GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance
Abstract
1. Introduction
2. Results
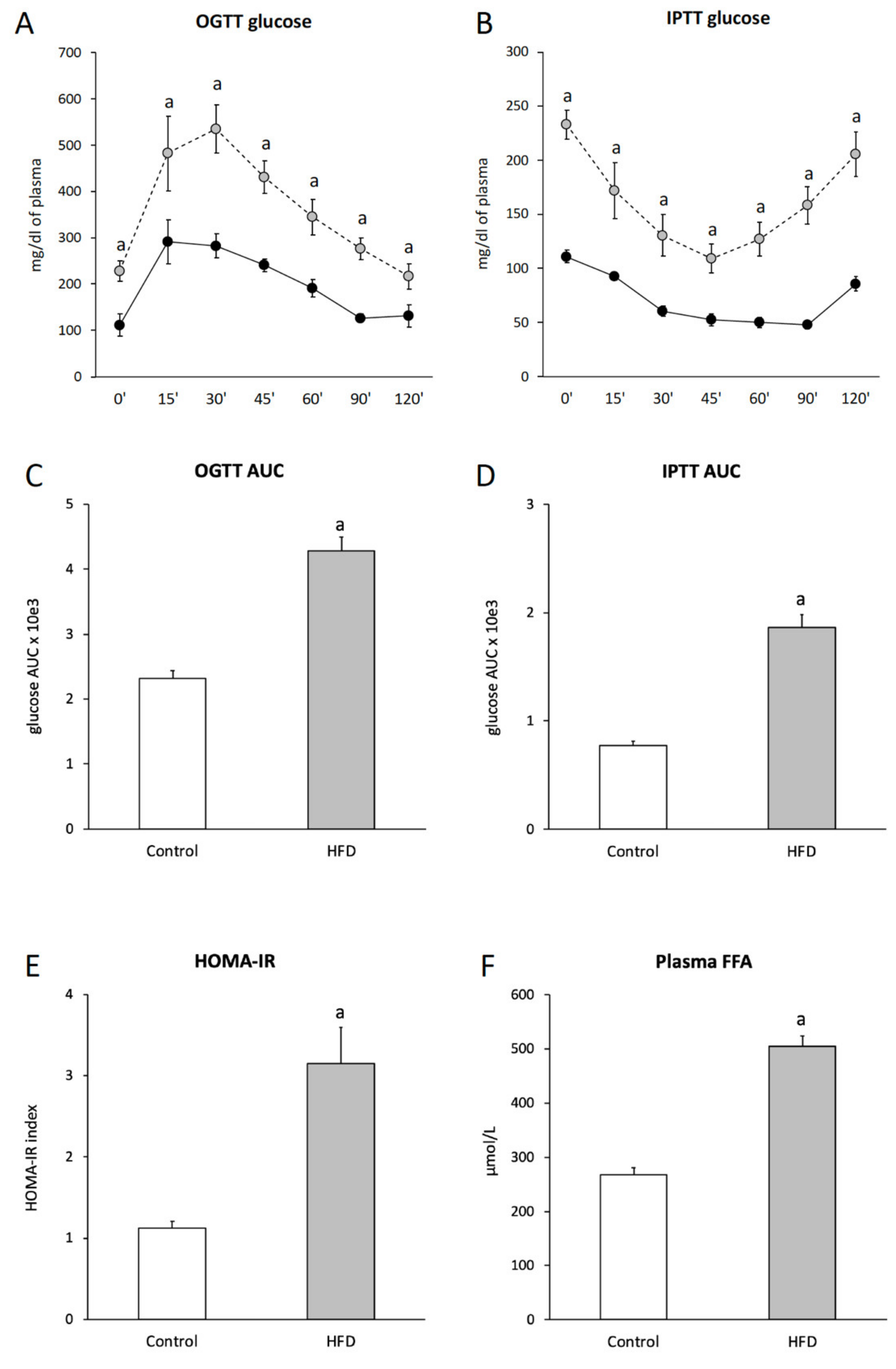
2.1. Insulin Sensitivity
2.2. Plasma Free Fatty Acids (FFA) Concentration
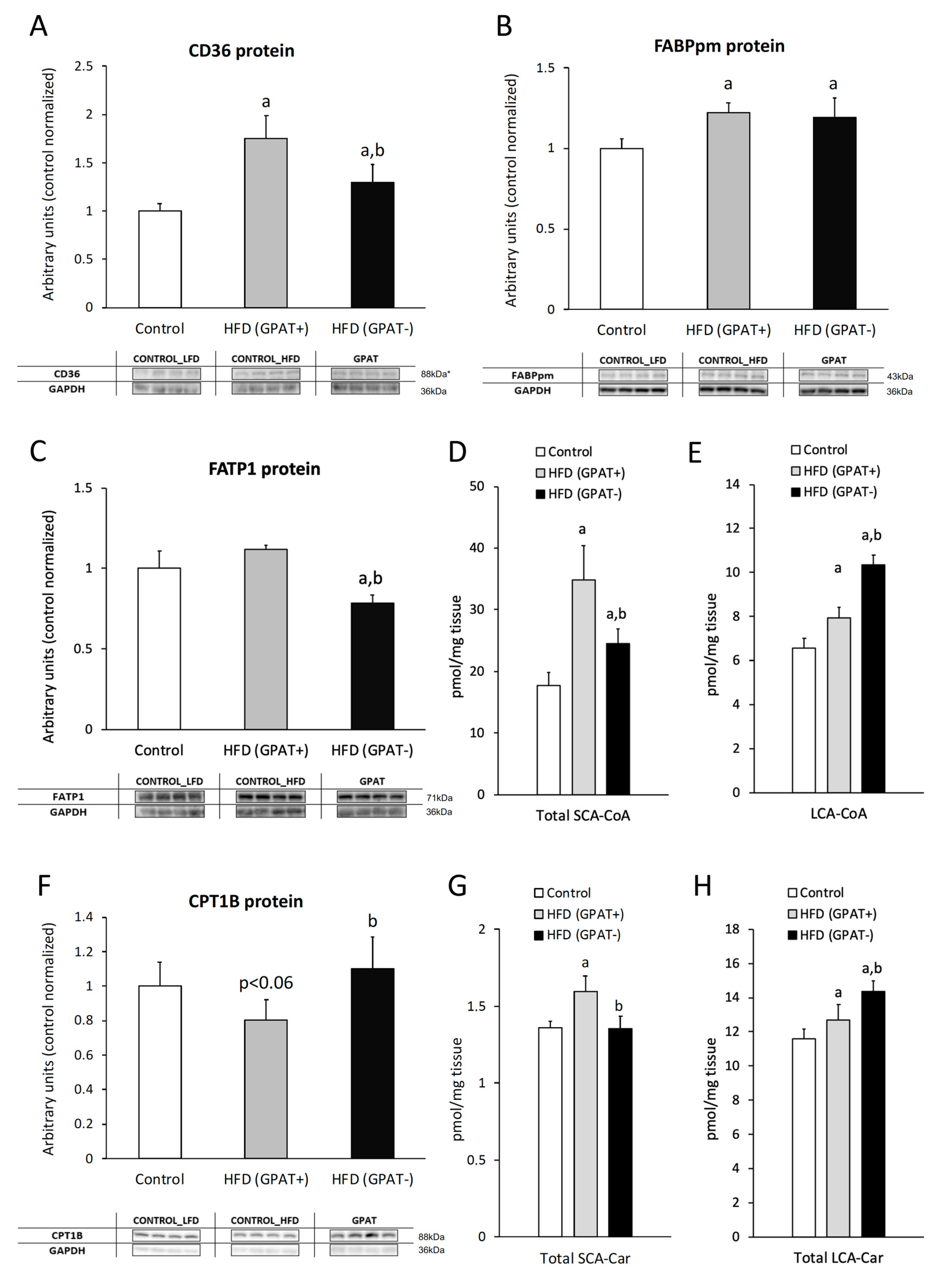
2.3. Fatty Acid Transporters
2.3.1. CD36
2.3.2. FABPpm
2.3.3. FATP1
2.4. Skeletal Muscle Short and Long Chain Acyl-CoA
2.5. Skeletal Muscle CPT1B Content.
2.6. Skeletal Muscle Short and Long-Chain Acyl-Carnitine Content
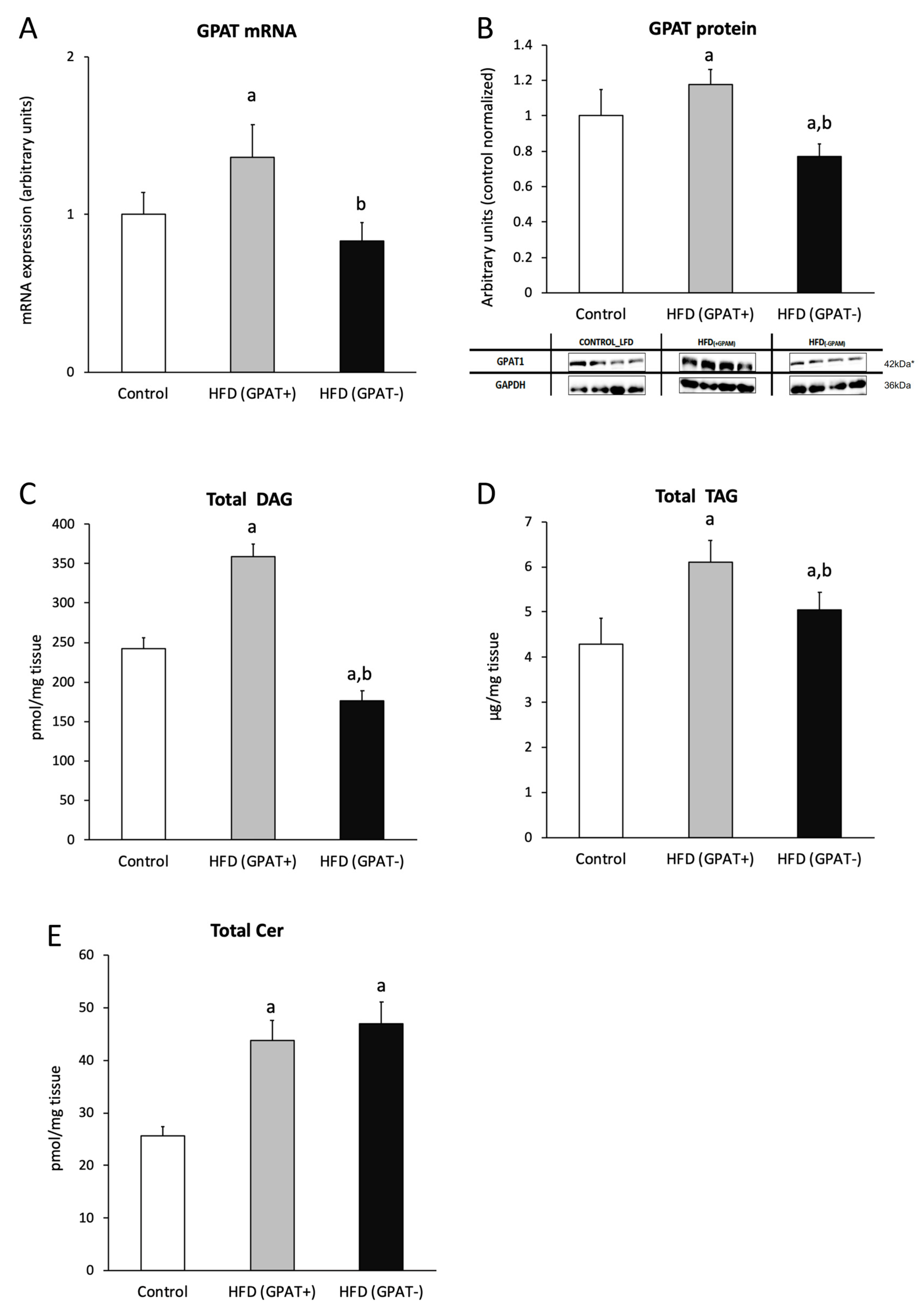
2.7. Skeletal Muscle mRNA and Protein Content of GPAT
2.8. Skeletal Muscle DAG Level
2.9. Skeletal Muscle TAG Level
2.10. Skeletal Muscle Ceramide Content
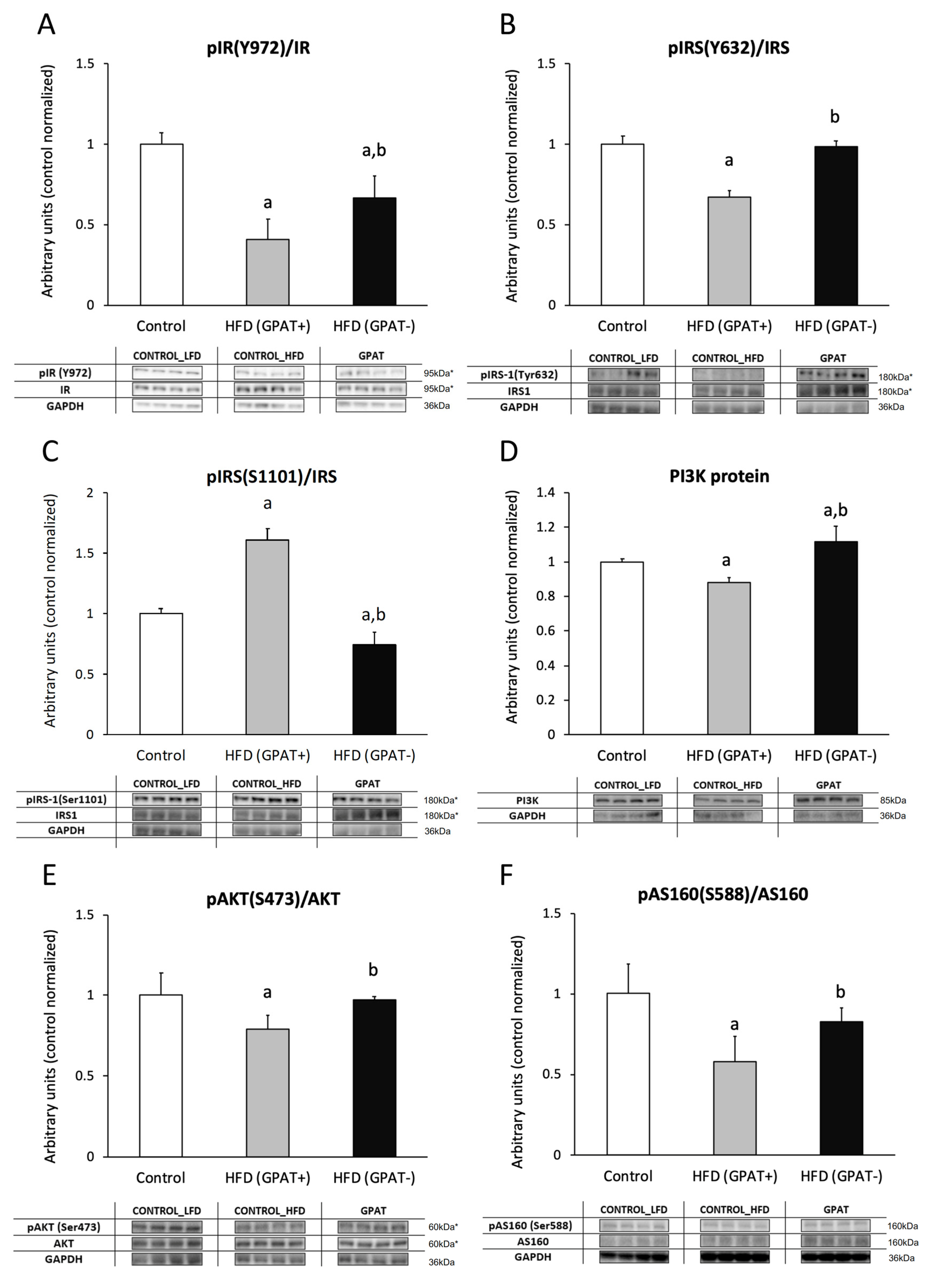
2.11. Insulin Cascade Activation/Inhibition
2.11.1. IR
2.11.2. IRS-1
2.11.3. PI3K
2.11.4. Akt Phosphorylation
2.11.5. AS160
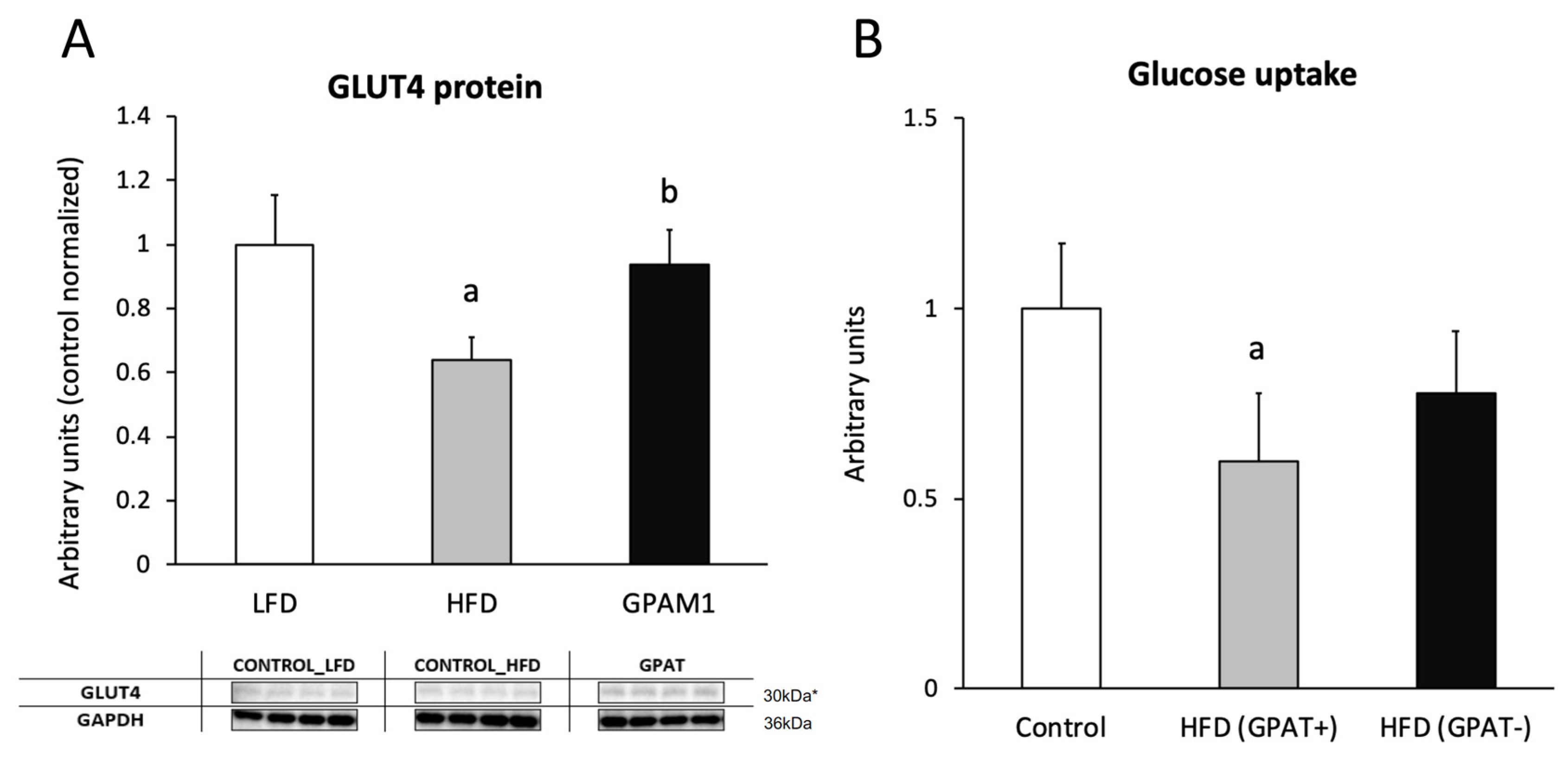
2.12. Glucotransporter 4 (GLUT4)
2.13. Skeletal Muscle Glucose Uptake
3. Discussion
4. Materials and Methods
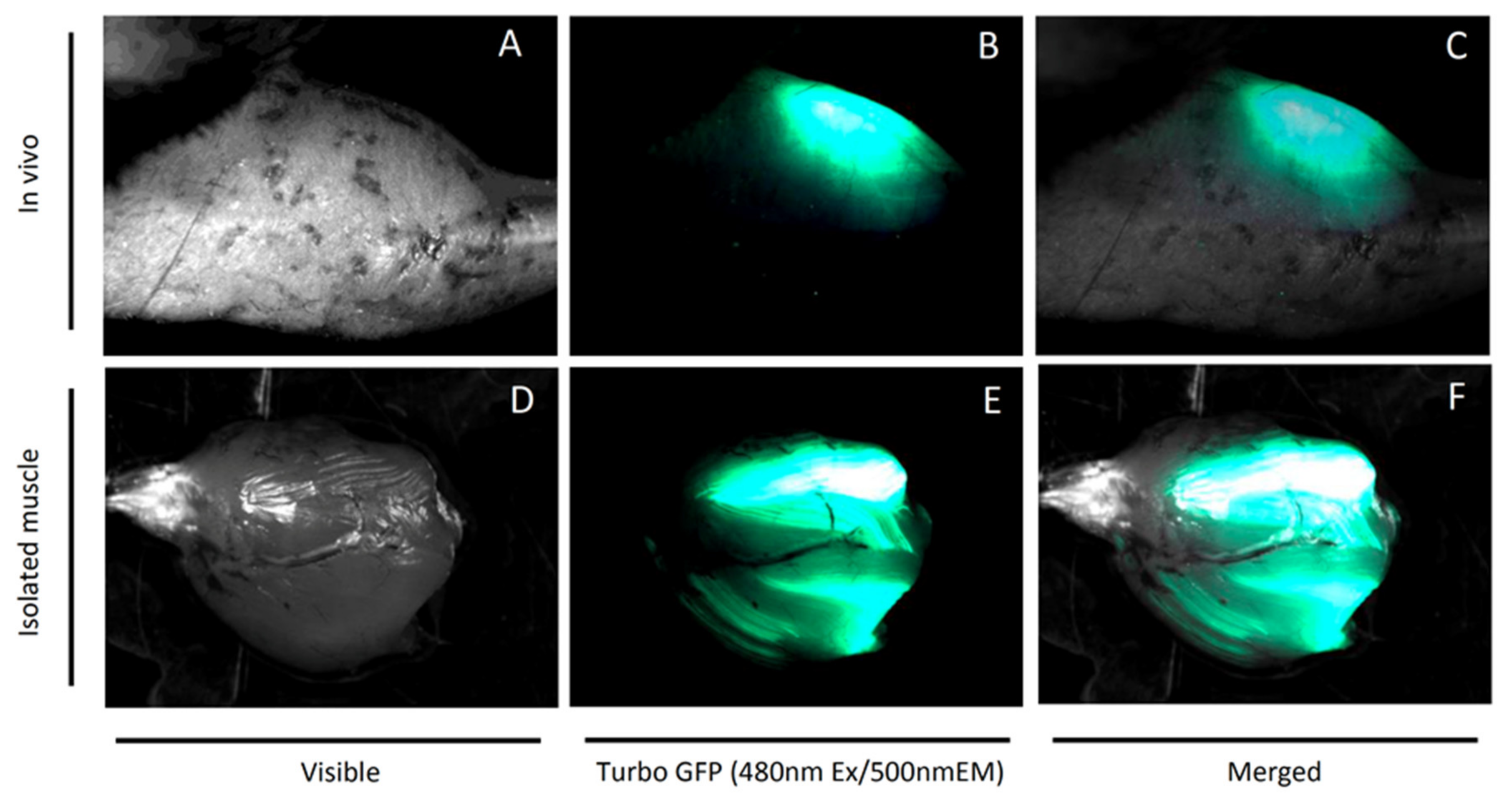
4.1. Plasmids and In Vivo Electroporation
4.2. Lipid Measurements
4.2.1. Plasma FFA
4.2.2. Sphingolipids
4.2.3. Diacylglycerols
4.2.4. Malonyl-CoA and Long-Chain Acyl-CoA
4.2.5. Acyl-carnitines
4.2.6. Triacylglycerols
4.3. Western Blotting
4.4. Real-Time PCR
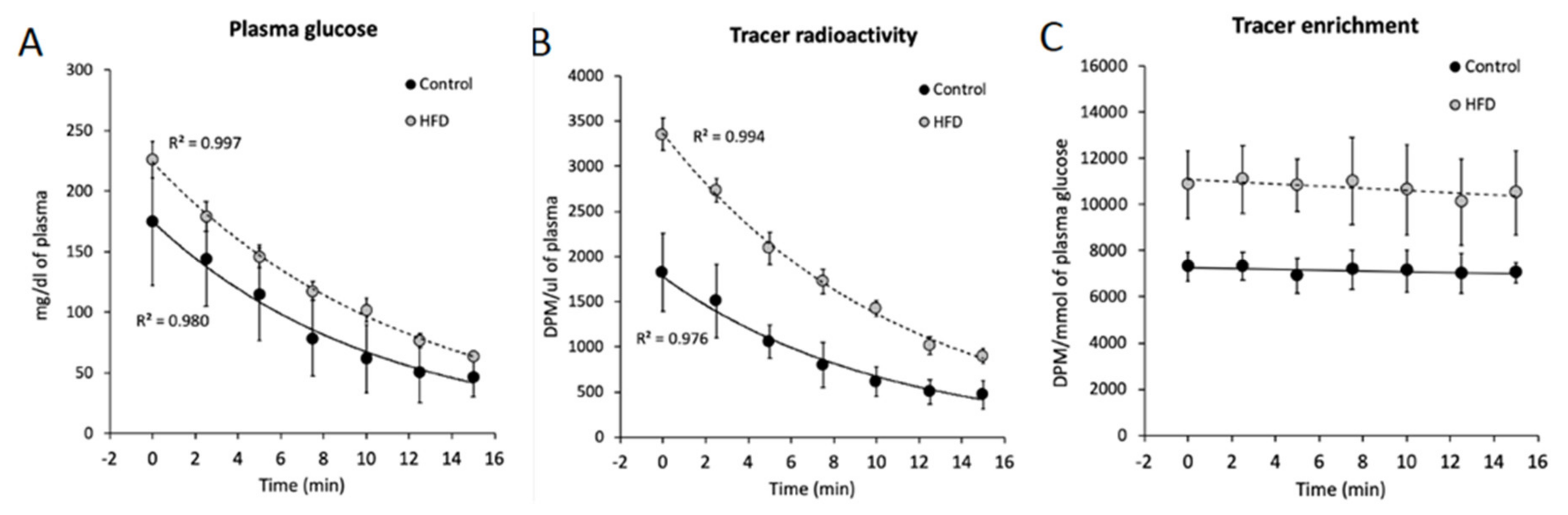
4.5. Insulin-Stimulated Glucose Uptake
4.6. Plasma Insulin and Glucose Concentration
4.7. Oral Glucose Tolerance Test (OGTT)
4.8. Insulin Tolerance Test (ITT)
4.9. HOMA-IR (Homeostatic Model Assessment of Insulin Resistance)
