Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis
Abstract
1. Introduction
2. Results
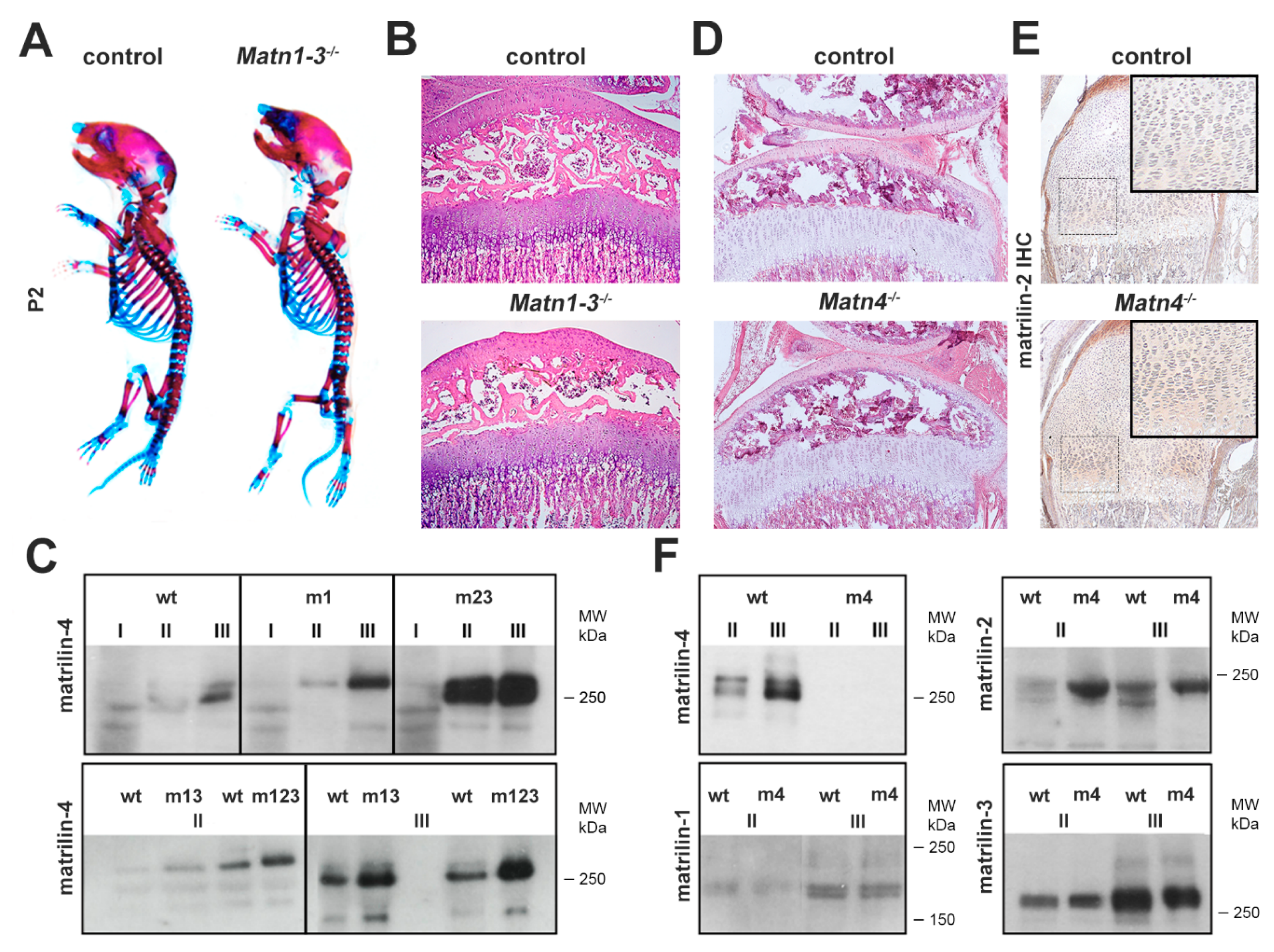
2.1. Biochemical Compensation in Cartilage of Mice Lacking Matrilins
2.2. Loss of Matrilins Results in Modulation of Lumbosacral Identity of the Vertebrae
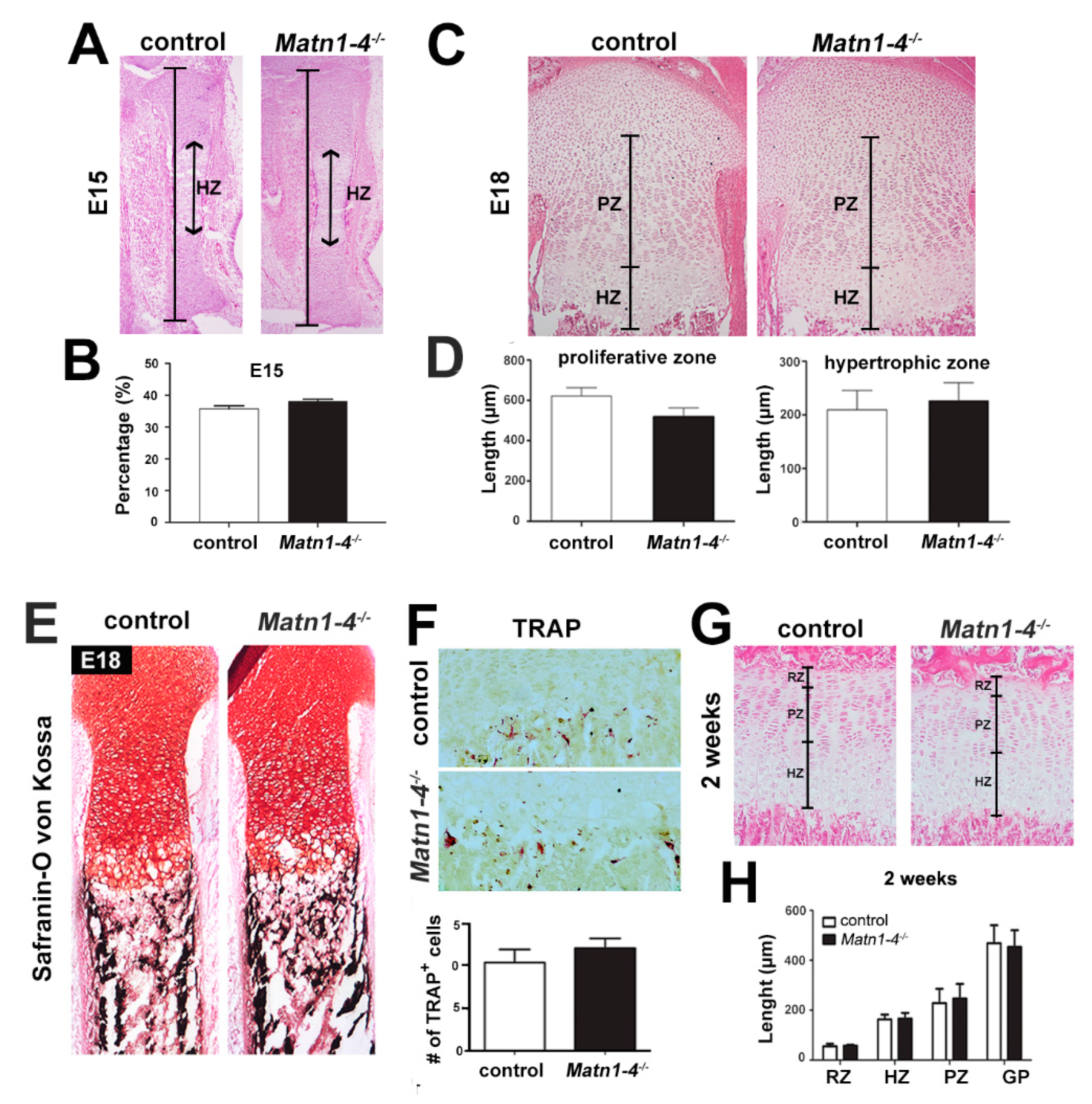
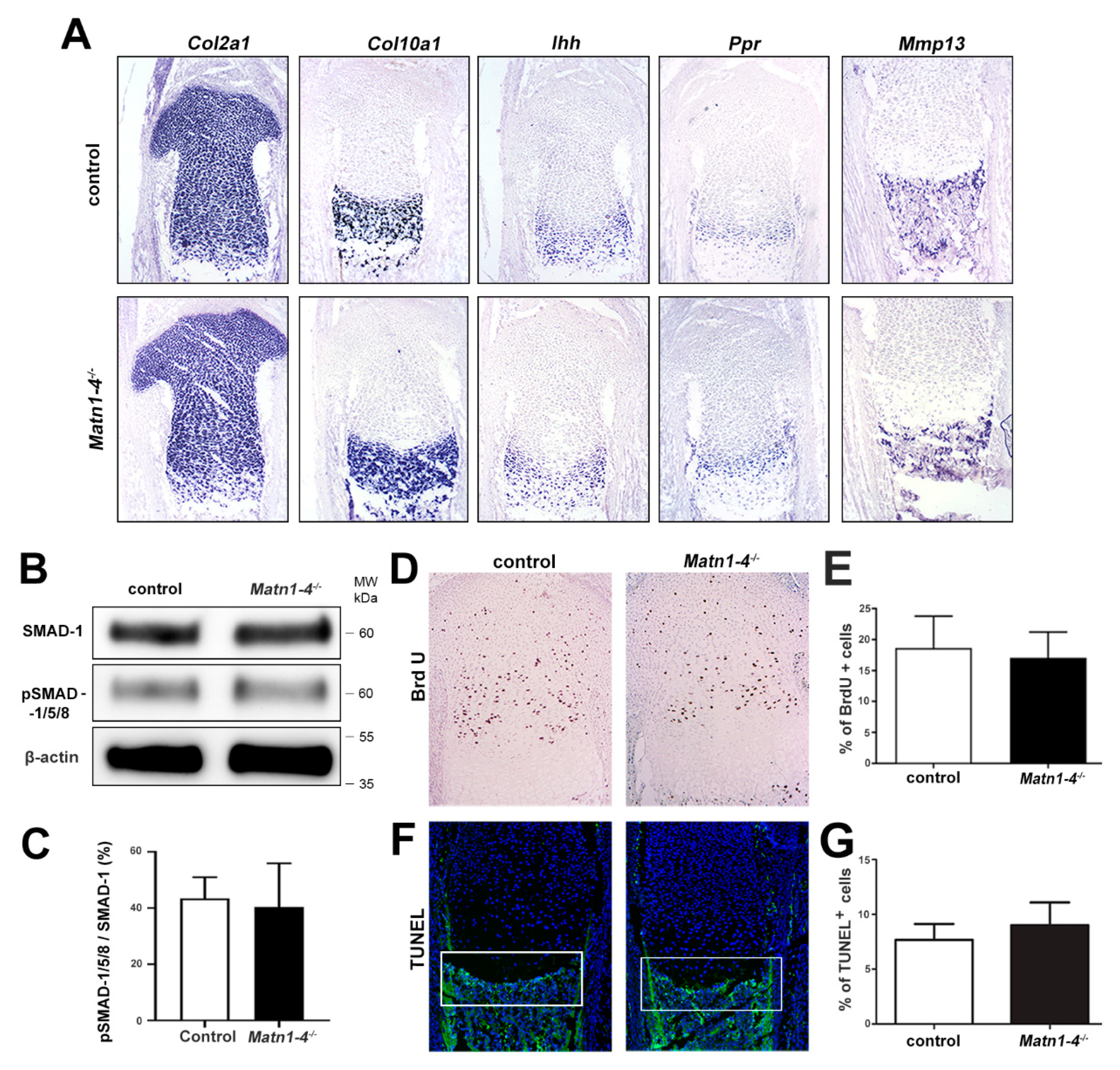
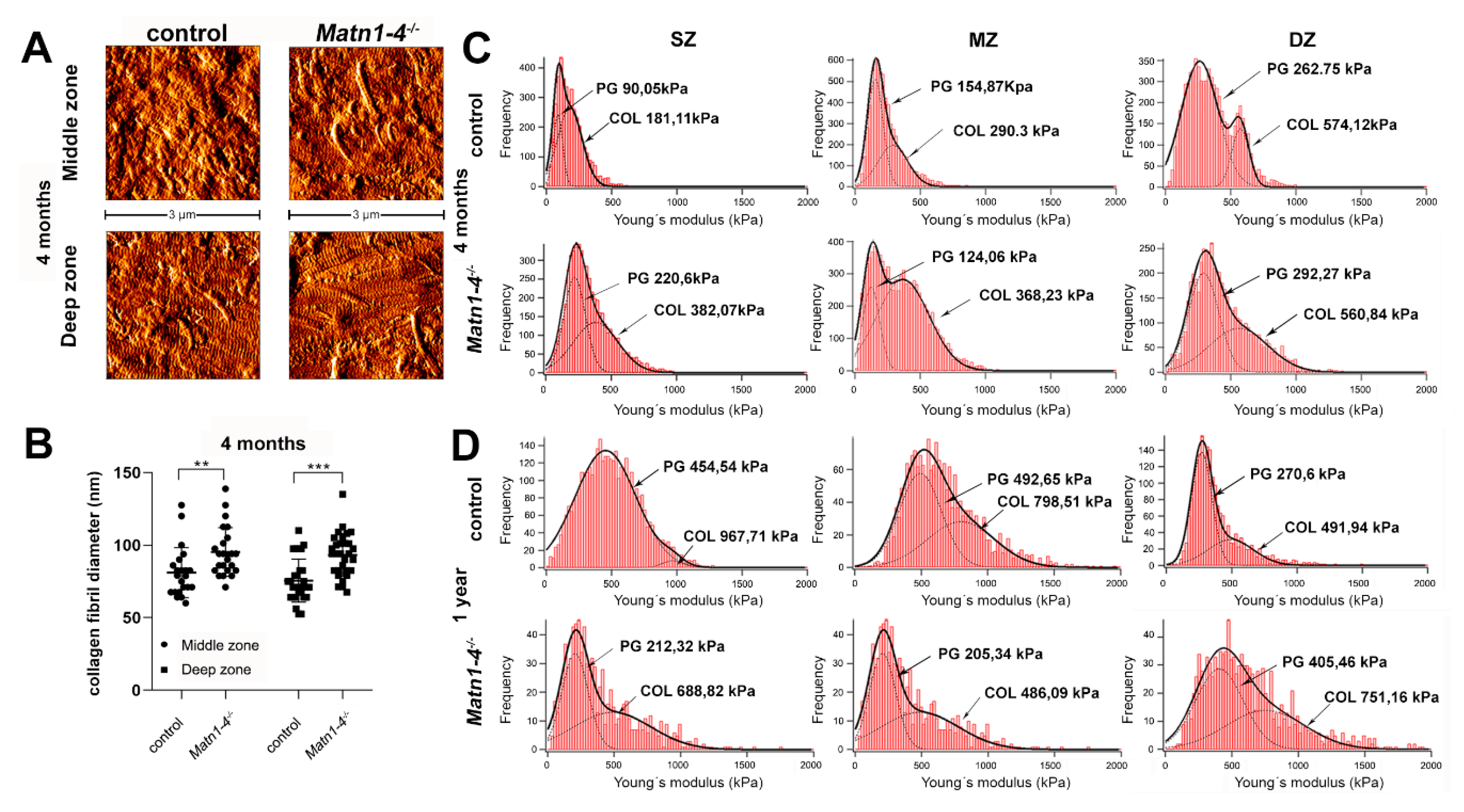
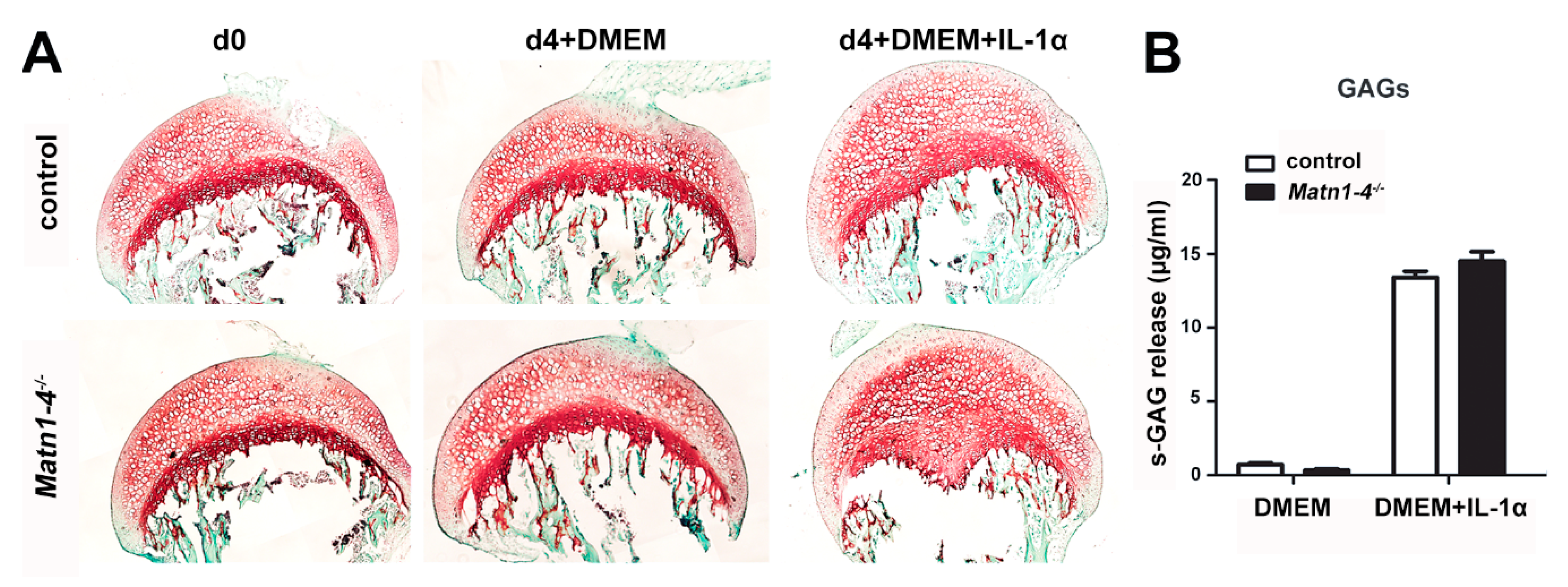
2.3. Lack of Matrilins Has No Adverse Effect on Structural and Functional Properties of the Cartilaginous Growth Plate in Long Bones
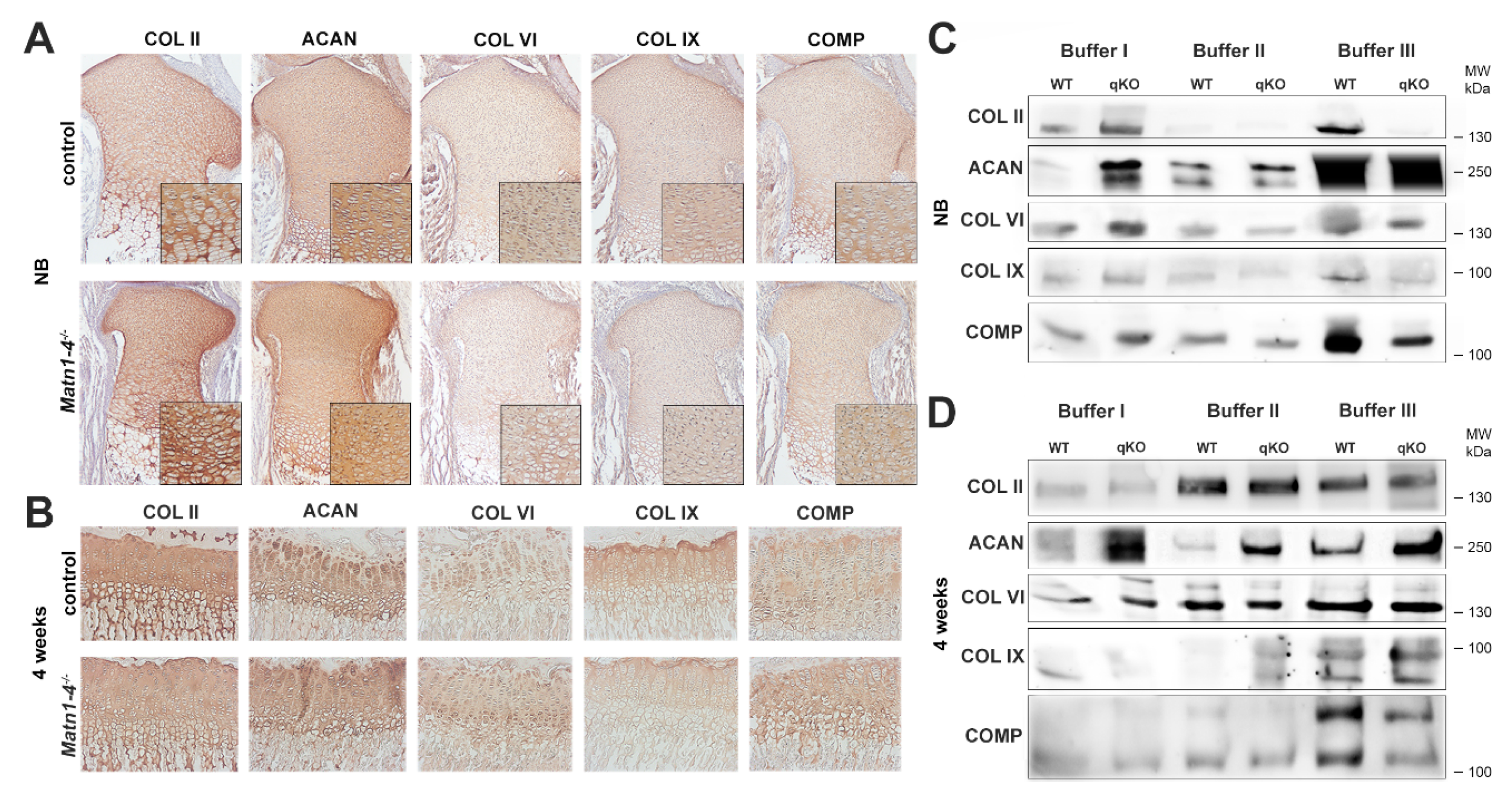
2.4. Normal Deposition but Altered Extractability of Binding Partners in the Cartilage ECM of Quadruple Knockout Mice
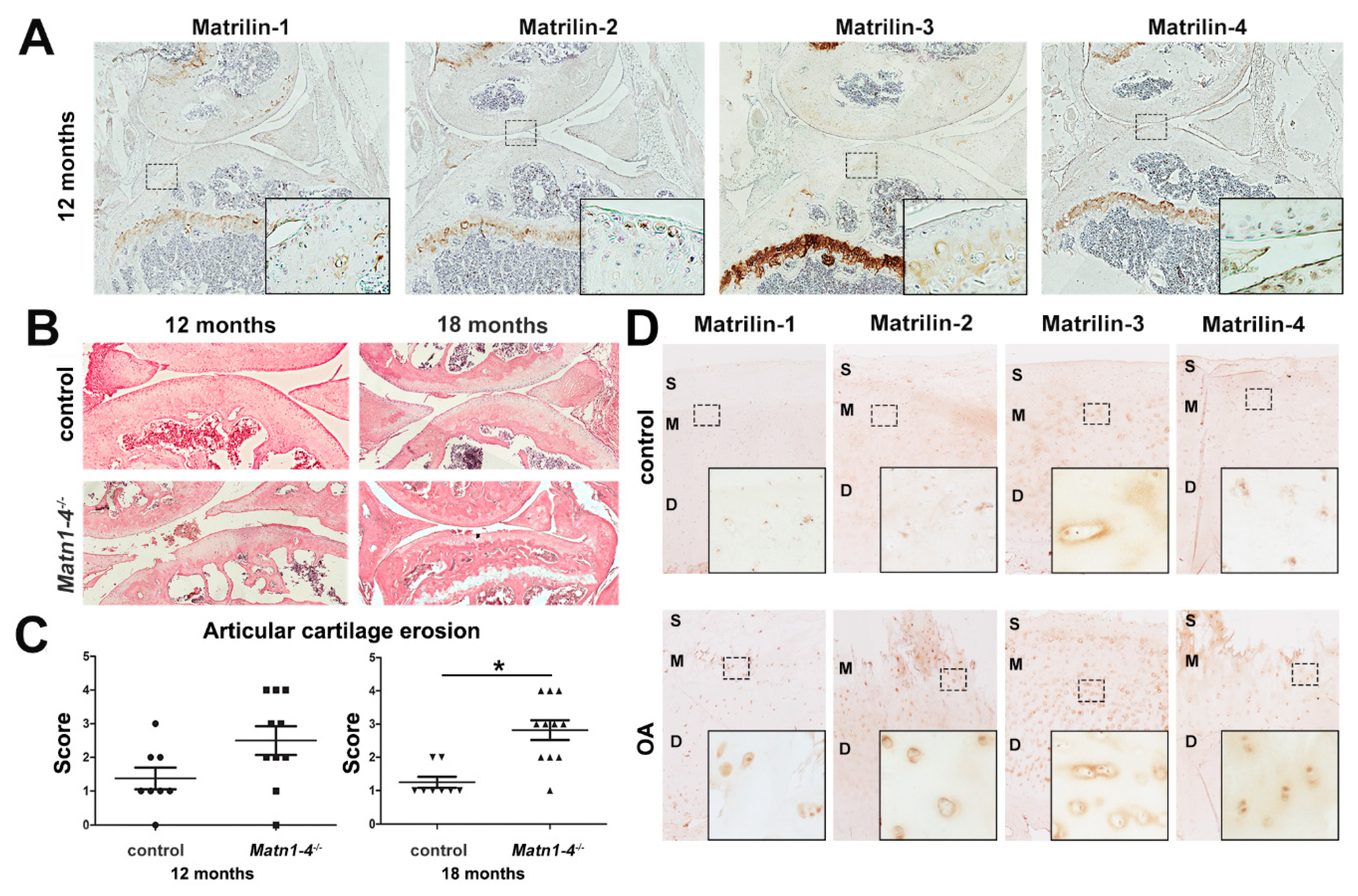
2.5. Depletion of All Matrilins in the Articular Cartilage Leads to Severe Spontaneous Osteoarthritis in Mice
2.6. MATN4-Deficiency Also Enhances Age-Associated Articular Cartilage Degradation
3. Discussion
4. Materials and Methods
4.1. Knockout Mice
4.2. Human Samples
4.3. Antibodies
4.4. Whole-Mount Skeletal Staining and X-ray Analysis
4.5. Histology, Immunohistochemistry and In Situ Hybridization
4.6. Cell Proliferation and Cell Death Assays
4.7. Protein Extraction and Western Blotting
4.8. Explant Culture
4.9. Atomic Force Microscopy (AFM)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| AFM | Atomic force microscopy |
| BMP-2 | Bone morphogenetic protein-2 |
| BrdU | Bromodeoxyuridine |
| COL | Collagen |
| COMP | Cartilage oligomeric matrix protein |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetracetic acid |
| GAG | Glycosaminoglycan |
| HE | Hematoxylin and eosin |
| IL-1 | Interleukin-1 |
| ITM | Interterritorial matrix |
| MATN | Matrilin |
| MED | Multiple epiphyseal dysplasia |
| OA | Osteoarthritis |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| P2 | Postnatal day 2 |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEMD | Spondylo-meta-epiphyseal dysplasia |
| TRAP | Tartrate-resistant acid phosphatase |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Klatt, A.R.; Becker, A.K.; Neacsu, C.D.; Paulsson, M.; Wagener, R. The matrilins: Modulators of extracellular matrix assembly. Int. J. Biochem. Cell. Biol. 2011, 43, 320–330. [Google Scholar] [CrossRef]
- Aszodi, A.; Hauser, N.; Studer, D.; Paulsson, M.; Hiripi, L.; Bosze, Z. Cloning, sequencing and expression analysis of mouse cartilage matrix protein cDNA. Eur. J. Biochem. 1996, 236, 970–977. [Google Scholar] [CrossRef]
- Klatt, A.R.; Nitsche, D.P.; Kobbe, B.; Morgelin, M.; Paulsson, M.; Wagener, R. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J. Biol. Chem. 2000, 275, 3999–4006. [Google Scholar] [CrossRef]
- Piecha, D.; Muratoglu, S.; Morgelin, M.; Hauser, N.; Studer, D.; Kiss, I.; Paulsson, M.; Deak, F. Matrilin-2, a large, oligomeric matrix protein, is expressed by a great variety of cells and forms fibrillar networks. J. Biol. Chem. 1999, 274, 13353–13361. [Google Scholar] [CrossRef]
