Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-Mediated Apoptosis
Abstract
1. Introduction
2. Results
2.1. Protective Effects of Simvastatin on the LPS-Induced Acute Renal Injury
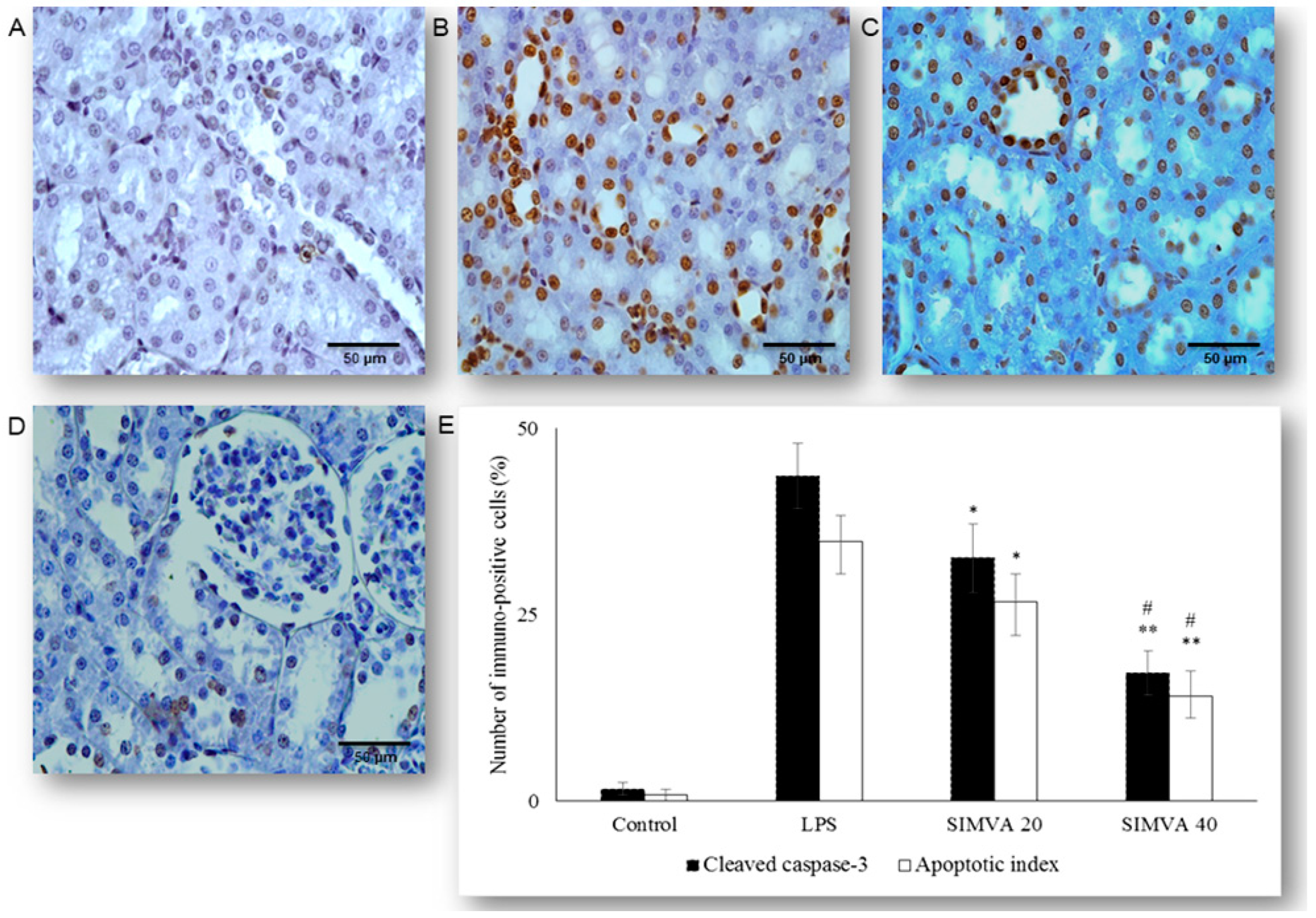
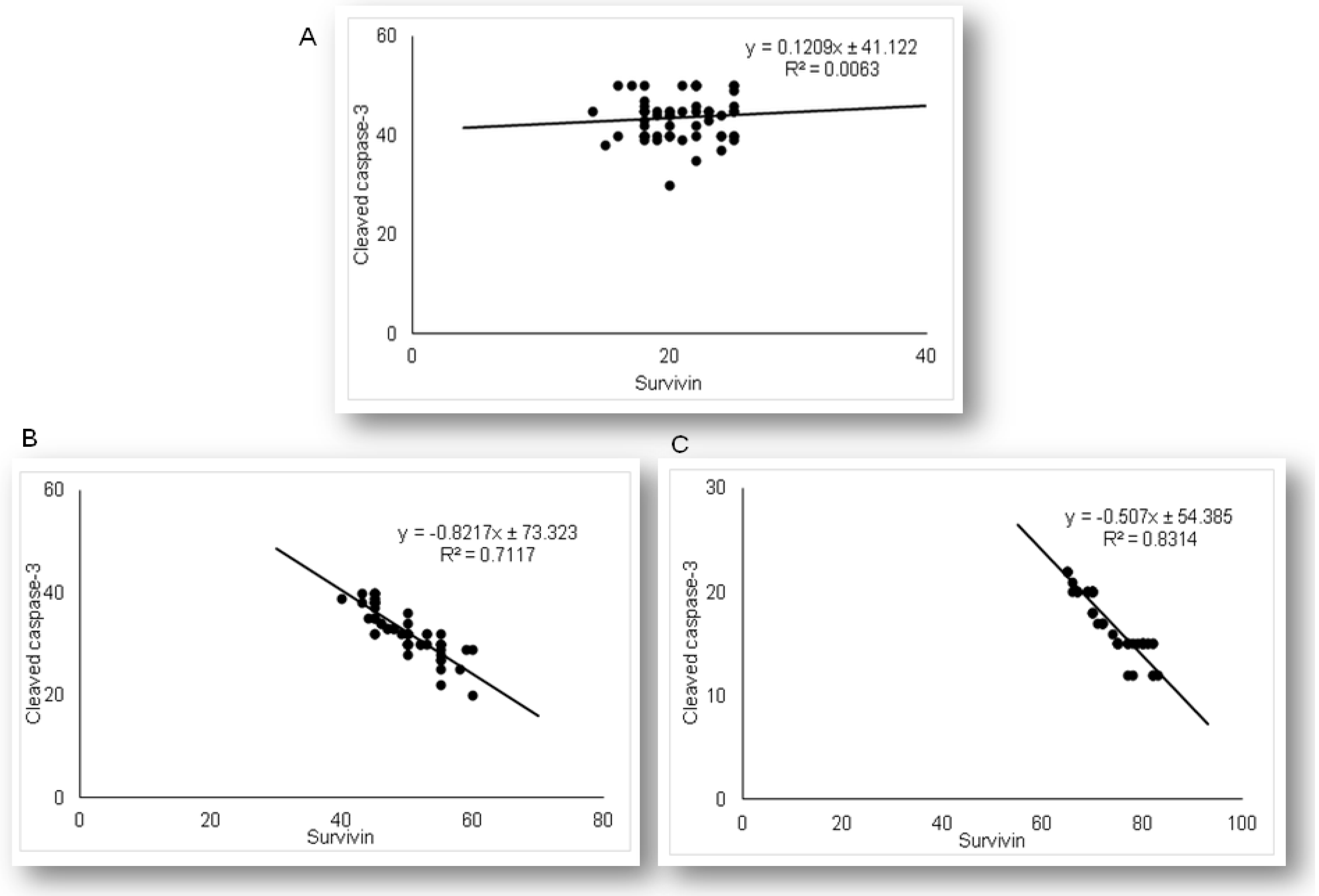
2.2. Simvastatin Inhibited Cleaved Caspase-3 Expression and Apoptotic Cell Death of Renal Tubular Epithelial Cells Induced by LPS
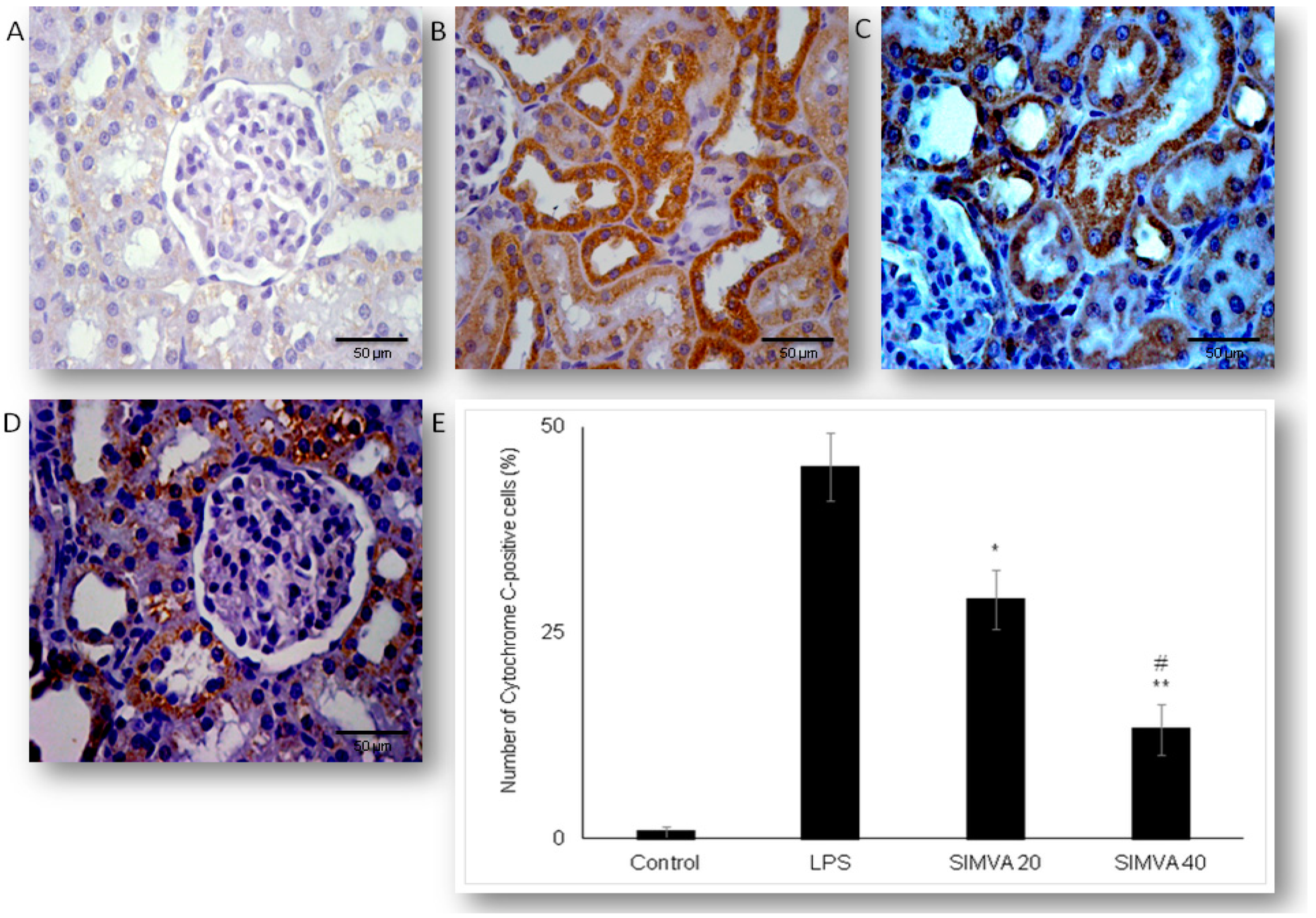
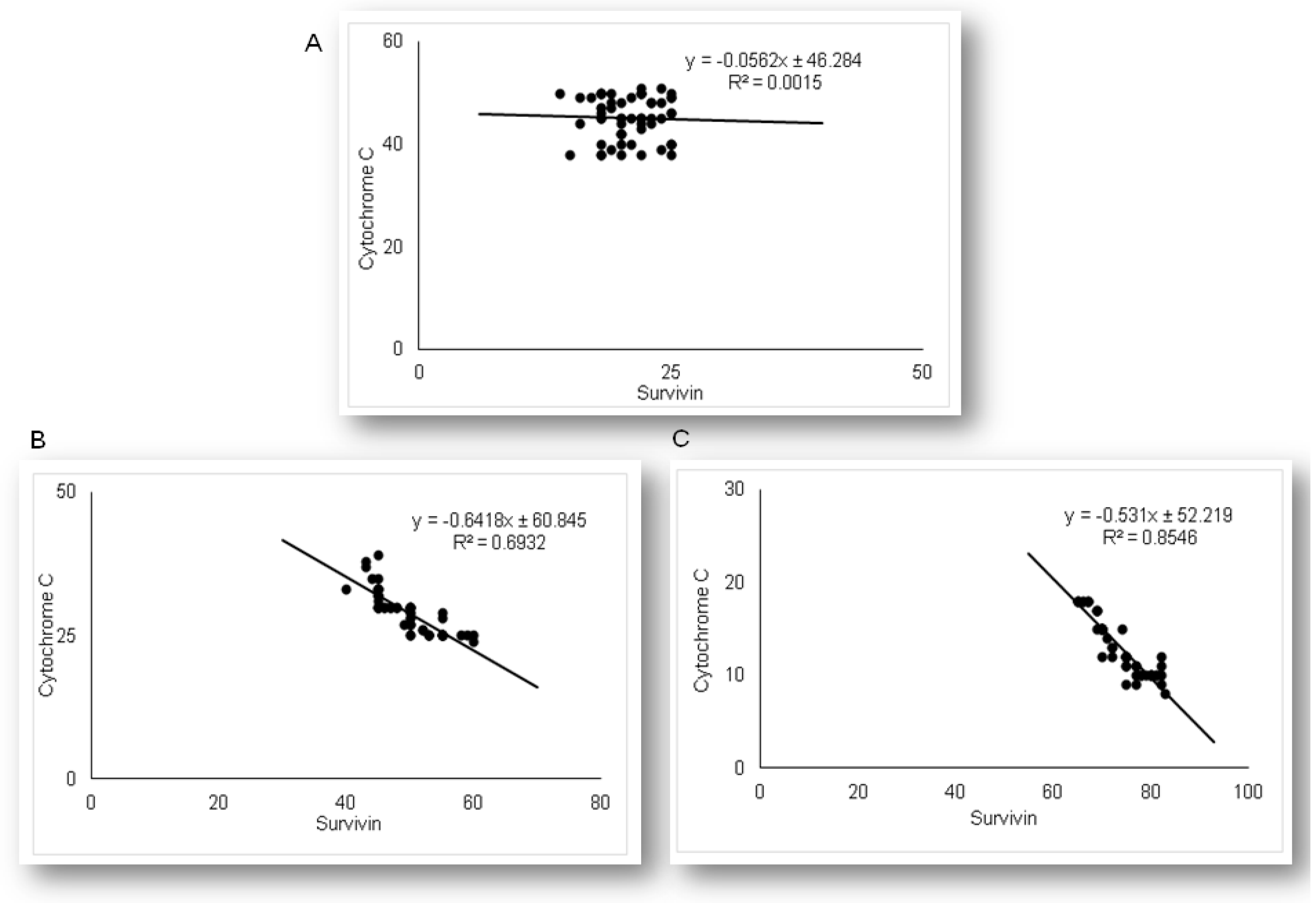
2.3. Simvastatin Attenuated Expression of Pro-Apoptotic Cytochrome C in Renal Tubular Epithelial Cells after LPS Administration
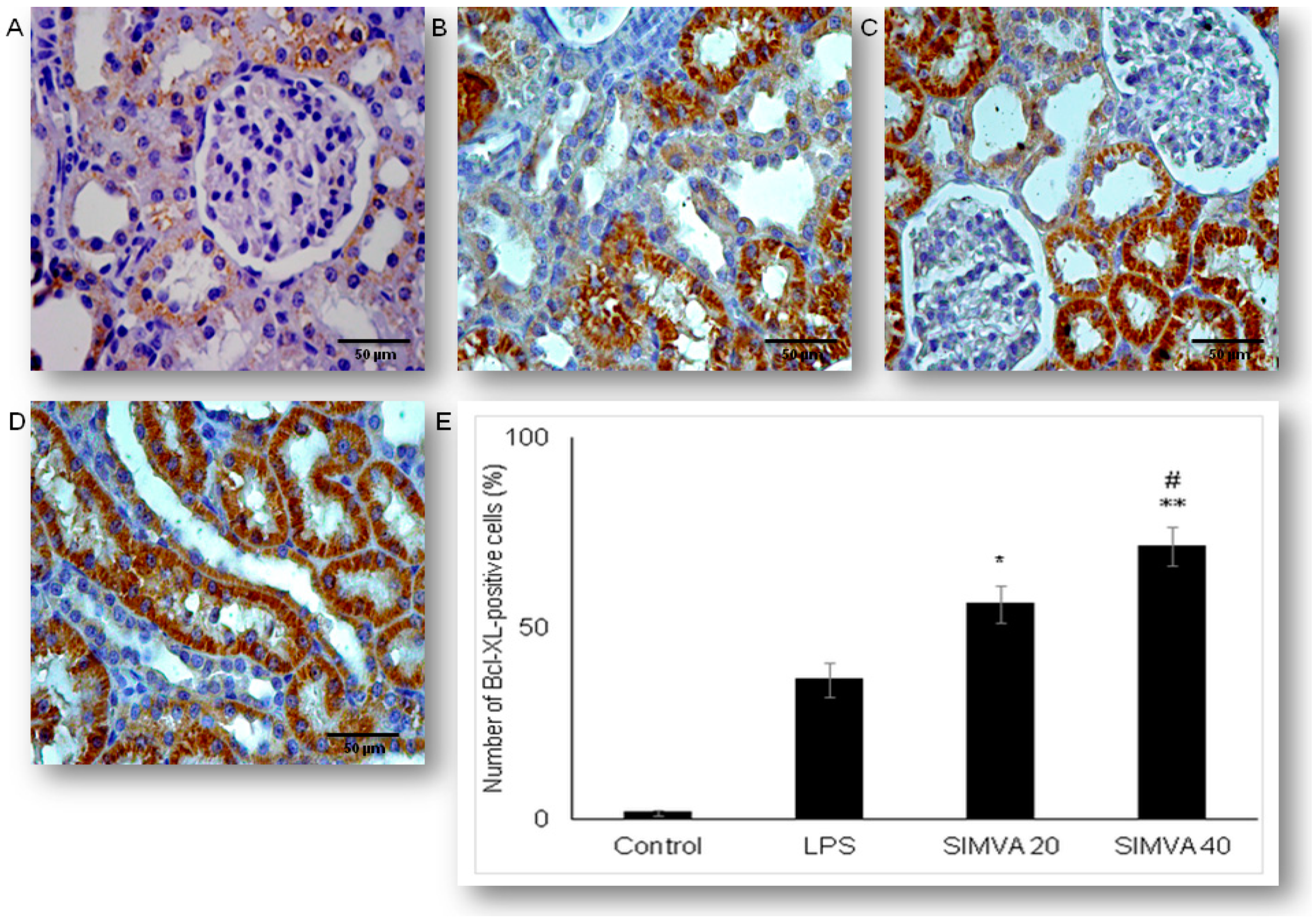
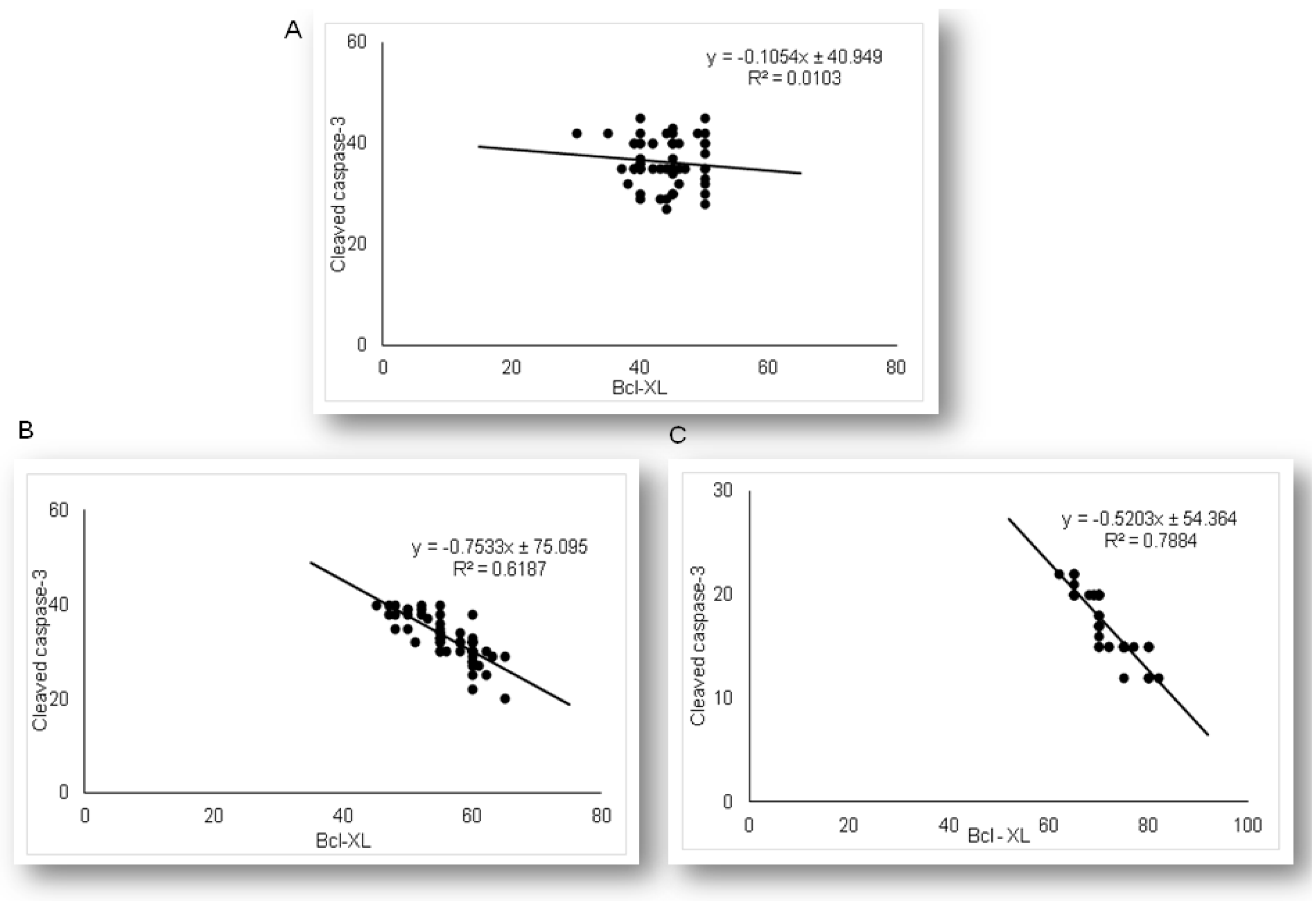
2.4. Expression of Anti-Apoptotic Bcl-XL in Renal Tubular Epithelial Cells after Simvastatin and LPS Administration
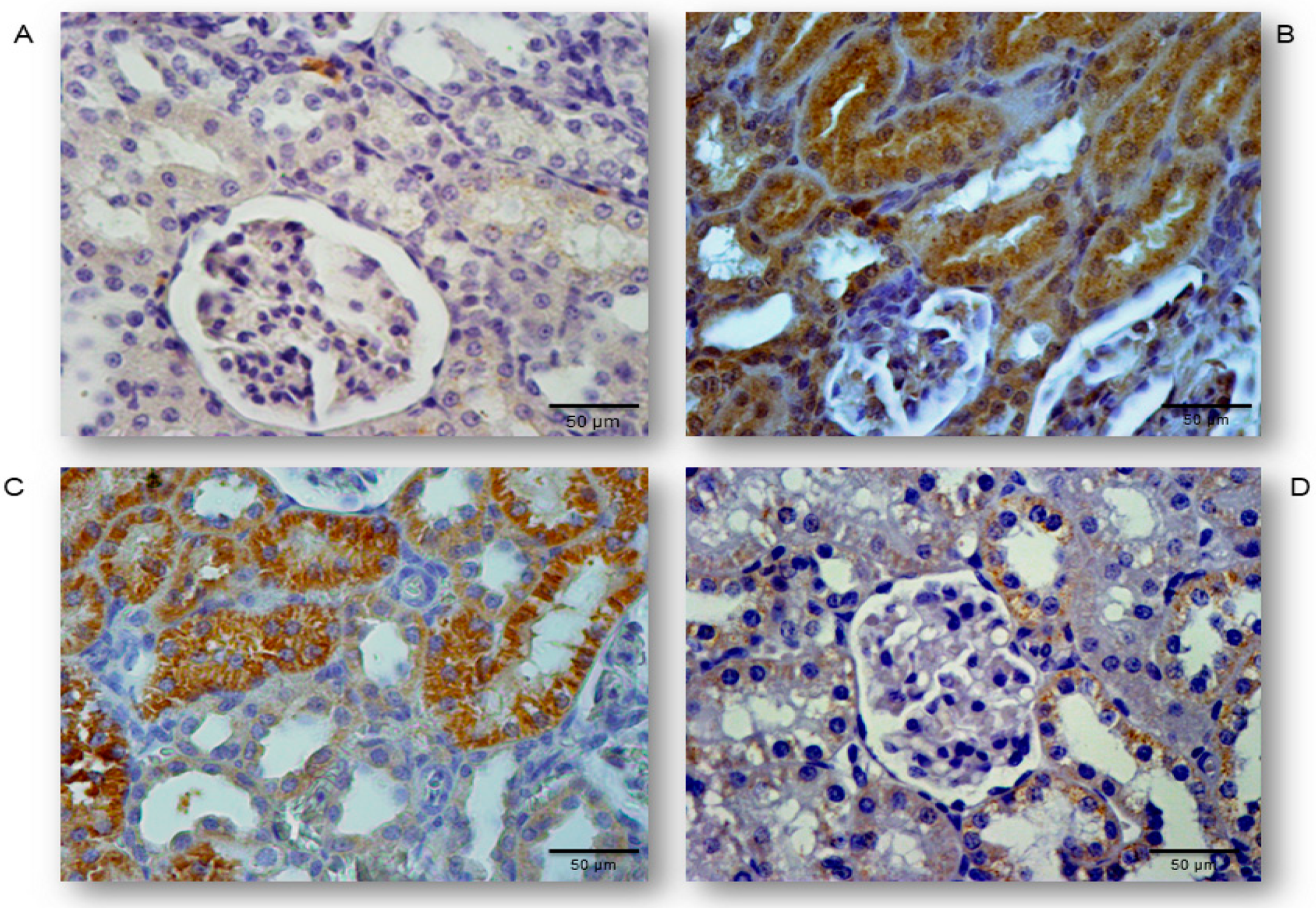
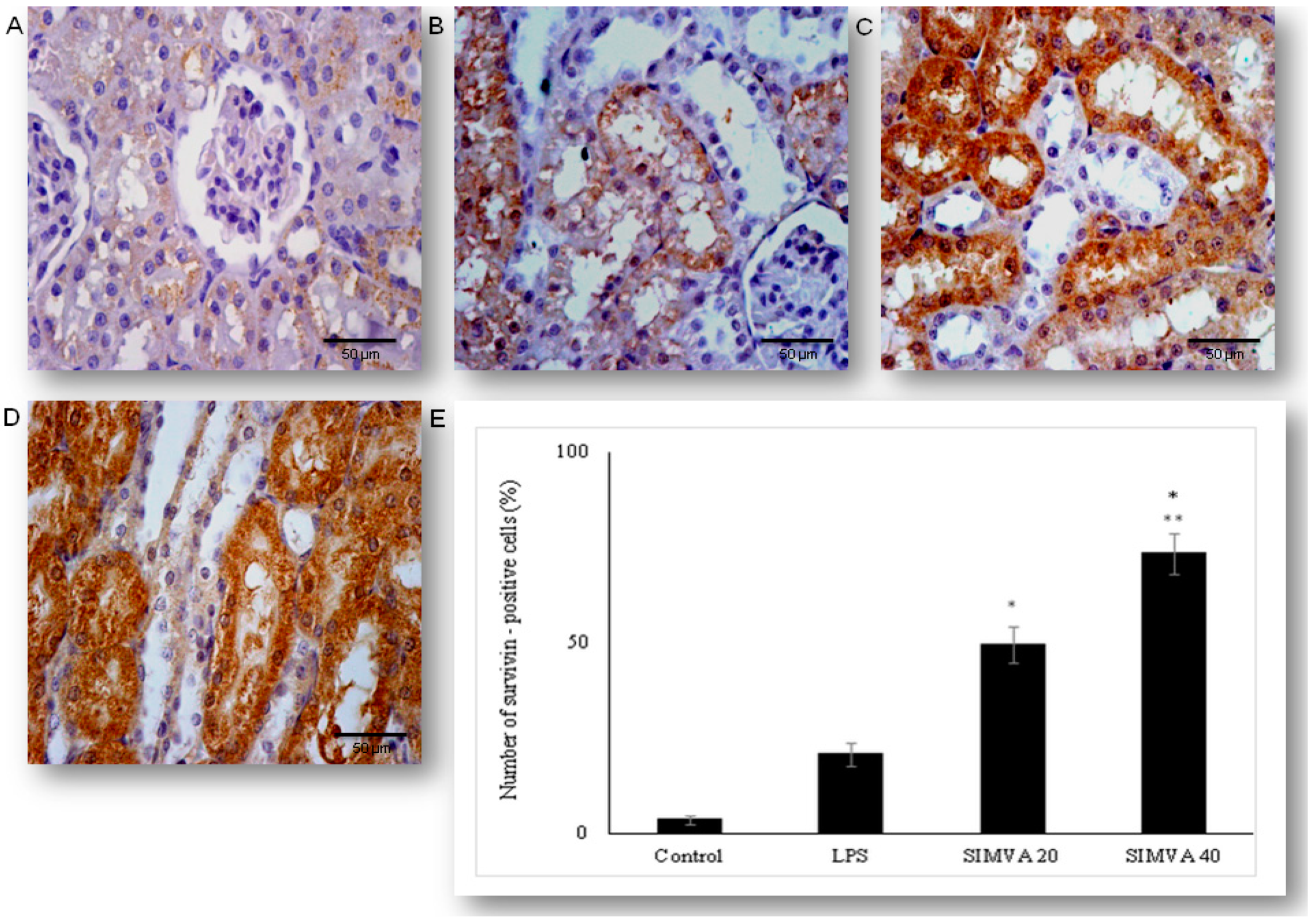
2.5. Simvastatin Enhanced Survivin Expression in Renal Tubular Epithelial Cells after LPS Administration
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Drugs
4.3. Experimental Design
4.4. Histopathological Examination and Semiquantitative Analysis of Renal Damage Score
4.5. Detection and Quantification of Tubular Cell Apoptosis in Situ by TUNEL Method
4.6. Detection and Quantification of Apoptosis Regulating Molecules by Immunohistochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care. 2016, 22, 546–553. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Brezgunova, A.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Popkov, V.A.; Silachev, D.N.; Zorov, D.B. Mechanisms of LPS-Induced acute kidney injury in neonatal and adult rats. Antioxidants 2018, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transpl. 2017, 32, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Cha, D.R.; Cho, W.Y.; Kim, H.K.; Chang, K.H.; Yun, S.Y.; Won, N.H. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron 2002, 91, 406–415. [Google Scholar] [CrossRef]