4.10. Protein Concentration
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HFD | High-fat diet |
| GPAT | Glycerol-3-phosphate acyltransferase |
| DAG | Diacylglycerols |
| T2D | Type 2 diabetes |
| FFA | Free fatty acids |
| FA | Fatty acids |
| FAT/CD36 | Fatty acid translocase |
| FABPpm | Fatty acid binding protein |
| FATP1–6 | Fatty acid transport protein family |
| ACS | Acyl-Co synthase |
| LCA-CoA | Long chain acyl-CoA |
| TAG | Triacylglycerols |
| Cer | Ceramides |
| CPT1 | Carnitine palmitoyltransferase 1 |
| IMCL | Intramyocellular lipids |
| PKC | Protein kinase C |
| Akt/PKB | Protein kinase B |
| PPA2 | Phosphatase A2 |
| LC/MS | Liquid chromatography mass spectrometry |
| HOMA-IR | Homeostatic model assessment |
References
- Belfort, R.; Mandarino, L.; Kashyap, S.; Wirfel, K.; Pratipanawatr, T.; Berria, R.; Defronzo, R.A.; Cusi, K. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 2005, 54, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Hady, H.R.; Markowski, A.R.; Kurianiuk, A.; Karwowska, A.; Górski, J.; Zabielski, P. Inhibition of Ceramide De Novo Synthesis Affects Adipocytokine Secretion and Improves Systemic and Adipose Tissue Insulin Sensitivity. Int. J. Mol. Sci. 2018, 19, 3995. [Google Scholar] [CrossRef]
- Grycel, S.; Markowski, A.R.; Hady, H.R.; Zabielski, P.; Kojta, I.; Imierska, M.; Górski, J.; Blachnio-Zabielska, A.U. Metformin treatment affects adipocytokine secretion and lipid composition in adipose tissues of diet-induced insulin-resistant rats. Nutrition 2019, 63-64, 126–133. [Google Scholar] [CrossRef]