- Klatt, A.R.; Nitsche, D.P.; Kobbe, B.; Macht, M.; Paulsson, M.; Wagener, R. Molecular structure, processing, and tissue distribution of matrilin-4. J. Biol. Chem. 2001, 276, 17267–17275. [Google Scholar] [CrossRef]
- Hauser, N.; Paulsson, M.; Heinegård, D.; Mörgelin, M. Interaction of Cartilage Matrix Protein with Aggrecan. Increased covalent cross-linking with tissue maturation. J. Biol. Chem. 1996, 271, 32247–32252. [Google Scholar] [CrossRef][Green Version]
- Winterbottom, N.; Tondravi, M.M.; Harrington, T.L.; Klier, F.G.; Vertel, B.M.; Goetinck, P.F. Cartilage matrix protein is a component of the collagen fibril of cartilage. Dev. Dyn. 1992, 193, 266–276. [Google Scholar] [CrossRef]
- Budde, B.; Blumbach, K.; Ylostalo, J.; Zaucke, F.; Ehlen, H.W.; Wagener, R.; Ala-Kokko, L.; Paulsson, M.; Bruckner, P.; Grassel, S. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol. Cell. Biol. 2005, 25, 10465–10478. [Google Scholar] [CrossRef]
- Mann, H.H.; Ozbek, S.; Engel, J.; Paulsson, M.; Wagener, R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem. 2004, 279, 25294–25298. [Google Scholar] [CrossRef]
- Wiberg, C.; Klatt, A.R.; Wagener, R.; Paulsson, M.; Bateman, J.F.; Heinegard, D.; Morgelin, M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 2003, 278, 37698–37704. [Google Scholar] [CrossRef]
- Wagener, R.; Ehlen, H.W.; Ko, Y.P.; Kobbe, B.; Mann, H.H.; Sengle, G.; Paulsson, M. The matrilins—Adaptor proteins in the extracellular matrix. FEBS Lett. 2005, 579, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Mates, L.; Nicolae, C.; Morgelin, M.; Deak, F.; Kiss, I.; Aszodi, A. Mice lacking the extracellular matrix adaptor protein matrilin-2 develop without obvious abnormalities. Matrix Biol. 2004, 23, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Paulsson, M.; Wagener, R. Expression of matrilins during maturation of mouse skeletal tissues. Matrix Biol. 2002, 21, 289–296. [Google Scholar] [CrossRef]
- Briggs, M.D.; Chapman, K.L. Pseudoachondroplasia and multiple epiphyseal dysplasia: Mutation review, molecular interactions, and genotype to phenotype correlations. Hum. Mutat. 2002, 19, 465–478. [Google Scholar] [CrossRef]
- Chapman, K.L.; Mortier, G.R.; Chapman, K.; Loughlin, J.; Grant, M.E.; Briggs, M.D. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat. Genet. 2001, 28, 393–396. [Google Scholar] [CrossRef]
- Mabuchi, A.; Haga, N.; Maeda, K.; Nakashima, E.; Manabe, N.; Hiraoka, H.; Kitoh, H.; Kosaki, R.; Nishimura, G.; Ohashi, H.; et al. Novel and recurrent mutations clustered in the von Willebrand factor A domain of MATN3 in multiple epiphyseal dysplasia. Hum. Mutat. 2004, 24, 439–440. [Google Scholar] [CrossRef]
- Jackson, G.C.; Barker, F.S.; Jakkula, E.; Czarny-Ratajczak, M.; Makitie, O.; Cole, W.G.; Wright, M.J.; Smithson, S.F.; Suri, M.; Rogala, P.; et al. Missense mutations in the beta strands of the single A-domain of matrilin-3 result in multiple epiphyseal dysplasia. J. Med. Genet. 2004, 41, 52–59. [Google Scholar] [CrossRef]
