Abstract
Bradykinin (BK), a hormone inducing pain and inflammation, is known to inhibit potassium M-currents (IM) and to increase the excitability of the superior cervical ganglion (SCG) neurons by activating the Ca2+-calmodulin pathway. M-current is also reduced by muscarinic agonists through the depletion of membrane phosphatidylinositol 4,5-biphosphate (PIP2). Similarly, the activation of muscarinic receptors inhibits the current through two-pore domain potassium channels (K2P) of the “Tandem of pore-domains in a Weakly Inward rectifying K+ channel (TWIK)-related channels” (TREK) subfamily by reducing PIP2 in mouse SCG neurons (mSCG). The aim of this work was to test and characterize the modulation of TREK channels by bradykinin. We used the perforated-patch technique to investigate riluzole (RIL) activated currents in voltage- and current-clamp experiments. RIL is a drug used in the palliative treatment of amyotrophic lateral sclerosis and, in addition to blocking voltage-dependent sodium channels, it also selectively activates the K2P channels of the TREK subfamily. A cell-attached patch-clamp was also used to investigate TREK-2 single channel currents. We report here that BK reduces spike frequency adaptation (SFA), inhibits the riluzole-activated current (IRIL), which flows mainly through TREK-2 channels, by about 45%, and reduces the open probability of identified single TREK-2 channels in cultured mSCG cells. The effect of BK on IRIL was precluded by the bradykinin receptor (B2R) antagonist HOE-140 (d-Arg-[Hyp3, Thi5, d-Tic7, Oic8]BK) but also by diC8PIP2 which prevents PIP2 depletion when phospholipase C (PLC) is activated. On the contrary, antagonizing inositol triphosphate receptors (IP3R) using 2-aminoethoxydiphenylborane (2-APB) or inhibiting protein kinase C (PKC) with bisindolylmaleimide did not affect the inhibition of IRIL by BK. In conclusion, bradykinin inhibits TREK-2 channels through the activation of B2Rs resulting in PIP2 depletion, much like we have demonstrated for muscarinic agonists. This mechanism implies that TREK channels must be relevant for the capture of information about pain and visceral inflammation.
1. Introduction
Bradykinin (BK) is released during tissue damage, it is one of the main mediators of inflammation and is able to activate and sensitize the nociceptor neurons mainly through the activation of B2 receptors (B2Rs) [1,2,3]. B2Rs are constitutively expressed in most tissues while B1 receptors (B1Rs) are induced by the inflammatory process [2,3,4,5,6,7,8].
It seems coherent that the sensation of pain produced by BK must be mediated by its action on sensory neurons (nociceptors), whether somatic [2,3] or visceral [9,10]. However, the presence of bradykinin receptors (BRs) in motor neurons of the sympathetic superior cervical ganglion (SCG) was described a long time ago. When studying the effect of BK on the movement of the nictitating membrane of cats, BK was shown to be a potent stimulator of the SCG [11]. Consistently, BK increases the excitability (membrane depolarization at rest and reduction of the spike frequency adaptation (SFA)) of rat SCG (rSCG) neurons [4,12,13,14]. Depolarization by activation of B2Rs has also been reported in the entire mouse ganglion [13,15,16]; however, changes in excitability have not been investigated in isolated cultured mouse SCG (mSCG) neurons. The importance of BR expression on SCG neurons is not completely understood. Studies have suggested that depolarization of presynaptic sympathetic-neuron terminals by BK induces the release of prostanoids which acting on nociceptor terminals may induce hyperalgesia. Consistently sympathectomy strongly reduces chloroform-induced hyperalgesia and its exacerbation by BK [4,17].
BK-induced excitability has been ascribed to the inhibition of the potassium M-current and such inhibition was reported to be due to B2R and Gαq/11 protein activation in rat and Gα11 in mouse SCG neurons [5,12,18]. The participation of phospholipase C (PLC), inositol triphosphate (IP3), Ca2+ released from IP3 stores and activation of Ca2+-Calmodulin in this process was also reported in rSCG [14,19,20]. Traditionally, the regulation of the potassium M-current by muscarinic agonists has been considered the main physiological pathway to modulate the excitability of SCG neurons [21,22,23,24]. However, researchers have also reported that the same agonists do modulate background two-pore domain potassium (K2P) channels of the “Tandem of pore-domains in a Weakly Inward rectifying K+ channel (TWIK)-related channels” (TREK) subfamily in these cells [25]. The two most expressed K2P channels in mSCG neurons are “TWIK-related spinal cord potassium channel” (TRESK) and TREK-2 [26,27] and it was reasonable to hypothesize that if the activation of muscarinic receptors inhibits M and TREK currents (ITREK) in mSCG, the activation of their bradykinin counterparts could do the same. Indeed, both types of channels, but also both types of receptors, have been thoroughly studied due to their important role on the setting of the resting membrane potential (RMP) and on the modulation of membrane excitability in many cell types.
The study of TREK currents in native neurons is challenging because the activity of these channels is reduced at room temperature and atmospheric pressure [28,29,30]. We take advantage of the neuroprotective agent riluzole, which activates the three members of the TREK subfamily (TREK-1, TREK-2 and TRAAK (TWIK-related arachidonic acid-stimulated potassium channel)) [31,32,33,34]. Riluzole does not activate other K2P channels and it was demonstrated that the outward current evoked by riluzole in mSCG neurons is mainly driven through TREK-2 channels [25,26,35]. TREK-1 and TREK-2, but not TRAAK, are well known to be modulated through G-protein coupled receptors and PLC activation [36,37]. In fact, it has been proposed that basal G protein activity may have a constant down regulating effect on these channels [38,39].
We have reported before that, in mSCG neurons, the muscarinic inhibition of TREK-2 currents following the PLC pathway requires a reduction of phosphatidylinositol 4,5-biphosphate (PIP2) levels [25]. In the current study we demonstrate that, much like it was reported in rSCG neurons, BK depolarizes and increases the excitability of mSCG neurons. A good amount of evidence is given to show that BK inhibits a riluzole activated TREK-like current by reducing the membrane PIP2 levels, via the same pathway than muscarinic agonists in the same preparation. The presence of this newly-described current could explain, at least in part, the effects of BK in SCG neurons. The main achievement of this study was to discover a new target for the control of sympathetic excitability by bradykinin. TREK channels are known to regulate the resting membrane potential in a good number of cellular types [34].
2. Results
Bradykinin has been shown to strongly modulate neuronal excitability by reducing M-currents in several preparations [40] including rat SCG neurons but, to our knowledge, only one study [41] has indirectly related bradykinin with TREK channels. Voltage-clamp whole-cell experiments were performed at room temperature (22–24 °C) and holding the neurons at −30 mV. A blocking “cocktail” containing tetraethylammonium (TEA, 15 mM), tetrodotoxin (TTX, 0.5 µM), and CsCl (1 mM) was used to block M-currents, voltage-dependent Na+ currents, and the hyperpolarization activated cationic h-current (Ih), respectively [42,43]. This blocking cocktail reduced the basal outward current found at −30 mV (I-30) by about 40% [25] and does not affect the riluzole-activated outward current as previously demonstrated [26].
2.1. Bradykinin Increases the Excitability of mSCG Neurons
The resting membrane potential of mSCG neurons recorded in culture was −62.6 ± 1.3 mV (n = 33), and in order to reduce variability, we manually clamped neurons at −60 mV before applying any treatment or protocol. For the same reason, only neurons firing less than 10 action potentials in response to maximal 1 s depolarizing pulses (adapting cells), were analyzed in this study [44].
In those conditions, bath application of bradykinin 250 nM depolarized mSCG neurons by 6.3 ± 0.7 mV (n = 18, p < 0.001, Figure 1(a1)) and in three of them, BK induced cell firing (not shown). As expected, application of the recently discovered activator of TREK channels BL1249 (BL, (5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine) [45,46,47] had an opposite effect on the resting membrane potential hyperpolarizing mSCG neurons by −5.7 ± 0.9 mV (n = 7, p < 0.01) and −17.6 ± 1.5 mV (n = 8, p < 0.001, Figure 1(a2)), when applied at 3 µM and 10 µM respectively. When BL (3 and 10 µM) was applied in the presence of bradykinin 250 nM, it produced a similar and significant hyperpolarization: −8.4 ± 0.7 mV (n = 10, p < 0.001) and −18.7 ± 1.5 mV (n = 8, p < 0.001), respectively (not shown). Interestingly, when applied in the presence of BL (3 and 10 µM), the depolarization produced by BK 250 nM was not statistically different between both groups (6.5 ± 1.6 and 4.5 ± 0.8 mV, p > 0.05, n = 7 and 8 respectively) and they were also not different from the control (only BK, p > 0.05). BL1249 has been shown to activate TREK-1 and TREK-2 channels but no other K2P channels [45].
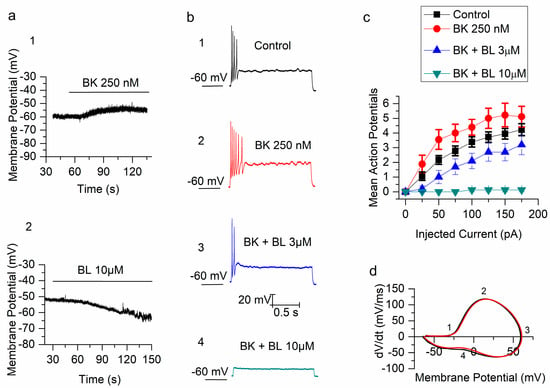
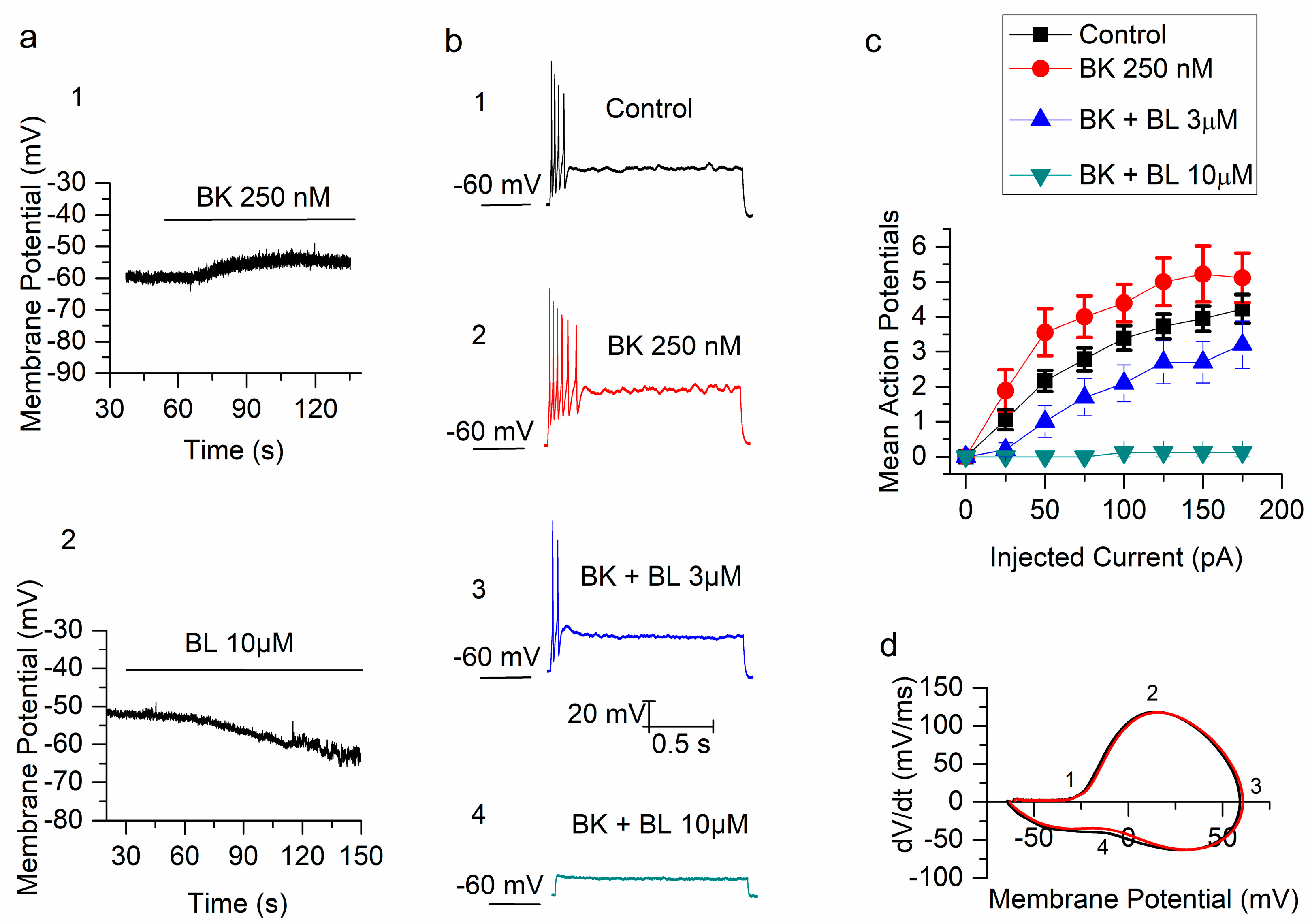
Figure 1.
