Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites
Abstract
1. Introduction
2. Results
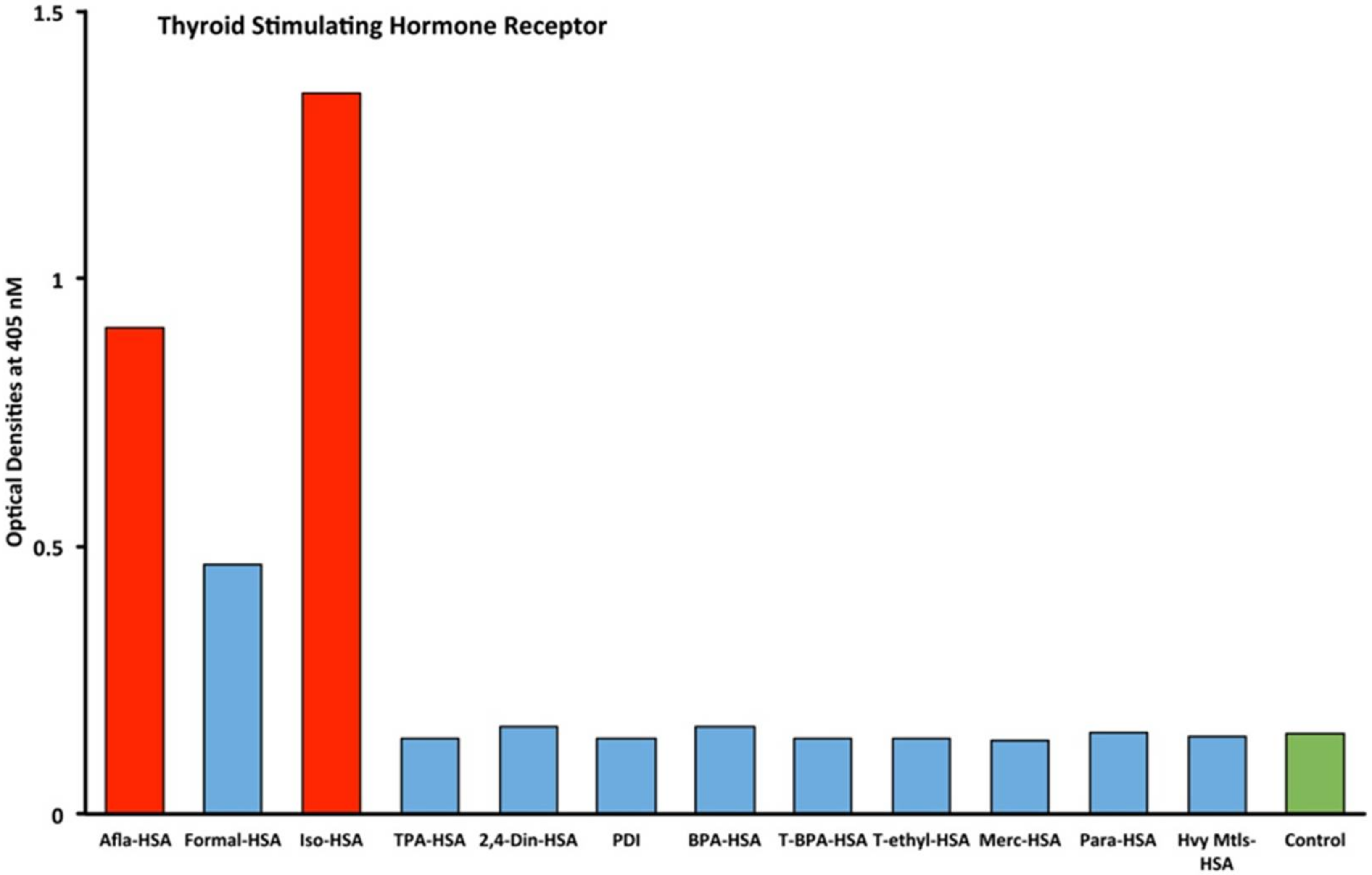
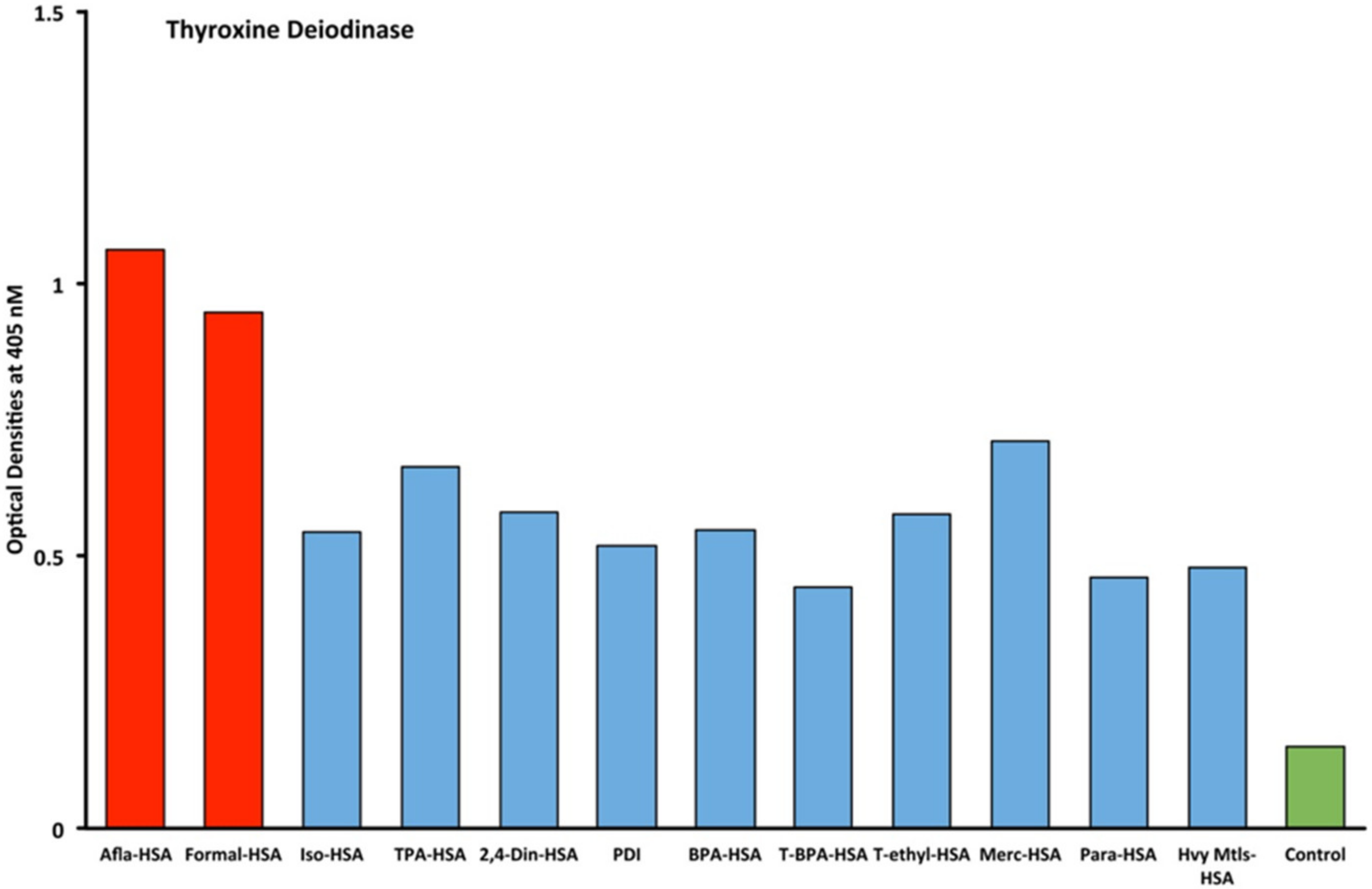
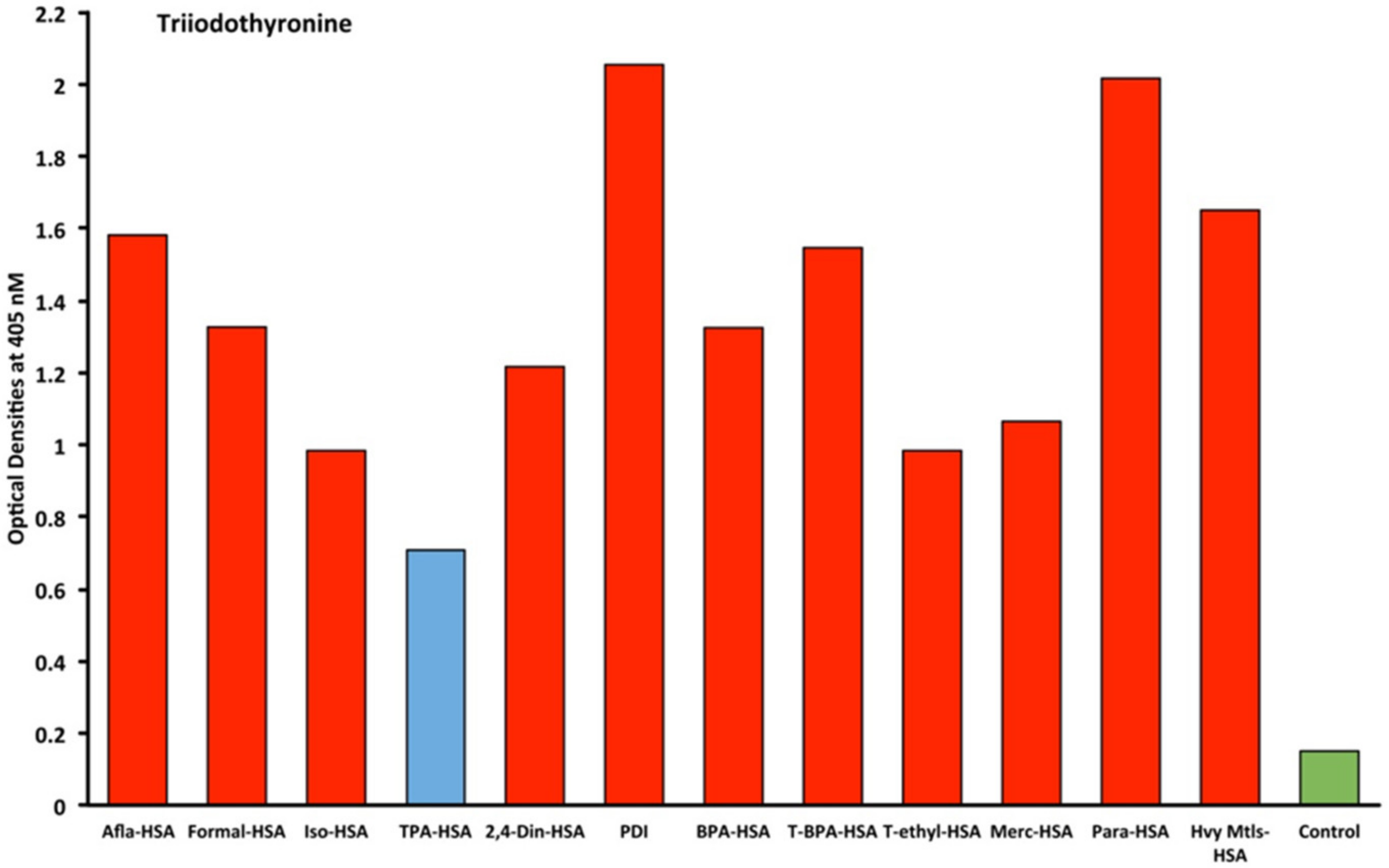
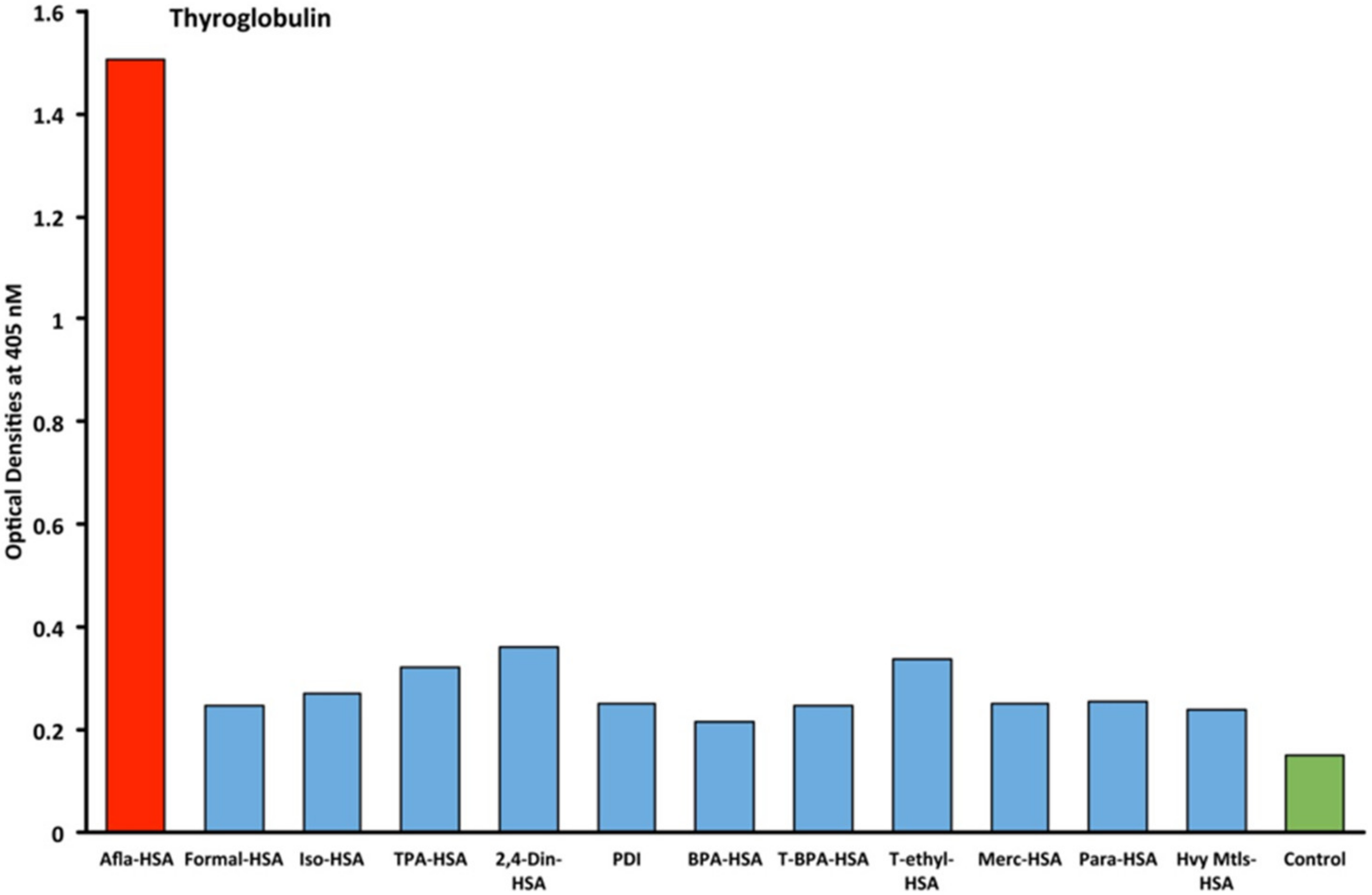
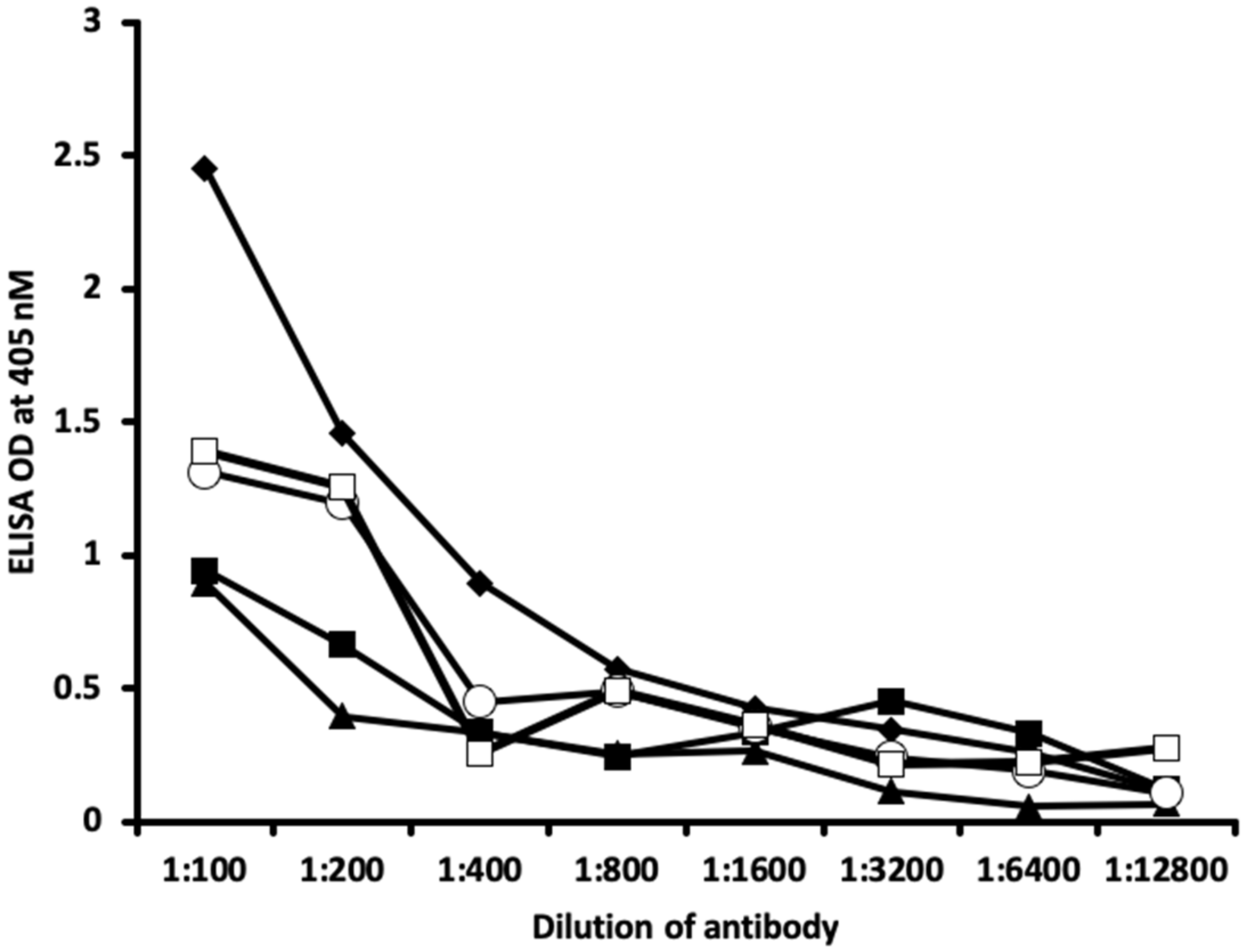
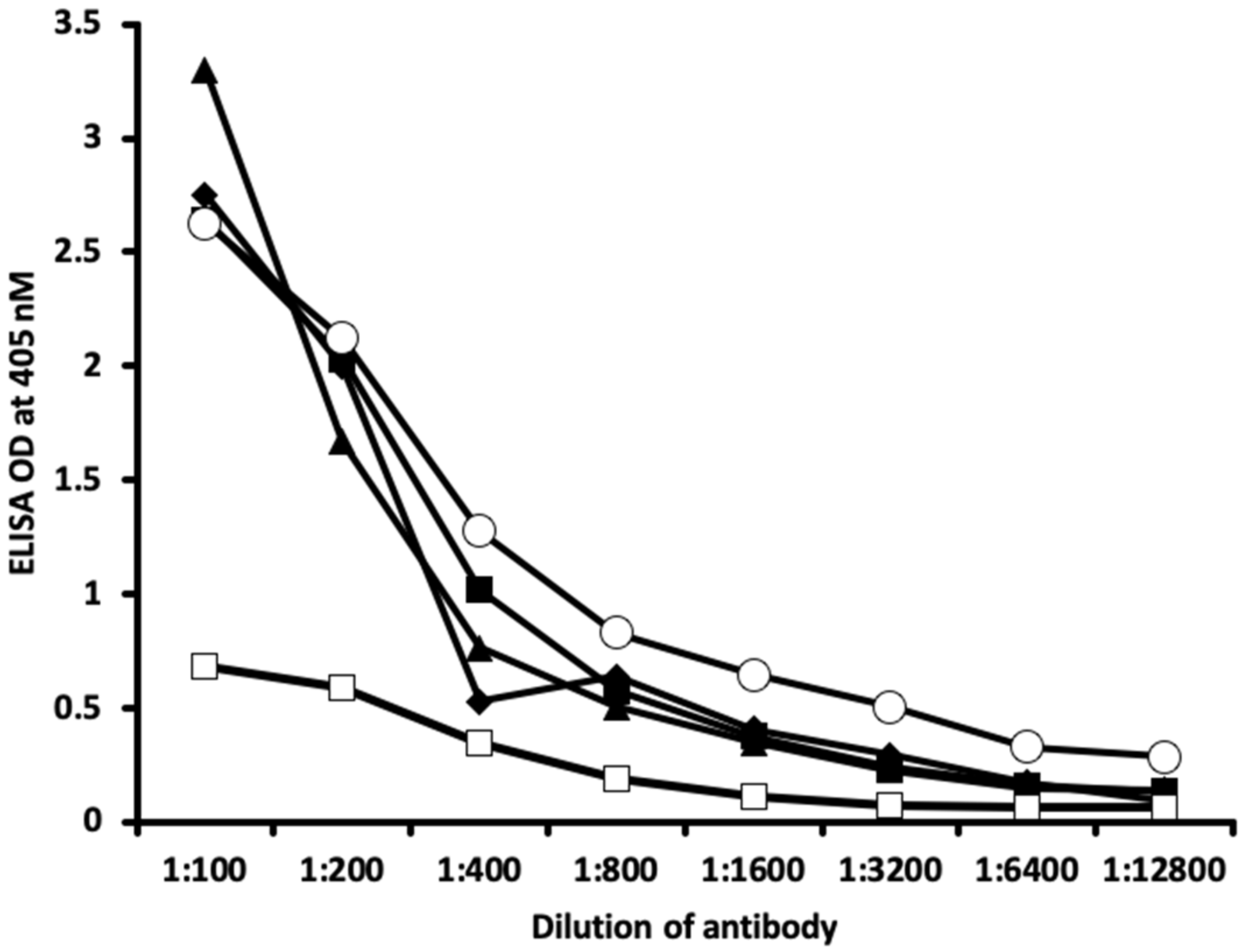
2.1. Results of ELISA
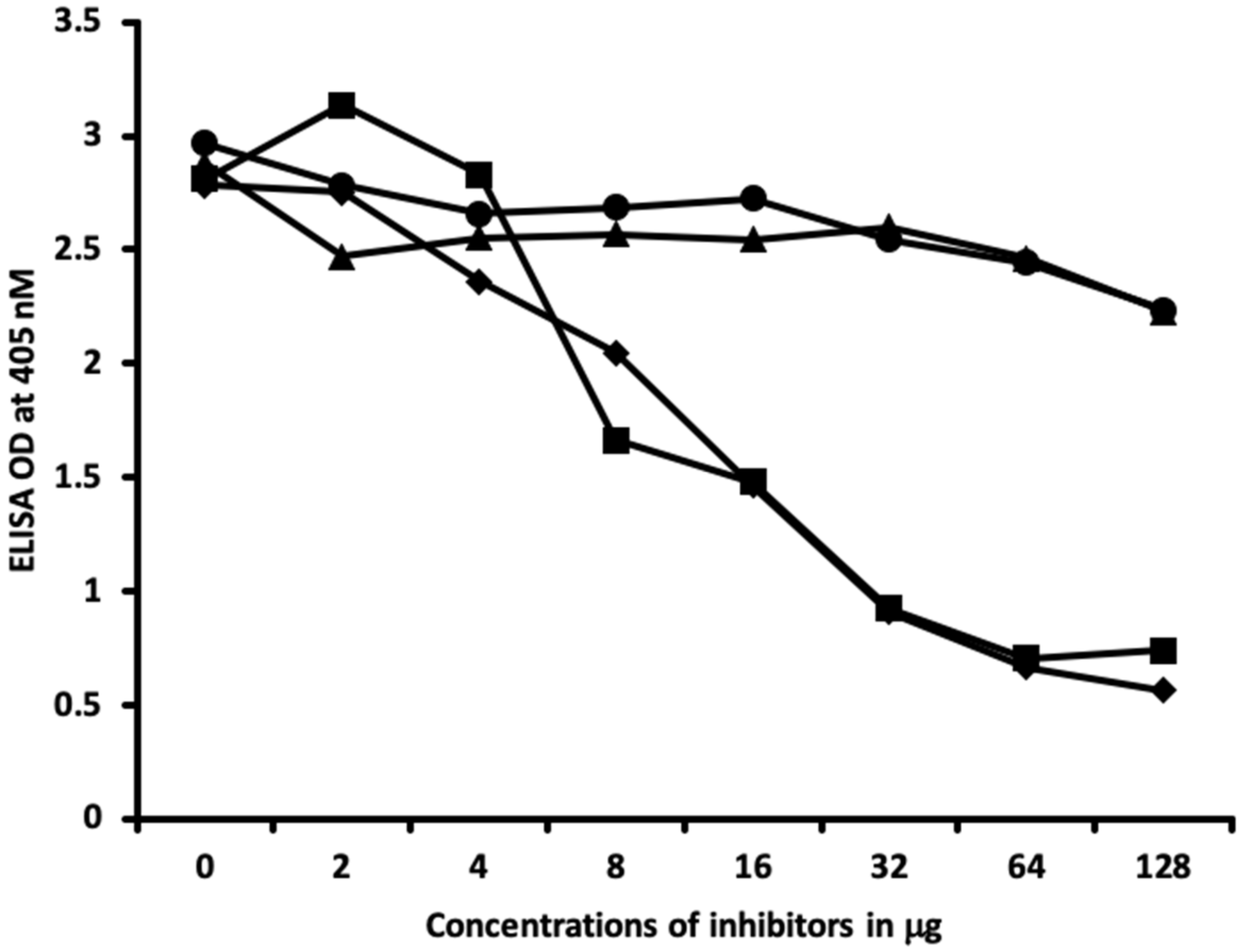
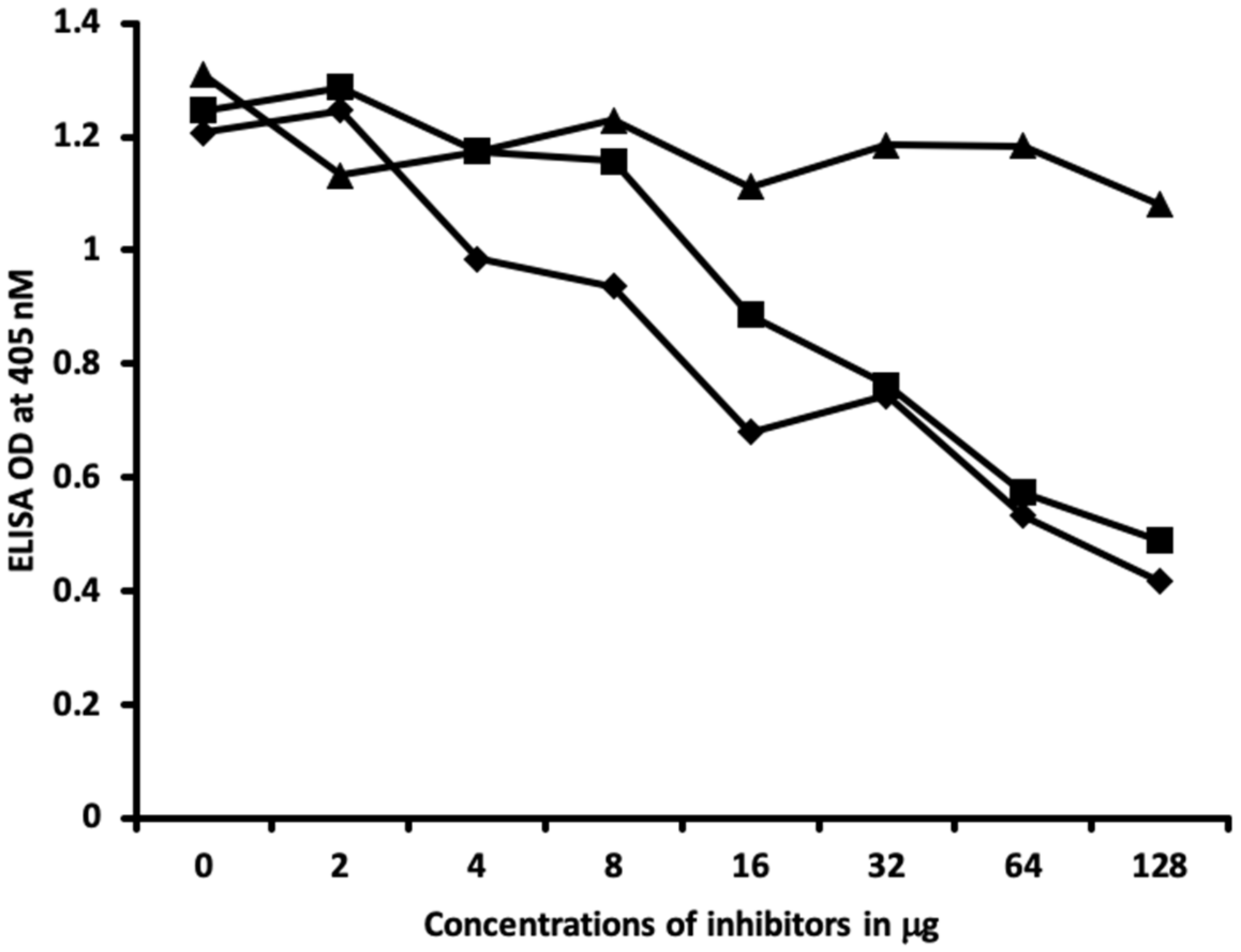
2.2. Demonstration of Analytical Specificity of Thyroid Antibodies Binding to Different Haptenic Chemicals to HSA
3. Discussion
4. Materials and Methods
4.1. Polyclonal and Monoclonal Antibodies
4.2. Proteins and Chemicals
4.3. Preparation of Chemicals Bound to HSA
4.4. Demonstration of Anti-Thyroid Antibody Binding to Various Chemicals Bound to HSA Using ELISA
4.5. Binding of Serially Diluted Anti-Thyroid Antibodies with Different Haptenic Chemicals Bound to HSA
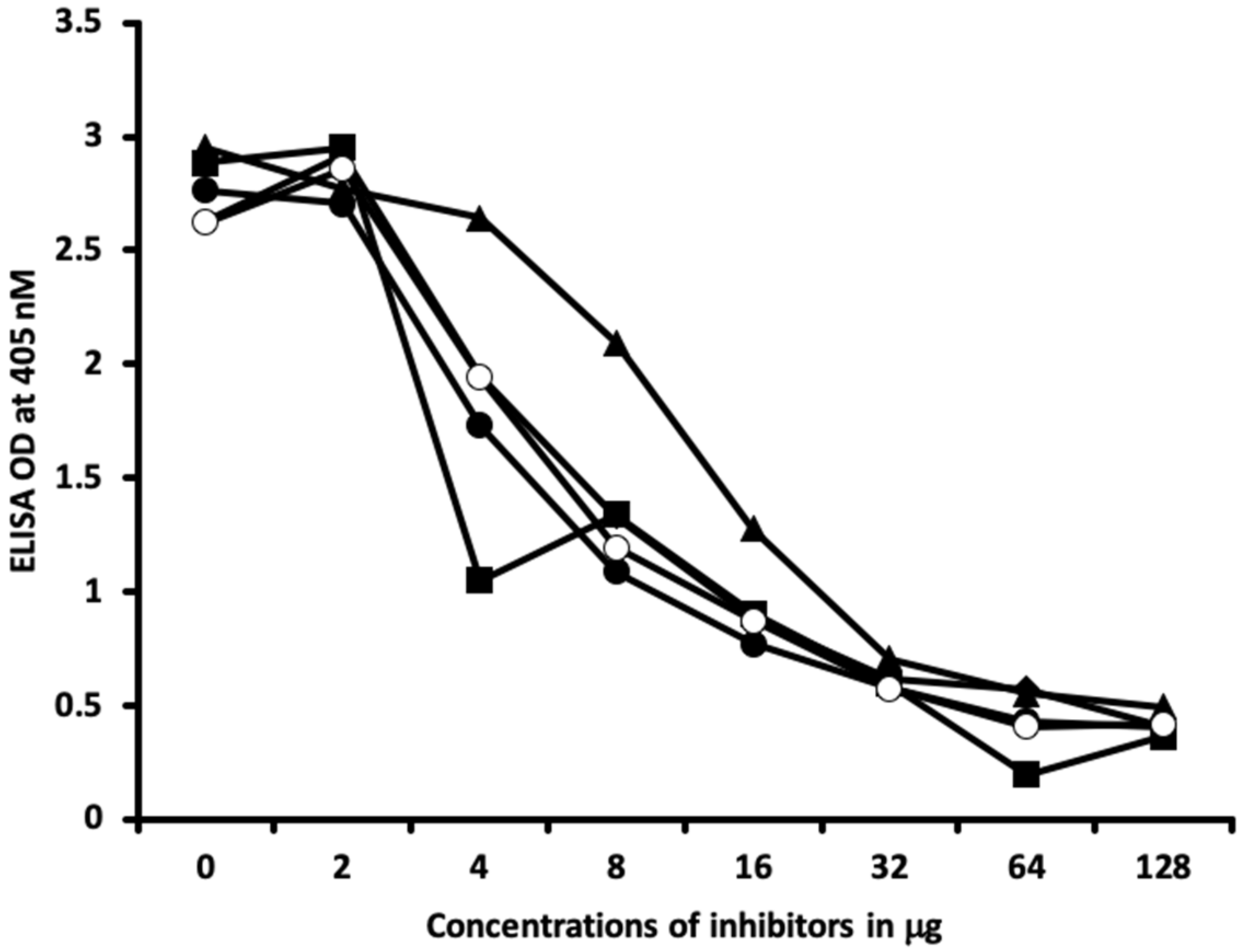
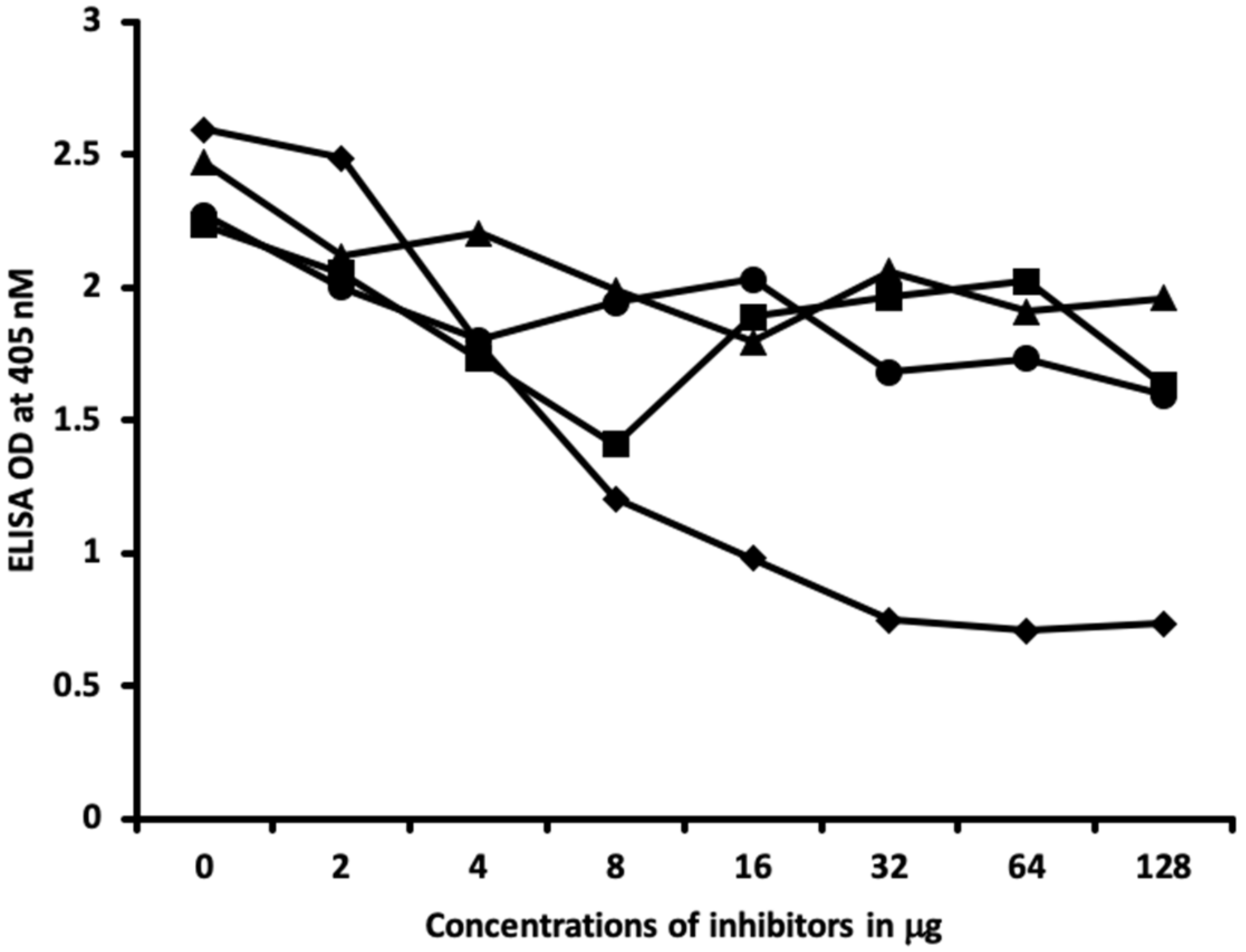
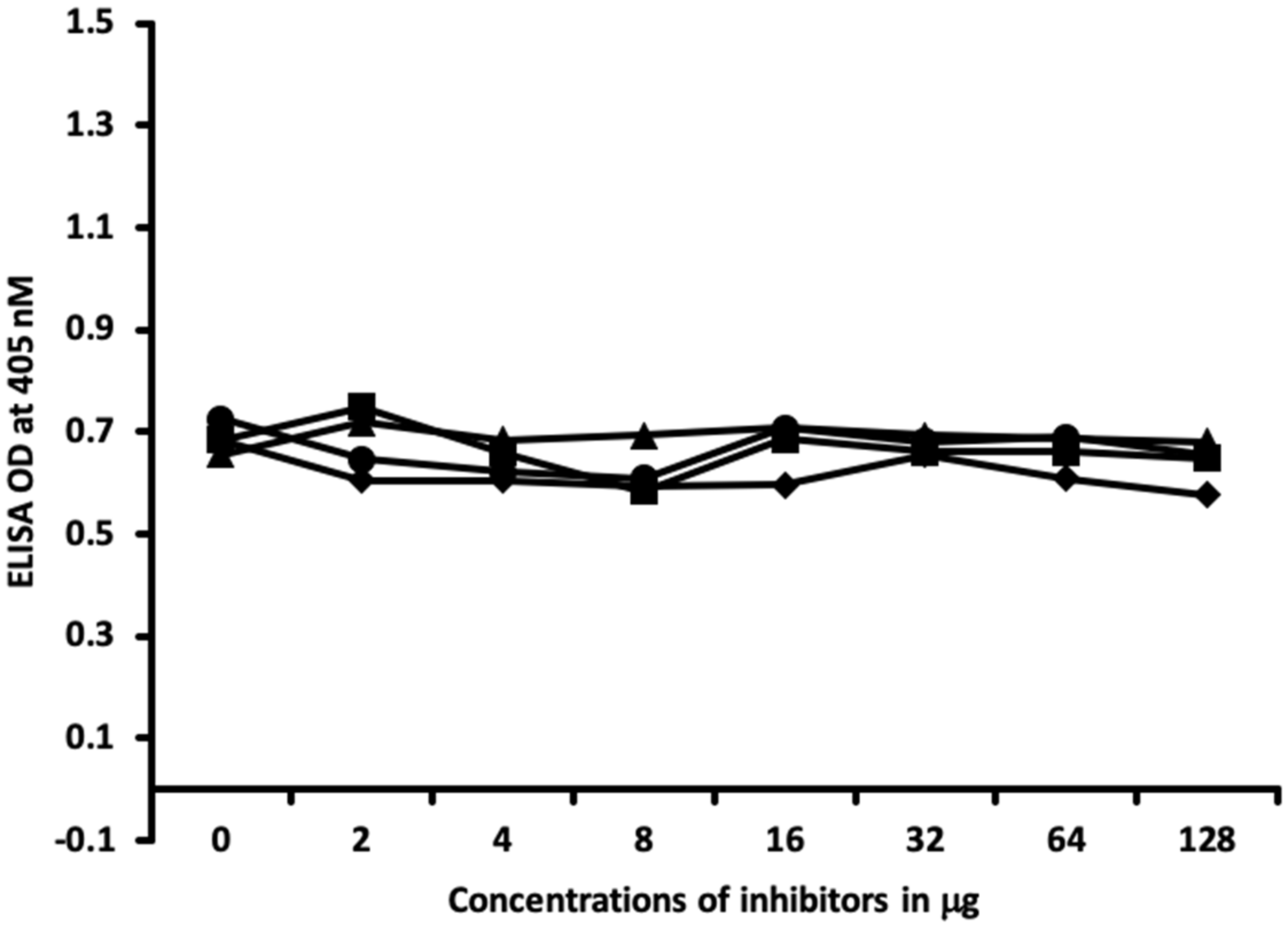
4.6. Inhibition of Anti-Thyroid Antibodies Binding to Different Haptenic Chemicals Bound to HSA-Coated Plates with the Same Haptenic Chemicals in Liquid Phase
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeki, Y.; Ishihara, K. Infection-immunity liaison: Pathogen-driven autoimmune-mimicry (PDAIM). Autoimmun. Rev. 2014, 13, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Fairweather, D. Complexities in the relationship between infection and autoimmunity. Curr. Allergy Asthma Rep. 2014, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M. Molecular