- Zabielski, P.; Chacinska, M.; Charkiewicz, K.; Baranowski, M.; Gorski, J.; Blachnio-Zabielska, A.U. Effect of metformin on bioactive lipid metabolism in insulin-resistant muscle. J. Endocrinol. 2017, 233, 329–340. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef]
- Zabielski, P.; Daniluk, J.; Hady, H.R.; Markowski, A.R.; Imierska, M.; Górski, J.; Blachnio-Zabielska, A.U. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J. Cell Physiol. 2019, 234, 1851–1861. [Google Scholar] [CrossRef]
- Roepstorff, C.; Helge, J.W.; Vistisen, B.; Kiens, B. Studies of plasma membrane fatty acid-binding protein and other lipid-binding proteins in human skeletal muscle. Proc. Nutr. Soc. 2004, 63, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, J.A.; Veerkamp, J.H.; Turcotte, L.P.; Kelley, D.E. Markers of capacity to utilize fatty acids in human skeletal muscle: Relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999, 13, 2051–2060. [Google Scholar] [CrossRef]
- Bruce, C.R.; Anderson, M.J.; Carey, A.L.; Newman, D.G.; Bonen, A.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J. Clin. Endocrinol. Metab. 2003, 88, 5444–5451. [Google Scholar] [CrossRef]
- Abumrad, N.; Harmon, C.; Ibrahimi, A. Membrane transport of long-chain fatty acids: Evidence for a facilitated process. J. Lipid Res. 1998, 39, 2309–2318. [Google Scholar] [PubMed]
