Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms
Abstract
1. Introduction
2. Photosynthesis: Generating Energy for Biopolymer Synthesis
3. Unusual TCA Cycle as a Source of Key Biopolymer Intermediates
4. Glycogen and Carbon Storage in Cyanobacteria
5. Production of PHA in Cyanobacteria
6. EPS Produced by Cyanobacteria
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATP | Adenosine 5′-triphosphate |
| CBB | Calvin-Benson-Bassham cycle |
| CCM | Carbon concentration mechanism |
| Chl | Chlorophyll |
| CPS | Capsular polysaccharides |
| Cyclic di-GMP | Cyclic diguanylate |
| DCW | Dry cell weight |
| EPS | Extracellular polymeric substances |
| GABA | γ-aminobutyric acid |
| GT | Glycosyltransferases |
| LPS | Lipopolysaccharide |
| NAD+/NADH | Nicotinamide adenine dinucleotide |
| NADP+/NADPH | Nicotinamide adenine dinucleotide phosphate |
| NPQ | Non-photochemical quenching |
| OGDH | 2-oxogluterate dehydrogenase |
| OPP | Oxidative pentose phosphate |
| OPX | Extra-membrane polysaccharides |
| PCC | Pasteur Collection of Cyanobacteria |
| PCP | Polysaccharide copolymerase |
| PHA | Polyhydroxyalkanoate |
| PHB | Polyhydroxybutyrate |
| PSI | Photosystem I |
| PSII | Photosystem II |
| RPS | Released polymer substances |
| TCA | Tricarboxylic acid cycle |
| WT | Wild type |
References
- Zhang, W.; Song, X. (Eds.) Synthetic Biology of Cyanobacteria; Springer: Singapore, 2018; ISBN 9789811308536. [Google Scholar]
- Xue, X.M.; Yan, Y.; Xiong, C.; Raber, G.; Francesconi, K.; Pan, T.; Ye, J.; Zhu, Y.G. Arsenic biotransformation by a cyanobacterium Nostoc sp. PCC 7120. Environ. Pollut. 2017, 228, 111–117. [Google Scholar] [CrossRef]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.B.; Li, X.; Daroch, M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef]
- Tang, J.; Jiang, D.; Luo, Y.; Liang, Y.; Li, L.; Shah, M.M.R.; Daroch, M. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018, 31, 14–20. [Google Scholar] [CrossRef]
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Gale, G.A.R.; Osorio, A.A.S.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging species and genome editing tools: Future prospects in cyanobacterial synthetic biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef]
- Du, W.; Burbano, P.C.; Hellingwerf, K.J.; Branco dos Santos, F. Challenges in the application of synthetic biology toward synthesis of commodity products by cyanobacteria via “direct conversion”. Adv. Exp. Med. Biol. 2018, 1080, 3–26. [Google Scholar]
- Jones, P.R. Genetic instability in cyanobacteria—An elephant in the room? Front. Bioeng. Biotechnol. 2014, 2, 12. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Tyagi, M.B.; Kumar, A. Cyanobacteria: Applications in Biotechnology. In Cyanobacteria: From Basic Science to Applications; Elsevier: London, UK, 2019; ISBN 9780128146682. [Google Scholar]
- Soule, T.; Garcia-Pichel, F. Cyanobacteria. In Encyclopedia of Microbiology; Elsevier: San Diego, CA, USA, 2019; ISBN 9780128117378. [Google Scholar]
