Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice
Abstract
1. Introduction
2. Results
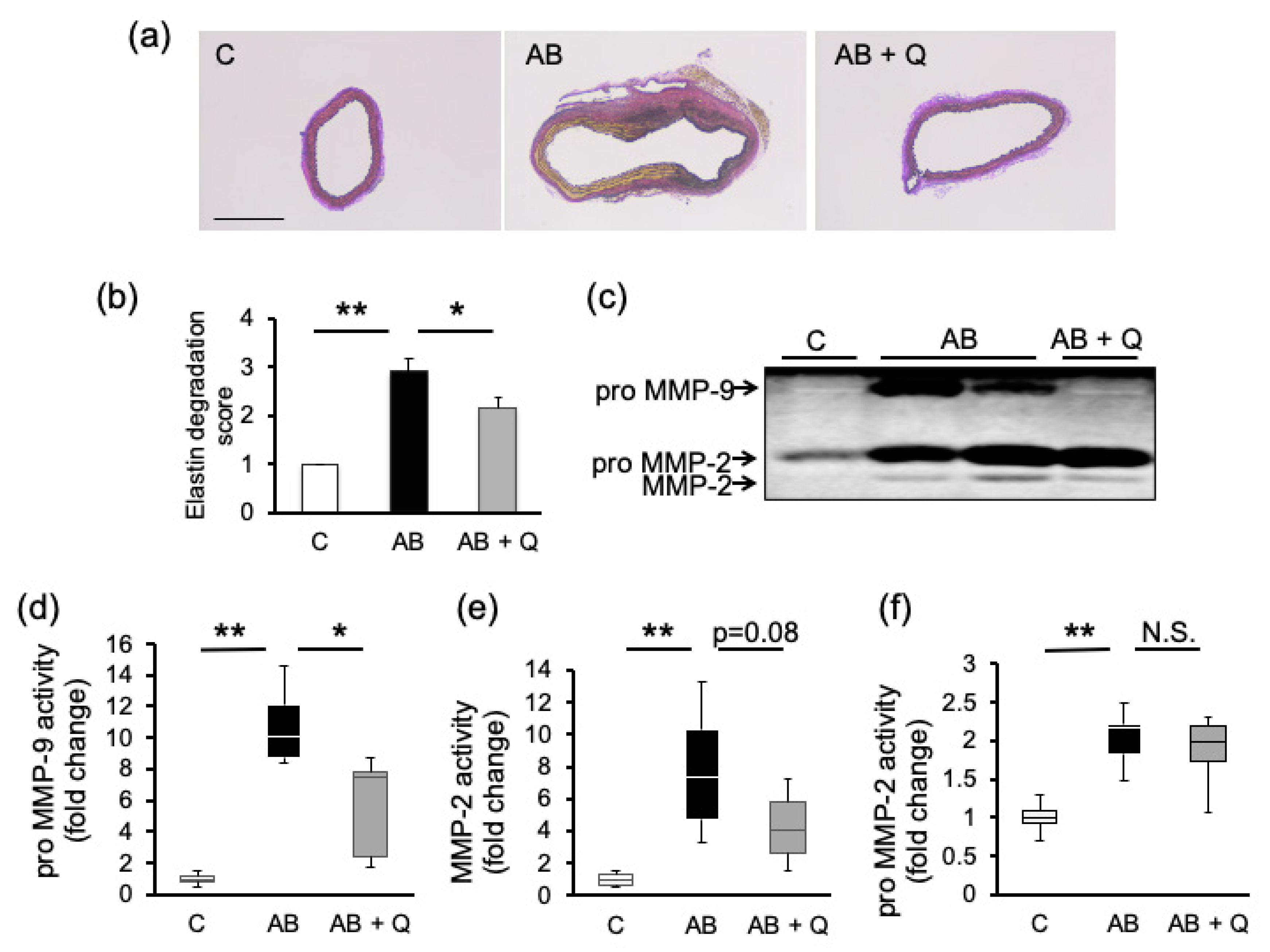
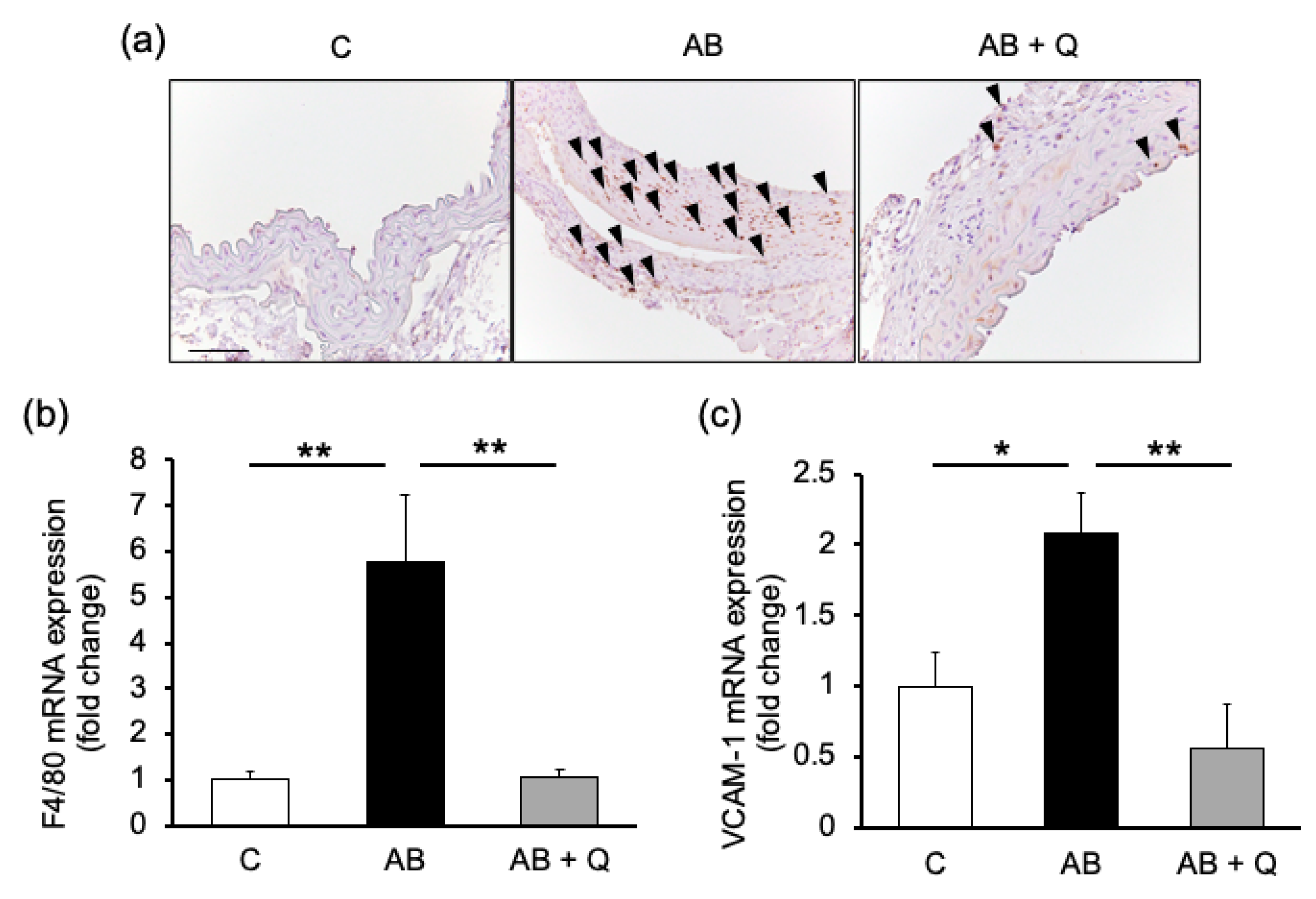
2.1. Quercetin Suppresses Aortic Aneurysm Onset via Anti-Inflammatory Effects
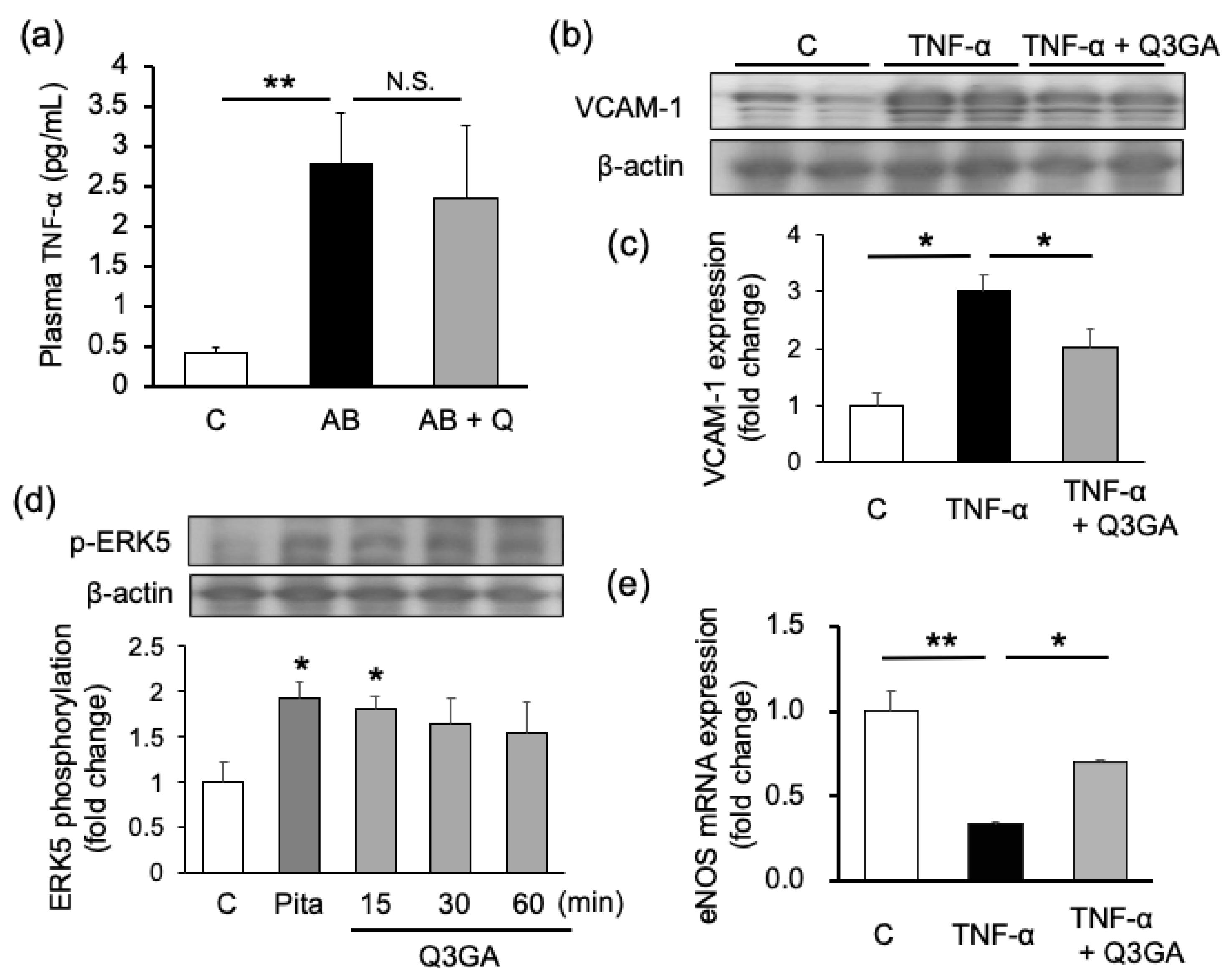
2.2. Quercetin Shows Endothelial Cell-Protective Effects in Cultured Human Umbilical Vein Endothelial Cells (HUVECs)
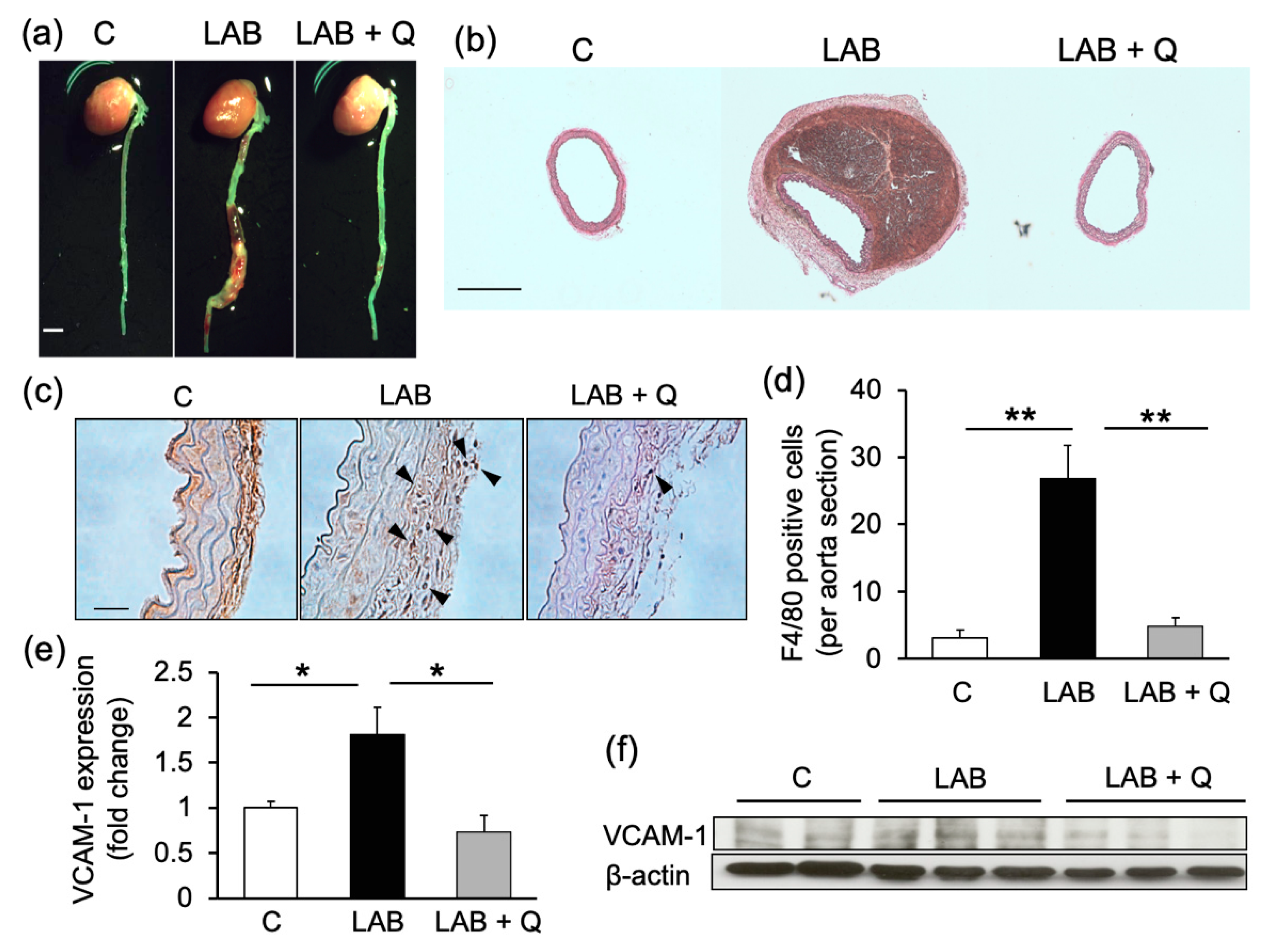
2.3. Quercetin Suppresses the Incidence of Aortic Dissection and Mortality in Mouse Models of Dissection
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents
4.3. Mice and In Vivo Experimental Strategies
4.4. Systolic Blood Pressure (SBP)
4.5. Tissue Sampling and Measurement of Aortic Diameter
4.6. Histology and Immunohistochemistry
4.7. Quantitative Real-Time PCR
4.8. Zymography Assay
4.9. ELISA for TNF-α Measurement
4.10. Cell Culture
4.11. Western Blotting
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Komatsu, S.; Takahashi, S.; Yutani, C.; Ohara, T.; Takewa, M.; Hirayama, A.; Kodama, K. Spontaneous ruptured aortic plaque and injuries: Insights for aging and acute aortic syndrome from non-obstructive general angioscopy. J. Cardiol. 2020, 75, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; O’Gara, P.T. Diagnosis and management of acute aortic syndromes: Dissection, intramural hematoma, and penetrating aortic ulcer. Curr. Cardiol. Rep. 2014, 16, 536. [Google Scholar] [CrossRef] [PubMed]