Bradykinin (BK) increases excitability in mouse superior cervical ganglion (mSCG) neurons. (a) The bradykinin depolarized (1) and BL1249 (5,6,7,8-tetrahydro-naphthalen-1-yl) -[2-(1H-tetrazol-5-yl)-phenyl]-amine) hyperpolarized (2) membrane potential of mSCG neurons. (b) The firing pattern evoked by a 125 pA current injection, in a mSCG neuron, in control (1) and after the application of BK (2), BK + BL − 3 µM (3) and BK + BL − 10 µM (4). (c) The number of action potentials (mean ± SEM) evoked by depolarizing step currents from 25 to 175 pA in 25 pA increments in four conditions: control (squares), BK 250 nM (circles), BK + BL 3 µM (triangles) and BK + BL 10 µM (inverted triangles). (d) Phase plot for two action potentials before and after BK 250 nM.
mSCG neurons are well known for their strong SFA in response to injections of depolarizing current (Figure 1(b1)) in standard solutions. Bradykinin (250 nM) provoked a small but significant increment (p < 0.05, n = 18) of the number of action potentials (Figure 1(b2)) in response to depolarizing current injections from 25 to 175 pA (Figure 1c). In the presence of BK, BL1249 reduced the firing at both 3 and 10 µM (n = 10 and 8 respectively; p < 0.05; Figure 1(b3,b4),c). The effect of BL 10 µM was so dramatic that neurons were unable to respond at all (Figure 1(b4),c). Also when BL 10 µM was applied first, mSCG neurons stopped firing at any current injection (n = 8, p < 0.05) and subsequent application of BK (in the presence of BL) did not increase the excitability (not shown). The effect of BL 3 µM on the firing was not significant but interestingly it precluded the increase of firing normally produced by BK 250 nM (n = 5; p > 0.05).
In order to investigate the effect of BK on the action potentials of mSCG neurons, we constructed phase plots calculating the derivative of voltage with respect to time and plotting it against membrane voltage. Figure 1d shows that the action potential threshold (1), maximal up-stroke velocity (2), maximal positive voltage reached (3), and hump decrease in velocity (4) were indistinguishable before and after application of BK 250 nM. In fact, the amplitude of the action potential (121.75 ± 3.01 vs. 120.72 ± 2.78 mV, n = 17, p = 0.1), the half-amplitude duration (2.37 ± 0.15 vs. 2.24 ± 0.12 ms, n = 17, p = 0.16), the threshold (−35.07 ± 1.16 vs. −35.14 ± 1.12 mV, n = 17, p = 0.88) and the latency to the first action potential in response to depolarizing current injections at 50 pA (28.53 ± 8.94 vs. 20.61 ± 1.98 ms, n = 17, p = 0.39) were all not statistically different.
2.2. Bradykinin Inhibits Whole-Cell and Single-Channel TREK-2 Currents
To obtain the control IRIL (200.2 ± 39.3 pA; n = 7; Figure 2a) we applied riluzole (300 µM for at least 3 min) to neurons clamped at −30 mV in the whole-cell perforated-patch mode and in the presence of the blocking cocktail. After washing out riluzole we applied BK (250 nM) during 4 min before the second application of riluzole, which evoked an IRIL significantly smaller than IRIL control (129.8 ± 32.2 pA, n = 7; Figure 2b), showing a significant reduction of 43.2 ± 6.1% (n = 7; p < 0.01; Figure 2c). Additionally, BK reduced the I-30 in 83.8 ± 24.1 pA (n = 7; Figure 2b). We have demonstrated before that repetitive application of riluzole does not desensitize IRIL [25].
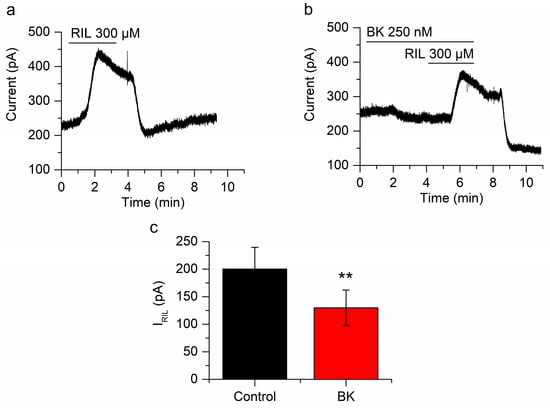
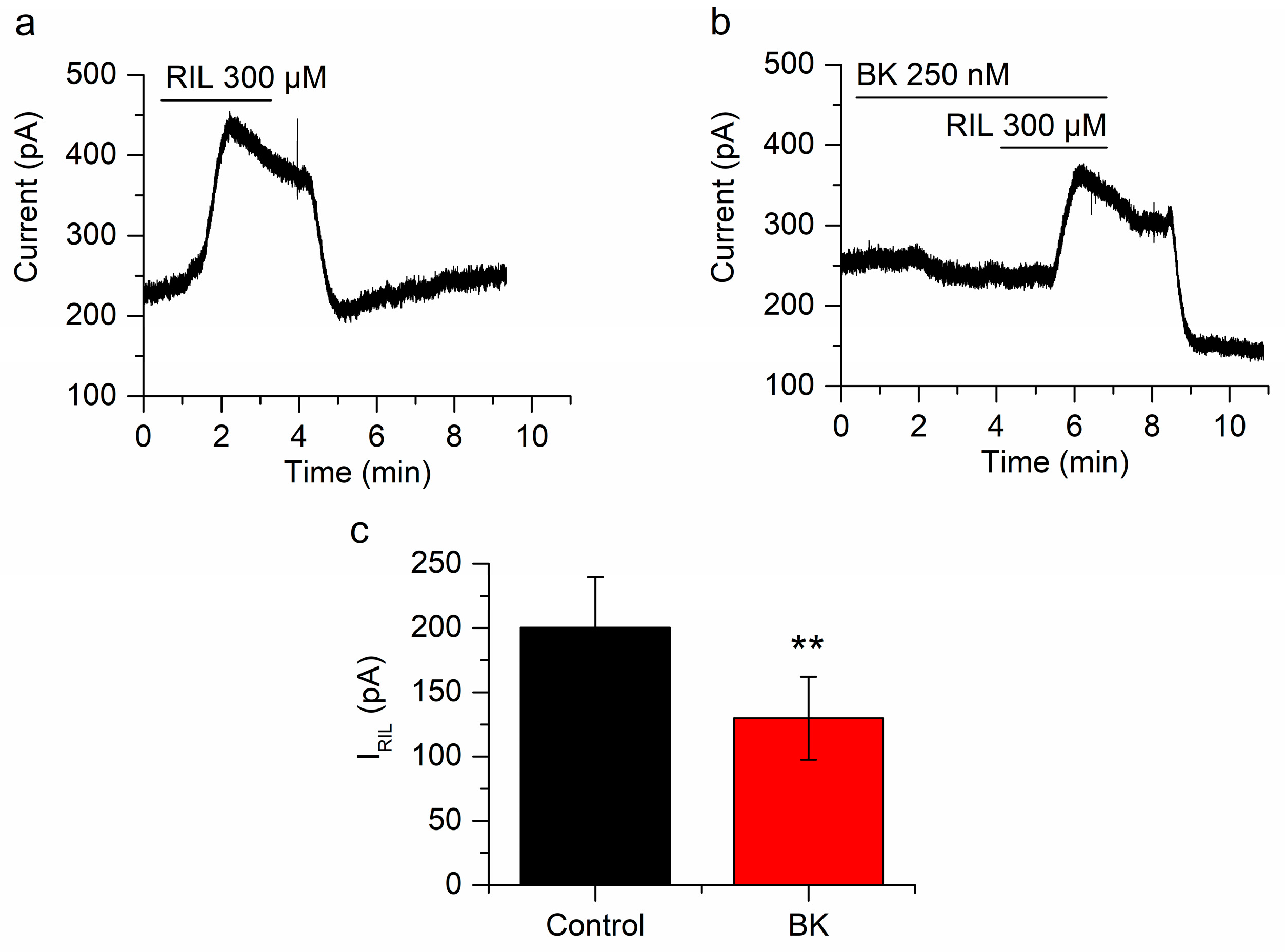
Figure 2.
Bradykinin inhibits IRIL. (a) Outward current induced by the application of riluzole (RIL) (300 µM) in mSCG neurons fixed at −30 mV (IRIL). (b) Both, the current at −30 mV and IRIL are reduced in the presence of BK (250 nM). (c) The difference between IRIL control (200.2 ± 39.3 pA, n = 7, black bar) and IRIL in the presence of bradykinin (129.8 ± 32.24 pA, n = 7, red bar) was clear and significant (p < 0.01). Recordings in (a,b) belong to the same neuron. ** p < 0.01.
Single TREK-2 channels were identified by using voltage ramps from −100 to +100 mV in SCG neurons. Cell-attached recordings showed a high conductance at negative potentials, which was clearly reduced at positive voltages, which is characteristic of TREK-2 channels (Figure 3a). Holding the patch (cell-attached) at −60 mV we applied BK (750 nM) in order to investigate whether BK can affect TREK-2 channels using an indirect pathway through second messengers (Figure 3b). The amplitude, open dwell time (duration of channel openings) and open probability (NPo) were measured from one minute recordings. These recordings were taken before BK application (control) and at least 3 min after BK application (BK), when the effect of the drug was stabilized. Figure 3e shows that BK induced a reduction in the NPo (from 7,72E-4 ± 1,56E-4 to 4,17E-4 ± 1,63E-4, p < 0.05, n = 11) without affecting the current amplitude (from 7.9 ± 0.3 pA to 7.9 ± 0.3 pA, p = 0.979, n = 11, Figure 3c) nor the open dwell time (from 0.88 ± 0.14 ms to 0.71 ± 0.12 ms, p = 0.290, n = 11, Figure 3d), indicating that BK reduced IRIL by reducing the open probability of the TREK-2 channels. These experiments strongly indicated that BK can inhibit TREK-2 channels and hence IRIL through the activation of a second messenger cascade.