- Otten, C.; Wagener, R.; Paulsson, M.; Zaucke, F. Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not. J. Med. Genet. 2005, 42, 774–779. [Google Scholar] [CrossRef]
- Cotterill, S.L.; Jackson, G.C.; Leighton, M.P.; Wagener, R.; Makitie, O.; Cole, W.G.; Briggs, M.D. Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3. Hum. Mutat. 2005, 26, 557–565. [Google Scholar] [CrossRef]
- Nundlall, S.; Rajpar, M.H.; Bell, P.A.; Clowes, C.; Zeeff, L.A.; Gardner, B.; Thornton, D.J.; Boot-Handford, R.P.; Briggs, M.D. An unfolded protein response is the initial cellular response to the expression of mutant matrilin-3 in a mouse model of multiple epiphyseal dysplasia. Cell Stress Chaperones 2010, 15, 835–849. [Google Scholar] [CrossRef]
- Mostert, A.K.; Dijkstra, P.F.; Jansen, B.R.; van Horn, J.R.; de Graaf, B.; Heutink, P.; Lindhout, D. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: Further delineation of the phenotype including 40 years follow-up. Am. J. Med. Genet. A 2003, 120, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Borochowitz, Z.U.; Scheffer, D.; Adir, V.; Dagoneau, N.; Munnich, A.; Cormier-Daire, V. Spondylo-epi-metaphyseal dysplasia (SEMD) matrilin 3 type: Homozygote matrilin 3 mutation in a novel form of SEMD. J. Med. Genet. 2004, 41, 366–372. [Google Scholar] [CrossRef]
- Stefansson, S.E.; Jonsson, H.; Ingvarsson, T.; Manolescu, I.; Jonsson, H.H.; Olafsdottir, G.; Palsdottir, E.; Stefansdottir, G.; Sveinbjornsdottir, G.; Frigge, M.L.; et al. Genomewide scan for hand osteoarthritis: A novel mutation in matrilin-3. Am. J. Hum. Genet. 2003, 72, 1448–1459. [Google Scholar] [CrossRef]
- Min, J.L.; Meulenbelt, I.; Riyazi, N.; Kloppenburg, M.; Houwing-Duistermaat, J.J.; Seymour, A.B.; van Duijn, C.M.; Slagboom, P.E. Association of matrilin-3 polymorphisms with spinal disc degeneration and osteoarthritis of the first carpometacarpal joint of the hand. Ann. Rheum. Dis. 2006, 65, 1060–1066. [Google Scholar] [CrossRef]
- Pullig, O.; Tagariello, A.; Schweizer, A.; Swoboda, B.; Schaller, P.; Winterpacht, A. MATN3 (matrilin-3) sequence variation (pT303M) is a risk factor for osteoarthritis of the CMC1 joint of the hand, but not for knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 279–280. [Google Scholar] [CrossRef]
- Meulenbelt, I.; Bijkerk, C.; Breedveld, F.C.; Slagboom, P.E. Genetic linkage analysis of 14 candidate gene loci in a family with autosomal dominant osteoarthritis without dysplasia. J. Med. Genet. 1997, 34, 1024–1027. [Google Scholar] [CrossRef]
- Loughlin, J.; Dowling, B.; Mustafa, Z.; Smith, A.; Sykes, B.; Chapman, K. Analysis of the association of the matrillin-1 gene (CRTM) with osteoarthritis: Comment on the article by Meulenbelt et al. Arthritis Rheum. 2000, 43, 1423–1425. [Google Scholar] [CrossRef]
- Montanaro, L.; Parisini, P.; Greggi, T.; Di Silvestre, M.; Campoccia, D.; Rizzi, S.; Arciola, C.R. Evidence of a linkage between matrilin-1 gene (MATN1) and idiopathic scoliosis. Scoliosis 2006, 1, 21. [Google Scholar] [CrossRef]