- Flusberg, D.A.; Sorger, P.K. Surviving apoptosis: Life-death signalling in single cells. Trends Cell. Biol. 2015, 25, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, R.; Dong, W.; Yang, H.; Zhang, L.; Chen, Y.; Wang, W.; Li, C.; Wu, Y.; Ye, Z.; et al. RIPK3 mediates renal tubular epithelial cell apoptosis in endotoxin-induced acute kidney injury. Mol. Med. Rep. 2019, 20, 1613–1620. [Google Scholar] [CrossRef]
- Jacobs, R.; Honore, P.M.; Joannes-Boyau, O.; Boer, W.; De Regt, J.; De Waele, E.; Collin, V.; Spapen, H.D. Septic acute kidney injury: The culprit is inflammatory apoptosis rather than ischemic necrosis. Blood Purif. 2011, 32, 262–265. [Google Scholar] [CrossRef]
- Parikh, S.M.; Yang, Y.; He, L.; Tang, C.; Zhan, M.; Dong, Z. Mitochondrial function and disturbances in the septic kidney. Semin. Nephrol. 2015, 35, 108–119. [Google Scholar] [CrossRef]
- Liu, J.X.; Yang, C.; Zhang, W.H.; Su, H.Y.; Liu, Z.J.; Pan, Q.; Liu, H.F. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 2019, 235, 116828. [Google Scholar] [CrossRef]
- Zhong, X.; He, J.; Zhang, X.; Li, C.; Tian, X.; Xia, W.; Gan, H.; Xia, Y. UCP2 alleviates tubular epithelial cell apoptosis in lipopolysaccharide-induced acute kidney injury by decreasing ROS production. Biomed. Pharm. 2019, 115, 108914. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.J.; Maeng, Y.I.; Leem, J.; Park, K.K. Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice. Biology 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.; Scindia, Y.; Loi, V.; Wlazlo, E.; Ghias, E.; Cechova, S.; Portilla, D.; Ledesma, J.; Swaminathan, S. Protective Role of DJ-1 in Endotoxin-induced Acute Kidney Injury. Am. J. Physiol. Renal. Physiol 2020. [Google Scholar] [CrossRef] [PubMed]
