Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites
Abstract
1. Introduction
2. Results
2.1. Renal Outcome in Mother Rats
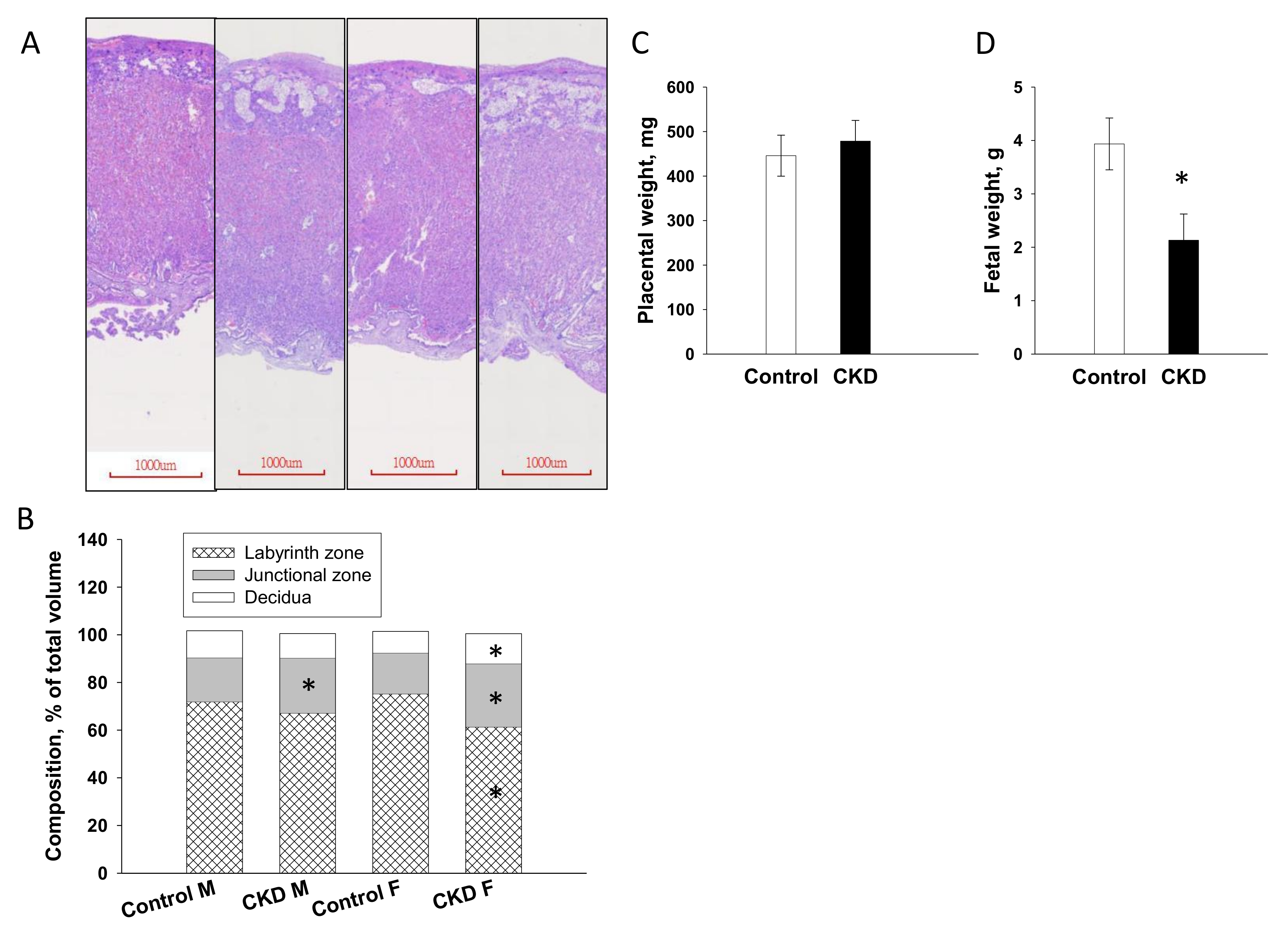
2.2. Placenta and Fetal Weight
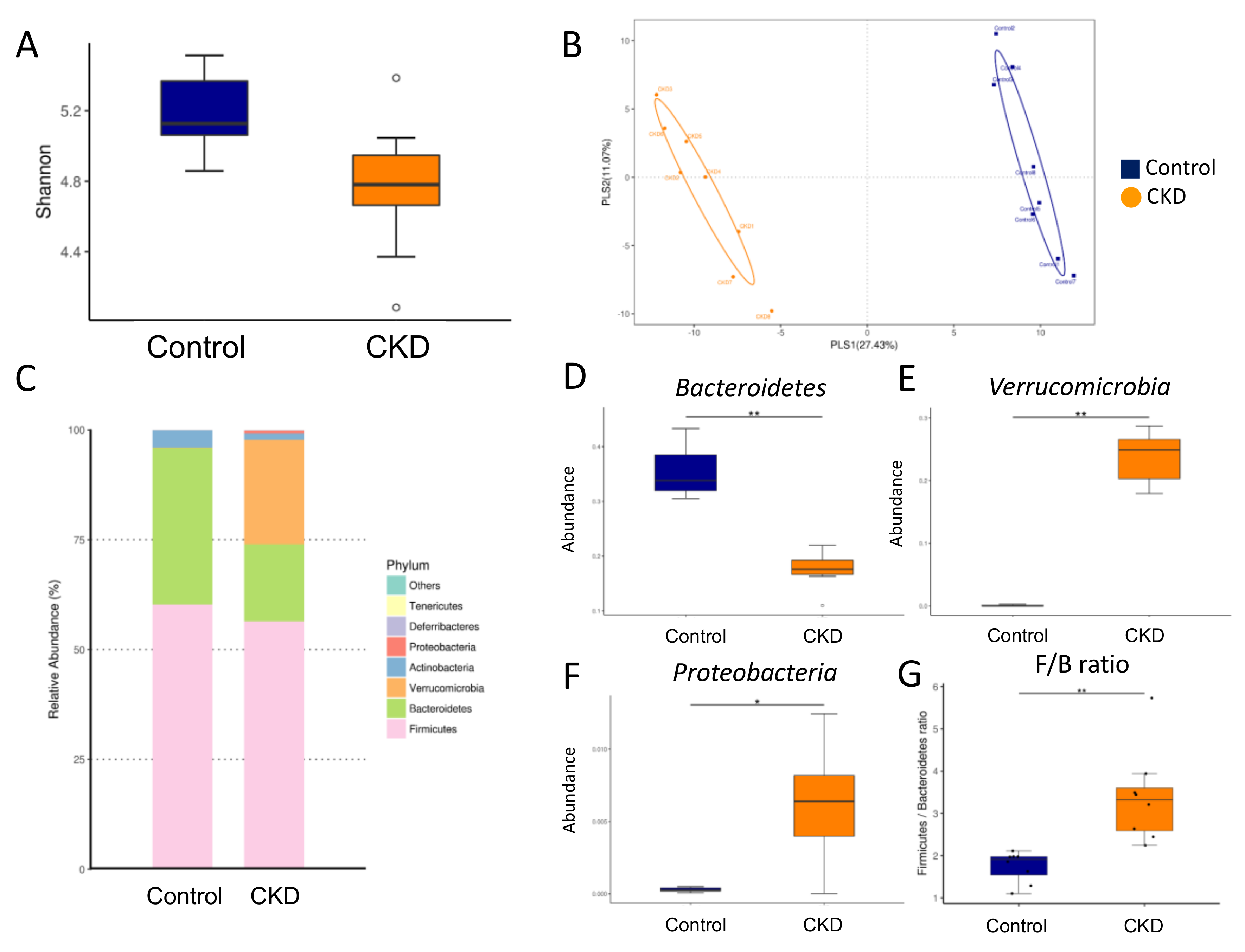
2.3. Gut Microbiota Composition in Mother Rats
2.4. NO Pathway
2.5. TMAO Pathway
2.6. Short Chain Fatty Acids in Plasma and Feces
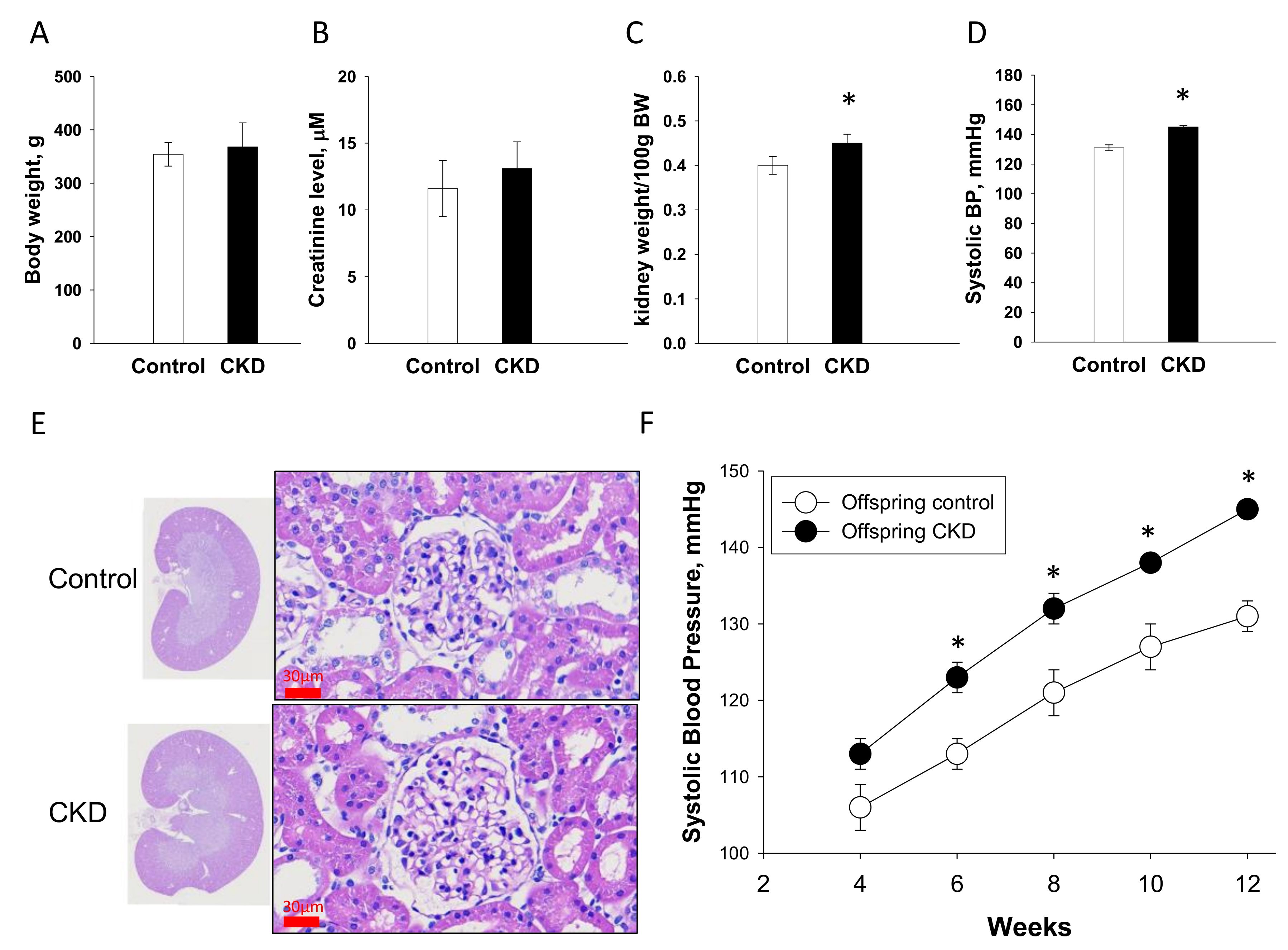
2.7. Renal Outcome in Offspring
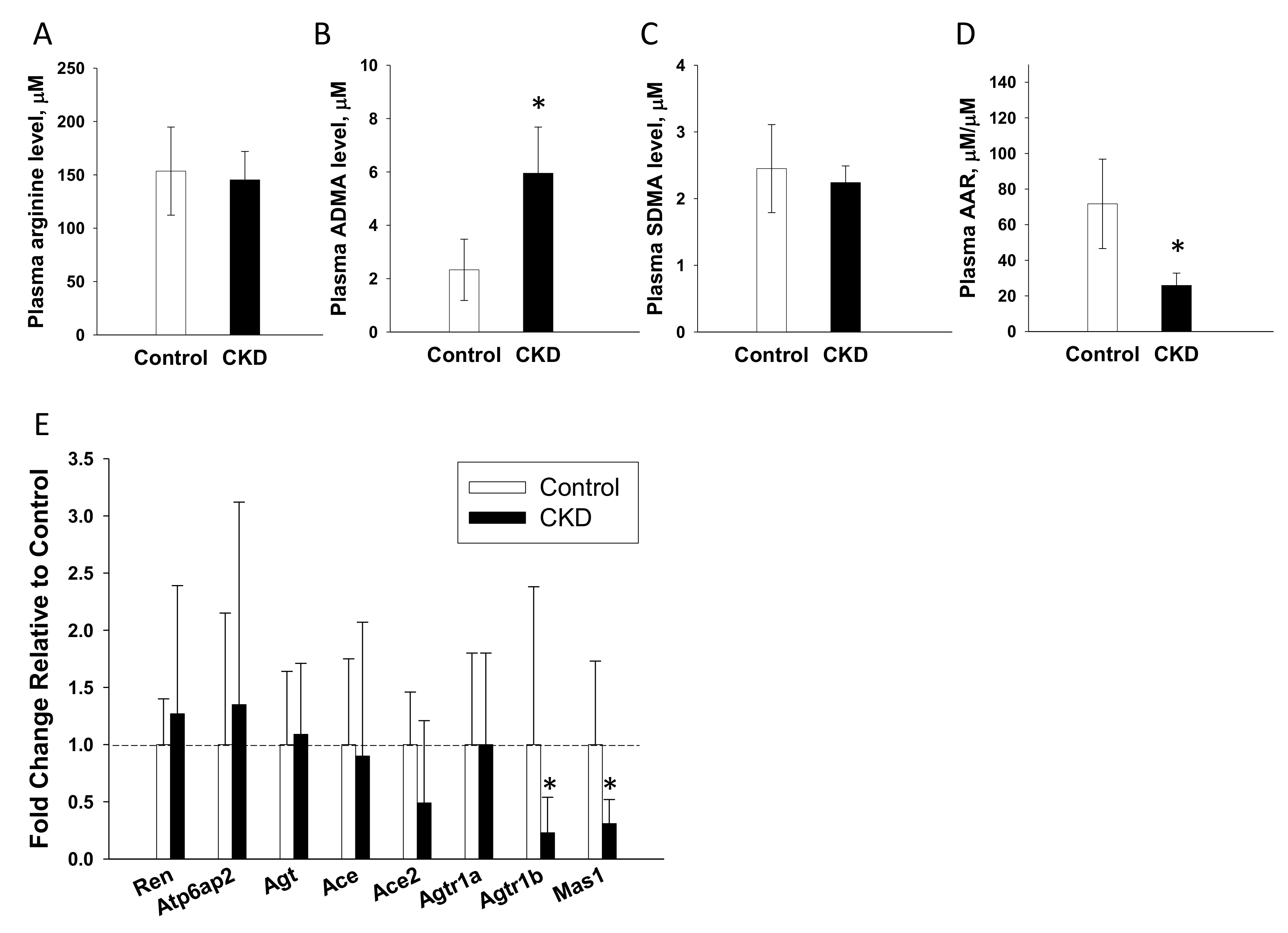
2.8. NO Pathway and the RAS in Offspring
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. High-Performance Liquid Chromatography (HPLC)
4.3. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
4.4. Gas Chromatography-Flame Ionization Detector (GC-FID)
4.5. Analysis of Gut Microbiota Composition
4.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.7. Histology and Morphometric Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| ACE2 | Angiotensin converting enzyme 2 |
| ADMA | Asymmetric dimethylarginine |
| ANOSIM | Analysis of Similarities |
| AT1R | Angiotensin II type 1 receptor |
| AT2R | Angiotensin II type 2 receptor |
| CKD | Chronic kidney disease |
| DMA | Dimethylamine |
| DOHaD | Developmental origins of health and disease |
| FID | Flame ionization detector |
| GD | Gestational day |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| MAS1 | Angiotensin (1–7) receptor |
| OTU | Operational taxonomic unit |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| RAS | Renin-angiotensin system |
| SCFA | Short chain fatty acid |
| SD | Sprague–Dawley |
| SDMA | Symmetric dimethylarginine |
| TMA | Trimethylamine |