- Schwieterman, W.; Sorrentino, D.; Potter, B.J.; Rand, J.; Kiang, C.L.; Stump, D.; Berk, P.D. Uptake of oleate by isolated rat adipocytes is mediated by a 40-kDa plasma membrane fatty acid binding protein closely related to that in liver and gut. Proc. Natl. Acad. Sci. USA 1988, 85, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 2014, 34, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef]
- Dohm, G.L.; Tapscott, E.B.; Pories, W.J.; Dabbs, D.J.; Flickinger, E.G.; Meelheim, D.; Fushiki, T.; Atkinson, S.M.; Elton, C.W.; Caro, J.F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J. Clin. Investig. 1988, 82, 486–494. [Google Scholar] [CrossRef]
- Hulver, M.W.; Berggren, J.R.; Cortright, R.N.; Dudek, R.W.; Thompson, R.P.; Pories, W.J.; MacDonald, K.G.; Cline, G.W.; Shulman, G.I.; Dohm, G.L.; et al. Skeletal muscle lipid metabolism with obesity. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E741–E747. [Google Scholar] [CrossRef]
- Nishizuka, Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995, 9, 484–496. [Google Scholar] [CrossRef]
- Turinsky, J.; O’Sullivan, D.M.; Bayly, B.P. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J. Biol. Chem. 1990, 265, 16880–16885. [Google Scholar]
- Adams, J.M., 2nd; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef]
- Timmers, S.; Schrauwen, P.; de Vogel, J. Muscular diacylglycerol metabolism and insulin resistance. Physiol. Behav. 2008, 94, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Browne, C.L.; Oakes, N.D.; Watkinson, A.; Chisholm, D.J.; Kraegen, E.W.; Biden, T.J. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 1997, 46, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Oakes, N.D.; Kennedy, C.J.; Jenkins, A.B.; Laybutt, D.R.; Chisholm, D.J.; Kraegen, E.W. A new antidiabetic agent, BRL 49653, reduces lipid availability and improves insulin action and glucoregulation in the rat. Diabetes 1994, 43, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Muller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Kappe, C.; Zhang, Q.; Nyström, T.; Sjöholm, A. Effects of high-fat diet and the anti-diabetic drug metformin on circulating GLP-1 and the relative number of intestinal L-cells. Diabetol. Metab. Syndr. 2014, 6, 70. [Google Scholar] [CrossRef]
- Guitart, M.; Osorio-Conles, O.; Pentinat, T.; Cebrià, J.; García-Villoria, J.; Sala, D.; Sebastián, D.; Zorzano, A.; Ribes, A.; Jiménez-Chillarón, J.C.; et al. Fatty acid transport protein 1 (FATP1) localizes in mitochondria in mouse skeletal muscle and regulates lipid and ketone body disposal. PLoS ONE 2014, 9, e98109. [Google Scholar] [CrossRef]
- Yun, H.Y.; Lee, T.; Jeong, Y. High-Fat Diet Increases Fat Oxidation and Promotes Skeletal Muscle Fatty Acid Transporter Expression in Exercise-Trained Mice. J. Med. Food 2020, 23, 281–288. [Google Scholar] [CrossRef]
- Chabowski, A.; Chatham, J.C.; Tandon, N.N.; Calles-Escandon, J.; Glatz, J.F.; Luiken, J.J.; Bonen, A. Fatty acid transport and FAT/CD36 are increased in red but not in white skeletal muscle of ZDF rats. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E675–E682. [Google Scholar] [CrossRef]