- Du., J.; Jiang, S.; Hu, Z.; Tang, S.; Sun, Y.; He, J.; Li, Z.; Yi, B.; Wang, I.; Zhang, H.; et al. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am. J. Physiol. Ren. Physiol. 2019, 316, F1068–F1077. [Google Scholar] [CrossRef] [PubMed]
- Nežić, L.; Škrbić, R.; Dobrić, S.; Stojiljković, M.P.; jaćevič, V.; Stoisavljević, S.; Milovanović, Z.A.; Stojaković, N. Simvastatin and indomethacin have similar anti-inflammatory activity in a rat model of acute local inflammation. Basic Clin. Pharm. Toxicol. 2009, 104, 185–191. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, Y.M.; Kaneki, M.; Bonab, A.A.; Tompkins, R.G.; Fischman, A.J. Simvastatin reduces burn injury-induced splenic apoptosis via down-regulation of the TNF-α/ NF-κB pathway. Ann. Surg. 2015, 261, 1006–1012. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Zhao, X.; Zhang, R. Experimental study of the protective effect of simvastatin on lung injury in rats with sepsis. Inflammation 2018, 41, 104–113. [Google Scholar] [CrossRef]
- He, X.; Yang, J.; Li, L.; Tan, H.; Wu, Y.; Ran, P.; Sun, S.; Chen, J.; Zhou, Y. Atorvastatin protects against contrast-induced nephropathy via anti-apoptosis by the upregulation of Hsp27 in vivo and in vitro. Mol. Med. Rep. 2017, 15, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tomar, A.; Puthanmadhom Narayanan, S.; Nag, T.C.; Arya, D.C.; Bhatia, J. Pitavastatin attenuates cisplatin--induced renal injury by targeting MAPK and apoptotic pathways. J. Pharm. Pharm. 2019, 71, 1072–1081. [Google Scholar] [CrossRef]
- Nežić, L.; Škrbić, R.; Dobrić, S.; Stojiljković, M.P.; Šatara, S.S.; Milovanović, Z.A.; Stojaković, N. Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. Gen. Physiol. Biophys. 2009, 28, 119–126. [Google Scholar]