- Jang, J.Y.; Park, E.K.; Ryoo, H.M.; Shin, H.I.; Kim, T.H.; Jang, J.S.; Park, H.S.; Choi, J.Y.; Kwon, T.G. Polymorphisms in the Matrilin-1 gene and risk of mandibular prognathism in Koreans. J. Dent. Res. 2010, 89, 1203–1207. [Google Scholar] [CrossRef]
- Chimusa, E.R.; Beighton, P.; Kumuthini, J.; Ramesar, R.S. Detecting genetic modifiers of spondyloepimetaphyseal dysplasia with joint laxity in the Caucasian Afrikaner community. Hum. Mol. Genet. 2019, 28, 1053–1063. [Google Scholar] [CrossRef]
- Aszodi, A.; Bateman, J.F.; Hirsch, E.; Baranyi, M.; Hunziker, E.B.; Hauser, N.; Bosze, Z.; Fassler, R. Normal skeletal development of mice lacking matrilin 1: Redundant function of matrilins in cartilage? Mol. Cell. Biol. 1999, 19, 7841–7845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ko, Y.; Kobbe, B.; Nicolae, C.; Miosge, N.; Paulsson, M.; Wagener, R.; Aszodi, A. Matrilin-3 is dispensable for mouse skeletal growth and development. Mol. Cell. Biol. 2004, 24, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Birk, D.E.; Goetinck, P.F. Mice lacking matrilin-1 (cartilage matrix protein) have alterations in type II collagen fibrillogenesis and fibril organization. Dev. Dyn. 1999, 216, 434–441. [Google Scholar] [CrossRef]
- Chen, Y.; Cossman, J.; Jayasuriya, C.T.; Li, X.; Guan, Y.; Fonseca, V.; Yang, K.; Charbonneau, C.; Yu, H.; Kanbe, K.; et al. Deficient mechanical activation of anabolic transcripts and post-traumatic cartilage degeneration in Matrilin-1 knockout mice. PLoS ONE 2016, 11, e0156676. [Google Scholar] [CrossRef]
- van der Weyden, L.; Wei, L.; Luo, J.; Yang, X.; Birk, D.E.; Adams, D.J.; Bradley, A.; Chen, Q. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am. J. Pathol. 2006, 169, 515–527. [Google Scholar] [CrossRef]
- Nicolae, C.; Ko, Y.P.; Miosge, N.; Niehoff, A.; Studer, D.; Enggist, L.; Hunziker, E.B.; Paulsson, M.; Wagener, R.; Aszodi, A. Abnormal collagen fibrils in cartilage of matrilin-1/matrilin-3-deficient mice. J. Biol. Chem. 2007, 282, 22163–22175. [Google Scholar] [CrossRef]
- Uckelmann, H.; Blaszkiewicz, S.; Nicolae, C.; Haas, S.; Schnell, A.; Wurzer, S.; Wagener, R.; Aszodi, A.; Essers, M.A. Extracellular matrix protein Matrilin-4 regulates stress-induced HSC proliferation via CXCR4. J. Exp. Med. 2016, 213, 1961–1971. [Google Scholar] [CrossRef]
- Malin, D.; Sonnenberg-Riethmacher, E.; Guseva, D.; Wagener, R.; Aszodi, A.; Irintchev, A.; Riethmacher, D. The extracellular-matrix protein matrilin 2 participates in peripheral nerve regeneration. J. Cell. Sci. 2009, 122, 995–1004. [Google Scholar] [CrossRef]
- Green, E.L. Genetic and non-genetic factors which influence the type of the skeleton in an inbred strain of mice. Genetics 1941, 26, 192–222. [Google Scholar]
- Mc, L.A.; Michie, D. Factors affecting vertebral variation in mice. 4. Experimental proof of the uterine basis of a maternal effect. Development 1958, 6, 645–659. [Google Scholar]