- Kirilovsky, D. Modulating energy arriving at photochemical reaction centers: Orange carotenoid protein-related photoprotection and state transitions. Photosynth. Res. 2015, 126, 3–17. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauß, N. Three-dimensional structure of cyanobaoterial photosystem I at 2.5 Å resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Q. Photoresponse mechanism in cyanobacteria: Key factor in photoautotrophic chassis. Adv. Exp. Med. Biol. 2018, 1080, 75–96. [Google Scholar]
- Muramatsu, M.; Sonoike, K.; Hihara, Y. Mechanism of downregulation of photosystem I content under high-light conditions in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 2009, 155, 989–996. [Google Scholar] [CrossRef]
- Czarnecki, O.; Grimm, B. Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. J. Exp. Bot. 2012, 63, 1675–1687. [Google Scholar] [CrossRef]
- Wilson, A.; Ajlani, G.; Verbavatz, J.M.; Vass, I.; Kerfeld, C.A.; Kirilovsky, D. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 2006, 18, 992–1007. [Google Scholar] [CrossRef]
- Karradt, A.; Sobanski, J.; Mattow, J.; Lockau, W.; Baier, K. NblA, a key protein of phycobilisome degradation, interacts with ClpC, a HSP100 chaperone partner of a cyanobacterial Clp protease. J. Biol. Chem. 2008, 283, 32394–32403. [Google Scholar] [CrossRef]
- Liang, F.; Lindblad, P. Effects of overexpressing photosynthetic carbon flux control enzymes in the cyanobacterium Synechocystis PCC 6803. Metab. Eng. 2016, 38, 56–64. [Google Scholar] [CrossRef]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef]
- Tamoi, M.; Miyazaki, T.; Fukamizo, T.; Shigeoka, S. The Calvin cycle in cyanobacteria is regulated by CP12 via the NAD(H)/NADP(H) ratio under light/dark conditions. Plant J. 2005, 42, 504–513. [Google Scholar] [CrossRef]
- Makowka, A.; Nichelmann, L.; Schulze, D.; Spengler, K.; Wittmann, C.; Forchhammer, K.; Gutekunst, K. Glycolytic Shunts Replenish the Calvin–Benson–Bassham Cycle as Anaplerotic Reactions in Cyanobacteria. Mol. Plant 2020, 13, 471–482. [Google Scholar] [CrossRef]
- Thompson, L.R.; Zeng, Q.; Kelly, L.; Huang, K.H.; Singer, A.U.; Stubbe, J.A.; Chisholm, S.W. Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism. Proc. Natl. Acad. Sci. USA 2011, 108, E757–E764. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, D.; Fernie, A.R.; Araújo, W.L. Unusual cyanobacterial TCA cycles: Not broken just different. Trends Plant Sci. 2012, 17, 503–509. [Google Scholar] [CrossRef]
- Mills, L.A.; McCormick, A.J.; Lea-Smith, D.J. Current knowledge and recent advances in understanding metabolism of the model cyanobacterium Synechocystis sp. PCC 6803. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Broddrick, J.T.; Rubin, B.E.; Welkie, D.G.; Du, N.; Mih, N.; Diamond, S.; Lee, J.J.; Golden, S.S.; Palsson, B.O. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA 2016, 113, E8344–E8353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bryant, D.A. The tricarboxylic acid cycle in cyanobacteria. Science 2011, 334, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qian, X.; Chang, S.; Dismukes, G.C.; Bryant, D.A. Natural and synthetic variants of the tricarboxylic acid cycle in cyanobacteria: Introduction of the GABA Shunt into Synechococcus sp. PCC 7002. Front. Microbiol. 2016, 7, 1972. [Google Scholar] [CrossRef]
- Knoop, H.; Zilliges, Y.; Lockau, W.; Steuer, R. The metabolic network of Synechocystis sp. PCC 6803: Systemic properties of autotrophic growth. Plant Physiol. 2010, 154, 410–422. [Google Scholar] [CrossRef]