- Nežić, L.; Škrbić, R.; Amidžić, L.j.; Gajanin, R.; Kuča, K.; Jaćević, V. Simvastatin protects cardiomyocytes against endotoxin-induced apoptosis and up-regulates survivin/NF-κB/p65 expression. Sci. Rep. 2018, 8, 14652. [Google Scholar] [CrossRef]
- Nežić, L.; Amidžić, L.j.; Škrbić, R.; Gajanin, R.; Nepovimova, E.; Vališ, M.; Kuča, K.; Jaćević, V. Simvastatin inhibits endotoxin-induced apoptosis in liver and spleen through up-regulation of survivin/NF-kB/p65 expression. Front. Pharm. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Yuen, P.S.; Hu, X.; Zhou, H.; Star, R.A. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidn. Int. 2006, 69, 1535–1542. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, R.P.; Wu, W.T.; Liao, K.W.; Hsu, N.; Hsu, B.G. Fluvastatin ameliorates endotoxin-induced multiple organ failure in conscious rats. Resuscitation 2007, 74, 166–174. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhao, X.; Yang, W.; Zhang, R. An experimental study of the protective effect of simvastatin on sepsis-induced myocardial depression in rats. Biomed. Pharm. 2017, 94, 705–711. [Google Scholar] [CrossRef]
- Apaya, M.K.; Lin, C.Y.; Chiou, C.Y.; Yang, C.C.; Ting, C.Y.; Shyur, L.F. Simvastatin and a plant galactolipid protect animals from septic shock by regulating oxylipin mediator dynamics through the MAPK-cPLA2 signalling pathway. Mol. Med. 2016, 21, 988–1001. [Google Scholar] [CrossRef]
- Özkök, E.; Yorulmaz, H.; Ateş, G.; Aydın, I.; Ergüven, M.; Tamer, Ş. The impact of pretreatment with simvastatin on kidney tissue of rats with acute sepsis. Physiol. Int. 2017, 104, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; Dong, Z. Renoprotective approaches and strategies in acute kidney injury. Pharm. Ther. 2016, 163, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, S.; Inoue, Y.; Yang, W.; Fukaya, M.; Carter, E.A.; Yu, Y.; Fishman, A.; Tompkins, R.; Kanrki, M. Farnesyltransferase inhibitor improved survival following endotoxin challenge in mice. Biochem. Biophys. Res. Commun. 2010, 391, 1459–1464. [Google Scholar] [CrossRef]
