Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis
Abstract
1. Introduction
2. Results
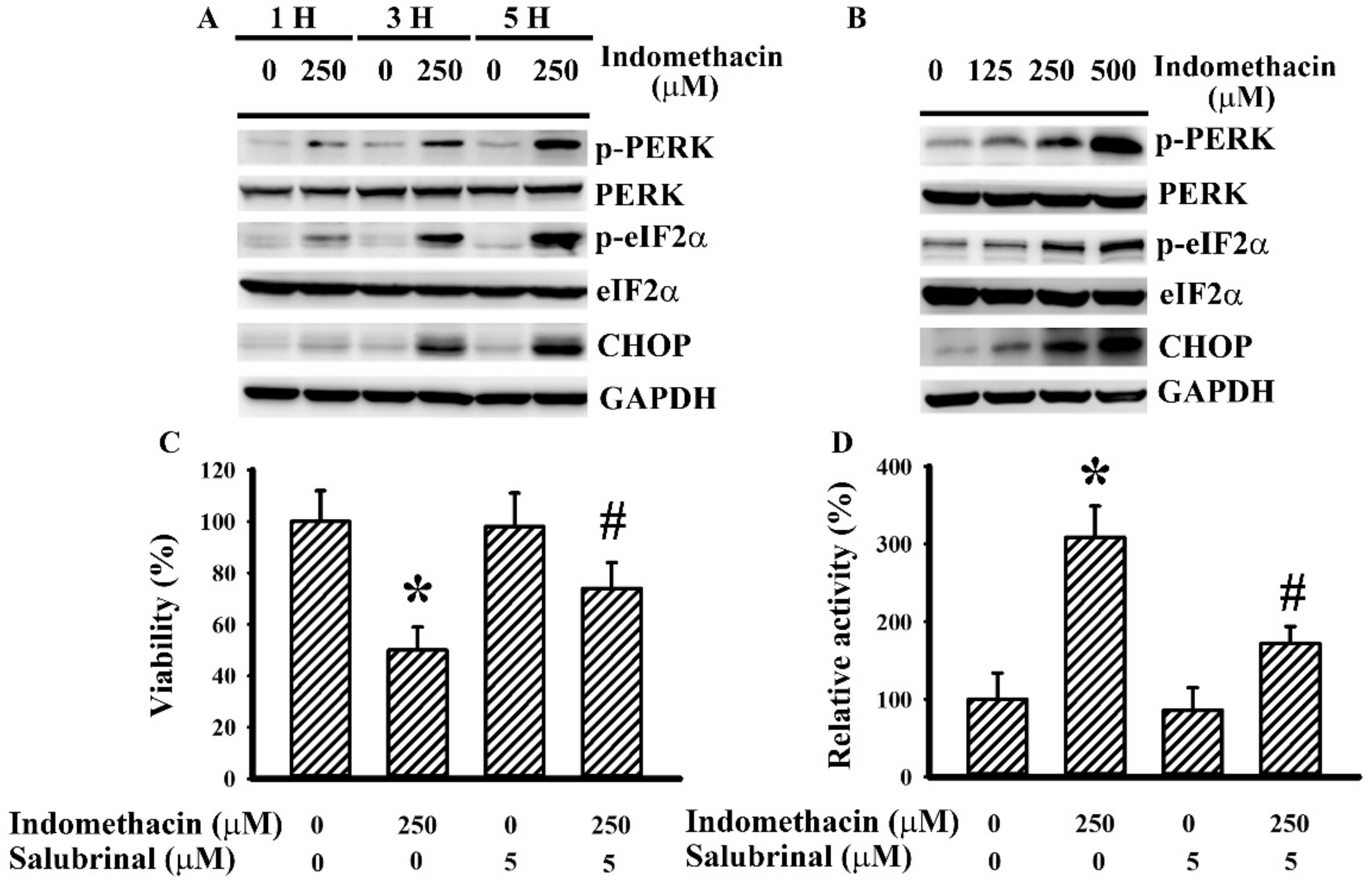
2.1. Indomethacin Induced ER Stress in H4 Cells
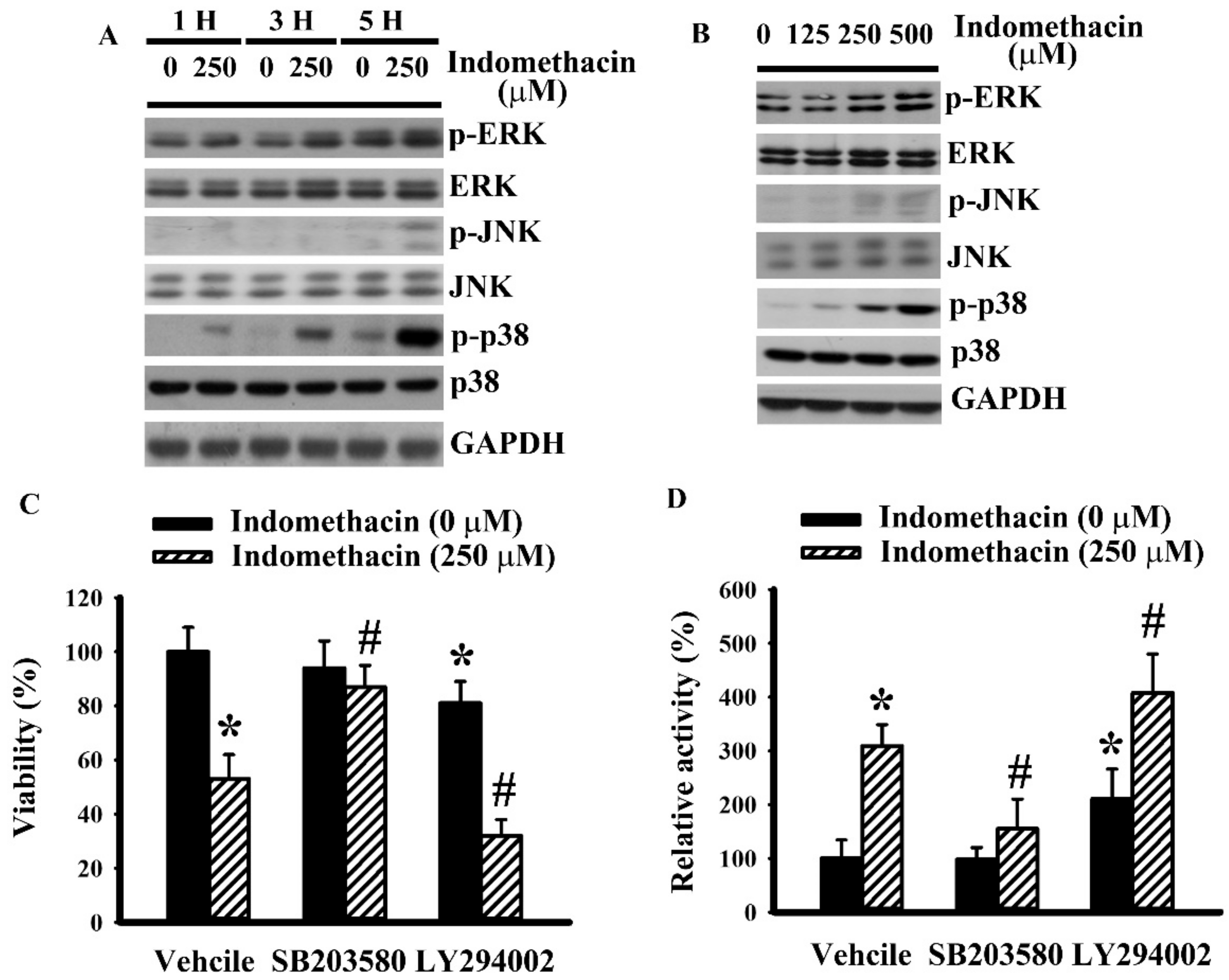
2.2. Indomethacin Altered Mitogen-Activated Protein Kinases (MAPKs) Phosphorylation in H4 Cells
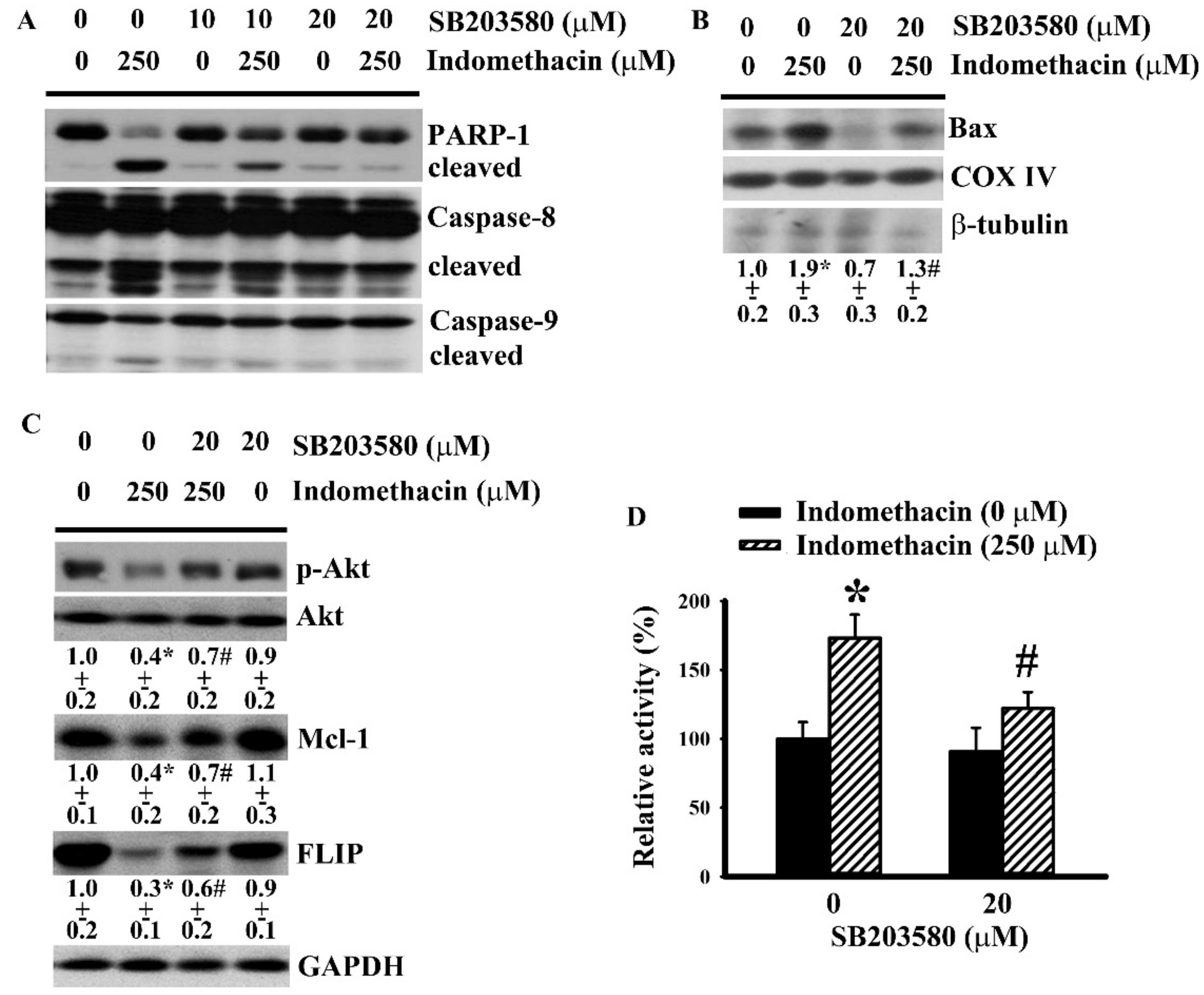
2.3. p38 Mediated Indomethacin-Induced Apoptotic Execution in H4 Cells
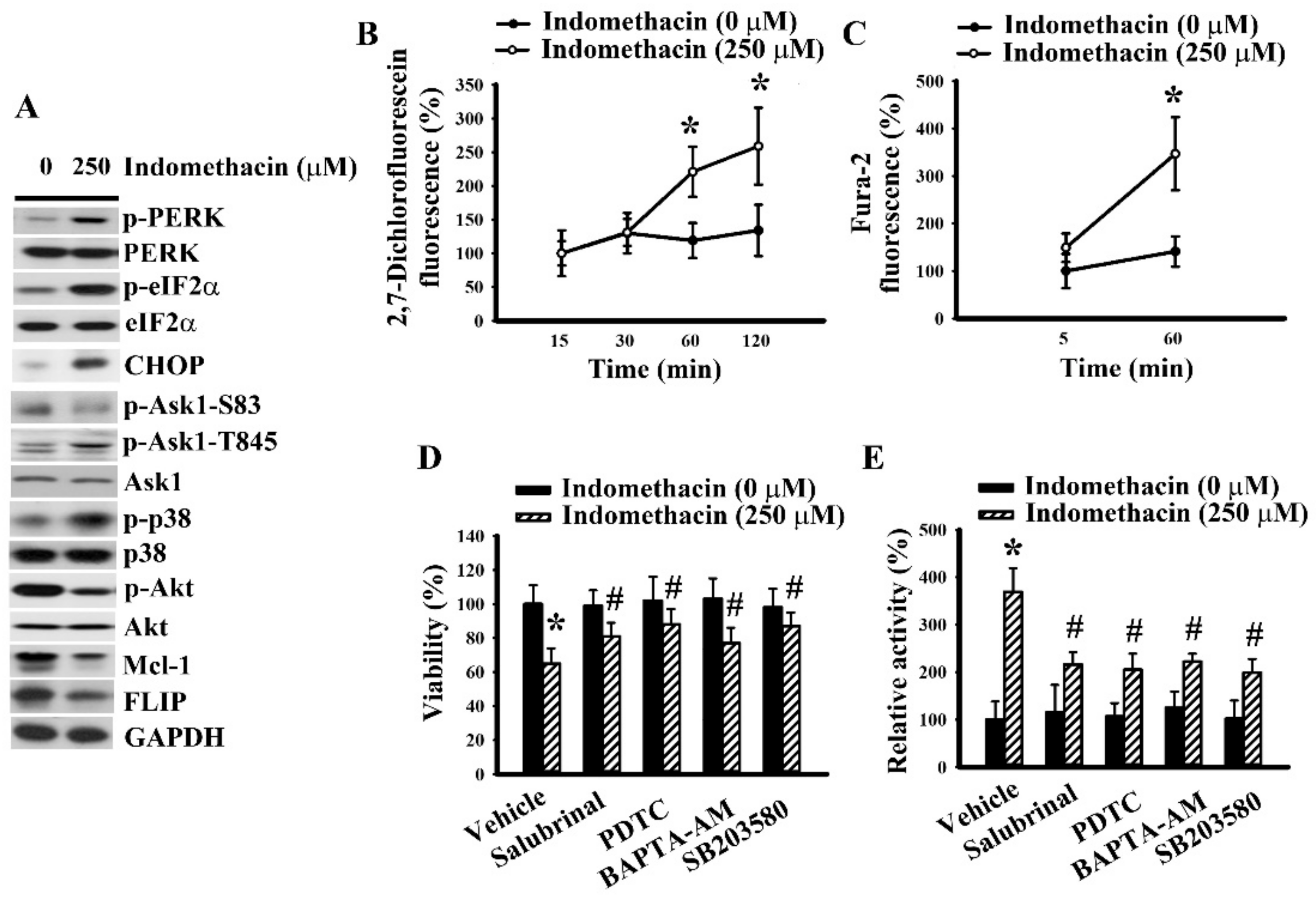
2.4. ER Stress Had a Role in Indomethacin-Induced p38 Phosphorylation in H4 Cells.
2.5. Indomethacin Induced Apoptotic Program in U87 Glioma Cells.
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cell Viability Assay
4.3. Caspase 3 Activity Assay
4.4. Phosphatase Assay
4.5. Mitochondrial Protein Isolation
4.6. Measurement of Reactive Oxygen Species
4.7. Measurement of Cytosolic Ca2+
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| Ask1 | Apoptosis signal-regulating kinase-1 |
| JNK | c-Jun N-terminal kinase |
| COX IV | Cytochrome oxidase IV |
| DMEM | Dulbecco’s modified Eagle medium |
| ER | Endoplasmic reticulum |
| ECL | Enhanced chemiluminescence |
| ERK | Extracellular signal-regulated kinase |
| FBS | Fetal bovine serum |
| FLIP | FLICE-inhibiting protein |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PARP-1 | Poly(ADP-ribose) polymerase 1 |
| PP2A | Protein phosphatase 2A |
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Cartron, P.F.; Loussouarn, D.; Campone, M.; Martin, S.A.; Vallette, F.M. Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis. 2012, 3, e421. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Krajewska, M.; Ehrmann, J.; Sikorska, M.; Lach, B.; Chatten, J.; Reed, J.C. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1, and Bax in tumors of central and peripheral nervous system origin. Am. J. Pathol. 1997, 150, 805–814. [Google Scholar]