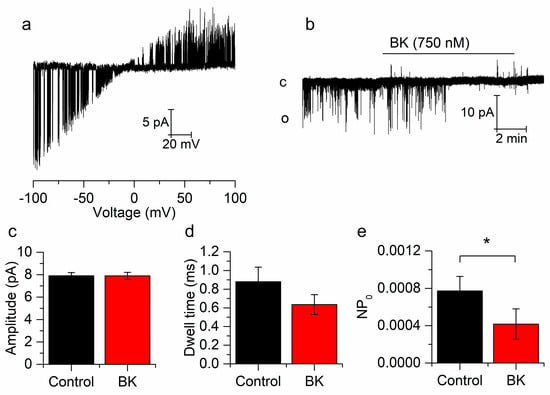
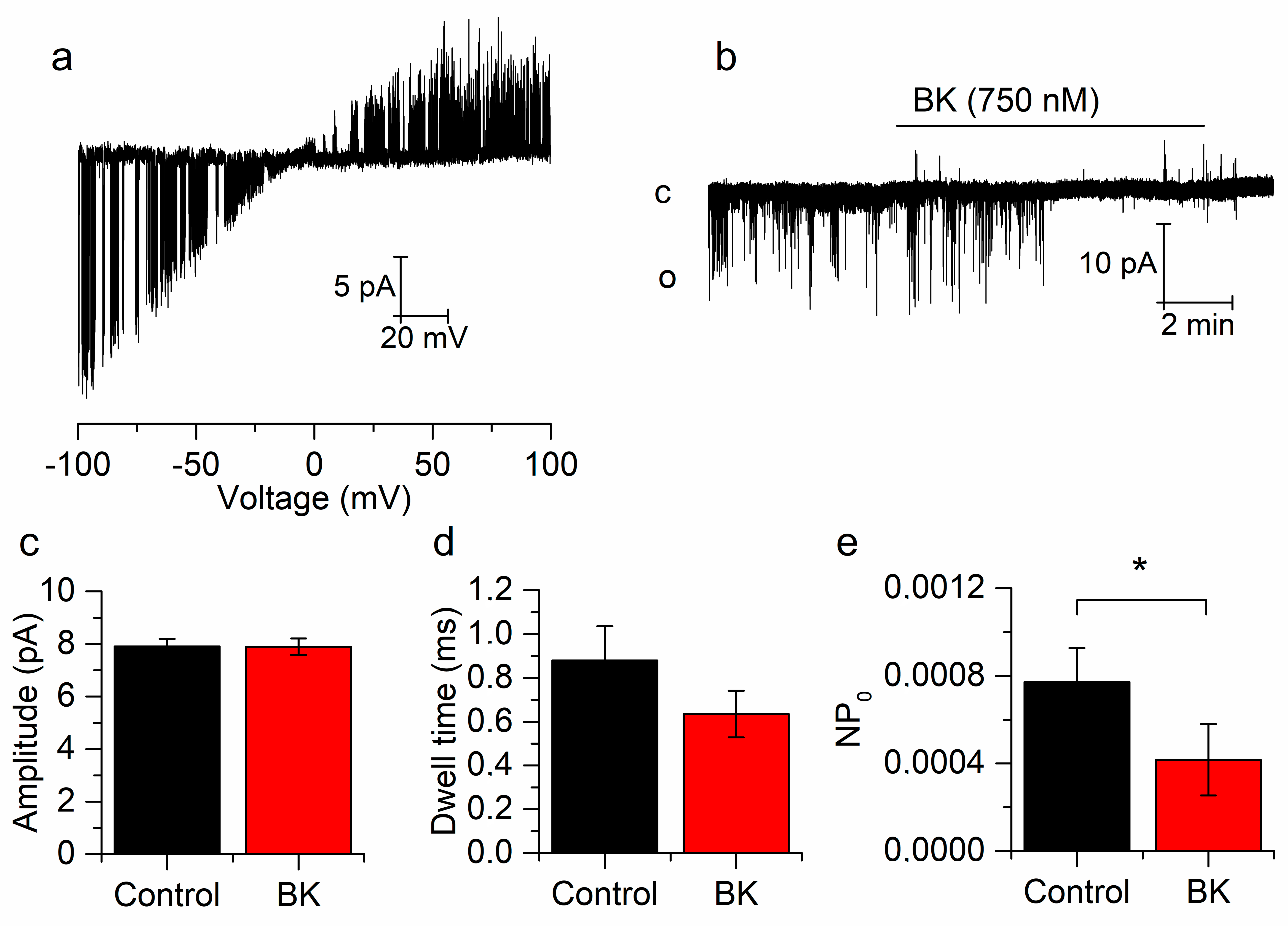
Figure 3.
Bradykinin reduces the open probability of single TREK-2 channels. (a) Representative cell-attached, TREK-2 single-channel behavior in response to voltage ramps from −100 to +100 pA in mSCG neurons. (b) Effect of BK on a single TREK-2 channel voltage-clamped at −60 mV. (c–e) BK reduces the open probability (e) without affecting dwell time (d) or current amplitude (c). Recordings belong to the same patch. * p < 0.05.
2.3. The Inhibition of IRIL by BK Is Mediated through B2 Receptors
The binding of BK to specific receptors, mainly B1R and B2R, provokes a signalling cascade starting with G-protein activation. In rat sympathetic neurons, Gq/11 is the main G-protein implicated in BK modulation [12]. In turn, Gq/11 activates the enzyme PLC which hydrolyzes PIP2 to give IP3 and diacylglycerol (DAG). IP3 binds to the IP3 endoplasmic reticulum receptors releasing Ca2+ and therefore increasing its intracellular concentration [48]. Finally, the Ca2+ increase, together with DAG, activates the PKC, which can phosphorylate other proteins [49]. All intermediate products of this cascade have been shown to directly modulate ion channels in different preparations.
To investigate the participation of BK receptors in the inhibition of IRIL, we used the selective B2R antagonist HOE-140 (d-Arg-[Hyp3, Thi5, d-Tic7, Oic8]BK). In a first step we applied riluzole (300 µM for 3 min in the presence of cocktail + HOE) to obtain IRIL in control conditions (158.3 ± 44.9 pA; n = 4; Figure 4a). Interestingly, the application of HOE (300 nM) to the bath solution slightly increased the steady-state current at −30 mV in 12.2 ± 1.2 pA (n = 4; not shown). Bradykinin (250 nM) was then applied in the presence of cocktail + HOE, it is relevant that in the presence of the B2 receptor antagonist, BK did not reduce the outward current at −30 mV (See panels b in Figure 5, Figure 6 and Figure 7). Finally, a second application of riluzole (300 µM, 3 min) in cocktail + HOE + BK (Figure 4b), induced an outward IRIL showing no significant difference with the IRIL control (142.7 ± 36.8 pA; n = 4; p = 0.2804; Figure 4c). The percentage of inhibition of IRIL by BK in presence of HOE was insignificant (5.2 ± 8.3%; Figure 4d), clearly indicating that the inhibition of IRIL by BK can be entirely explained by the activation of B2 receptors.
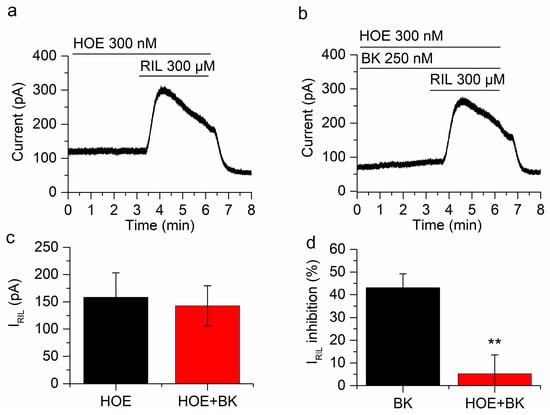
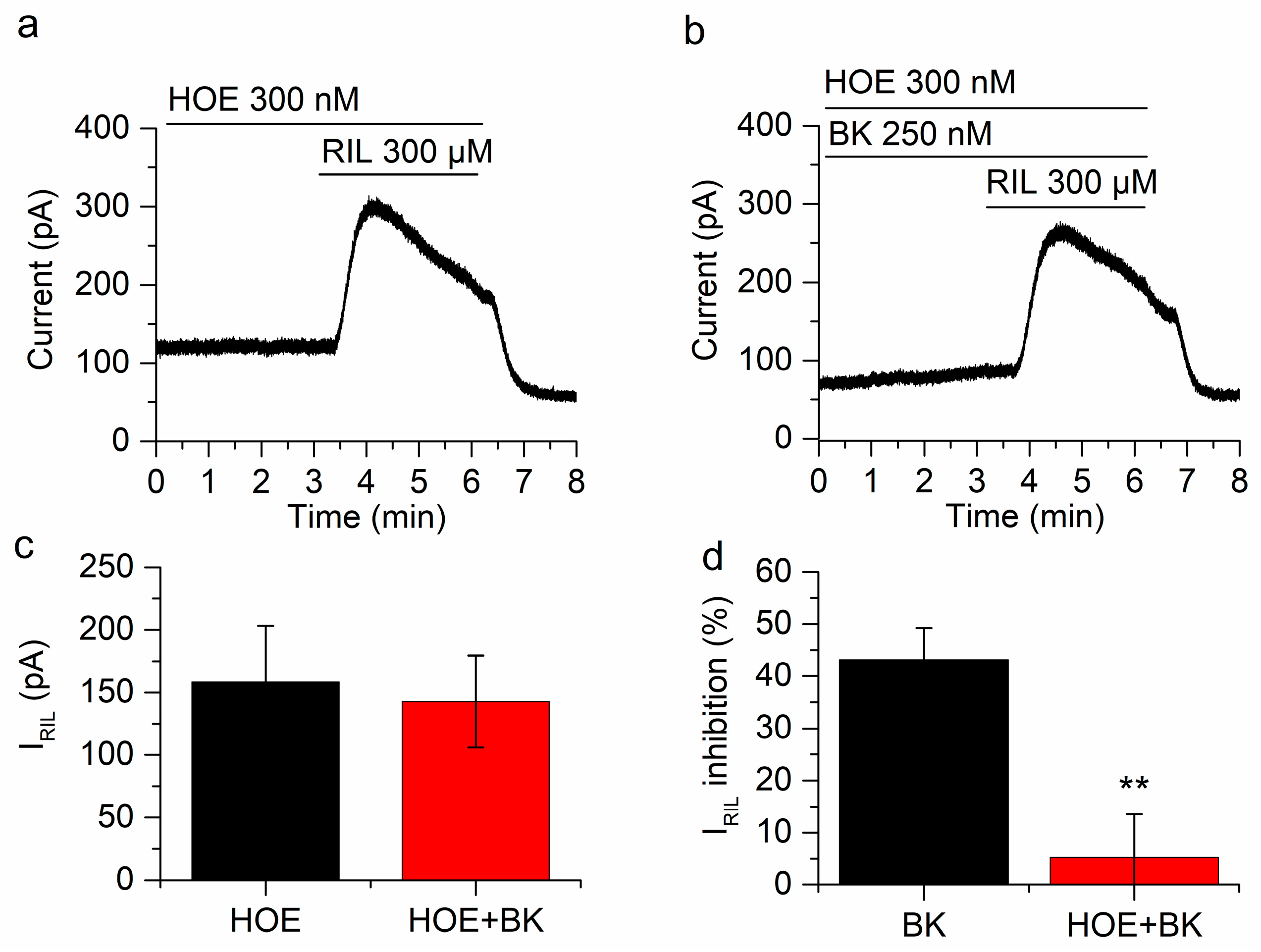
Figure 4.