mimicry: Can epitope mimicry induce autoimmune disease? Immunol. Cell Biol. 1997, 75, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Houliston, R.S.; Yuki, N.; Hirama, T.; Khieu, N.H.; Brisson, J.R.; Gilbert, M.; Jarrell, H.C. Recognition characteristics of monoclonal antibodies that are cross-reactive with gangliosides and lipooligosaccharide from Campylobacter jejuni strains associated with Guillain-Barre and Fisher syndromes. Biochemistry 2007, 46, 36–44. [Google Scholar] [CrossRef]
- Hargreaves, C.E.; Grasso, M.; Hampe, C.S.; Stenkova, A.; Atkinson, S.; Joshua, G.W.; Wren, B.W.; Buckle, A.M.; Dunn-Walters, D.; Banga, J.P. Yersinia enterocolitica provides the link between thyroid-stimulating antibodies and their germline counterparts in Graves’ disease. J. Immunol. 2013, 190, 5373–5381. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Lu, J.; Jiang, F.; Zhang, H.; Gao, L.; Zhao, J. Identification of outer membrane porin f protein of Yersinia enterocolitica recognized by antithyrotopin receptor antibodies in Graves’ disease and determination of its epitope using mass spectrometry and bioinformatics tools. J. Clin. Endocrinol. Metab. 2010, 95, 4012–4020. [Google Scholar] [CrossRef]
- Tozzoli, R.; Barzilai, O.; Ram, M.; Villalta, D.; Bizzaro, N.; Sherer, Y.; Shoenfeld, Y. Infections and autoimmune thyroid diseases: Parallel detection of antibodies against pathogens with proteomic technology. Autoimmun. Rev. 2008, 8, 112–115. [Google Scholar] [CrossRef]