- Placzek, W.J.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, e40. [Google Scholar] [CrossRef]
- Limonta, P.; Moretti, R.M.; Marzagalli, M.; Fontana, F.; Raimondi, M.; Montagnani Marelli, M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int. J. Mol. Sci. 2019, 20, 961. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef]
- Chen, Y.H.; Cimino, P.J.; Luo, J.; Dahiya, S.; Gutmann, D.H. ABCG1 maintains high-grade glioma survival in vitro and in vivo. Oncotarget 2016, 7, 23416–23424. [Google Scholar] [CrossRef]
- He, Y.; Su, J.; Lan, B.; Gao, Y.; Zhao, J. Targeting off-target effects: Endoplasmic reticulum stress and autophagy as effective strategies to enhance temozolomide treatment. Onco Targets Ther. 2019, 12, 1857–1865. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X. Bufothionine promotes apoptosis via triggering ER stress and synergizes with temozolomide in glioblastoma multiforme cells. Anat. Rec. 2019, 302, 1950–1957. [Google Scholar] [CrossRef]
- Xipell, E.; Aragón, T.; Martínez-Velez, N.; Vera, B.; Idoate, M.A.; Martínez-Irujo, J.J.; Garzón, A.G.; Gonzalez-Huarriz, M.; Acanda, A.M.; Jones, C.; et al. Endoplasmic reticulum stress-inducing drugs sensitize glioma cells to temozolomide through downregulation of MGMT, MPG, and Rad51. Neuro Oncol. 2016, 18, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pusch, S.; Innes, J.; Sidlauskas, K.; Ellis, M.; Lau, J.; El-Hassan, T.; Aley, N.; Launchbury, F.; Richard-Loendt, A.; et al. Mutant IDH sensitizes gliomas to endoplasmic reticulum stress and triggers apoptosis via miR-183-mediated inhibition of semaphorin 3E. Cancer Res. 2019, 79, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chu, J.; Wang, F. Expression and clinical significance of cyclooxygenase 2 and survivin in human gliomas. Oncol. Lett. 