BK inhibition of IRIL is mediated by B2R. (a) IRIL was not affected in the presence of HOE-140 (d-Arg-[Hyp3, Thi5, d-Tic7, Oic8]BK) in mSCG neurons held at −30 mV. (b) In the presence of HOE-140 the inhibition of IRIL by BK was significantly reduced. (c) There is no difference between IRIL control (158.3 ± 44.9 pA, black bar) and IRIL in the presence of BK and HOE-140 (142.7 ± 36.8 pA). (d) The inhibition by BK in the presence of HOE-104 was 5.2 ± 8.3% (red bar), which is significantly different from the inhibition in the absence of HOE-140 (43.2 ± 6.1%, black bar, p < 0.01). Recordings in (a,b) belong to the same neuron. ** p < 0.01.
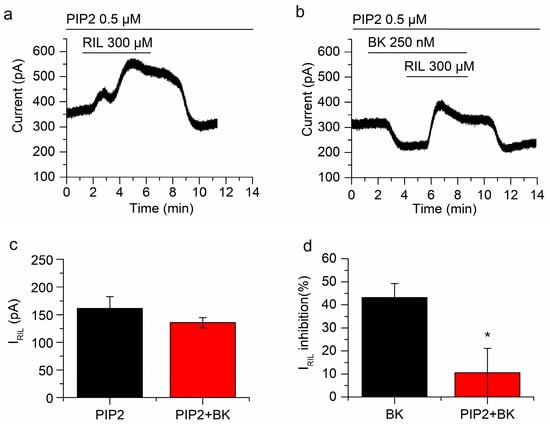
Figure 5.
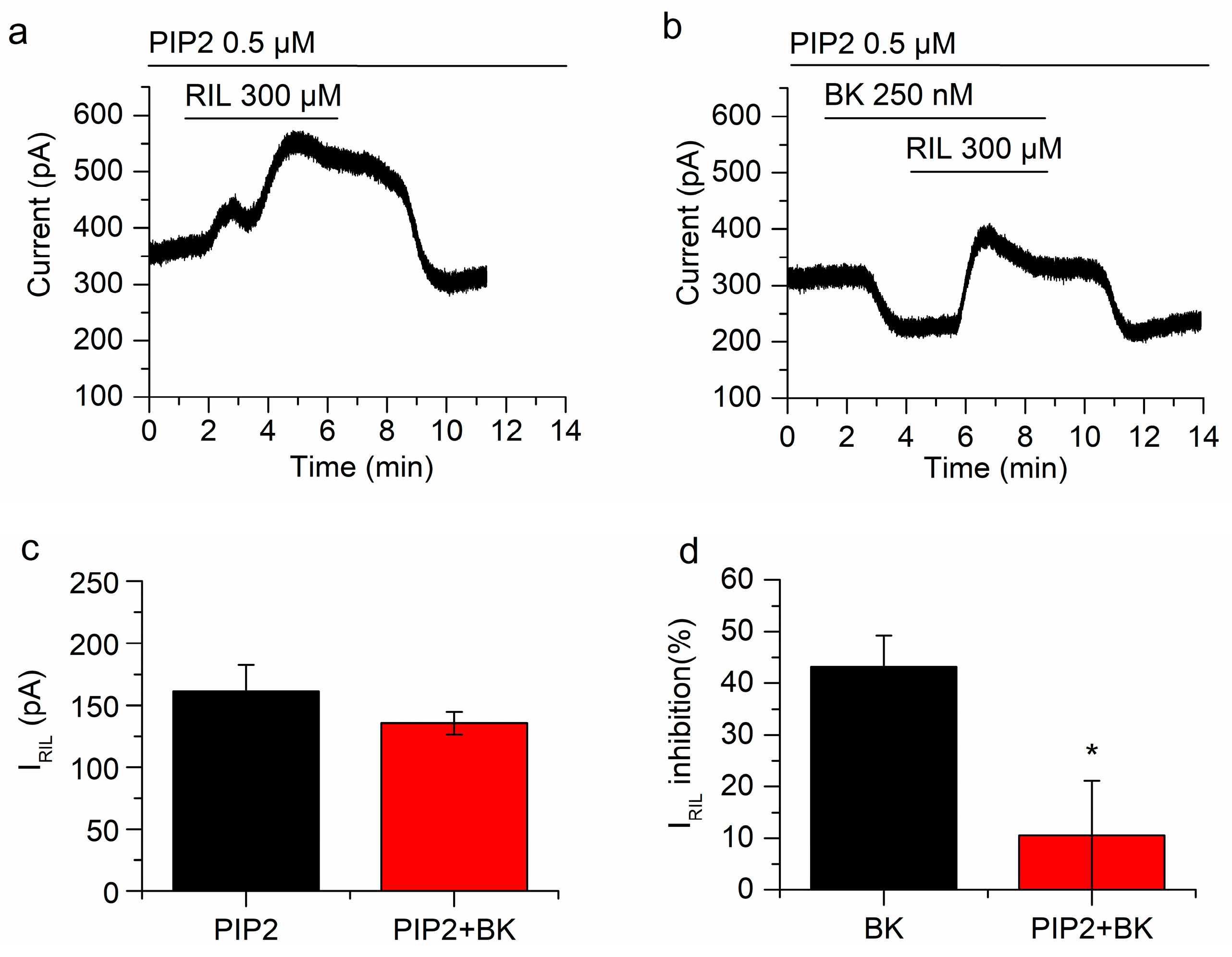
Keeping phosphatidylinositol 4,5-biphosphate (PIP2) concentration precludes the inhibition of TREK-2 by BK. (a) Riluzole-activated current after incubation of neurons with diC8PIP2 for 1 h. (b) In the presence of a PIP2 excess, BK clearly reduced I-30 but the inhibition of IRIL by BK was abolished. (c) There was no difference between IRIL (black bar; n = 5) and IRIL in the presence of BK (red bar; n = 5) when cells were pre-incubated with diC8PIP2. (d) The inhibition of IRIL by BK in the presence of diC8PIP2 (red bar; n = 5) is significantly different (p < 0.05) from the inhibition by BK alone (black bar; n = 7). Recordings in (a,b) belong to the same neuron. * p < 0.05.
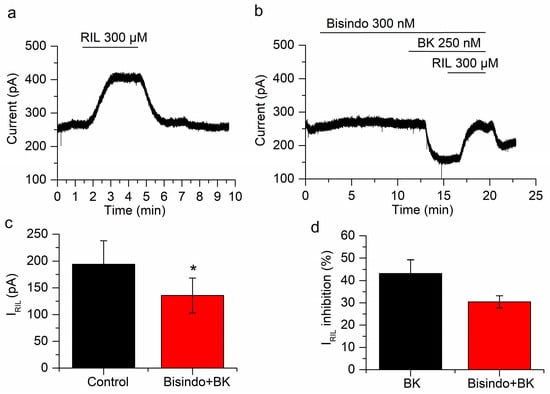
Figure 6.
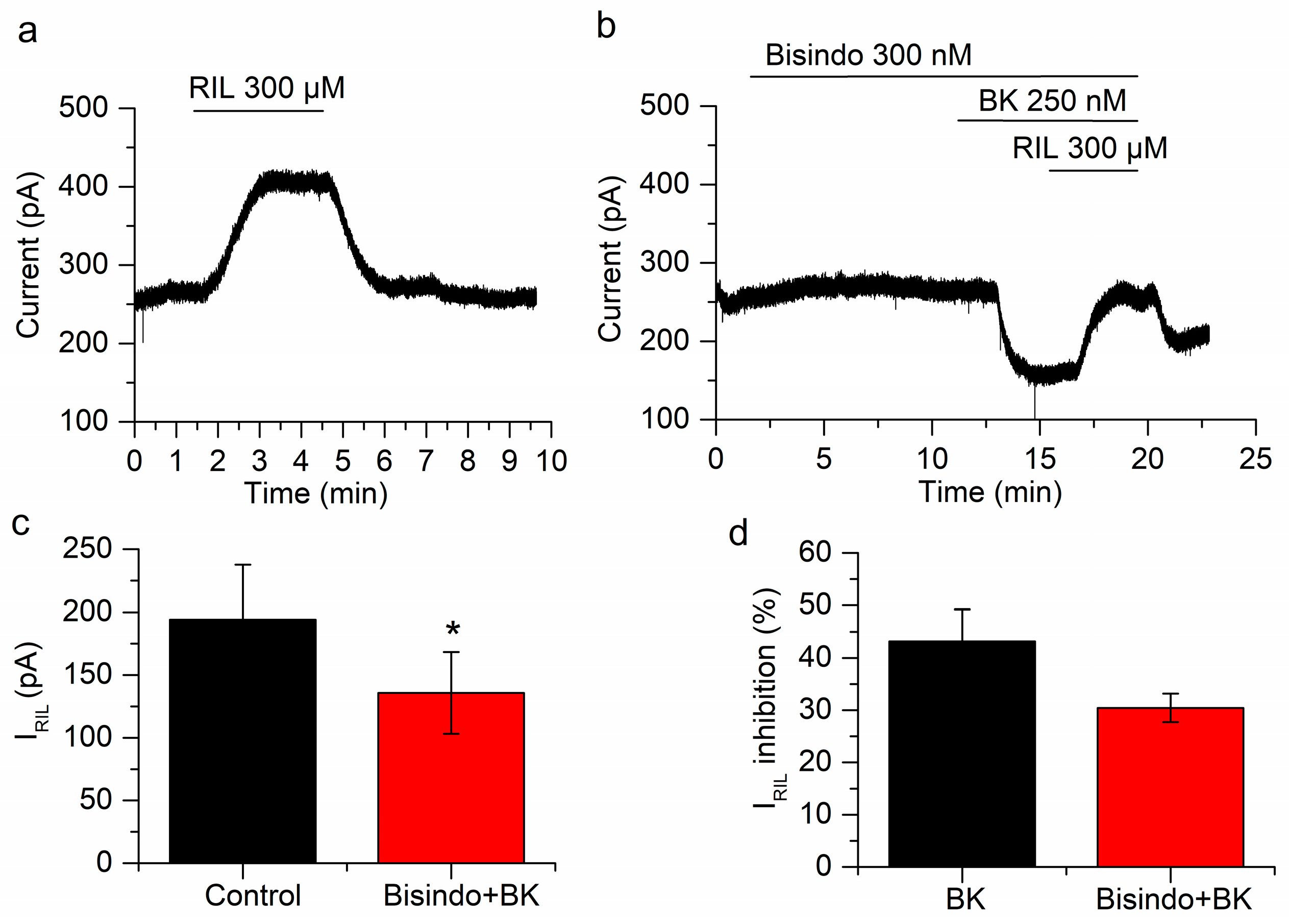
Protein kinase C (PKC) does not affect the inhibition of IRIL by BK. (a) Current activated by riluzole in control conditions. (b) In the presence of the PKC blocker bisindolylmaleimide both I-30 and IRIL are inhibited by BK. (c) The difference between IRIL (black bar) and IRIL in the presence of bisindolylmaleimide and BK (red bar) is statistically significant (p < 0.05; n = 4). (d) The inhibition of IRIL by BK in presence of bisindolylmaleimide (red bar) is similar to the inhibition by BK alone (black bar; p = 0.17). Recordings in (a,b) belong to the same neuron. * p < 0.05.