- Slotta, J.E.; Laschke, M.W.; Schilling, M.K.; Menger, M.D.; Jeppsson, B.; Thorlacius, H. Simvastatin attenuate hepatic sensitization to lipopolysaccharide after partial hepatectomy. J. Surg. Res. 2010, 162, 184–192. [Google Scholar] [CrossRef]
- Lee, E.F.; Fairlie, W.D. The Structural Biology of Bcl-xL. Int. J. Mol. Sci. 2019, 20, 2234. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Shen, H.; Jin, S.; Zhang, S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharm. 2016, 781, 92–99. [Google Scholar] [CrossRef]
- Dohi, T.; Beltrami, E.; Wall, N.R.; Plescia, J.; Altieri, D.C. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Inv. 2004, 114, 1117–1127. [Google Scholar] [CrossRef]
- Tsang, T.J.; Hsueh, Y.C.; Wei, E.I.; Lundy, D.J.; Cheng, B.; Chen, Y.T.; Wang, S.S.; Hsieh, P.C.H. Subcellular localization of survivin determines its function in cardiomyocytes. Theranostics 2017, 7, 4577–4590. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Menzebach, A.; Van de Wouwer, M.; Betz, I.; De Vriese, A.; Conway, E.M. Protective role of the inhibitor of apoptosis protein, survivin, in toxin-induced acute renal failure. Faseb. J. 2008, 22, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, Y.; Huang, T.S.; Zhao, J.; Huang, X.J.; Tang, H.X.; An, N.; Pan, Q.; Xu, Y.-Z.; Liu, H.-F. Asiatic acid protects against cisplatin-induced acute kidney injury via anti-apoptosis and anti-inflammation. Biomed. Pharmacother. 2018, 107, 1354–1362. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Conway, E.M.; Harris, R.S. Survivin mediates renal proximal tubule recovery from AKI. J. Am. Soc. Nephrol. 2013, 24, 2023–2033. [Google Scholar] [CrossRef]