- Soveid, M.; Asl, K.H.; Omrani, G.R. Infection by CagA positive strains of Helicobacter pylori is associated with autoimmune thyroid disease in Iranian patients. Iran J. Immunol. 2012, 9, 48–52. [Google Scholar]
- Figura, N.; di Cairano, G.; Lore, F.; Guarino, E.; Gragnoli, A.; Cataldo, D.; Giannace, R.; Vaira, D.; Bianciardi, L.; Kristodhullu, S.; et al. The infection by Helicobacter pylori strains expressing CagA is highly prevalent in women with autoimmune thyroid disorders. J. Physiol. Pharmacol. 1999, 50, 817–826. [Google Scholar]
- Vojdani, A.; Rahimian, P.; Kalhor, H.; Mordechai, E. Immunological cross reactivity between Candida albicans and human tissue. J. Clin. Lab. Immunol. 1996, 48, 1–15. [Google Scholar]
- Gregoric, E.; Gregoric, J.A.; Guarneri, F.; Benvenga, S. Injections of Clostridium botulinum neurotoxin A may cause thyroid complications in predisposed persons based on molecular mimicry with thyroid autoantigens. Endocrine 2011, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.H.; Donadi, E.A.; Tambascia, M.A.; Magna, L.A.; Prigenzi, L.S. Relationship between HLA antigens and infectious agents in contributing towards the development of Graves’ disease. Immunol. Investig. 1998, 27, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Guarneri, F.; Vaccaro, M.; Santarpia, L.; Trimarchi, F. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid 2004, 14, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Rizzo, M.; Citarrella, R.; Rappa, F.; Campanella, C.; Bucchieri, F.; Patti, A.; Nikolic, D.; Cabibi, D.; Amico, G.; et al. Elevated blood Hsp60, its structural similarities and cross-reactivity with thyroid molecules, and its presence on the plasma membrane of oncocytes point to the chaperonin as an immunopathogenic factor in hashimoto’s thyroiditis. Cell Stress Chaperones 2014, 19, 343–353. [Google Scholar] [CrossRef]