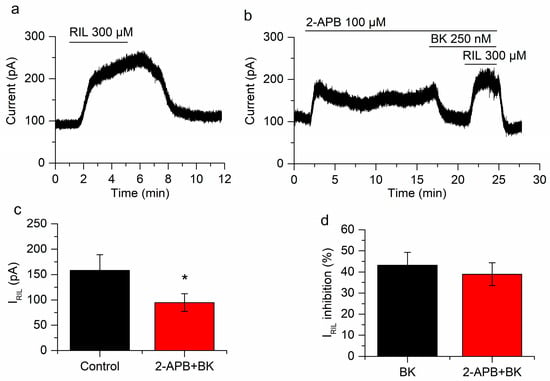
Figure 7.
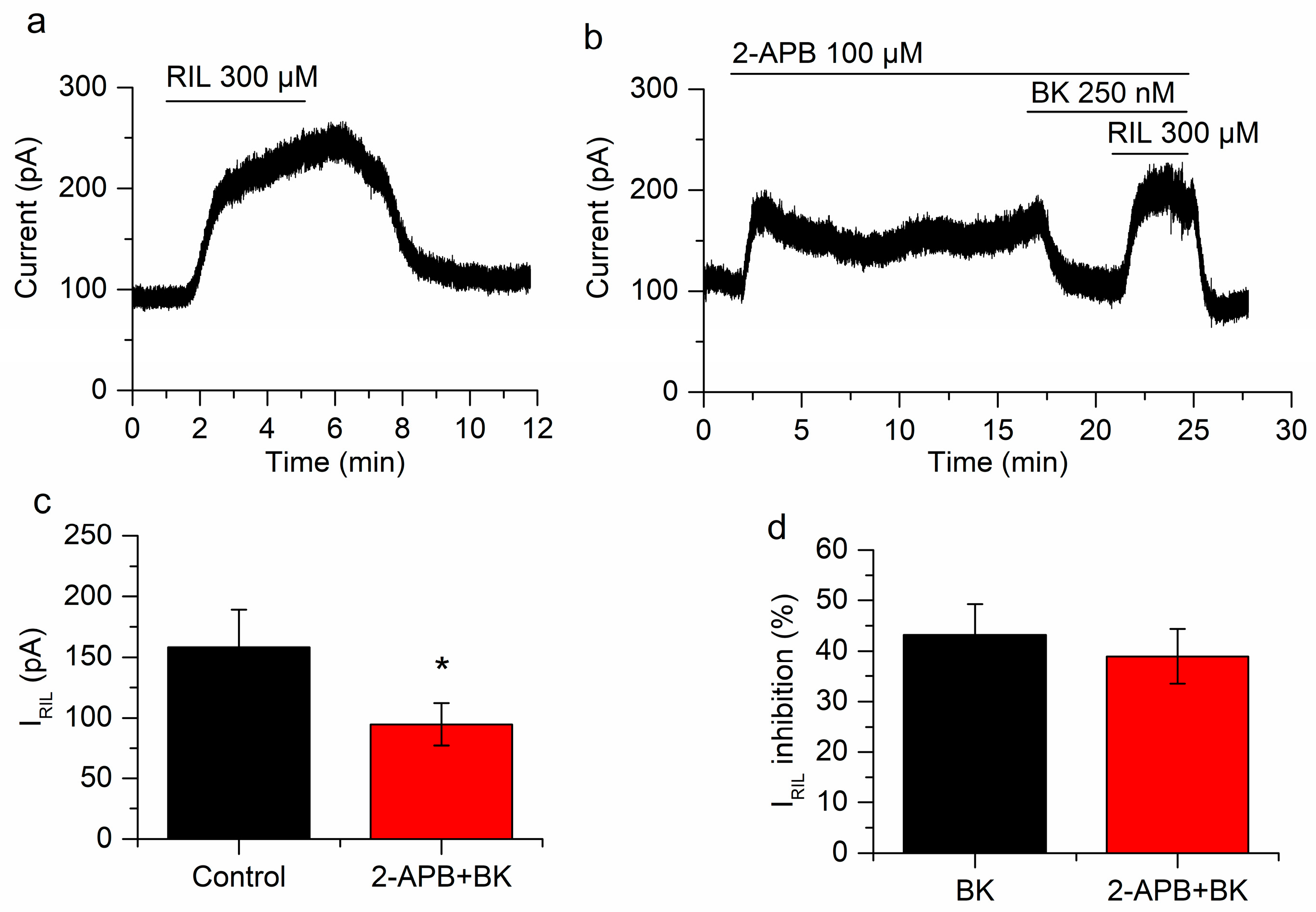
Ca2+ release does not play a role in the inhibition of IRIL by BK. (a) IRIL in control conditions. (b) Application of 2-aminoethoxydiphenylborane (2-APB) provokes an outward current at −30 mV and the inhibition of IRIL by BK is not affected in the presence of this drug. (c) The difference between IRIL control (black bar) and IRIL in presence of BK and 2-APB (red bar) is significant (p = 0.029; n = 5). (d) The percentages of BK inhibition in absence (black bar) and presence of 2-APB (red bar) are similar (p = 0.79). Recordings in (a,b) belong to the same neuron. * p < 0.05.
2.4. Manipulating PIP2 Concentration Affects the Inhibition of IRIL by Bradykinin
We have recently reported that the inhibition of TREK-2 channels by muscarinic agonists in mSCG neurons greatly depends on PIP2 depletion [25]. As B2 receptors also activate Gq/11 and PLC, the reduction of PIP2 seemed a good candidate to explain the inhibition of IRIL by BK. To investigate this issue, we incubated mSCG neurons with diC8PIP2 (0.5 µM, saturating concentration) and a histone carrier, for 1 h, before starting current recordings. As expected, in this situation, the application of BK did not affect IRIL significantly (From 161.3 ± 21.4 pA to 135.5 ± 9.3 pA, p = 0.20, n = 5; Figure 5a,b). Consistently, the percentage of current reduction (10.5 ± 10.6%) was irrelevant when compared with the control (43.2 ± 6.1%; without diC8PIP2; Figure 5c,d). Nevertheless, BK inhibited I-30 significantly (−113.4 ± 22 pA; p = 0.0067; n = 5; Figure 5b) in presence of diC8PIP2 indicating distinct pathways involved in the inhibition of IRIL and I-30 (experiments carried out in cocktail). Altogether these results demonstrate that the inhibition of the riluzole activated current by bradykinin is mediated by the reduction of PIP2 but the inhibition of I-30 is not.
2.5. Neither Protein Kinase C nor Ca2+ Are Involved in the Inhibition of IRIL by Bradykinin
In the PLC pathway, concomitant with the reduction of PIP2 there is an increase of IP3 and DAG. DAG and the Ca2+ released by the binding of IP3 to its receptor finally activate PKC. Indeed, research has shown that the inhibition of the M-current by BK depends on the increase of Ca2+ produced in this way [19,50]. We investigated the role of these last messengers, on the inhibition of TREK-2 currents by BK, by inhibiting PKC and antagonizing IP3 receptors.
In order to inhibit PKC we applied bisindolylmaleimide (300 nM) for 10 min. In these conditions, bradykinin inhibited IRIL by 30.4 ± 2.7%, from 194.1 ± 43.5 to 135.7 ± 32.6 pA (p = 0.023; n = 4; Figure 6a–c). This inhibition is not different (p = 0.17) from that obtained in the absence of bisindolylmaleimide (43.2 ± 6.1%; Figure 6d). The application of bisindolylmaleimide had no effect on I-30 (7.4 ± 4.3 pA; n = 4; p = 0.18; Figure 6b) but BK application reduced significantly this current (−81 ± 22.1 pA; p = 0.035; n = 4; Figure 6b). This experiment indicated that PKC is not involved in the inhibition of IRIL or I-30 by BK.
To test the other limb of the PLC pathway we antagonized the IP3R using 2-APB (100 µM) and hence hampered the increase in Ca2+ that is normally produced by the activation of BK receptors. Bradykinin still reduced IRIL when the increase in Ca2+ was prevented (from 158.4 ± 30.7 pA to 94.6 ± 17.5 pA; p = 0.029; n = 5; Figure 7). The inhibition (39 ± 5.4%; Figure 7d) was similar to that found in the absence of 2-APB (43.2 ± 6.1%; p = 0.79; Figure 7d). We previously performed control experiments comparing riluzole activation in the presence and absence of 2-APB and differences were not found [26]. Interestingly, the application of 2-APB produced a fast increase of I-30 (71.4 ± 19.3 pA; p = 0.020; Figure 7b), this was a transient effect that slowly decreased in −29.9 ± 9.7 pA (p = 0.037). This was in agreement with 2-APB acting as a TREK-2 activator [51]. The application of BK (250 nM) still reduced I-30 in the presence of 2-APB (−48.3 ± 1.5 pA; p = 5.18 × 10−6; n = 5; Figure 7b), probably reflecting the inhibition of the TREK-2 current, previously activated by 2-APB.
3. Discussion
In addition to being a potent vasodilator and a pain and inflammation activator [1], bradykinin depolarizes the resting membrane potential and reduces the spike frequency adaptation in rSCG neurons [12,13]. This modulation has been related to the M-current that is well known to be inhibited by BK [12,50,52]. On the other hand, the potassium channels of the TREK subfamily have a similar role to the M channels in some excitable cells, contributing to the control of the RMP, the firing and therefore to the control of excitability [26,29]. In the present work we demonstrated, for the first time, that bradykinin also modulates TREK currents in mSCG neurons by reducing PIP2. The inhibition of these channels could contribute to the increase in excitability produced by BK in sympathetic neurons.
3.1. I-30 Includes ITREK and IM
When the membrane of SCG neurons is depolarized to −30 mV, a characteristic and constant outward current emerges, this current was initially attributed to the opening of voltage-activated, non-inactivating potassium M-channels and it explains why most voltage protocols used to study the M-current start by fixing neurons at −30 mV [53]. Our group has demonstrated the expression of background potassium TREK-2 currents in mSCG neurons but although these are considered essentially voltage-independent, its macroscopic intensity-voltage curve is almost indistinguishable from that of the M-current [25,35]. The best tool we have to isolate the TREK current from the M-current is to selectively activate TREK currents with riluzole in the presence of TEA, a strong blocker of the M-currents but not affecting TREK channels [26]. We have previously demonstrated that the current activated by riluzole is not affected by blockers of other channels like voltage-gated and persistent Na+, voltage-gated Ca2+, hyperpolarization-activated cation, transient-receptor- potential cation, voltage-gated A and DR type K+, M type K+, and Ca2+-activated K+ (Big K+ and Small K+) channels [26]. As riluzole activates the TREK subfamily exclusively [26,31,32], and, within the TREK subfamily, TREK-2 is largely more expressed than the other TREK subfamily channels in mSCG neurons [27], we can propose that IRIL is mainly transported through TREK-2 channels in these neurons. Assuming that the outward current observed at −30 mV is composed mainly of IM and ITREK, when TEA and other blockers not affecting TREK currents are applied, we expect the remaining current to be mainly ITREK. Even so, we call this current I-30 because we suspect that the cocktail may not be capable of removing the IM completely.