| TMAO | Trimethylamine-N-oxide |
References
- Piccoli, G.B.; Alrukhaimi, M.; Liu, Z.H.; Zakharova, E.; Levin, A. World Kidney Day Steering Committee. What we do and do not know about women and kidney diseases; Questions unanswered and answers unquestioned: Reflection on World Kidney Day and International Woman’s Day. Physiol. Int. 2018, 105, 1–18. [Google Scholar] [CrossRef]
- Munkhaugen, J.; Lydersen, S.; Romundstad, P.R.; Widerøe, T.-E.; Vikse, B.E.; Hallan, S. Kidney function and future risk for adverse pregnancy outcomes: A population-based study from HUNT II, Norway. Nephrol. Dial. Transplant. 2009, 24, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Yosypiv, I.V. Developmental Programming of Hypertension and Kidney Disease. Int. J. Nephrol. 2012, 2012, 760580. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Hsu, C.-N. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int. J. Mol. Sci. 2017, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Nevis, I.F.; Reitsma, A.; Dominic, A.; McDonald, S.; Thabane, L.; Akl, E.A.; Hladunewich, M.; Akbari, A.; Joseph, G.; Sia, W.; et al. Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin. J. Am. Soc. Nephrol. 2011, 6, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Hladunewich, M.A. Chronic Kidney Disease and Pregnancy. Semin. Nephrol. 2017, 37, 337–346. [Google Scholar] [CrossRef]
- Chu, D.M.; Meyer, K.M.; Prince, A.L.; Aagaard, K.M. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 2016, 7, 459–470. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv. Nutr. 2019, 11, 66–76. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Lee, C.