2017, 14, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Eberstål, S.; Sandén, E.; Fritzell, S.; Darabi, A.; Visse, E.; Siesjö, P. Intratumoral COX-2 inhibition enhances GM-CSF immunotherapy against established mouse GL261 brain tumors. Int. J. Cancer 2014, 134, 2748–2753. [Google Scholar] [CrossRef]
- Guenzle, J.; Garrelfs, N.W.C.; Goeldner, J.M.; Weyerbrock, A. Cyclooxygenase (COX) inhibition by acetyl salicylic acid (ASA) enhances antitumor effects of nitric oxide in glioblastoma in vitro. Mol. Neurobiol. 2019, 56, 6046–6055. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, Q.; Bell, K.A.; Yao, X.; Du, Y.; Zhang, E.; Yu, J.J.; Yu, Y.; Shi, Z.; Jiang, J. Small-molecule inhibition of prostaglandin E receptor 2 impairs cyclooxygenase-associated malignant glioma growth. Br. J. Pharmacol. 2019, 176, 1680–1699. [Google Scholar] [CrossRef]
- Cha, W.; Park, S.W.; Kwon, T.K.; Hah, J.H.; Sung, M.W. Endoplasmic reticulum stress response as a possible mechanism of cyclooxygenase-2-independent anticancer effect of celecoxib. Anticancer Res. 2014, 34, 1731–1735. [Google Scholar]
- Kim, B.; Kim, J.; Kim, Y.S. Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress. Anat. Cell. Biol. 2017, 50, 293–300. [Google Scholar] [CrossRef]
- Chiou, S.K.; Hoa, N.; Hodges, A.; Ge, L.; Jadus, M.R. Indomethacin promotes apoptosis in gastric cancer cells through concomitant degradation of Survivin and Aurora B kinase proteins. Apoptosis 2014, 19, 1378–1388. [Google Scholar] [CrossRef]
- Mazumder, S.; De, R.; Debsharma, S.; Bindu, S.; Maity, P.; Sarkar, S.; Saha, S.J.; Siddiqui, A.A.; Banerjee, C.; Nag, S.; et al. Indomethacin impairs mitochondrial dynamics by activating the PKCζ-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J. Biol. Chem. 2019, 294, 8238–8258. [Google Scholar] [CrossRef]