- Gryszczynska, B.; Budzyn, M.; Formanowicz, D.; Wanic-Kossowska, M.; Formanowicz, P.; Majewski, W.; Iskra, M.; Kasprzak, M.P. Selected atherosclerosis-related diseases may differentially affect the relationship between plasma advanced glycation end products, receptor sRAGE, and uric acid. J. Clin. Med. 2020, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Group, J.C.S.J.W. Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011): Digest version. Circ. J. 2013, 77, 789–828. [Google Scholar] [CrossRef]
- Eckstein, H.H.; Bockler, D.; Flessenkamper, I.; Schmitz-Rixen, T.; Debus, S.; Lang, W. Ultrasonographic screening for the detection of abdominal aortic aneurysms. Dtsch. Arztebl. Int. 2009, 106, 657–663. [Google Scholar] [CrossRef]
- Izawa-Ishizawa, Y.; Imanishi, M.; Zamami, Y.; Toya, H.; Nagao, T.; Morishita, M.; Tsuneyama, K.; Horinouchi, Y.; Kihira, Y.; Takechi, K.; et al. Development of a novel aortic dissection mouse model and evaluation of drug efficacy using in-vivo assays and database analyses. J. Hypertens. 2019, 37, 73–83. [Google Scholar] [CrossRef]
- Pan, L.; Lin, Z.; Tang, X.; Tian, J.; Zheng, Q.; Jing, J.; Xie, L.; Chen, H.; Lu, Q.; Wang, H.; et al. S-Nitrosylation of plastin-3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. Arterioscler Thromb. Vasc. Biol. 2020, 40, 175–188. [Google Scholar] [CrossRef]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tsou, T.L.; Nuki, Y.; Liang, E.I.; Makino, H.; Hashimoto, T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension 2010, 55, 1267–1274. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Konta, T.; Watanabe, M.; Fujimoto, S.; Sato, Y.; Asahi, K.; Yamagata, K.; Tsuruya, K.; Narita, I.; et al. Effect of hypertension on aortic artery disease-related mortality-3.8-year nationwide community-based prospective cohort study. Circ. J. 2018, 82, 2776–2782. [Google Scholar] [CrossRef]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martinez-Gonzalez, M.A.; Medina-Remon, A.; Castaner, O.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.M. Effects of polyphenol, measured by a biomarker of total polyphenols in urine, on cardiovascular risk factors after a long-term follow-up in the predimed study. Oxid. Med. Cell Longev. 2016, 2016, 2572606. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Quifer-Rada, P.; Rinaldi de Alvarenga, J.F.; Perez-Fernandez, S.; Tresserra-Rimbau, A.; Lamuela-Raventos, R.M. Changing to a low-polyphenol diet alters vascular biomarkers in healthy men after only two weeks. Nutrients 2018, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Simic, A.; Manojlovic, D.; Segan, D.; Todorovic, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in health food: Metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J. Pharmacol. Sci. 2011, 115, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian. Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef]
- Ishisaka, A.; Kawabata, K.; Miki, S.; Shiba, Y.; Minekawa, S.; Nishikawa, T.; Mukai, R.; Terao, J.; Kawai, Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS ONE 2013, 8, e80843. [Google Scholar] [CrossRef]
- Ishizawa, K.; Izawa-Ishizawa, Y.; Ohnishi, S.; Motobayashi, Y.; Kawazoe, K.; Hamano, S.; Tsuchiya, K.; Tomita, S.; Minakuchi, K.; Tamaki, T.; et al. Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J. Pharmacol. Sci. 2009, 109, 257–264. [Google Scholar] [CrossRef]
- Iademarco, M.F.; Barks, J.L.; Dean, D.C. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-α in cultured endothelial cells. J. Clin. Invest. 1995, 95, 264–271. [Google Scholar] [CrossRef]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Alexander, R.W.; Medford, R.M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J. Clin. Invest. 1993, 92, 1866–1874. [Google Scholar] [CrossRef]
- Le, N.T.; Takei, Y.; Izawa-Ishizawa, Y.; Heo, K.S.; Lee, H.; Smrcka, A.V.; Miller, B.L.; Ko, K.A.; Ture, S.; Morrell, C.; et al. Identification of activators of ERK5 transcriptional activity by high-throughput screening and the role of endothelial ERK5 in vasoprotective effects induced by statins and antimalarial agents. J. Immunol. 2014, 193, 3803–3815. [Google Scholar] [CrossRef]