- Wilson, R.L.; Selvaraju, V.; Lakshmanan, R.; Thirunavukkarasu, M.; Campbell, J.; McFadden, D.W.; Maulik, D.W. Thioredoxin-1 attenuates sepsis-induced cardiomyopathy after cecal ligation and puncture in mice. J. Surg. Res. 2017, 220, 68–78. [Google Scholar] [CrossRef]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef]
- Kosaka, J.; Lankadeva, Y.R.; May, C.N.; Bellomo, R. Histopathology of septic acute kidney injury: A systematic review of experimental data. Crit. Care Med. 2016, 44, e897–e903. [Google Scholar] [CrossRef]
- Kita, T.; Brown, M.S.; Goldstein, J.L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J. Clin. Inv. 1980, 66, 1094–1100. [Google Scholar] [CrossRef]
- Morel, J.; Hargreaves, I.; Brealey, D.; Neergheen, V.; Backman, J.T.; Lindig, S.; Bläss, M.; Bauer, M.; McAuley, D.F.; Singer, M. Simvastatin pre-treatment improves survival and mitochondrial function in a 3-day fluid-resuscitated rat model of sepsis. Clin. Sci. 2017, 131, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Jovic, D.; Kuča, K.; Dragojevic-Simic, V.; Dobric, S.; Trajkovic, S.; Borisev, I.; Segrt, Z.; Milovanovic, Z.; Bokonjic, D.; et al. Effects of Fullerenol nanoparticles and Amifostine on radiation-induced tissue damages: Histopathological analysis. J. Appl. Biomed. 2016, 14, 285–297. [Google Scholar] [CrossRef]
- Jaćević, V.; Djordjevic, A.; Srdjenovic, B.; Milic-Tores, V.; Segrt, Z.; Dragojevic-Simic, V.; Kuca, K. Fullerenol nanoparticles prevent doxorubicin-induced acute hepatotoxicity in rats. Exp. Mol. Pathol. 2017, 102, 360–369. [Google Scholar] [CrossRef]
- Jaćević, V.; Dragojević-Simić, V.; Tatomirović, Ž.; Dobrić, S.; Bokonjić, D.; Kovačević, A.; Nepovimova, E.; Vališ, M.; Kuča, K. The efficacy of amifostine against multiple-dose doxorubicin-induced toxicity in rats. Int. J. Mol. Sci. 2018, 19, 2370. [Google Scholar] [CrossRef] [PubMed]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Toxic injury to the muscle tissue of rats following acute oximes exposure. Sci. Rep. 2019, 9, 1457. [Google Scholar] [CrossRef]