- Pantovic, A.; Bosnjak, M.; Arsikin, K.; Kosic, M.; Mandic, M.; Ristic, B.; Tosic, J.; Grujicic, D.; Isakovic, A.; Micic, N.; et al. In vitro antiglioma action of indomethacin is mediated via AMP-activated protein kinase/mTOR complex 1 signalling pathway. Int. J. Biochem. Cell Biol. 2017, 83, 84–96. [Google Scholar] [CrossRef]
- Tse, A.K.; Cao, H.H.; Cheng, C.Y.; Kwan, H.Y.; Yu, H.; Fong, W.F.; Yu, Z.L. Indomethacin sensitizes TRAIL-resistant melanoma cells to TRAIL-induced apoptosis through ROS-mediated upregulation of death receptor 5 and downregulation of survivin. J. Investig. Dermatol. 2014, 134, 1397–1407. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Ren, Y.; Wu, Y.; Yang, S.; Zhangm, Y.; Chen, H.; Li, W.; Zhu, Y. Antiproliferative and apoptotic effects of indomethacin on human retinoblastoma cell line Y79 and the involvement of β-catenin, nuclear factor-κB and Akt signaling pathways. Ophthalmic Res. 2014, 51, 109–115. [Google Scholar] [CrossRef]
- Bernardi, A.; Jacques-Silva, M.C.; Delgado-Cañedo, A.; Lenz, G.; Battastini, A.M. Nonsteroidal anti-inflammatory drugs inhibit the growth of C6 and U138-MG glioma cell lines. Eur. J. Pharmacol. 2006, 532, 214–222. [Google Scholar] [CrossRef]
- Bernardi, A.; Bavaresco, L.; Wink, M.R.; Jacques-Silva, M.C.; Delgado-Cañedo, A.; Lenz, G.; Battastini, A.M. Indomethacin stimulates activity and expression of ecto-5′-nucleotidase/CD73 in glioma cell lines. Eur. J. Pharmacol. 2007, 569, 8–15. [Google Scholar] [CrossRef]
- Bernardi, A.; Frozza, R.L.; Jäger, E.; Figueiró, F.; Bavaresco, L.; Salbego, C.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Selective cytotoxicity of indomethacin and indomethacin ethyl ester-loaded nanocapsules against glioma cell lines: An in vitro study. Eur. J. Pharmacol. 2008, 586, 24–34. [Google Scholar] [CrossRef]