- Zhang, S.; Bryant, D.A. Biochemical validation of the glyoxylate cycle in the cyanobacterium Chlorogloeopsis fritschii strain PCC 9212. J. Biol. Chem. 2015, 290, 14019–14030. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, Y.; Lun, D.S.; Dismukes, G.C. Rerouting of Metabolism into Desired Cellular Products by Nutrient Stress: Fluxes Reveal the Selected Pathways in Cyanobacterial Photosynthesis. ACS Synth. Biol. 2018, 7, 1465–1476. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Asención Diez, M.D.; Kuhn, M.L.; McEwen, S.; Salerno, G.L.; Iglesias, A.A.; Ballicora, M.A. The unique nucleotide specificity of the sucrose synthase from Thermosynechococcus elongatus. FEBS Lett. 2013, 587, 165–169. [Google Scholar] [CrossRef]
- Hickman, J.W.; Kotovic, K.M.; Miller, C.; Warrener, P.; Kaiser, B.; Jurista, T.; Budde, M.; Cross, F.; Roberts, J.M.; Carleton, M. Glycogen synthesis is a required component of the nitrogen stress response in Synechococcus elongatus PCC 7942. Algal Res. 2013, 2, 98–106. [Google Scholar] [CrossRef]
- Knoop, H.; Gründel, M.; Zilliges, Y.; Lehmann, R.; Hoffmann, S.; Lockau, W.; Steuer, R. Flux Balance Analysis of Cyanobacterial Metabolism: The Metabolic Network of Synechocystis sp. PCC 6803. PLoS Comput. Biol. 2013, 9, e1003081. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Wu, G.; Zhao, N. Changes in photosynthesis and pigmentation in an agp deletion mutant of the cyanobacterium Synechocystis sp. Biotechnol. Lett. 2003, 25, 391–396. [Google Scholar] [CrossRef]
- Fu, J.; Xu, X. The functional divergence of two glgP homologues in Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 2006, 260, 201–209. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Wang, M.; Lu, X. Progress and perspective on cyanobacterial glycogen metabolism engineering. Biotechnol. Adv. 2019, 37, 771–786. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, B.H.; Moon, Y.; Spalding, M.H.; Jane, J.L. Glycogen synthase isoforms in Synechocystis sp. PCC6803: Identification of different roles to produce glycogen by targeted mutagenesis. PLoS ONE 2014, 9, e91524. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Wu, G.; Zhao, N. Sucrose accumulation in salt-stressed cells of agp gene deletion-mutant in cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 2003, 218, 71–77. [Google Scholar] [CrossRef]
- Gründel, M.; Scheunemann, R.; Lockau, W.; Zilliges, Y. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology (UK) 2012, 158, 3032–3043. [Google Scholar] [CrossRef]
- Xu, Y.; Tiago Guerra, L.; Li, Z.; Ludwig, M.; Charles Dismukes, G.; Bryant, D.A. Altered carbohydrate metabolism in glycogen synthase mutants of Synechococcus sp. strain PCC 7002: Cell factories for soluble sugars. Metab. Eng. 2013, 16, 56–67. [Google Scholar] [CrossRef]
- Guerra, L.T.; Xu, Y.; Bennette, N.; McNeely, K.; Bryant, D.A.; Dismukes, G.C. Natural osmolytes are much less effective substrates than glycogen for catabolic energy production in the marine cyanobacterium Synechococcus sp. strain PCC 7002. J. Biotechnol. 2013, 166, 65–75. [Google Scholar] [CrossRef]