- Yang, X.; Trehan, S.K.; Guan, Y.; Sun, C.; Moore, D.C.; Jayasuriya, C.T.; Chen, Q. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J. Biol. Chem. 2014, 289, 34768–34779. [Google Scholar] [CrossRef] [PubMed]
- Gentili, C.; Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, H.; Hedbom, E.; Heineg rd, D.; Mengarelli-Widholm, S.; Reinholt, F.P.; Svensson, O. Association of the aggrecan keratan sulfate-rich region with collagen in bovine articular cartilage. J. Biol. Chem. 1999, 274, 5777–5781. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Wagener, R. Matrilins. Methods Cell. Biol. 2018, 143, 429–446. [Google Scholar] [PubMed]
- Murray, S.A.; Morgan, J.L.; Kane, C.; Sharma, Y.; Heffner, C.S.; Lake, J.; Donahue, L.R. Mouse gestation length is genetically determined. PLoS ONE 2010, 5, e12418. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Bachmanov, A.A.; Tordoff, M.G. Forty mouse strain survey of body composition. Physiol. Behav. 2007, 91, 593–600. [Google Scholar] [CrossRef]
- Jayasuriya, C.T.; Zhou, F.H.; Pei, M.; Wang, Z.; Lemme, N.J.; Haines, P.; Chen, Q. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-beta in chondroprogenitor cells. Int. J. Mol. Sci. 2014, 15, 14555–14573. [Google Scholar] [CrossRef]
- Chen, Q.; Johnson, D.M.; Haudenschild, D.R.; Tondravi, M.; Goetinck, P. Cartilage matrix protein forms a type II collagen-independent filamentous network: Analysis in primary cell cultures with a retrovirus expression system. Mol. Biol. Cell 1995, 6, 1743–1753. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Johnson, D.M.; Goetinck, P.F. Assembly of a novel cartilage matrix protein filamentous network: Molecular basis of differential requirement of von Willebrand factor A domains. Mol. Biol. Cell 1999, 10, 2149–2162. [Google Scholar] [CrossRef]
- Dreier, R.; Opolka, A.; Grifka, J.; Bruckner, P.; Grässel, S. Collagen IX-deficiency seriously compromises growth cartilage development in mice. Matrix Biol. 2008, 27, 319–329. [Google Scholar] [CrossRef]
- Blumbach, K.; Bastiaansen-Jenniskens, Y.; DeGroot, J.; Paulsson, M.; Van Osch, G.; Zaucke, F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: Cartilage oligomeric matrix protein counteracts type IX collagen–induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009, 60, 3676–3685. [Google Scholar] [CrossRef]
- Blumbach, K.; Niehoff, A.; Paulsson, M.; Zaucke, F. Ablation of collagen IX and COMP disrupts epiphyseal cartilage architecture. Matrix Biol. 2008, 27, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Brachvogel, B.; Zaucke, F.; Dave, K.; Norris, E.L.; Stermann, J.; Dayakli, M.; Koch, M.; Gorman, J.J.; Bateman, J.F.; Wilson, R. Comparative proteomic analysis of normal and collagen IX null mouse cartilage reveals altered extracellular matrix composition and novel components of the collagen IX interactome. J. Biol. Chem. 2013, 288, 13481–13492. [Google Scholar] [CrossRef] [PubMed]