3.2. IRIL is Due to the Activation of ITREK
As BK inhibits both the M and TREK currents, the question arises of whether IRIL can be contaminated with the M-current. BK applied in the cocktail reduces I-30 and inhibits IRIL, and both effects disappear when the BK receptors are antagonized, indicating that both depend on BK receptor activation. This suggests that part of the I-30 is due to ITREK as most, if not all, IM is blocked by TEA [43].
On the contrary, when we keep high levels of PIP2, BK is not inhibiting IRIL but still reduces I-30, suggesting that BK inhibits IRIL by depleting PIP2 but it is able to reduce part of the I-30 (probably IM) through a different pathway when PIP2 reduction is not possible. Taken together all these data indicate that IRIL is essentially a riluzole-activated TREK current and that I-30 has two components (ITREK and a residual IM when TEA is present). This hypothesis is strongly supported by the fact that IM has been reported to be inhibited by BK through the activation of Ca2+-calmodulin, but not by PIP2 depletion (like reported for muscarinic agonists), in SCG neurons [12,52,54,55]. This different mechanism could be due to the fact that muscarinic receptors are far away from IP3R and hence Ca2+ release from the ER becomes difficult. However, BK receptors are close enough to provoke Ca2+ release [52,56]. Keeping a high level of PIP2 does not impede IP3 production and hence Ca2+ release, when BK stimulates its receptors [19].
Moreover, as we reported before, when using the PI3/PI4-Kinase-inhibitor wortmannin to block the replenishment of PIP2 and to maintain PIP2 concentration very low it can be demonstrated that IRIL, and also I-30, need high concentrations of PIP2 to be activated. This is consistent with both M and TREK channels being inhibited by PIP2 depletion in SCG neurons [25,57]. Our results contrast with previous data showing that BK does not increase the concentration of PIP2 in rat SCG neurons [14,58,59]. This discrepancy could be related to the use of different species and ages for the extraction of neurons. In fact, it has also been shown that the regulation of the M current by muscarinic agonists is quite different in rat (Gq) and mouse (G11, Gq and PTX-sensitive G-proteins) and not less important, the inhibition of the M current by BK is mediated by Gα11 in mSCG but by Gαq/11 in rSCG neurons [18].
3.3. I-30 versus ITREK
When the activation of IP3 receptors was avoided with 2-APB and hence prevented the inhibition of IM, BK still inhibited I-30, indicating that besides the M component there exists a second component that can be inhibited through a pathway different from the Ca2+-calmodulin one, probably the TREK current activated by 2-APB contributes to this inhibition. Consistently, the reduction of the I-30 in these conditions resulted smaller than that obtained in cocktail alone (around 50 pA) as it represents the ITREK only while the inhibition of IRIL by BK was well preserved in those conditions.
3.4. Physiological Relevance and Conclusions
The TREK subfamily has been implicated in the transduction of mechanical, chemical, and thermal stimuli [60,61], but also related to pain transduction, as it is well expressed in dorsal root ganglia (DRG) small neurons and in trigeminal ganglia neurons [62,63,64,65]. Recent studies have proposed the TREK subfamily as a possible target for pain treatment [62,66,67] and TREK-1 seems to be involved in morphine analgesia without adverse effects in mice [68]. Also aristolochic acid, used traditionally as painkiller, enhanced TREK-1 and TREK-2 currents [69]. The present work shows that TREK-2 channels are inhibited by BK, an inductor of pain and inflammation, much like as described before for M-current inhibition [12]. The activation of TREK channels by the neuroprotector riluzole hyperpolarizes nociceptive DRG neurons resulting in a decrease of the effects of BK [70]. The TREK subfamily is well expressed in the DRG neurons [28,71,72], the TREK-2 channel being the most expressed, at least in neonatal rats [73]. Additionally, the blood vessel vasodilation produced by bradykinin is strongly attenuated in TREK-1 deleted animals [41]. These and other studies strongly relate BK with TREK channels and pain.
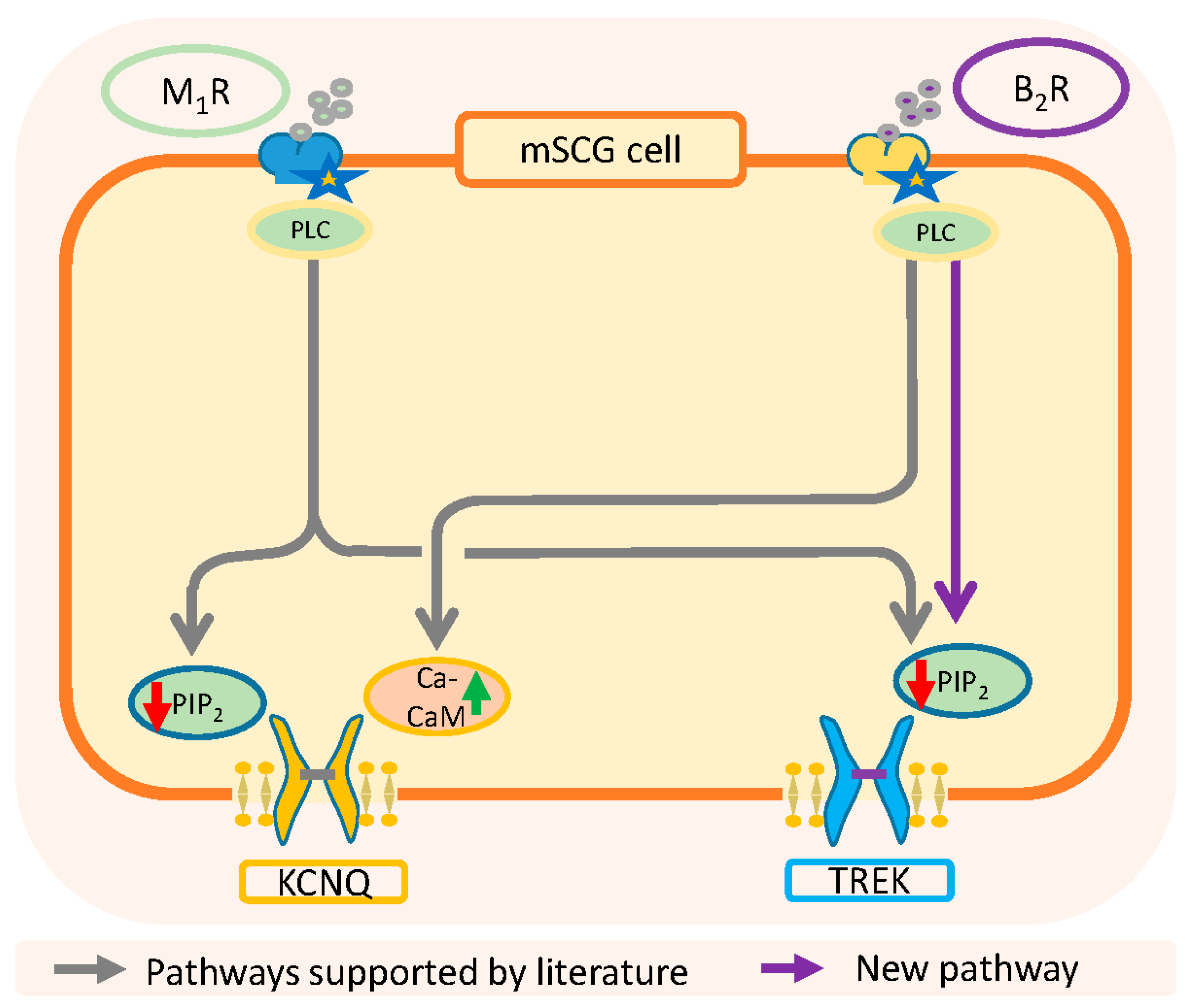
As summarized in Figure 8, the activation of muscarinic M1-receptors inhibits both IM and ITREK through the depletion of PIP2, while the activation of B2-receptors uses two different inhibitory pathways, PIP2 reduction for ITREK and Ca2+-calmodulin for IM. Importantly, it has been reported that TREK-1 channels have a group of cationic aminoacids in the C-terminal region that interacts electrostatically with the inner part of the membrane. A reduction in the concentration of PIP2 causes fluctuations in this electrostatic interaction resulting in the dissociation of the c-terminal domain from the membrane and a change in channel activity [74,75]. An important conclusion of this work is that the riluzole activated current and about half of the current at −30 (in the presence of Na+, K+ and h-current blockers) are carried through TREK channels. Both currents and also individual TREK-2 channels are inhibited by bradykinin through PIP2 depletion. These results also suggest that previous interpretations on the effects of BK on the nervous system may need a review.
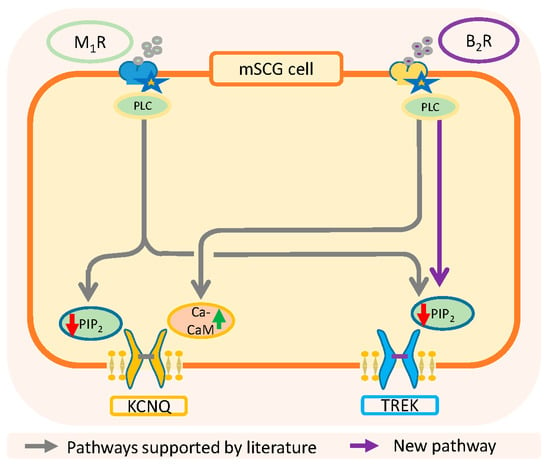
Figure 8.
Summary of classical and new pathways involved in the resting membrane potential and excitability of SCG neurons. KCNQ: gen codifying M-type voltage dependent K channels.
4. Materials and Methods
All protocols performed were approved by the Spanish Research Council and the Animal Welfare Committee of the University of Vigo (Code: 07/2014; Date: 24/10/2016). They accomplished the Spanish (RD 53/2013) and European (2010/63/EU) directives for the protection of animals used for experimental purposes.
4.1. Cell Culture
Swiss-CD1 mice of both sexes and 20 to 60-days-old were terminally anaesthetized with CO2 before decapitation. SCG ganglia were then quickly dissected and placed in L-15 culture medium to clean and disaggregate, first enzymatically and then mechanically. Then, neurons were placed in laminin-coated 35 mm Petri dishes and kept in culture (37 °C, 5% CO2) for 12 to 24 h before recording. Detailed dissection and culture procedures have been reported before [22,76].