- Yao, L.; Cengic, I.; Anfelt, J.; Hudson, E.P. Multiple Gene Repression in Cyanobacteria Using CRISPRi. ACS Synth. Biol. 2016, 5, 207–212. [Google Scholar] [CrossRef]
- Huang, C.H.; Shen, C.R.; Li, H.; Sung, L.Y.; Wu, M.Y.; Hu, Y.C. CRISPR interference (CRISPRi) for gene regulation and succinate production in cyanobacterium S. elongatus PCC 7942. Microb. Cell Fact. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Sun, T.; Li, S.; Song, X.; Pei, G.; Diao, J.; Cui, J.; Shi, M.; Chen, L.; Zhang, W. Re-direction of carbon flux to key precursor malonyl-CoA via artificial small RNAs in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2018, 11, 26. [Google Scholar] [CrossRef]
- Qiao, C.; Duan, Y.; Zhang, M.; Hagemann, M.; Luo, Q.; Lu, X. Effects of Reduced and Enhanced Glycogen Pools on Salt-Induced Sucrose Production in a Sucrose-Secreting Strain of Synechococcus sp. PCC 7942. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, S.; Sun, H.; Duan, Y.; Qiao, C.; Luan, G.; Lu, X. Adopting a theophylline-responsive riboswitch for flexible regulation and understanding of glycogen metabolism in Synechococcus elongatus PCC7942. Front. Microbiol. 2019, 10, 551. [Google Scholar] [CrossRef]
- Jacobsen, J.H.; Frigaard, N.U. Engineering of photosynthetic mannitol biosynthesis from CO2 in a cyanobacterium. Metab. Eng. 2014, 21, 60–70. [Google Scholar] [CrossRef]
- Suzuki, E.; Ohkawa, H.; Moriya, K.; Matsubara, T.; Nagaike, Y.; Iwasaki, I.; Fujiwara, S.; Tsuzuki, M.; Nakamura, Y. Carbohydrate metabolism in mutants of the cyanobacterium Synechococcus elongatus PCC 7942 defective in glycogen synthesis. Appl. Environ. Microbiol. 2010, 76, 3153–3159. [Google Scholar] [CrossRef]
- Carrieri, D.; Paddock, T.; Maness, P.C.; Seibert, M.; Yu, J. Photo-catalytic conversion of carbon dioxide to organic acids by a recombinant cyanobacterium incapable of glycogen storage. Energy Environ. Sci. 2012, 5, 9457–9461. [Google Scholar] [CrossRef]
- Van der Woude, A.D.; Angermayr, S.A.; Puthan Veetil, V.; Osnato, A.; Hellingwerf, K.J. Carbon sink removal: Increased photosynthetic production of lactic acid by Synechocystis sp. PCC6803 in a glycogen storage mutant. J. Biotechnol. 2014, 184, 100–102. [Google Scholar] [CrossRef]
- Work, V.H.; Melnicki, M.R.; Hill, E.A.; Davies, F.K.; Kucek, L.A.; Beliaev, A.S.; Posewitz, M.C. Lauric acid production in a glycogen-less strain of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Zhang, X.; Singapuri, S.P.; Kalra, I.; Liu, X.; Morgan-Kiss, R.M.; Wang, X. Glycogen metabolism supports photosynthesis start through the oxidative pentose phosphate pathway in cyanobacteria. Plant Physiol. 2020, 182, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Benson, P.J.; Purcell-Meyerink, D.; Hocart, C.H.; Truong, T.T.; James, G.O.; Rourke, L.; Djordjevic, M.A.; Blackburn, S.I.; Price, G.D. Factors altering pyruvate excretion in a glycogen storage mutant of the cyanobacterium, Synechococcus PCC7942. Front. Microbiol. 2016, 7, 475. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, C.R.; Huang, C.H.; Sung, L.Y.; Wu, M.Y.; Hu, Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab. Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Shimakawa, G.; Hasunuma, T.; Kondo, A.; Matsuda, M.; Makino, A.; Miyake, C. Respiration accumulates Calvin cycle intermediates for the rapid start of photosynthesis in Synechocystis sp. PCC 6803. Biosci. Biotechnol. Biochem. 2014, 78, 1997–2007. [Google Scholar] [CrossRef]