-T.; Chan, J.Y.H.; Hsu, C.-N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal NG-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636.e1–636.e72. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Chan, J.Y.; Lee, C.-T.; Tain, Y.-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Wu, K.L.H.; Lee, W.-C.; Leu, S.; Chan, J.Y.H.; Tain, Y.-L. Aliskiren Administration during Early Postnatal Life Sex-Specifically Alleviates Hypertension Programmed by Maternal High Fructose Consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Zheng, P.D.; Oura, H.; Koizumi, F. Animal Model of Adenine-Induced Chronic Renal Failure in Rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, D.; Gil-Peña, H.; Fuente, R.; García-López, E.; Loredo, V.; Frías, O.H.; Ordoñez, F.A.; Rodríguez, J.; Santos, F. Chronic kidney disease induced by adenine: A suitable model of growth retardation in uremia. Am. J. Physiol. Physiol. 2015, 309, F57–F62. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Centron, P.; Barrows, I.; Dwivedi, R.; Raj, D.S. Gut Microbiota and Cardiovascular Uremic Toxicities. Toxins 2018, 10, 287. [Google Scholar] [CrossRef]
- Huang, L.-T.; Hsieh, C.-S.; Chang, K.-A.; Tain, Y.-L. Roles of Nitric Oxide and Asymmetric Dimethylarginine in Pregnancy and Fetal Programming. Int. J. Mol. Sci. 2012, 13, 14606–14622. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Huang, L.-T. Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension. Int. J. Mol. Sci. 2014, 15, 11773–11782. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Tain, Y.-L. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef]