4.2. Perforated-Patch Recording
Electrophysiological data were recorded using an Axopatch 200B amplifier (Molecular Devices, Union City, CA, USA) controlled through a DIGIDATA 1440A digitizer (Molecular Devices). The software pClamp 10.0 (Molecular Devices) was used to design the protocols. The standard bath solution was composed of (mM): 140 NaCl, 3 KCl, 1 MgCl2, 2 CaCl2, 10 D-glucose, 10 HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); pH 7.2 adjusted with Tris (Tris(hydroxymethyl)-aminomethane). The intracellular solution contained (mM): 90 K-acetate, 20 KCl, 3 MgCl2, 1 CaCl2, 3 EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid), 40 HEPES, and ~20 NaOH to achieve a pH of 7.2. Amphotericin-B (75 µg/mL) was freshly prepared every day and added to the intracellular solution to obtain the perforated patch configuration. Borosilicate glass was used to produce 4–5 MΩ electrode resistances. After 15–20 min, since the gigaseal was attained, access resistance reached values below 20 MΩ and recording started. Cells with higher access resistance were discarded. Voltage-clamp recordings were sampled at 2 KHz and low-pass filtered at 1 KHz while current-clamp (bridge-mode like) signals were sampled at 10 KHz filtering at 5 KHz. The data obtained (mean ± SEM) were analyzed and plotted using pClamp 10.0 and Origin 9.0 (OriginLab Corporation, Northampton, MA, USA) and the statistical significance was assumed when paired Student’s t-test gave p-values less than 0.05.
4.3. Single-Channel Recording
Single-channel recordings were performed in the cell-attached configuration. Bath and pipette solutions were identical and contained (mM): 150 KCl, 1 MgCl2, 5 EGTA, and 10 HEPES, pH 7.2 was achieved with KOH. The electrode resistance for single-channel experiments ranged from 10 to 12 MΩ. Data were sampled at 20 KHz and low-pass filtered at 2 KHz using the amplifier built-in filter. Single-channel openings faster than 50 µs were discarded. The threshold detection for single-channel openings was set at 50% of the amplitude. Single-channel parameters: amplitude, open dwell time and NPo were measured using pClamp 10.0 software. The NPo was calculated according to the following equation: NPo = to/T, where N is the number of channels, Po is the open state probability, to was the total time that the channel was found in the open state, and T is the total observation time. The data obtained (mean ± SEM) were analyzed and plotted using Clampfit 10 and Origin 9.0 applying Paired Student’s t-test and considering significant p-values < 0.05.
4.4. Drugs
All drugs were applied directly to the bath solution (10 mL/min) during the protocol, except for diC8PIP2 with a histone carrier, which was previously added to the culture dishes.
TTX and HOE-140 were purchased from Tocris Bioscience (Bristol, UK), diC8PIP2 and the histone carrier were purchased from Echelon Biosciences (Salt Lake City, UT, USA), and all the other chemicals were obtained from Sigma-Aldrich (Madrid, Spain).
Author Contributions
Conceptualization, A.R. and J.A.L.; Formal analysis, P.R.-R., A.R., L.R.-R. and S.H.-P.; Funding acquisition, J.A.L.; Investigation, P.R.-R., A.R., L.R.-R., S.H.-P. and J.A.L.; Methodology, P.R.-R., A.R. and J.A.L.; Project administration, J.A.L.; Supervision, J.A.L.; Writing—original draft, P.R.-R. and A.R.; Writing—review and editing, J.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish government (grant references: CONSOLIDER CSD2008-00005, BFU2014-58999-P), the regional government “Xunta de Galicia” (grant numbers INBIOMED CN2012/273, INB1-131H-2) and it was partially funded by FEDER. All the funding was awarded to J Antonio Lamas.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 2-APB | 2-aminoethoxydiphenylborane |
| BK | bradykinin |
| BL | BL1249 |
| BRs | bradykinin receptors |
| B1R | bradykinin receptor 1 |
| B2R | bradykinin receptor 2 |
| DAG | diacylglycerol |
| DRG | dorsal root ganglia |
| HOE | HOE-140. d-Arg-[Hyp3, Thi5, d-Tic7, Oic8]BK |
| Ih | hyperpolarization activated cationic current |
| IM | potassium M-current |
| IP3 | inositol triphosphate |
| IP3R | inositol triphosphate receptor |
| IRIL | riluzole-activated current |
| ITREK | potassium current through TREK channels |
| I-30 | basal outward current at -30 mV |
| K2P | two pore domain potassium channels |
| mSCG | mouse superior cervical ganglion |
| NPo | open probability |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKC | protein kinase C |
| PLC | phospholipase C |
| RMP | resting membrane potential |
| rSCG | Rat superior cervical ganglion |
| SCG | superior cervical ganglion |
| SFA | spike frequency adaptation |
| TEA TRAAK | Tetraethylammonium TWIK-related arachidonic acid-stimulated potassium channel |
| TREK TRESK TWIK | TWIK-related potassium channel TWIK-related spinal cord potassium channel Tandem of pore-domains in a Weakly Inward rectifying K+ channel |
| TTX | tetrodotoxin |
References
- Greaves, M.; Shuster, S. Responses of skin blood vessels to bradykinin, histamine and 5-hydroxytryptamine. J. Physiol Lond. 1967, 193, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Dray, A.; Bettaney, J.; Forster, P.; Perkins, M.N. Activation of a bradykinin receptor in peripheral nerve and spinal cord in the neonatal rat in vitro. Br. J. Pharmacol. 1988, 95, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Naeem, S.; Phagoo, S.B.; Campbell, E.A.; Urban, L.; Burgess, G.M. B1 bradykinin receptors and sensory neurones. Br. J. Pharmacol. 1996, 118, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Babbedge, R.; Dray, A.; Urban, L. Bradykinin depolarises the rat isolated superior cervical ganglion via B2 receptor activation. Neurosci. Lett. 1995, 193, 161–164. [Google Scholar] [CrossRef]
- Prado, G.N.; Taylor, L.; Zhou, X.; Ricupero, D.; Mierke, D.F.; Polgar, P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J. Cell Physiol. 2002, 193, 275–286. [Google Scholar] [CrossRef]
- Bascands, J.L.; Emond, C.; Pecher, C.; Regoli, D.; Girolami, J.P. Bradykinin stimulates production of inositol (1,4,5) trisphosphate in cultured mesangial cells of the rat via a BK2-kinin receptor. Br. J. Pharmacol. 1991, 102, 962–965. [Google Scholar] [CrossRef]
- Innis, R.B.; Manning, D.C.; Stewart, J.M.; Snyder, S.H. [3H]Bradykinin receptor binding in mammalian tissue membranes. Proc. Natl. Acad. Sci. USA 1981, 78, 2630–2634. [Google Scholar] [CrossRef]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar]
- McGuire, C.; Boundouki, G.; Hockley, J.R.F.; Reed, D.; Cibert-Goton, V.; Peiris, M.; Kung, V.; Broad, J.; Aziz, Q.; Chan, C.; et al. Ex vivo study of human visceral nociceptors. Gut 2018, 67, 86–96. [Google Scholar] [CrossRef]
- Brunsden, A.M.; Grundy, D. Sensitization of visceral afferents to bradykinin in rat jejunum in vitro. J. Physiol Lond. 1999, 521, 517–527. [Google Scholar] [CrossRef]
- Lewis, G.P.; Reit, E. The action of angiotensin and bradykinin on the superior cervical ganglion of the cat. J. Physiol Lond. 1965, 179, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Brown, D.A.; Milligan, G.; Willer, E.; Buckley, N.J.; Caulfield, M.P. Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 Receptors and Gaq/11. Neuron 1995, 14, 399–405. [Google Scholar] [CrossRef]
- Seabrook, G.R.; Bowery, B.J.; Hill, R.G. Bradykinin receptors in mouse and rat isolated superior cervical ganglia. Br. J. Pharmacol. 1995, 115, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Zaika, O.; Tolstykh, G.P.; Jaffe, D.B.; Shapiro, M.S. Inositol triphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels. J. Neurosci. 2007, 27, 8914–8926. [Google Scholar] [CrossRef] [PubMed]
- Seabrook, G.R.; Bowery, B.J.; Heavens, R.; Brown, N.; Ford, H.; Sirinathsinghi, D.J.; Borkowski, J.A.; Hess, J.F.; Strader, C.D.; Hill, R.G. Expression of B1 and B2 bradykinin receptor mRNA and their functional roles in sympathetic ganglia and sensory dorsal root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology 1997, 36, 1009–1017. [Google Scholar] [CrossRef]
- Borkowski, J.A.; Ransom, R.W.; Seabrook, G.R.; Trumbauer, M.; Chen, H.; Hill, R.G.; Strader, C.D.; Hess, J.F. Targeted disruption of a B2 bradykinin receptor gene in mice eliminates bradykinin action in smooth muscle and neurons. J. Biol. Chem. 1995, 270, 13706–13710. [Google Scholar] [CrossRef]
- Levine, J.D.; Taiwo, Y.O.; Collins, S.D.; Tam, J.K. Noradrenaline hyperalgesia is mediated through interaction with sympathetic postganglionic neurone terminals rather than activation of primary afferent nociceptors. Nature 1986, 323, 158–160. [Google Scholar] [CrossRef]
- Haley, J.E.; Delmas, P.; Offermanns, S.; Abogadie, F.C.; Simon, M.I.; Buckley, N.J.; Brown, D.A. Muscarinic inhibition of calcium current and M current in Galpha q-deficient mice. J. Neurosci. 2000, 20, 3973–3979. [Google Scholar] [CrossRef]
- Cruzblanca, H.; Koh, D.S.; Hille, B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 7151–7156. [Google Scholar] [CrossRef]
- Haley, J.E.; Abogadie, F.C.; Fernandez, F.; Dayrell, M.; Vallis, Y.; Buckley, N.J.; Brown, D.A. Bradykinin, but not muscarinic, inhibition of M-current in rat sympathetic ganglion neurons involves phospholipase C-beta 4. J. Neurosci. 2000, 20, RC105. [Google Scholar] [CrossRef]
- Brown, D.A.; Abogadie, F.C.; Allen, T.G.; Buckley, N.J.; Caulfield, M.P.; Delmas, P.; Haley, J.E.; Lamas, J.A.; Selyanko, A.A. Muscarinic mechanisms in nerve cells. Life Sci. 1997, 60, 1137–1144. [Google Scholar] [CrossRef]
- Romero, M.; Reboreda, A.; Sanchez, E.; Lamas, J.A. Newly developed blockers of the M-current do not reduce spike frequency adaptation in cultured mouse sympathetic neurons. Eur. J. Neurosci. 2004, 19, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Constanti, A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br. J. Pharmacol. 1980, 70, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Adams, P.R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 1980, 283, 673–676. [Google Scholar] [CrossRef]
- Rivas-Ramirez, P.; Cadaveira-Mosquera, A.; Lamas, J.A.; Reboreda, A. Muscarinic modulation of TREK currents in mouse sympathetic superior cervical ganglion neurons. Eur. J. Neurosci. 2015, 42, 1797–1807. [Google Scholar] [CrossRef]
- Cadaveira-Mosquera, A.; Ribeiro, S.J.; Reboreda, A.; Perez, M.; Lamas, J.A. Activation of TREK currents by the neuroprotective agent riluzole in mouse sympathetic neurons. J. Neurosci. 2011, 31, 1375–1385. [Google Scholar] [CrossRef]