- Jacobsen, J.H.; Rosgaard, L.; Sakuragi, Y.; Frigaard, N.U. One-step plasmid construction for generation of knock-out mutants in cyanobacteria: Studies of glycogen metabolism in Synechococcus sp. PCC 7002. Photosynth. Res. 2011, 107, 215–221. [Google Scholar] [CrossRef]
- Sarkar, D.; Mueller, T.J.; Liu, D.; Pakrasi, H.B.; Maranas, C.D. A diurnal flux balance model of Synechocystis sp. PCC 6803 metabolism. PLoS Comput. Biol. 2019, 15, e1006692. [Google Scholar] [CrossRef]
- Andreas Angermayr, S.; van Alphen, P.; Hasdemir, D.; Kramer, G.; Iqbal, M.; van Grondelle, W.; Hoefsloot, H.C.; Choi, Y.H.; Hellingwerf, K.J. Culturing synechocystis sp. Strain pcc 6803 with N2 and CO2 in a diel regime reveals multiphase glycogen dynamics with low maintenance costs. Appl. Environ. Microbiol. 2016, 82, 4180–4189. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Jabbur, M.L.; Johnson, C.H.; van Sebille, E.; Sasaki, H. Circadian clock helps cyanobacteria manage energy in coastal and high latitude ocean. ISME J. 2020, 14, 560–568. [Google Scholar] [CrossRef]
- Cano, M.; Holland, S.C.; Artier, J.; Burnap, R.L.; Ghirardi, M.; Morgan, J.A.; Yu, J. Glycogen Synthesis and Metabolite Overflow Contribute to Energy Balancing in Cyanobacteria. Cell Rep. 2018, 23, 667–672. [Google Scholar] [CrossRef]
- Tan, X.; Hou, S.; Song, K.; Georg, J.; Klähn, S.; Lu, X.; Hess, W.R. The primary transcriptome of the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Biotechnol. Biofuels 2018, 11, 218. [Google Scholar] [CrossRef]
- Heilmann, B.; Hakkila, K.; Georg, J.; Tyystjärvi, T.; Hess, W.R.; Axmann, I.M.; Dienst, D. 6S RNA plays a role in recovery from nitrogen depletion in Synechocystis sp. PCC 6803. BMC Microbiol. 2017, 17, 229. [Google Scholar] [CrossRef]
- Alonso-Casajús, N.; Dauvillée, D.; Viale, A.M.; Muñoz, F.J.; Baroja-Fernández, E.; Morán-Zorzano, M.T.; Eydallin, G.; Ball, S.; Pozueta-Romero, J. Glycogen phosphorylase, the product of the glgP gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J. Bacteriol. 2006, 188, 5266–5272. [Google Scholar] [CrossRef]
- Liberton, M.; Bandyopadhyay, A.; Pakrasi, H.B. Enhanced nitrogen fixation in a glgX-deficient strain of Cyanothece sp. strain ATCC 51142, a unicellular nitrogen-fixing cyanobacterium. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Xu, X. Effects of a Type I RM System on Gene Expression and Glycogen Catabolism in Synechocystis sp. PCC 6803. Front. Microbiol. 2020, 11, 1258. [Google Scholar] [CrossRef]
- Li, X.; Shen, C.R.; Liao, J.C. Isobutanol production as an alternative metabolic sink to rescue the growth deficiency of the glycogen mutant of Synechococcus elongatus PCC 7942. Photosynth. Res. 2014, 120, 301–310. [Google Scholar] [CrossRef]
- Oliver, N.J.; Rabinovitch-Deere, C.A.; Carroll, A.L.; Nozzi, N.E.; Case, A.E.; Atsumi, S. Cyanobacterial metabolic engineering for biofuel and chemical production. Curr. Opin. Chem. Biol. 2016, 35, 43–50. [Google Scholar] [CrossRef]
- Koch, M.; Doello, S.; Gutekunst, K.; Forchhammer, K. PHB is produced from Glycogen turn-over during nitrogen starvation in Synechocystis sp. PCC 6803. Int. J. Mol. Sci. 2019, 20, 1942. [Google Scholar] [CrossRef]