- Lane, D.; Koprowski, H. Molecular recognition and the future of monoclonal antibodies. Nature 1982, 296, 200–201. [Google Scholar] [CrossRef]
- Kubicka-Muranyi, M.; Goebels, R.; Goebel, C.; Uetrecht, J.; Gleichmann, E. T lymphocytes ignore procainamide, but respond to its reactive metabolites in peritoneal cells: Demonstration by the adoptive transfer popliteal lymph node assay. Toxicol. Appl. Pharmacol. 1993, 122, 88–94. [Google Scholar] [CrossRef]
- Novotny, J.; Bruccoleri, R.; Newell, J.; Murphy, D.; Haber, E.; Karplus, M. Molecular anatomy of the antibody binding site. J. Biol. Chem. 1983, 258, 14433–14437. [Google Scholar]
- Feldmann, M. Induction of immunity and tolerance in vitro by hapten protein conjugates. 3. Hapten inhibition studies of antigen binding to B cells in immunity and tolerance. J. Exp. Med. 1972, 136, 532–545. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D.; Mukherjee, P.S. Elevated levels of antibodies against xenobiotics in a subgroup of healthy subjects. J. Appl. Toxicol. 2015, 35, 383–397. [Google Scholar] [CrossRef]
- Kharrazian, D.; Vojdani, A. Correlation between antibodies to bisphenol A, its target enzyme protein disulfide isomerase and antibodies to neuron-specific antigens. J. Appl. Toxicol. 2017, 37, 479–484. [Google Scholar] [CrossRef]