- Kamper, M.; Hamann, N.; Prein, C.; Clausen-Schaumann, H.; Farkas, Z.; Aszodi, A.; Niehoff, A.; Paulsson, M.; Zaucke, F. Early changes in morphology, bone mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX−/− mice. Matrix Biol. 2016, 49, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.; Hecht, J. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets 2008, 9, 869–877. [Google Scholar] [CrossRef]
- Pullig, O.; Weseloh, G.; Klatt, A.; Wagener, R.; Swoboda, B. Matrilin-3 in human articular cartilage: Increased expression in osteoarthritis. Osteoarthr. Cartil. 2002, 10, 253–263. [Google Scholar] [CrossRef]
- Firner, S.; Zaucke, F.; Michael, J.; Dargel, J.; Schiwy-Bochat, K.-H.; Heilig, J.; Rothschild, M.A.; Eysel, P.; Brüggemann, G.-P.; Niehoff, A. Extracellular Distribution of Collagen II and Perifibrillar Adapter Proteins in Healthy and Osteoarthritic Human Knee Joint Cartilage. J. Histochem. Cytochem. 2017, 65, 593–606. [Google Scholar] [CrossRef]
- Hosseininia, S.; Önnerfjord, P.; Dahlberg, L.E. Targeted proteomics of hip articular cartilage in OA and fracture patients. J. Orthop. Res. 2019, 37, 131–135. [Google Scholar] [CrossRef]
- Vincourt, J.B.; Etienne, S.; Grossin, L.; Cottet, J.; Bantsimba-Malanda, C.; Netter, P.; Mainard, D.; Libante, V.; Gillet, P.; Magdalou, J. Matrilin-3 switches from anti- to pro-anabolic upon integration to the extracellular matrix. Matrix Biol. 2012, 31, 290–298. [Google Scholar] [CrossRef]
- Vincourt, J.B.; Vignaud, J.M.; Lionneton, F.; Sirveaux, F.; Kawaki, H.; Marchal, S.; Lomazzi, S.; Plenat, F.; Guillemin, F.; Netter, P. Increased expression of matrilin-3 not only in osteoarthritic articular cartilage but also in cartilage-forming tumors, and down-regulation of SOX9 via epidermal growth factor domain 1-dependent signaling. Arthritis Rheum. 2008, 58, 2798–2808. [Google Scholar] [CrossRef]
- Klatt, A.R.; Klinger, G.; Paul-Klausch, B.; Kuhn, G.; Renno, J.H.; Wagener, R.; Paulsson, M.; Schmidt, J.; Malchau, G.; Wielckens, K. Matrilin-3 activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009, 583, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.; Paul-Klausch, B.; Klinger, G.; Hillebrand, U.; Kühn, G.; Kobbe, B.; Renno, J.; Johannis, W.; Paulsson, M.; Wagener, R. The matrilin-3 VWA1 domain modulates interleukin-6 release from primary human chondrocytes. Osteoarthr. Cartil. 2013, 21, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Goldring, M.B.; Terek, R.; Chen, Q. Matrilin-3 induction of IL-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res. Ther. 2012, 14, R197. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, D.; Li, H.; Chen, N.; Shang, Y. Downregulation of microRNA-448 inhibits IL-1β-induced cartilage degradation in human chondrocytes via upregulation of matrilin-3. Cell. Mol. Biol. Lett. 2018, 23, 7. [Google Scholar] [CrossRef]
- Okimura, A.; Okada, Y.; Makihira, S.; Pan, H.; Yu, L.; Tanne, K.; Imai, K.; Yamada, H.; Kawamoto, T.; Noshiro, M. Enhancement of cartilage matrix protein synthesis in arthritic cartilage. Arthritis Rheum. 1997, 40, 1029–1036. [Google Scholar] [CrossRef]