- Jaćević, V.; Nepovimova, E.; Kuča, K. Acute toxic injuries of rat’s visceral tissues induced by different oximes. Sci. Rep. 2019, 9, 16425. [Google Scholar] [CrossRef]
- Jaćević, V.; Wu, Q.; Nepovimova, E.; Kuča, K. Efficacy of methylprednisolone on T-2 toxin-induced cardiotoxicity in vivo: A pathohistological study. Environ. Toxicol. Pharm. 2019, 71, 103221. [Google Scholar] [CrossRef]
- Jaćević, V.; Wu, Q.; Nepovimova, E.; Kuča, K. Cardiomiopythy induced by T-2 toxin. Food Chem. Toxicol. 2020, 137, 111138. [Google Scholar] [CrossRef]
- Wang, K.; Brems, J.J.; Gamelli, R.L.; Holterman, X.A. Survivin signalling is regulated through the nuclear factor-kappa B pathway during glycochenodeoxycholate-induced hepatocyte apoptosis. Biochim. Biophys. Acta 2010, 1803, 1368–1375. [Google Scholar] [CrossRef]
- Scheer, A.; Knauer, S.K.; Verhaegh, R. Survivin expression pattern in the intestine of normoxic and ischemic rats. BMC Gastroenterol. 2017, 17, 76. [Google Scholar] [CrossRef]


| Treatment (mg/kg) | Renal Damage Score (6 Kidneys/Group × 6 Slices/Kidney) | ± S.D. | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Control | 30 | 6 | 0 | 0 | 0 | 0.67 ± 0.48 |
| LPS | 0 | 0 | 0 | 15 | 21 | 3.58 ± 0.50 a3 |
| Simvastatin 10 group | 0 | 0 | 12 | 24 | 0 | 2.67 ± 0.48 a1 |
| Simvastatin 20 group | 0 | 0 | 24 | 12 | 0 | 2.33 ± 0.47 a1 |
| Simvastatin 40 group | 0 | 21 | 15 | 0 | 0 | 1.42 ± 0.50 b2 |
| Degree | Description |
|---|---|
| 0 | Normal finding. |
| 1 | Mild damage: Single glomerular cells slightly enlarged. Mild dilatation of small blood vessels. A few foci of inflammatory cell infiltrates. |