- Kharrazian, D.; Herbert, M.; Vojdani, A. The Associations between Immunological Reactivity to the Haptenation of Unconjugated Bisphenol A to Albumin and Protein Disulfide Isomerase with Alpha-Synuclein Antibodies. Toxics 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Kharrazian, D.; Herbert, M.; Vojdani, A. Immunological Reactivity Using Monoclonal and Polyclonal Antibodies of Autoimmune Thyroid Target Sites with Dietary Proteins. J. Thyroid Res. 2017, 2017, 4354723. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Guarneri, F. Molecular mimicry and autoimmune thyroid disease. Rev. Endocr. Metab. Disord. 2016, 17, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid disrupting chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef]
- Chipinda, I.; Hettick, J.M.; Siegel, P.D. Haptenation: Chemical reactivity and protein binding. J. Allergy 2011, 2011, 839682. [Google Scholar] [CrossRef]
- Plan, L.C.; Zeiss, C.R.; Leach, C.L.; Hatoum, N.S.; Levitz, D.; Garvin, P.J.; Patterson, R. Antibody response to trimellityl hemoglobin in trimellitic anhydride-induced lung injury. J. Allergy Clin. Immunol. 1988, 82, 1098–1103. [Google Scholar] [CrossRef]
- Pezzini, A.; Riviera, A.; Paggiaro, P.; Spiazzi, A.; Gerosa, F.; Filieri, M.; Toma, G.; Tridente, G. Specific IgE antibodies in twenty-eight workers with diisocyanate-induced bronchial asthma. Clin. Allergy 1984, 14, 453–461. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Verdoliva, A.; Ruvo, M.; Villain, M.; Cassani, G.; Fassina, G. Antigenicity of topochemically related peptides. Biochim. Biophys. Acta 1995, 1253, 57–62. [Google Scholar] [CrossRef]