- Sampson, S.R.; Cooper, D.R. Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol. Genet. Metab. 2006, 89, 32–47. [Google Scholar] [CrossRef]
- Turban, S.; Hajduch, E. Protein kinase C isoforms: Mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett. 2011, 585, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.A.; Li, L.O.; Li, Y.; Cline, G.W.; Shulman, G.I.; Coleman, R.A. Glycerol-3-phosphate acyltransferase 1 deficiency in ob/ob mice diminishes hepatic steatosis but does not protect against insulin resistance or obesity. Diabetes 2010, 59, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Baranowski, M.; Nikolajuk, A.; Otziomek, E.; Zabielski, P.; Adamska, A.; Blachnio, A.; Gorski, J.; Gorska, M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 2007, 50, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, E.; Błachnio-Zabielska, A.U. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2776. [Google Scholar] [CrossRef] [PubMed]
- Persson, X.M.; Blachnio-Zabielska, A.U.; Jensen, M.D. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J. Lipid Res. 2010, 51, 2761–2765. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Persson, X.M.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. 2012, 26, 1134–1140. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Zabielski, P.; Jensen, M.D. Intramyocellular diacylglycerol concentrations and [U-13C]palmitate isotopic enrichment measured by LC/MS/MS. J. Lipid Res. 2013, 54, 1705–1711. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Jensen, M.D. Measuring long-chain acyl-coenzyme A concentrations and enrichment using liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Rapid Commun. Mass Spectrom. 2011, 25, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Minkler, P.E.; Kerner, J.; Ingalls, S.T.; Hoppel, C.L. Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue. Anal. Biochem. 2008, 376, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Ecker, J.; Haag, A.; Spanier, B.; Daniel, H. An LC-MS/MS method to quantify acylcarnitine species including isomeric and odd-numbered forms in plasma and tissues. J. Lipid Res. 2015, 56, 2029–2039. [Google Scholar] [CrossRef]
- Fueger, P.T.; Shearer, J.; Bracy, D.P.; Posey, K.A.; Pencek, R.R.; McGuinness, O.P.; Wasserman, D.H. Control of muscle glucose uptake: Test of the rate-limiting step paradigm in conscious, unrestrained mice. J. Physiol. 2005, 562, 925–935. [Google Scholar] [CrossRef]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
| Control | HFD | |
|---|---|---|
| Glucose (mg/dL) | 109 ± 14 | 184 ± 22 a |
| Insulin (μU/mL) | 25.1 ± 2.9 | 41.3 ± 2.2 a |
| OGTT AUC (×103) | 2.32 ± 0.12 | 4.27 ± 0.21 a |
| ITT AUC (×103) | 0.78 ± 0.04 | 1.86 ± 0.12 a |
| HOMA-IR | 1.12 ± 0.09 | 3.14 ± 0.45 a |
| 16:0/16:0 | 16:0/18:0 | 16:0/18:1 | 16:0/18:2 | 18:0/18:0 | 18:0/18:1 | 18:0/18:2 | 18:1/18:1 | 18:2/18:2 | 18:0/20:0 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 13.41 ± 1.87 | 83.47 ± 11.87 | 32.18 ± 4.97 | 38.09 ± 5.91 | 2.10 ± 0.23 | 21.88 ± 3.11 | 0.96 ± 0.19 | 20.98 ± 2.31 | 24.91 ± 2.63 | 3.85 ± 0.58 |