- Ohno, S.; Murakami, K.; Tanimoto, K.; Sugiyama, H.; Makihira, S.; Shibata, T.; Yoneno, K.; Kato, Y.; Tanne, K. Immunohistochemical study of matrilin-1 in arthritic articular cartilage of the mandibular condyle. J. Oral Pathol. Med. 2003, 32, 237–242. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, J.; Guo, Y.; Javidiparsijani, S.; Wang, G.; Wang, Y.; Liu, H.; Liu, J.; Luo, J. Matrilin-2 is a widely distributed extracellular matrix protein and a potential biomarker in the early stage of osteoarthritis in articular cartilage. BioMed Res. Int. 2014, 2014, 986127. [Google Scholar] [CrossRef]
- Chou, C.-H.; Lee, M.T.M.; Song, I.-W.; Lu, L.-S.; Shen, H.-C.; Lee, C.-H.; Wu, J.-Y.; Chen, Y.-T.; Kraus, V.B.; Wu, C.-C. Insights into osteoarthritis progression revealed by analyses of both knee tibiofemoral compartments. Osteoarthr. Cartil. 2015, 23, 571–580. [Google Scholar] [CrossRef]
- Aszódi, A.; Módis, L.; Páldi, A.; Rencendorj, A.; Kiss, I.; Bösze, Z. The zonal expression of chicken cartilage matrix protein in the developing skeleton of transgenic mice. Matrix Biol. 1994, 14, 181–190. [Google Scholar] [CrossRef]
- Hyde, G.; Dover, S.; Aszodi, A.; Wallis, G.A.; Boot-Handford, R.P. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev. Biol. 2007, 304, 825–833. [Google Scholar] [CrossRef]
- Alberton, P.; Dugonitsch, H.C.; Hartmann, B.; Li, P.; Farkas, Z.; Saller, M.M.; Clausen-Schaumann, H.; Aszodi, A. Aggrecan hypomorphism compromises articular cartilage biomechanical properties and is associated with increased incidence of spontaneous osteoarthritis. Int. J. Mol. Sci. 2019, 20, 1008. [Google Scholar] [CrossRef] [PubMed]
- Gronau, T.; Krüger, K.; Prein, C.; Aszodi, A.; Gronau, I.; Iozzo, R.V.; Mooren, F.C.; Clausen-Schaumann, H.; Bertrand, J.; Pap, T. Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin. Ann. Rheum. Dis. 2017, 76, 442–449. [Google Scholar] [CrossRef]
- Mieloch, A.A.; Richter, M.; Trzeciak, T.; Giersig, M.; Rybka, J.D. Osteoarthritis Severely Decreases the Elasticity and Hardness of Knee Joint Cartilage: A Nanoindentation Study. J. Clin. Med. 2019, 8, 1865. [Google Scholar] [CrossRef] [PubMed]
- Leighton, M.P.; Nundlall, S.; Starborg, T.; Meadows, R.S.; Suleman, F.; Knowles, L.; Wagener, R.; Thornton, D.J.; Kadler, K.E.; Boot-Handford, R.P. Decreased chondrocyte proliferation and dysregulated apoptosis in the cartilage growth plate are key features of a murine model of epiphyseal dysplasia caused by a matn3 mutation. Hum. Mol. Genet. 2007, 16, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.A.; Pirog, K.A.; Fresquet, M.; Thornton, D.J.; Boot-Handford, R.P.; Briggs, M.D. Loss of matrilin 1 does not exacerbate the skeletal phenotype in a mouse model of multiple epiphyseal dysplasia caused by a Matn3 V194D mutation. Arthritis Rheum. 2012, 64, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Sun, Q.; Wang, Y.; Yang, J.; Yang, J.; Zhang, T.; Luo, S.; Wang, L.; Jiang, Y. Intra-articular delivery of antago-miR-483-5p inhibits osteoarthritis by modulating matrilin 3 and tissue inhibitor of metalloproteinase 2. Mol. Ther. 2017, 25, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Runner, M.N. Inheritance of susceptibility to congenital deformity—Embryonic instability. J. Natl. Cancer Inst. 1954, 15, 637–649. [Google Scholar]