- Marchalonis, J.J.; Adelman, M.K.; Robey, I.F.; Schluter, S.F.; Edmundson, A.B. Exquisite specificity and peptide epitope recognition promiscuity, properties shared by antibodies from sharks to humans. J. Mol. Recognit. 2001, 14, 110–121. [Google Scholar] [CrossRef]
- Hirano, T.; Kagechika, H. Thyromimetics: A review of recent reports and patents (2004–2009). Expert Opin. Ther. Pat. 2010, 20, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Cody, V. Molecular conformation of thyroid hormones: Structure and binding interactions of thyroxine. Endocr. Res. Commun. 1979, 6, 123–134. [Google Scholar] [CrossRef] [PubMed]
- McKinney, J.D.; Waller, C.L. Molecular determinants of hormone mimicry: Halogenated aromatic hydrocarbon environmental agents. J. Toxicol. Environ. Health B Crit. Rev. 1998, 1, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Kharrazian, D. The potential roles of bisphenol a (BPA) pathogenesis in autoimmunity. Autoimmune Dis. 2014, 2014, 743616. [Google Scholar] [CrossRef]
- Delom, F.; Mallet, B.; Carayon, P.; Lejeune, P.J. Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein. J. Biol. Chem. 2001, 276, 21337–21342. [Google Scholar] [CrossRef]
- Liu, X.W.; Sok, D.E. Multimerization of bovine thyroglobulin, partially unfolded or partially unfolded/reduced; involvement of protein disulfide isomerase and glutathionylated disulfide linkage. Arch. Pharm. Res. 2004, 27, 1275–1283. [Google Scholar] [CrossRef]
- Farwell, A.P.; Lynch, R.M.; Okulicz, W.C.; Comi, A.M.; Leonard, J.L. The actin cytoskeleton mediates the hormonally regulated translocation of type II iodothyronine 5′-deiodinase in astrocytes. J. Biol. Chem. 1990, 265, 18546–18553. [Google Scholar]
