the human primary motor cortex? Brain 2005, 128, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Higo, N.; Oishi, T.; Isa, T. Increased expression of the growth-associated protein-43 gene after primary motor cortex lesion in macaque monkeys. Neurosci. Res. 2015, 98, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Higo, N.; Hayashi, T.; Nishimura, Y.; Sugiyama, Y.; Oishi, T.; Tsukada, H.; Isa, T.; Onoe, H. Temporal plasticity involved in recovery from manual dexterity deficit after motor cortex lesion in macaque monkeys. J. Neurosci. 2015, 35, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Higo, N.; Oishi, T.; Yamashita, A.; Matsuda, K.; Hayashi, M.; Yamane, S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J. Neurophysiol. 2008, 99, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Higo, N.; Yoshino-Saito, K.; Murata, Y.; Nishimura, Y.; Oishi, T.; Isa, T. Effects of early versus late rehabilitative training on manual dexterity after corticospinal tract lesion in macaque monkeys. J. Neurophysiol. 2013, 109, 2853–2865. [Google Scholar] [CrossRef][Green Version]
- Allred, R.P.; Maldonado, M.A.; Hsu And, J.E.; Jones, T.A. Training the “less-affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restor. Neurol. Neurosci. 2005, 23, 297–302. [Google Scholar]
- Girgis, J.; Merrett, D.; Kirkland, S.; Metz, G.A.S.; Verge, V.; Fouad, K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 2007, 130, 2993–3003. [Google Scholar] [CrossRef]
- Carmel, J.B.; Kimura, H.; Berrol, L.J.; Martin, J.H. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur. J. Neurosci. 2013, 37, 1090–1102. [Google Scholar] [CrossRef]
- Carmel, J.B.; Martin, J.H. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front. Integr. Neurosci. 2014, 8, 4935. [Google Scholar] [CrossRef]
- Carmel, J.B.; Kimura, H.; Martin, J.H. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J. Neurosci. 2014, 34, 462–466. [Google Scholar] [CrossRef]
- Kataoka, H.; Miyatake, N.; Kitayama, N.; Murao, S.; Tanaka, S. A pilot study of short-term toe resistance training in patients with type 2 diabetes mellitus. Diabetol. Int. 2017, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Miyatake, N.; Murao, S.; Tanaka, S. A randomized controlled trial of short-term toe resistance training to improve toe pinch force in patients with type 2 diabetes. Acta Med. Okayama 2018, 72, 9–15. [Google Scholar] [PubMed]
- Zhou, S. Chronic neural adaptations to unilateral exercise: Mechanisms of cross education. Exerc. Sport Sci. Rev. 2000, 28, 177–184. [Google Scholar] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Krivickas, L.S.; Kim, S.-K.; Foldvari, M.; Roubenoff, R. Strength training in older women: Early and late changes in whole muscle and single cells. Muscle Nerve 2003, 28, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Jitsuki, S.; Nakajima, W.; Murata, Y.; Jitsuki-Takahashi, A.; Katsuno, Y.; Tada, H.; Sano, A.; Suyama, K.; Mochizuki, N.; et al. CRMP2-binding compound, edonerpic maleate, accelerates motor function recovery from brain damage. Science 2018, 360, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Chopp, M.; Ye, X.; Liu, Z.; Zacharek, A.; Cui, Y.; Roberts, C.; Buller, B.; Chen, J. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol. Dis. 2012, 46, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Biophys. Rev. 2020, 18, 62. [Google Scholar] [CrossRef]
- Nagayach, A.; Patro, N.; Patro, I. Experimentally induced diabetes causes glial activation, glutamate toxicity and cellular damage leading to changes in motor function. Front. Cell. Neurosci. 2014, 8, 224. [Google Scholar] [CrossRef]
- Mark, L.P.; Prost, R.W.; Ulmer, J.L.; Smith, M.M.; Daniels, D.L.; Strottmann, J.M.; Brown, W.D.; Hacein-Bey, L. Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. Am. J. Neuroradiol. 2001, 22, 1813–1824. [Google Scholar]
- Baydas, G.; Nedzvetskii, V.S.; Tuzcu, M.; Yasar, A.; Kirichenko, S.V. Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: Effects of vitamin E. Eur. J. Pharmacol. 2003, 462, 67–71. [Google Scholar] [CrossRef]
- Baydas, G.; Reiter, R.J.; Yasar, A.; Tuzcu, M.; Akdemir, S.; Nedzvetskii, V.S. Melatonin reduces glial reactivity in the hippocampus, cortex, and cerebellum of streptozotocin-induced diabetic rats. Free Radic. Biol. Med. 2003, 35, 797–804. [Google Scholar] [CrossRef]
| Model | Induction Mechanism | Type of Diabetes |
|---|---|---|
| STZ rats | Chemical induction | Type 1 diabetes |
| Bio-Breeding (BB) rats | Spontaneous | Type 1 diabetes |
| Zucker rats | Spontaneous | Obesity model of type 2 diabetes |
| Goto–Kakizaki rats | Spontaneous | Lean model of type 2 diabetes |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muramatsu, K. Diabetes Mellitus-Related Dysfunction of the Motor System. Int. J. Mol. Sci. 2020, 21, 7485. https://doi.org/10.3390/ijms21207485
Muramatsu K. Diabetes Mellitus-Related Dysfunction of the Motor System. International Journal of Molecular Sciences. 2020; 21(20):7485. https://doi.org/10.3390/ijms21207485
Chicago/Turabian StyleMuramatsu, Ken. 2020. "Diabetes Mellitus-Related Dysfunction of the Motor System" International Journal of Molecular Sciences 21, no. 20: 7485. https://doi.org/10.3390/ijms21207485
APA StyleMuramatsu, K. (2020). Diabetes Mellitus-Related Dysfunction of the Motor System. International Journal of Molecular Sciences, 21(20), 7485. https://doi.org/10.3390/ijms21207485