- Patel, R.; Varghese, J.F.; Singh, R.P.; Yadav, U.C.S. Induction of endothelial dysfunction by oxidized low-density lipoproteins via downregulation of Erk-5/Mef2c/KLF2 signaling: Amelioration by fisetin. Biochimie 2019, 163, 152–162. [Google Scholar] [CrossRef]
- Deng, Y.; Lei, T.; Li, H.; Mo, X.; Wang, Z.; Ou, H. ERK5/KLF2 activation is involved in the reducing effects of puerarin on monocyte adhesion to endothelial cells and atherosclerotic lesion in apolipoprotein E-deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yun, S.; Lee, H.; Yang, J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa, B. Int. J. Mol. Sci. 2017, 18, 2497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, X.; Liang, T.; Bao, D.; Wang, Y.; Du, Y.; Li, H.; Du, J.; Chen, A.; Fu, Z.; et al. Effect of hyperbaric oxygen on tissue damage and expression of adhesion molecules and C3 in a rat model of renal ischemia-reperfusion injury after kidney transplantation. Ann. Transplant. 2020, 25, e919385. [Google Scholar] [CrossRef]
- Chen, Y.; Waqar, A.B.; Nishijima, K.; Ning, B.; Kitajima, S.; Matsuhisa, F.; Chen, L.; Liu, E.; Koike, T.; Yu, Y.; et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J. Cell Mol. Med. 2020, 24, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M.; et al. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals; National Institute of Health: Washington, DC, USA, 2011.