- Hauf, W.; Watzer, B.; Roos, N.; Klotz, A.; Forchhammer, K. Photoautotrophic polyhydroxybutyrate granule formation is regulated by cyanobacterial phasin PhaP in Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2015, 81, 4411–4422. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364, 1–13. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Hauf, W.; Schlebusch, M.; Hüge, J.; Kopka, J.; Hagemann, M.; Forchhammer, K. Metabolic changes in Synechocystis PCC6803 upon nitrogen-starvation: Excess NADPH sustains polyhydroxybutyrate accumulation. Metabolites 2013, 3, 101. [Google Scholar] [CrossRef]
- Osanai, T.; Shirai, T.; Iijima, H.; Kuwahara, A.; Suzuki, I.; Kondo, A.; Hirai, M.Y. Alteration of cyanobacterial sugar and amino acid metabolism by overexpression hik8, encoding a KaiC-associated histidine kinase. Environ. Microbiol. 2015, 17, 2430–2440. [Google Scholar] [CrossRef]
- Hirai, K.; Nojo, M.; Sato, Y.; Tsuzuki, M.; Sato, N. Contribution of protein synthesis depression to poly-β-hydroxybutyrate accumulation in Synechocystis sp. PCC 6803 under nutrient-starved conditions. Sci. Rep. 2019, 9, 19944. [Google Scholar] [CrossRef]
- Koch, M.; Berendzen, K.W.; Forchhammer, K. On the role and production of polyhydroxybutyrate (Phb) in the cyanobacterium synechocystis sp. pcc 6803. Life 2020, 10, 47. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yao, C.; Cao, X.; Tian, J.; Xue, S. FabG can function as PhaB for poly-3-hydroxybutyrate biosynthesis in photosynthetic cyanobacteria Synechocystis sp. PCC 6803. Bioengineered 2017, 8, 707–715. [Google Scholar] [CrossRef]
- Osanai, T.; Numata, K.; Oikawa, A.; Kuwahara, A.; Iijima, H.; Doi, Y.; Tanaka, K.; Saito, K.; Hirai, M.Y. Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in synechocystis sp. PCC 6803. DNA Res. 2013, 20, 525–535. [Google Scholar] [CrossRef]
- Osanai, T.; Oikawa, A.; Numata, K.; Kuwahara, A.; Iijima, H.; Doi, Y.; Saito, K.; Hirai, M.Y. Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 2014, 164, 1831–1841. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Disruption of polyhydroxybutyrate synthesis redirects carbon flow towards glycogen synthesis in Synechocystis sp. PCC 6803 Overexpressing glgC/glgA. Plant Cell Physiol. 2018, 59, 2020–2029. [Google Scholar] [CrossRef]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef]
- Koch, M.; Orthwein, T.; Alford, J.T.; Forchhammer, K. The Slr0058 Protein From Synechocystis sp. PCC 6803 Is a Novel Regulatory Protein Involved in PHB Granule Formation. Front. Microbiol. 2020, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Carpine, R.; Du, W.; Olivieri, G.; Pollio, A.; Hellingwerf, K.J.; Marzocchella, A.; Branco dos Santos, F. Genetic engineering of Synechocystis sp. PCC6803 for poly-β-hydroxybutyrate overproduction. Algal Res. 2017, 25, 117–127. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Kovacs, T.; Pflügl, S.; Druzhinina, I.; Kroll, P.; Lackner, M.; Herwig, C. Increased poly-Β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour. Technol. 2018, 266, 34–44. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Bryant, D.A. Metabolic engineering of Synechococcus sp. PCC 7002 to produce poly-3-hydroxybutyrate and poly-3-hydroxybutyrate-co-4-hydroxybutyrate. Metab. Eng. 2015, 32, 174–183. [Google Scholar] [CrossRef]