- Safran, M.; Leonard, J.L. Characterization of a N-bromoacetyl-L-thyroxine affinity-labeled 55-kilodalton protein as protein disulfide isomerase in cultured glial cells. Endocrinology 1991, 129, 2011–2016. [Google Scholar] [CrossRef][Green Version]
- Hiroi, T.; Okada, K.; Imaoka, S.; Osada, M.; Funae, Y. Bisphenol A binds to protein disulfide isomerase and inhibits its enzymatic and hormone-binding activities. Endocrinology 2006, 147, 2773–2780. [Google Scholar] [CrossRef][Green Version]
- Grasselli, E.; Cortese, K.; Fabbri, R.; Smerilli, A.; Vergani, L.; Voci, A.; Gallo, G.; Canesi, L. Thyromimetic actions of tetrabromobisphenol A (TBBPA) in steatotic FaO rat hepatoma cells. Chemosphere 2014, 112, 511–518. [Google Scholar] [CrossRef]
- den Besten, C.; Vet, J.J.; Besselink, H.T.; Kiel, G.S.; van Berkel, B.J.; Beems, R.; van Bladeren, P.J. The liver, kidney, and thyroid toxicity of chlorinated benzenes. Toxicol. Appl. Pharmacol. 1991, 111, 69–81. [Google Scholar] [CrossRef]
- Schreiber, V.; Pribyl, T.; Jahodova, J. Inhibition of thyroxine binding to adenohypophysial proteins in vitro by 2,4-dinitrophenol administered in vivo. Physiol. Bohemoslov. 1975, 24, 147–156. [Google Scholar] [PubMed]
- Chu, I.; Villeneuve, D.; Secours, V.; Valli, V.E. Comparative toxicity of 1,2,3,4-, 1,2,4,5-, and 1,2,3,5-tetrachlorobenzene in the rat: Results of acute and subacute studies. J. Toxicol. Environ. Health 1983, 11, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.J.; Danse, L.H.; van Leeuwen, F.X.; Loeber, J.G. Four-week toxicity study of phenyl isothiocyanate in rats. Food Chem. Toxicol. 1985, 23, 1015–1017. [Google Scholar] [CrossRef]