- Racasan, S.; Braam, B.; Koomans, H.A.; Joles, J.A. Programming blood pressure in adult SHR by shifting perinatal balance of NO and reactive oxygen species toward NO: The inverted Barker phenomenon. Am. J. Physiol. Physiol. 2005, 288, F626–F636. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef]
- Schmedes, M.; Brejnrod, A.; Aadland, E.K.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fecal Metabolites and Gut Microbiome: Results from a Randomized Crossover Intervention Study. Mol. Nutr. Food Res. 2018, 63, e1700976. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-E.; Park, J.I.; Cho, H.; Kwak, M.-J.; Kim, B.-Y.; Yang, S.H.; Lee, J.P.; Kim, D.K.; Joo, K.W.; et al. The Association between Gut Microbiota and Uremia of Chronic Kidney Disease. Microorganisms 2020, 8, 907. [Google Scholar] [CrossRef]
- Castillo-Rodríguez, E.; Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Fernandez-Fernandez, B.; Kanbay, M.; Tejedor, A.; Lázaro, A.; Ruiz-Ortega, M.; et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins 2018, 10, 300. [Google Scholar] [CrossRef]
- Hsu, C.; Chang-Chien, G.; Lin, S.; Hou, C.; Tain, Y.-L. Targeting on Gut Microbial Metabolite Trimethylamine-N-Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.W.; et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Chan, J.Y.H.; Yu, H.-R.; Lee, W.-C.; Wu, K.L.H.; Chang-Chien, G.-P.; Lin, S.; Hou, C.-Y.; Tain, Y.-L. Targeting on Gut Microbiota-Derived Metabolite Trimethylamine to Protect Adult Male Rat Offspring against Hypertension Programmed by Combined Maternal High-Fructose Intake and Dioxin Exposure. Int. J. Mol. Sci. 2020, 21, 5488. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Fu, P. Gut microbiota—Derived short-chain fatty acids and kidney diseases. Drug Des. Dev. Ther. 2017, 11, 3531–3542. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Huang, L.-T.; Tsai, C.-C.; Sheen, J.-M.; Tiao, M.-M.; Yu, H.-R.; Lin, I.-C.; Tain, Y.-L. Maternal high-fat diet sex-specifically alters placental morphology and transcriptome in rats: Assessment by next-generation sequencing. Placenta 2019, 78, 44–53. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Lin, Y.-J.; Yu, H.-R.; Yu, H.-R.; Tiao, M.-M.; Chen, C.-C.; Tsai, C.-C.; Huang, L.-T.; Hsu, C.-N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Lin, I.-C.; Yu, H.-R.; Huang, L.-T.; Tiao, M.-M.; Tain, Y.-L. Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]


| □ | Control | CKD |
|---|---|---|
| NO pathway | ||
| L-citrulline, μM | 56.2 ± 9.3 | 79.8 ± 20.4 * |
| L-arginine, μM | 283.4 ± 29.1 | 190.7 ± 36.4 * |
| Asymmetric dimethylarginine, μM | 1.59 ± 0.43 | 5.59 ± 1.54 * |
| Symmetric dimethylarginine, μM | 0.76 ± 0.44 | 1.48 ± 0.26 * |
| L-arginine-to-asymmetric dimethylarginine, μM/μM | 187.4 ± 44.9 | 35.6 ± 7.7 * |
| TMAO pathway | ||
| TMAO, ng/mL | 300.8 ± 44.9 | 1080.6 ± 162.1 * |
| Trimethylamine, ng/mL | 198.7 ± 40.1 | 165.5 ± 41.5 |
| Dimethylamine, ng/mL | 233.5 ± 56.6 | 680.3 ± 125.8 * |
| □ | Control | CKD |
|---|---|---|
| Plasma | ||
| Acetate, μM | 655.2 ± 297.7 | 413.9 ± 144.8 * |
| Propionate, μM | 51.8 ± 15 | 50.4 ± 26.2 |
| Butyrate, μM | 52.8 ± 20 | 27.6 ± 12.4 * |
| Feces | ||
| Acetate, mM/gm feces | 5.91 ± 1.11 | 3.1 ± 0.68 * |