- Bernardi, A.; Braganhol, E.; Jäger, F.; Figueiró, F.; Edelweiss, M.I.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Indomethacin-loaded nanocapsules treatment reduces in vivo glioblastoma growth in a rat glioma model. Cancer Lett. 2009, 281, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Frozza, R.L.; Hoppe, J.B.; Salbego, C.; Pohlmann, A.R.; Battastini, A.M.; Guterres, S.S. The antiproliferative effect of indomethacin-loaded lipid-core nanocapsules in glioma cells is mediated by cell cycle regulation, differentiation, and the inhibition of survival pathways. Int. J. Nanomed. 2013, 8, 711–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigues, S.F.; Fiel, L.A.; Shimada, A.L.; Pereira, N.R.; Guterres, S.S.; Pohlmann, A.R.; Farsky, S.H. Lipid-core nanocapsules act as a drug shuttle through the blood brain barrier and reduce glioblastoma after intravenous or oral administration. J. Biomed. Nanotechnol. 2016, 12, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Li, J.R.; Wu, C.C.; Wang, J.D.; Yang, C.P.; Chen, W.Y.; Wang, W.Y.; Chen, C.J. Indomethacin induced glioma apoptosis involving ceramide signals. Exp. Cell. Res. 2018, 365, 66–77. [Google Scholar] [CrossRef]
- Eom, K.S.; Kim, H.J.; So, H.S.; Park, R.; Kim, T.Y. Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction. Biol. Pharm. Bull. 2010, 33, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Song, G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol. Appl. Pharmacol. 2018, 360, 201–211. [Google Scholar] [CrossRef]
- Wang, R.; Deng, D.; Shao, N.; Xu, Y.; Xue, L.; Peng, Y.; Liu, Y.; Zhi, F. Evodiamine activates cellular apoptosis through suppressing PI3K/AKT and activating MAPK in glioma. Onco Targets Ther. 2018, 11, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Fu, X.Y.; Cao, W.Q.; Xiang, W.Z.; Hou, Y.J.; Ma, J.K.; Wang, Y.; Fan, C.D. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J. Agric. Food Chem. 2019, 67, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jeong, Y.J.; Yu, A.R.; Yoon, K.S.; Choe, W.; Ha, J.; Kim, S.S.; Yeo, E.J.; Kang, I. Fluoxetine induces apoptosis through endoplasmic reticulum stress via mitogen-activated protein kinase activation and histone hyperacetylation in SK-N-BE(2)-M17 human neuroblastoma cells. Apoptosis 2017, 22, 1079–1097. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.N.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Er, E.; Oliver, L.; Carton, P.F.; Juin, P.; Manon, S.; Vallette, F.M. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim. Biophys. Acta 2006, 1757, 1301–1311. [Google Scholar] [CrossRef]
- McDonald, F.E.; Ironside, J.W.; Gregor, A.; Wyatt, B.; Stewart, M.; Rye, R.; Adams, J.; Potts, H.W. The prognostic influence of bcl-2 in malignant glioma. Br. J. Cancer. 2002, 86, 1899–1904. [Google Scholar] [CrossRef]
- Rieger, L.; Weller, M.; Bornemann, A.; Schabet, M.; Dichgans, J.; Meyermann, R. BCL-2 family protein expression in human malignant glioma: A clinical-pathological correlative study. J. Neurol. Sci. 1998, 155, 68–75. [Google Scholar] [CrossRef]
- Wang, P.G.; Li, Y.T.; Pan, Y.; Gao, Z.Z.; Guan, X.W.; Jia, L.; Liu, F.T. Lower expression of Bax predicts poor clinical outcome in patients with glioma after curative resection and radiotherapy/chemotherapy. J. Neurooncol. 2019, 141, 71–81. [Google Scholar] [CrossRef]
- Ou, Y.C.; Kuan, Y.H.; Li, J.R.; Raung, S.L.; Wang, C.C.; Hung, Y.Y.; Chen, C.J. Induction of apoptosis by luteolin involving akt inactivation in human 786-O renal cell carcinoma cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 109105. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.C.; Li, J.R.; Kuan, Y.H.; Raung, S.L.; Wang, C.C.; Hung, Y.Y.; Pan, P.H.; Lu, H.C.; Chen, C.J. Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci. 2014, 100, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Shirai, K.; Oka, K.; Mobaraki, A.; Yoshida, Y.; Noda, S.E.; Okamoto, M.; Suzuki, Y.; Itoh, J.; Itoh, H.; et al. Higher pAkt expression predicts a significant worse prognosis in glioblastomas. J. Radiat. Res. 2010, 51, 343–348. [Google Scholar] [CrossRef]
- Liu, Q.; Hofmann, P.A. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2204–H2212. [Google Scholar] [CrossRef]
- Henderson, L.E.; Abdelmegeed, M.A.; Yoo, S.H.; Rhee, S.G.; Zhu, X.; Smith, M.A.; Nguyen, R.Q.; Perry, G.; Song, B.J. Enhanced phosphorylation of Bax and its translocation into mitochondria in the brains of individuals affiliated with Alzheimer’s disease. Open Neurol. J. 2017, 11, 48–58. [Google Scholar] [CrossRef]
- Liao, L.; Zhou, Q.; Song, Y.; Wu, W.; Yu, H.; Wang, S.; Chen, Y.; Ye, M.; Lu, L. Ceramide mediates Ox-LDL-induced human vascular smooth muscle cell calcification via p38 mitogen-activated protein kinase signaling. PLoS ONE 2013, 8, e82379. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Shen, F.J.; Tong, Y.Q.; Li, Y. PP2A inhibits cervical cancer cell migration by dephosphorylation of p-JNK, p-p38 and the p-ERK/MAPK signaling pathway. Curr. Med. Sci. 2018, 38, 115–123. [Google Scholar] [CrossRef]
- Tomita, T.; Sadakata, H.; Tamura, M.; Matsui, H. Indomethacin-induced generation of reactive oxygen species leads to epithelial cell injury before the formation of intestinal lesions in mice. J. Physiol. Pharmacol. 2014, 65, 435–440. [Google Scholar]
- Ahn, J.; Won, M.; Choi, J.H.; Kim, Y.S.; Jung, C.R.; Im, D.S.; Kyun, M.L.; Lee, K.; Song, K.B.; Chung, K.S. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21(Cip1) downregulation are required for shikonin-induced apoptosis. Apoptosis 2013, 18, 870–881. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Wan, J.; Wang, W.; Wang, L.; Yuan, Y.; Yang, Z.; Liu, X.; Mingm, L. Low glucose and metformin-induced apoptosis of human ovarian cancer cells is connected to ASK1 via mitochondrial and endoplasmic reticulum stress-associated pathways. J. Exp. Clin. Cancer Res. 2019, 38, 77. [Google Scholar] [CrossRef]
- Chang, C.Y.; Li, J.R.; Wu, C.C.; Ou, Y.C.; Chen, W.Y.; Kuan, Y.H.; Wang, W.Y.; Chen, C.J. Valproic acid sensitizes human glioma cells to gefitinib-induced autophagy. IUBMB Life 2015, 67, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Ou, Y.C.; Chang, C.Y.; Pan, H.C.; Liao, S.L.; Chen, S.Y.; Raung, S.L.; Lai, C.Y. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-α signaling and contributes to neuronal death. Glia 2012, 60, 487–501. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Li, J.-R.; Wu, C.-C.; Wang, J.-D.; Liao, S.-L.; Chen, W.-Y.; Wang, W.-Y.; Chen, C.-J. Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis. Int. J. Mol. Sci. 2020, 21, 557. https://doi.org/10.3390/ijms21020557
Chang C-Y, Li J-R, Wu C-C, Wang J-D, Liao S-L, Chen W-Y, Wang W-Y, Chen C-J. Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis. International Journal of Molecular Sciences. 2020; 21(2):557. https://doi.org/10.3390/ijms21020557
Chicago/Turabian StyleChang, Cheng-Yi, Jian-Ri Li, Chih-Cheng Wu, Jiaan-Der Wang, Su-Lan Liao, Wen-Ying Chen, Wen-Yi Wang, and Chun-Jung Chen. 2020. "Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis" International Journal of Molecular Sciences 21, no. 2: 557. https://doi.org/10.3390/ijms21020557
APA StyleChang, C.-Y., Li, J.-R., Wu, C.-C., Wang, J.-D., Liao, S.-L., Chen, W.-Y., Wang, W.-Y., & Chen, C.-J. (2020). Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis. International Journal of Molecular Sciences, 21(2), 557. https://doi.org/10.3390/ijms21020557