- Langer, P.; Greer, M.A. Antithyroid activity of some naturally occurring isothiocyanates in vitro. Metabolism 1968, 17, 596–605. [Google Scholar] [CrossRef]
- Schmutzler, C.; Gotthardt, I.; Hofmann, P.J.; Radovic, B.; Kovacs, G.; Stemmler, L.; Nobis, I.; Bacinski, A.; Mentrup, B.; Ambrugger, P.; et al. Endocrine disruptors and the thyroid gland—A combined in vitro and in vivo analysis of potential new biomarkers. Environ. Health Perspect. 2007, 115, 77–83. [Google Scholar] [CrossRef]
- Patel, K.G.; Bhatt, H.V.; Choudhury, A.R. Alteration in thyroid after formaldehyde (HCHO) treatment in rats. Ind. Health 2003, 41, 295–297. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharrazian, D.; Herbert, M.; Vojdani, A. Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites. Int. J. Mol. Sci. 2020, 21, 7324. https://doi.org/10.3390/ijms21197324
Kharrazian D, Herbert M, Vojdani A. Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites. International Journal of Molecular Sciences. 2020; 21(19):7324. https://doi.org/10.3390/ijms21197324
Chicago/Turabian StyleKharrazian, Datis, Martha Herbert, and Aristo Vojdani. 2020. "Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites" International Journal of Molecular Sciences 21, no. 19: 7324. https://doi.org/10.3390/ijms21197324
APA StyleKharrazian, D., Herbert, M., & Vojdani, A. (2020). Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites. International Journal of Molecular Sciences, 21(19), 7324. https://doi.org/10.3390/ijms21197324