- Pereira, S.B.; Mota, R.; Vieira, C.P.; Vieira, J.; Tamagnini, P. Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci. Rep. 2015, 5, 14835. [Google Scholar] [CrossRef]
- Singh, M.K.; Rai, P.K.; Rai, A.; Singh, S.; Singh, J.S. Poly-β-hydroxybutyrate production by the cyanobacterium scytonema geitleri bharadwaja under varying environmental conditions. Biomolecules 2019, 9, 198. [Google Scholar] [CrossRef]
- Flaibani, A.; Olsen, Y.; Painter, T.J. Polysaccharides in desert reclamation: Compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr. Res. 1989, 190, 235–248. [Google Scholar] [CrossRef]
- Mazor, G.; Kidron, G.J.; Vonshak, A.; Abeliovich, A. The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol. Ecol. 1996, 21, 121–130. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kant, C.; Yadav, R.K.; Reddy, Y.P.; Abraham, G. Cyanobacterial Exopolysaccharides: Composition, Biosynthesis, and Biotechnological Applications. In Cyanobacteria: From Basic Science to Applications; Elsevier Inc.: London, UK, 2018; ISBN 9780128146682. [Google Scholar]
- Kehr, J.C.; Dittmann, E. Biosynthesis and function of extracellular glycans in cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Philippis, R. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae. Developments in Applied Phycology; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 6, pp. 565–590. [Google Scholar] [CrossRef]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Morona, R.; Purins, L.; Tocilj, A.; Matte, A.; Cygler, M. Sequence-structure relationships in polysaccharide co-polymerase (PCP) proteins. Trends Biochem. Sci. 2009, 34, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Mainprize, I.L.; Naismith, J.H.; Whitfield, C. Pivotal Roles of the Outer Membrane Polysaccharide Export and Polysaccharide Copolymerase Protein Families in Export of Extracellular Polysaccharides in Gram-Negative Bacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 155–177. [Google Scholar] [CrossRef]
- Durai, P.; Batool, M.; Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef]
- Pereira, S.B.; Santos, M.; Leite, J.P.; Flores, C.; Eisfeld, C.; Büttel, Z.; Mota, R.; Rossi, F.; De Philippis, R.; Gales, L.; et al. The role of the tyrosine kinase Wzc (Sll0923) and the phosphatase Wzb (Slr0328) in the production of extracellular polymeric substances (EPS) by Synechocystis PCC 6803. Microbiologyopen 2019, 8, e00753. [Google Scholar] [CrossRef]
- Ohki, K.; Kanesaki, Y.; Suzuki, N.; Okajima, M.; Kaneko, T.; Yoshikawa, S. Physiological properties and genetic analysis related to exopolysaccharide (EPS) production in the fresh-water unicellular cyanobacterium aphanothece sacrum (suizenji nori). J. Gen. Appl. Microbiol. 2019, 65, 39–46. [Google Scholar] [CrossRef]