| 2 | Moderate damage: < 50% glomerular cells with proliferation. Severe vasodilatation associated with hyperemia and edema. Various numbers of inflammatory cells infiltrates. |
| 3 | Severe and focal damage: > 50% glomerular cells with proliferation. Transmural rupture of the blood vessels (up to 50%) associated with an accumulation of inflammatory cells. |
| 4 | Severe and diffuse damage: Complete loss of the normal glomerular architecture, and the basal membrane and endothelial cells of the blood vessels (>50%). High-intensity hemorrhages and diffuse accumulation of inflammatory cells. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nežić, L.; Škrbić, R.; Amidžić, L.; Gajanin, R.; Milovanović, Z.; Nepovimova, E.; Kuča, K.; Jaćević, V. Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-Mediated Apoptosis. Int. J. Mol. Sci. 2020, 21, 7236. https://doi.org/10.3390/ijms21197236
Nežić L, Škrbić R, Amidžić L, Gajanin R, Milovanović Z, Nepovimova E, Kuča K, Jaćević V. Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-Mediated Apoptosis. International Journal of Molecular Sciences. 2020; 21(19):7236. https://doi.org/10.3390/ijms21197236
Chicago/Turabian StyleNežić, Lana, Ranko Škrbić, Ljiljana Amidžić, Radoslav Gajanin, Zoran Milovanović, Eugenie Nepovimova, Kamil Kuča, and Vesna Jaćević. 2020. "Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-Mediated Apoptosis" International Journal of Molecular Sciences 21, no. 19: 7236. https://doi.org/10.3390/ijms21197236
APA StyleNežić, L., Škrbić, R., Amidžić, L., Gajanin, R., Milovanović, Z., Nepovimova, E., Kuča, K., & Jaćević, V. (2020). Protective Effects of Simvastatin on Endotoxin-Induced Acute Kidney Injury through Activation of Tubular Epithelial Cells’ Survival and Hindering Cytochrome C-Mediated Apoptosis. International Journal of Molecular Sciences, 21(19), 7236. https://doi.org/10.3390/ijms21197236