| HFD(+GPAT) | 16.15 ± 2.47 a | 144.52 ± 16.79 a | 39.36 ± 4.67 a | 66.52 ± 11.60 a | 2.92 ± 0.23 a | 22.90 ± 3.65 | 1.98 ± 0.33 a | 23.37 ± 2.21 a | 34.00 ± 5.15 a | 7.23 ± 0.96 a |
| HFD(-GPAT) | 11.52 ± 1.11 b | 59.86 ± 10.31 a,b | 24.15 ± 1.21 a,b | 21.31 ± 3.68 a,b | 0.91 ± 0.11 a,b | 16.74 ± 2.42 a,b | 1.53 ± 0.13 a,b | 16.73 ± 1.25 a,b | 18.68 ± 1.72 a,b | 4.90 ± 0.50 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojta, I.; Zabielski, P.; Roszczyc-Owsiejczuk, K.; Imierska, M.; Sokołowska, E.; Błachnio-Zabielska, A. GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 7369. https://doi.org/10.3390/ijms21197369
Kojta I, Zabielski P, Roszczyc-Owsiejczuk K, Imierska M, Sokołowska E, Błachnio-Zabielska A. GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance. International Journal of Molecular Sciences. 2020; 21(19):7369. https://doi.org/10.3390/ijms21197369
Chicago/Turabian StyleKojta, Iwona, Piotr Zabielski, Kamila Roszczyc-Owsiejczuk, Monika Imierska, Emilia Sokołowska, and Agnieszka Błachnio-Zabielska. 2020. "GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance" International Journal of Molecular Sciences 21, no. 19: 7369. https://doi.org/10.3390/ijms21197369
APA StyleKojta, I., Zabielski, P., Roszczyc-Owsiejczuk, K., Imierska, M., Sokołowska, E., & Błachnio-Zabielska, A. (2020). GPAT Gene Silencing in Muscle Reduces Diacylglycerols Content and Improves Insulin Action in Diet-Induced Insulin Resistance. International Journal of Molecular Sciences, 21(19), 7369. https://doi.org/10.3390/ijms21197369