| Propionate, mM/gm feces | 0.83 ± 0.35 | 0.38 ± 0.22 * |
| Butyrate, mM/gm feces | 0.36 ± 0.23 | 0.24 ± 0.11 |
| Gene | Forward | Reverse |
|---|---|---|
| Ren | 5 aacattaccagggcaactttcact 3 | 5 acccccttcatggtgatctg 3 |
| Atp6ap2 | 5 gaggcagtgaccctcaacat 3 | 5 ccctcctcacacaacaaggt 3 |
| Agt | 5 gcccaggtcgcgatgat 3 | 5 tgtacaagatgctgagtgaggcaa 3 |
| Ace | 5 caccggcaaggtctgctt 3 | 5 cttggcatagtttcgtgaggaa 3 |
| Ace2 | 5 acccttcttacatcagccctactg 3 | 5 tgtccaaaacctaccccacatat 3 |
| Agtr1a | 5 gctgggcaacgagtttgtct 3 | 5 cagtccttcagctggatcttca 3 |
| Agtr1b | 5 caatctggctgtggctgactt 3 | 5 tgcacatcacaggtccaaaga 3 |
| Mas1 | 5 catctctcctctcggctttgtg 3 | 5 cctcatccggaagcaaagg 3 |
| R18S | 5 gccgcggtaattccagctcca 3 | 5 cccgcccgctcccaagatc 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-N.; Yang, H.-W.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. https://doi.org/10.3390/ijms21197237
Hsu C-N, Yang H-W, Hou C-Y, Chang-Chien G-P, Lin S, Tain Y-L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. International Journal of Molecular Sciences. 2020; 21(19):7237. https://doi.org/10.3390/ijms21197237
Chicago/Turabian StyleHsu, Chien-Ning, Hung-Wei Yang, Chih-Yao Hou, Guo-Ping Chang-Chien, Sufan Lin, and You-Lin Tain. 2020. "Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites" International Journal of Molecular Sciences 21, no. 19: 7237. https://doi.org/10.3390/ijms21197237
APA StyleHsu, C.-N., Yang, H.-W., Hou, C.-Y., Chang-Chien, G.-P., Lin, S., & Tain, Y.-L. (2020). Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. International Journal of Molecular Sciences, 21(19), 7237. https://doi.org/10.3390/ijms21197237