- Imanishi, M.; Izawa-Ishizawa, Y.; Sakurada, T.; Kohara, Y.; Horinouchi, Y.; Sairyo, E.; Zamami, Y.; Takechi, K.; Chuma, M.; Fukushima, K.; et al. Nitrosonifedipine, a photodegradation product of nifedipine, suppresses pharmacologically induced aortic aneurysm formation. Pharmacology 2018, 102, 287–299. [Google Scholar] [CrossRef]
- Droguett, D.; Castillo, C.; Leiva, E.; Theoduloz, C.; Schmeda-Hirschmann, G.; Kemmerling, U. Efficacy of quercetin against chemically induced murine oral squamous cell carcinoma. Oncol. Lett. 2015, 10, 2432–2438. [Google Scholar] [CrossRef]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol. Biol. 2012, 878, 121–135. [Google Scholar]

| Control, n (%) | AB, n (%) | AB + Q, n (%) | p Value * | |
|---|---|---|---|---|
| TAA | 0/18 (0%) | 3/18 (17%) | 2/20 (10%) | 0.653 |
| AAA | 0/18 (0%) | 13/18 (72%) | 9/20 (45%) | 0.090 |
| total aneurysm | 0/18 (0%) | 13/18 (72%) | 9/20 (45%) | 0.090 |
| rupture | 0/18 (0%) | 6/18 (33%) | 3/20 (15%) | 0.26 |
| Control, n (%) | LAB, n (%) | LAB + Q, n (%) | p Value | |
|---|---|---|---|---|
| Dissection | 0/17 (0%) | 6/18 (17%) | 4/25 (10%) | 0.275 |
| rupture | 0/17 (0%) | 4/18 (33%) | 0/25 (15%) | 0.025 * |
| Target gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| mF4/80 | CTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAGTTTATCGTG |
| mVCAM1 | CCATTGAAGATACCGGGAAAT | TAGCTGTCTGCTCCACAGGAT |
| mβactin | AAGTGTGACGTTGACATCCG | GATCCACATCTGCTGGAAG |
| hVCAM1 | GGTGGGACACAAATAAGGGTTTTGG | CTTGCAATTCTTTTACAGCCTGCC |
| heNOS | TGGTACATGAGCACTGAGATCG | CCACGTTGATTTCCACTGCTG |
| hβactin | GCGGGAAATCGTGCGTGACATTA | ATGGAGTTGAAGGTAGTTTCGTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, M.; Izawa-Ishizawa, Y.; Goda, M.; Hosooka, M.; Kagimoto, Y.; Saito, N.; Matsuoka, R.; Zamami, Y.; Chuma, M.; Yagi, K.; et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. Int. J. Mol. Sci. 2020, 21, 7226. https://doi.org/10.3390/ijms21197226
Kondo M, Izawa-Ishizawa Y, Goda M, Hosooka M, Kagimoto Y, Saito N, Matsuoka R, Zamami Y, Chuma M, Yagi K, et al. Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. International Journal of Molecular Sciences. 2020; 21(19):7226. https://doi.org/10.3390/ijms21197226
Chicago/Turabian StyleKondo, Masateru, Yuki Izawa-Ishizawa, Mitsuhiro Goda, Mayuko Hosooka, Yuu Kagimoto, Naoko Saito, Rie Matsuoka, Yoshito Zamami, Masayuki Chuma, Kenta Yagi, and et al. 2020. "Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice" International Journal of Molecular Sciences 21, no. 19: 7226. https://doi.org/10.3390/ijms21197226
APA StyleKondo, M., Izawa-Ishizawa, Y., Goda, M., Hosooka, M., Kagimoto, Y., Saito, N., Matsuoka, R., Zamami, Y., Chuma, M., Yagi, K., Takechi, K., Tsuneyama, K., & Ishizawa, K. (2020). Preventive Effects of Quercetin against the Onset of Atherosclerosis-Related Acute Aortic Syndromes in Mice. International Journal of Molecular Sciences, 21(19), 7226. https://doi.org/10.3390/ijms21197226