- Cadaveira-Mosquera, A.; Perez, M.; Reboreda, A.; Rivas-Ramirez, P.; Fernandez-Fernandez, D.; Lamas, J.A. Expression of K2P channels in sensory and motor neurons of the autonomic nervous system. J. Mol. Neurosci. 2012, 48, 86–96. [Google Scholar] [CrossRef]
- Maingret, F.; Lauritzen, I.; Patel, A.J.; Heurteaux, C.; Reyes, R.; Lesage, F.; Lazdunski, M.; Honore, E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000, 19, 2483–2491. [Google Scholar] [CrossRef]
- Zhang, H.; Shepherd, N.; Creazzo, T.L. Temperature-sensitive TREK currents contribute to setting the resting membrane potential in embryonic atrial myocytes. J. Physiol Lond. 2008, 586, 3645–3656. [Google Scholar] [CrossRef]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Perez, S. Ion Channels and Thermosensitivity: TRP, TREK, or Both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef]
- Duprat, F.; Lesage, F.; Patel, A.J.; Fink, M.; Romey, G.; Lazdunski, M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 2000, 57, 906–912. [Google Scholar] [PubMed]
- Lesage, F.; Terrenoire, C.; Romey, G.; Lazdunski, M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J. Biol. Chem. 2000, 275, 28398–28405. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.A.; Fernandez-Fernandez, D. Tandem pore TWIK-related potassium channels and neuroprotection. Neural Regen. Res. 2019, 14, 1293–1308. [Google Scholar] [CrossRef]
- Lamas, J.A. Mechanosensitive K2P channels, TREKking through the autonomic nervous system. In Mechanically Gated Channels and their Regulation; Kamkin, A., Lozinsky, I., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2012; Volume 6, pp. 35–68. [Google Scholar]
- Chemin, J.; Girard, C.; Duprat, F.; Lesage, F.; Romey, G.; Lazdunski, M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003, 22, 5403–5411. [Google Scholar] [CrossRef]
- Kang, D.; Han, J.; Kim, D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am. J. Physiol. Cell Physiol. 2006, 291, C649–C656. [Google Scholar] [CrossRef]
- Cohen, A.; Sagron, R.; Somech, E.; Segal-Hayoun, Y.; Zilberberg, N. Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K(2P)2.1 channel. Mol. Cell Neurosci. 2009, 40, 382–389. [Google Scholar] [CrossRef]
- Xiao, Z.; Deng, P.Y.; Rojanathammanee, L.; Yang, C.; Grisanti, L.; Permpoonputtana, K.; Weinshenker, D.; Doze, V.A.; Porter, J.E.; Lei, S. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J. Biol. Chem. 2009, 284, 10980–10991. [Google Scholar] [CrossRef]
- Choi, S.I.; Hwang, S.W. Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception. Biomol. Ther. (Seoul) 2018, 26, 255–267. [Google Scholar] [CrossRef]
- Garry, A.; Fromy, B.; Blondeau, N.; Henrion, D.; Brau, F.; Gounon, P.; Guy, N.; Heurteaux, C.; Lazdunski, M.; Saumet, J.L. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: The endothelial link. EMBO Rep. 2007, 8, 354–359. [Google Scholar] [CrossRef]
- Lamas, J.A. A hyperpolarization-activated cation current (I-h) contributes to resting membrane potential in rat superior cervical sympathetic neurones. Pflugers Arch. Eur. J. Physiol. 1998, 436, 429–435. [Google Scholar] [CrossRef]
- Hadley, J.K.; Noda, M.; Selyanko, A.A.; Wood, I.C.; Abogadie, F.C.; Brown, D.A. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br. J. Pharmacol. 2000, 129, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.A.; Romero, M.; Reboreda, A.; Sanchez, E.; Ribeiro, S.J. A riluzole- and valproate-sensitive persistent sodium current contributes to the resting membrane potential and increases the excitability of sympathetic neurones. Pflugers Arch. Eur. J. Physiol. 2009, 458, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.; Arrigoni, C.; Lou, H.; Bryant, C.; Gallardo-Godoy, A.; Renslo, A.R.; Minor, D.L., Jr. Protein and Chemical Determinants of BL-1249 Action and Selectivity for K2P Channels. ACS Chem. Neurosci. 2018, 9, 3153–3165. [Google Scholar] [CrossRef] [PubMed]
- Tertyshnikova, S.; Knox, R.J.; Plym, M.J.; Thalody, G.; Griffin, C.; Neelands, T.; Harden, D.G.; Signor, L.; Weaver, D.; Myers, R.A.; et al. BL-1249 [(5,6,7,8-tetrahydro-naphthalen-1-yl)-[2-(1H-tetrazol-5-yl)-phenyl]-amine]: A putative potassium channel opener with bladder-relaxant properties. J. Pharmacol. Exp. Ther. 2005, 313, 250–259. [Google Scholar] [CrossRef]
- Veale, E.L.; Al-Moubarak, E.; Bajaria, N.; Omoto, K.; Cao, L.; Tucker, S.J.; Stevens, E.B.; Mathie, A. Influence of the N terminus on the biophysical properties and pharmacology of TREK1 potassium channels. Mol. Pharmacol. 2014, 85, 671–681. [Google Scholar] [CrossRef]
- Yano, K.; Higashida, H.; Hattori, H.; Nozawa, Y. Bradykinin-induced transient accumulation of inositol trisphosphate in neuron-like cell line NG108-15 cells. FEBS Lett. 1985, 181, 403–406. [Google Scholar] [CrossRef]
- Tippmer, S.; Quitterer, U.; Kolm, V.; Faussner, A.; Roscher, A.; Mosthaf, L.; Muller-Esterl, W.; Haring, H. Bradykinin induces translocation of the protein kinase C isoforms alpha, epsilon, and zeta. Eur. J. Biochem. 1994, 225, 297–304. [Google Scholar] [CrossRef]
- Delmas, P.; Brown, D.A. Pathways modulating neural KCNQ/M (Kv7) potassium. Nat. Rev. Neurosci. 2005, 6, 850–862. [Google Scholar] [CrossRef]
- Beltran, L.; Beltran, M.; Aguado, A.; Gisselmann, G.; Hatt, H. 2-Aminoethoxydiphenyl borate activates the mechanically gated human KCNK channels KCNK 2 (TREK-1), KCNK 4 (TRAAK), and KCNK 10 (TREK-2). Front. Pharmacol. 2013, 4, 63. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Zaika, O.; Tolstykh, G.P.; Shapiro, M.S. Regulation of neural KCNQ channels: Signalling pathways, structural motifs and functional implications. J. Physiol. 2008, 586, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.A. The role of calcium in M-current inhibition by muscarinic agonists in rat sympathetic neurons. Neuroreport 1999, 10, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, A.; Hoshi, N. A change in configuration of the calmodulin-KCNQ channel complex underlies Ca2+-dependent modulation of KCNQ channel activity. PLoS ONE 2013, 8, e82290. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Gomis-Perez, C.; Bernardo-Seisdedos, G.; Alaimo, A.; Malo, C.; Aldaregia, J.; Lopez-Robles, C.; Areso, P.; Butz, E.; Wahl-Schott, C.; et al. Uncoupling PIP2-calmodulin regulation of Kv7.2 channels by an assembly destabilizing epileptogenic mutation. J. Cell. Sci. 2015, 128, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.; Wanaverbecq, N.; Abogadie, F.C.; Mistry, M.; Brown, D.A. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron 2002, 34, 209–220. [Google Scholar] [CrossRef]
- Zhang, H.; Craciun, L.C.; Mirshahi, T.; Rohacs, T.; Lopes, C.M.; Jin, T.; Logothetis, D.E. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 2003, 37, 963–975. [Google Scholar] [CrossRef]
- Gamper, N.; Reznikov, V.; Yamada, Y.; Yang, J.; Shapiro, M.S. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 2004, 24, 10980–10992. [Google Scholar] [CrossRef]
- Zaika, O.; Zhang, J.; Shapiro, M.S. Combined Phosphoinositide and Ca2+ Signals Mediating Receptor Specificity toward Neuronal Ca2+ Channels. J. Biol. Chem. 2011, 286, 830–841. [Google Scholar] [CrossRef]
- Noel, J.; Sandoz, G.; Lesage, F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 2011, 5, 402–409. [Google Scholar] [CrossRef]
- Patel, A.J.; Honore, E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001, 24, 339–346. [Google Scholar] [CrossRef]
- Alloui, A.; Zimmermann, K.; Mamet, J.; Duprat, F.; Noel, J.; Chemin, J.; Guy, N.; Blondeau, N.; Voilley, N.; Rubat-Coudert, C.; et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006, 25, 2368–2376. [Google Scholar] [CrossRef]
- Acosta, C.; Djouhri, L.; Watkins, R.; Berry, C.; Bromage, K.; Lawson, S.N. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J. Neurosci. 2014, 34, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hatakeyama, T.; Taniguchi, K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci. Lett. 2009, 454, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Mathie, A.; Veale, E.L. Two-pore domain potassium channels: Potential therapeutic targets for the treatment of pain. Pflugers Arch. 2015, 467, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Busserolles, J.; Christin, M.; Devilliers, M.; Poupon, L.; Legha, W.; Alloui, A.; Aissouni, Y.; Bourinet, E.; Lesage, F.; et al. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. Pain 2014, 155, 2534–2544. [Google Scholar] [CrossRef]
- Vivier, D.; Soussia, I.B.; Rodrigues, N.; Lolignier, S.; Devilliers, M.; Chatelain, F.C.; Prival, L.; Chapuy, E.; Bourdier, G.; Bennis, K.; et al. Development of the first two-pore domain potassium channel TWIK-related K(+) channel 1-selective agonist possessing in vivo antinociceptive activity. J. Med. Chem. 2017, 60, 1076–1088. [Google Scholar] [CrossRef]
- Devilliers, M.; Busserolles, J.; Lolignier, S.; Deval, E.; Pereira, V.; Alloui, A.; Christin, M.; Mazet, B.; Delmas, P.; Noel, J.; et al. Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Veale, E.L.; Mathie, A. Aristolochic acid, a plant extract used in the treatment of pain and linked to Balkan endemic nephropathy, is a regulator of K2P channels. Br. J. Pharmacol. 2016, 173, 1639–1652. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Gigout, S.; Huang, D.; Yang, Y.; Li, L.; Wang, C.; Sundt, D.; Jaffe, D.B.; Zhang, H.; et al. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 2014, 155, 2306–2322. [Google Scholar] [CrossRef]
- Cameron, K.; Bartle, E.; Roark, R.; Fanelli, D.; Pham, M.; Pollard, B.; Borkowski, B.; Rhoads, S.; Kim, J.; Rocha, M.; et al. Neurosteroid binding to the amino terminal and glutamate binding domains of ionotropic glutamate receptors. Steroids 2012, 77, 774–779. [Google Scholar] [CrossRef]
- Kang, D.; Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 2006, 291, C138–C146. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Choe, C.; Kim, D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 2005, 564, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Chemin, J.; Patel, A.J.; Duprat, F.; Lauritzen, I.; Lazdunski, M.; Honoré, E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005, 24, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; Bell, S.C.; Isacoff, E.Y. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc. Natl. Acad. Sci. USA 2011, 108, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pinna, J.; Lamas, J.A.; Gallego, R. Calcium current components in intact and dissociated adult mouse sympathetic neurons. Brain Res. 2002, 951, 227–236. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).