- Russell, W.L. Maternal influence on number of lumbar vertebrae in mice raised from transplanted ovaries. Genetics 1948, 33, 627. [Google Scholar]
- Yap, K.L.; Sysa-Shah, P.; Bolon, B.; Wu, R.-C.; Gao, M.; Herlinger, A.L.; Wang, F.; Faiola, F.; Huso, D.; Gabrielson, K. Loss of NAC1 expression is associated with defective bony patterning in the murine vertebral axis. PLoS ONE 2013, 8, e69099. [Google Scholar] [CrossRef]
- Pei, M.; Luo, J.; Chen, Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthr. Cartil. 2008, 16, 1110–1117. [Google Scholar] [CrossRef]
- Aszodi, A.; Hunziker, E.B.; Brakebusch, C.; Fassler, R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003, 17, 2465–2479. [Google Scholar] [CrossRef] [PubMed]
- Prein, C.; Warmbold, N.; Farkas, Z.; Schieker, M.; Aszodi, A.; Clausen-Schaumann, H. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. 2016, 50, 1–15. [Google Scholar] [CrossRef] [PubMed]
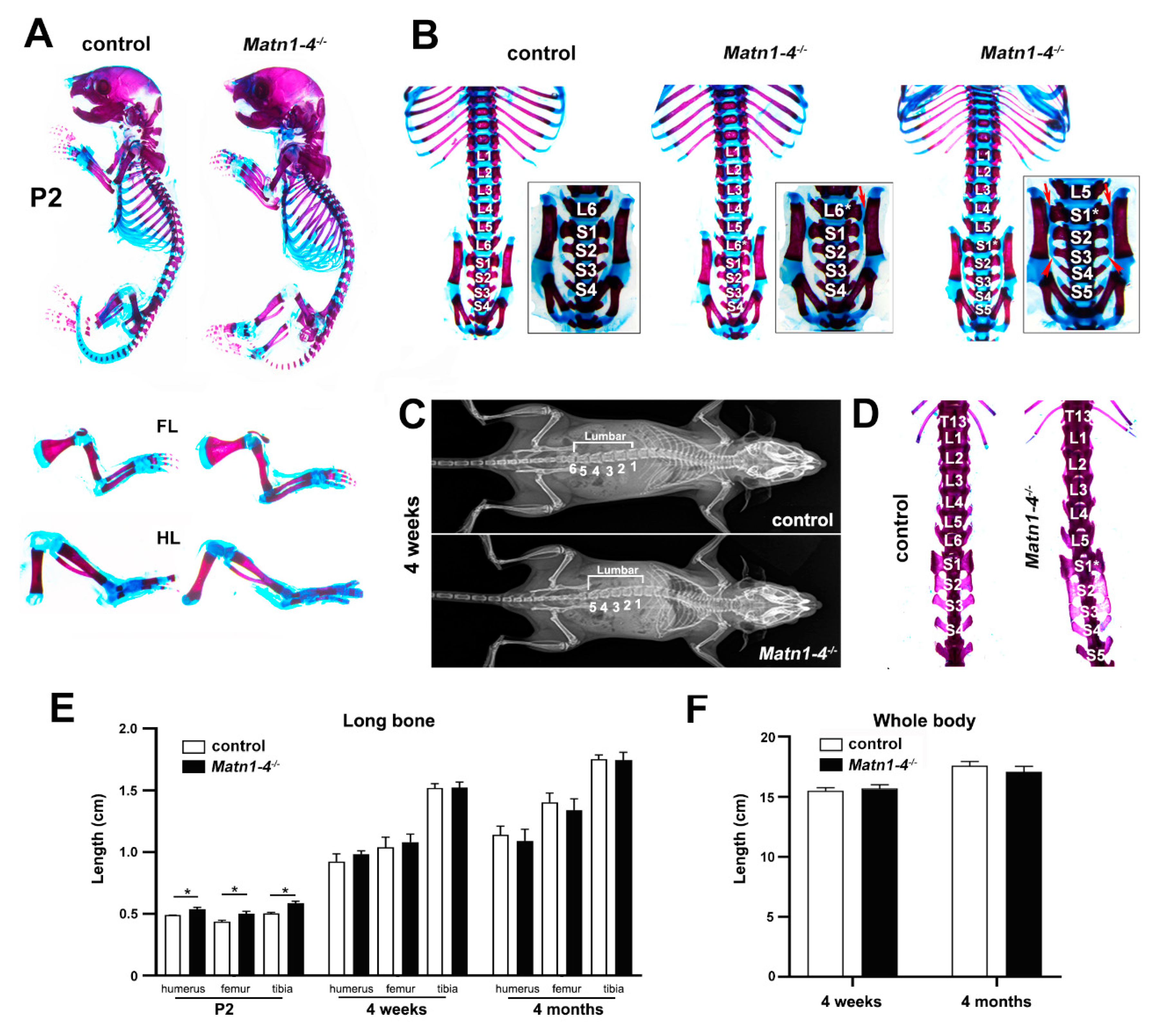


| Lumbosacral Pattern | Control | Matn1-4+/− | Matn1-4−/− |
|---|---|---|---|
| (n = 45) | (n = 7) | (n = 40) | |
| L6/S4 | 42 (93.3%) | 3 (42.8%) | 6 (12.5%) |
| L6*/S4 | 0 | 2 (28.6%) | 0 |
| L5/S5 | 3 (6.7%) | 2 (28.6%) | 34 (87.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Fleischhauer, L.; Nicolae, C.; Prein, C.; Farkas, Z.; Saller, M.M.; Prall, W.C.; Wagener, R.; Heilig, J.; Niehoff, A.; et al. Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 666. https://doi.org/10.3390/ijms21020666
Li P, Fleischhauer L, Nicolae C, Prein C, Farkas Z, Saller MM, Prall WC, Wagener R, Heilig J, Niehoff A, et al. Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis. International Journal of Molecular Sciences. 2020; 21(2):666. https://doi.org/10.3390/ijms21020666
Chicago/Turabian StyleLi, Ping, Lutz Fleischhauer, Claudia Nicolae, Carina Prein, Zsuzsanna Farkas, Maximilian Michael Saller, Wolf Christian Prall, Raimund Wagener, Juliane Heilig, Anja Niehoff, and et al. 2020. "Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis" International Journal of Molecular Sciences 21, no. 2: 666. https://doi.org/10.3390/ijms21020666
APA StyleLi, P., Fleischhauer, L., Nicolae, C., Prein, C., Farkas, Z., Saller, M. M., Prall, W. C., Wagener, R., Heilig, J., Niehoff, A., Clausen-Schaumann, H., Alberton, P., & Aszodi, A. (2020). Mice Lacking the Matrilin Family of Extracellular Matrix Proteins Develop Mild Skeletal Abnormalities and Are Susceptible to Age-Associated Osteoarthritis. International Journal of Molecular Sciences, 21(2), 666. https://doi.org/10.3390/ijms21020666