- Gloaguen, V.; Morvan, H.; Hoffmann, L.; Sainte Catherine, O.; Kraemer, M.; Krausz, P. Bioactive capsular polysaccharide from the thermophilic cyanophyte/cyanobacterium Mastigodadus laminosus—Cytotoxic properties. Planta Med. 2007, 73, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.C.; Howell, P.L. Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 2013, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Komoda, K.; Sakurai, N.; Tajima, K.; Tanaka, I.; Yao, M. The c-di-GMP recognition mechanism of the PilZ domain of bacterial cellulose synthase subunit A. Biochem. Biophys. Res. Commun. 2013, 431, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Nobles, D.R.; Romanovicz, D.K.; Brown, J. Cellulose in cyanobacteria. Origin of vascular plant cellulose synthase? Plant Physiol. 2001, 127, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Z.; Li, T.; Zhang, Y.; Bryant, D.A.; Zhao, J. High-yield production of extracellular type-I cellulose by the cyanobacterium Synechococcus sp. PCC 7002. Cell Discov. 2015, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Saotome, T.; Ochiai, Y.; Katayama, M.; Narikawa, R.; Ikeuchi, M. Cellulose accumulation and a cellulose synthase gene are responsible for cell aggregation in the cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 2011, 52, 957–966. [Google Scholar] [CrossRef]
- Maeda, K.; Tamura, J.; Okuda, Y.; Narikawa, R.; Midorikawa, T.; Ikeuchi, M. Genetic identification of factors for extracellular cellulose accumulation in the thermophilic cyanobacterium Thermosynechococcus vulcanus: Proposal of a novel tripartite secretion system. Mol. Microbiol. 2018, 109, 121–134. [Google Scholar] [CrossRef]
- Milou Schuurmans, R.; Matthijs, H.C.P.; Stal, L.J.; Hellingwerf, K.J. Cyanobacterial cellulose synthesis in the light of the photanol concept. In Cyanobacteria: An Economic Perspective; John Wiley and Sons: Chichester, UK, 2013; ISBN 9781118402238. [Google Scholar]
- Trabelsi, L.; Ben Ouada, H.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Ge, H.; Xia, L.; Zhou, X.; Zhang, D.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J. Microbiol. 2014, 52, 179–183. [Google Scholar] [CrossRef]
- Vonshak, A. Spirulina Platensis (Arthropsira): Physiology, Cell-Biology and Biotechnology; Taylor and Francis: London, UK, 2002; ISBN 0748406743. [Google Scholar]
- Carvalho, A.P.; Monteiro, C.M.; Malcata, F.X. Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri. J. Appl. Phycol. 2009, 21, 543–552. [Google Scholar] [CrossRef]
- De Philippis, R.; Margheri, M.C.; Pelosi, E.; Ventura, S. Exopolysaccharide production by a unicellular cyanobacterium isolated from a hypersaline habitat. J. Appl. Phycol. 1993, 5, 387–394. [Google Scholar] [CrossRef]
- Huang, W.J.; Lai, C.H.; Cheng, Y.L. Evaluation of extracellular products and mutagenicity in cyanobacteria cultures separated from a eutrophic reservoir. Sci. Total Environ. 2007, 377, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Shen, S.G.; Wang, H.Y.; Sun, Y.; Dai, Y.J.; Jia, S.R. Comparative metabolomic analysis of the effects of light quality on polysaccharide production of cyanobacterium Nostoc flagelliforme. Algal Res. 2015, 9, 143–150. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Mugnai, G.; Simiani, A.; De Philippis, R. Soil Type and Cyanobacteria Species Influence the Macromolecular and Chemical Characteristics of the Polysaccharidic Matrix in Induced Biocrusts. Microb. Ecol. 2019, 78, 482–493. [Google Scholar] [CrossRef]
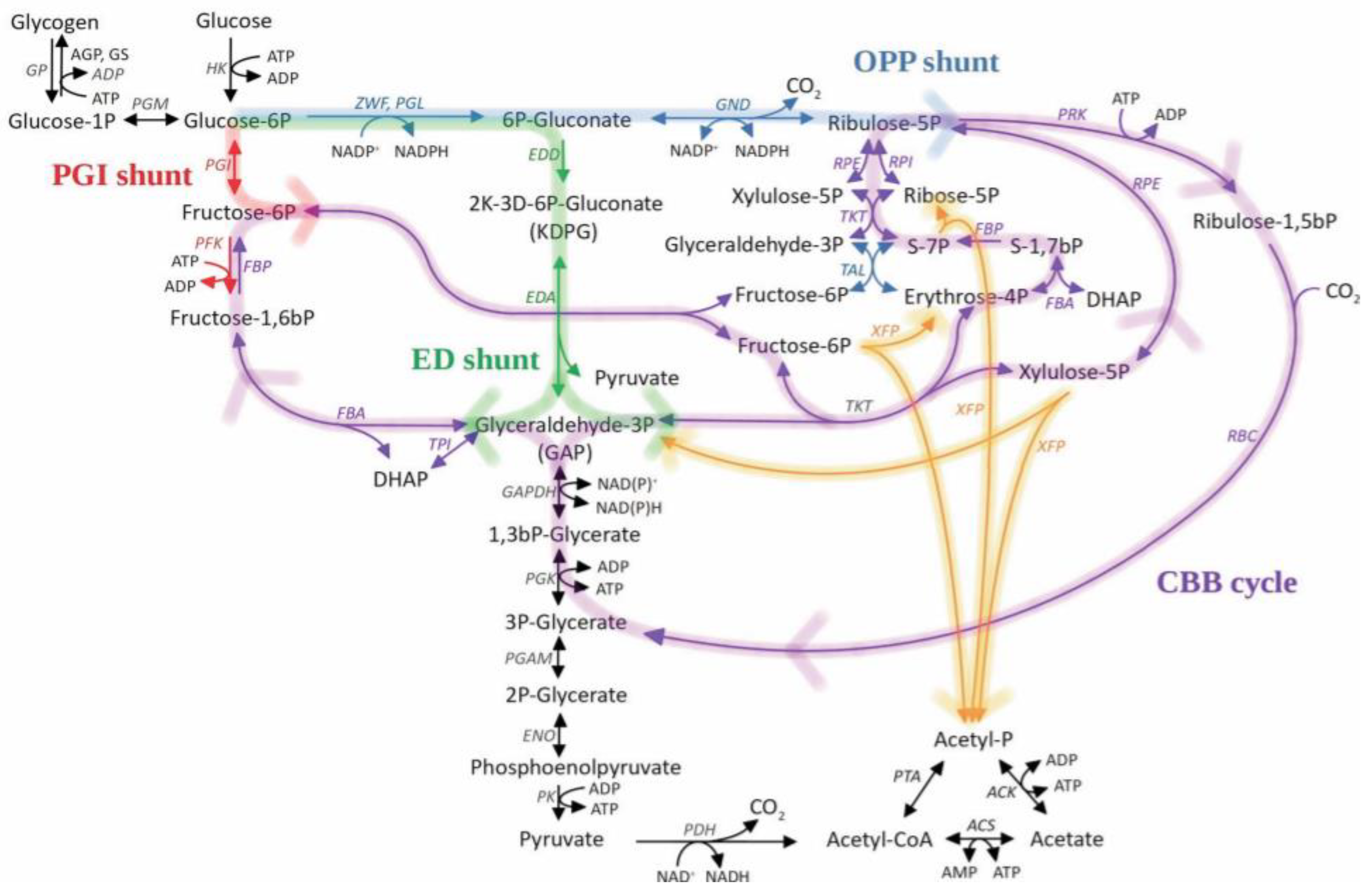
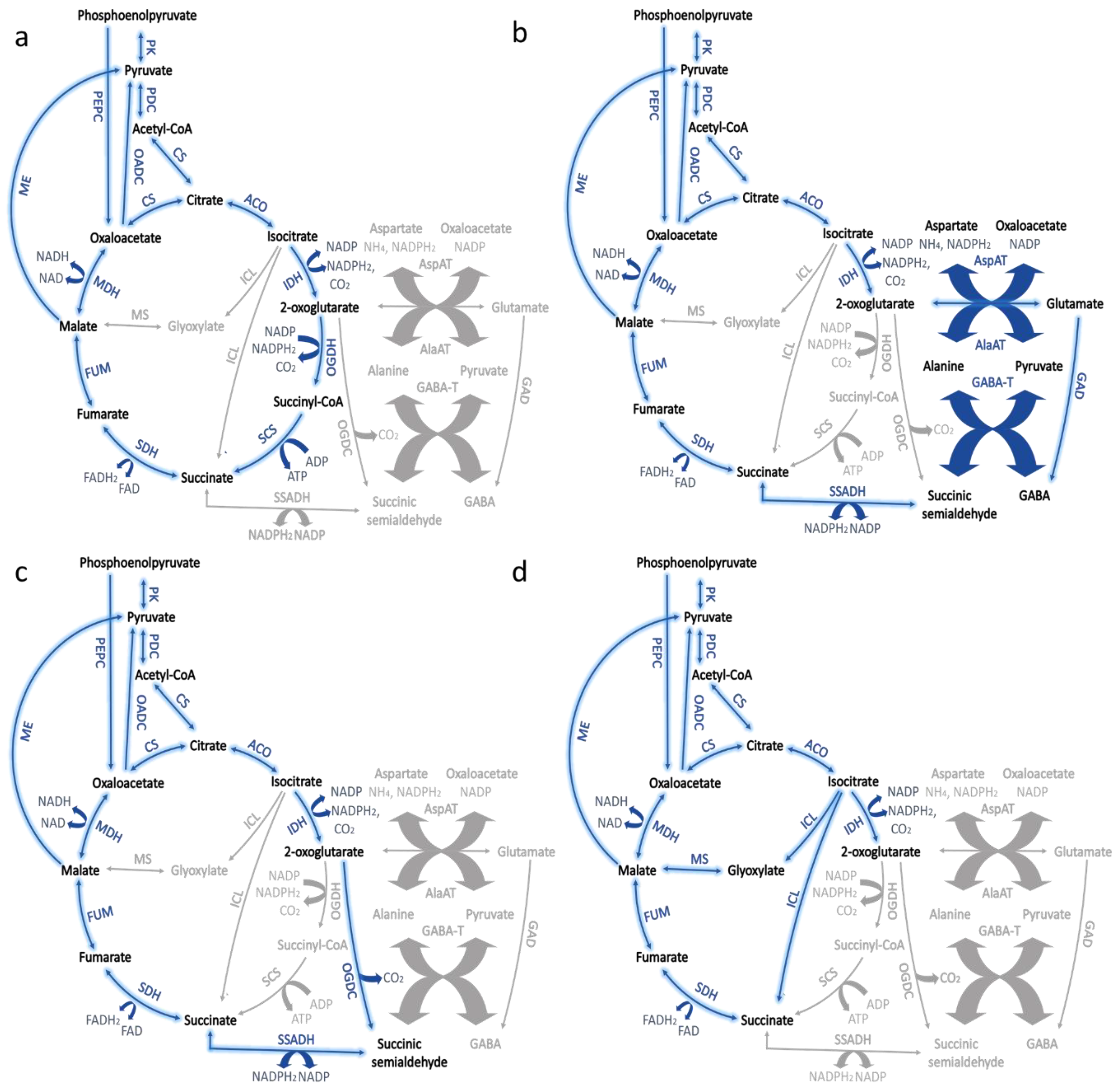
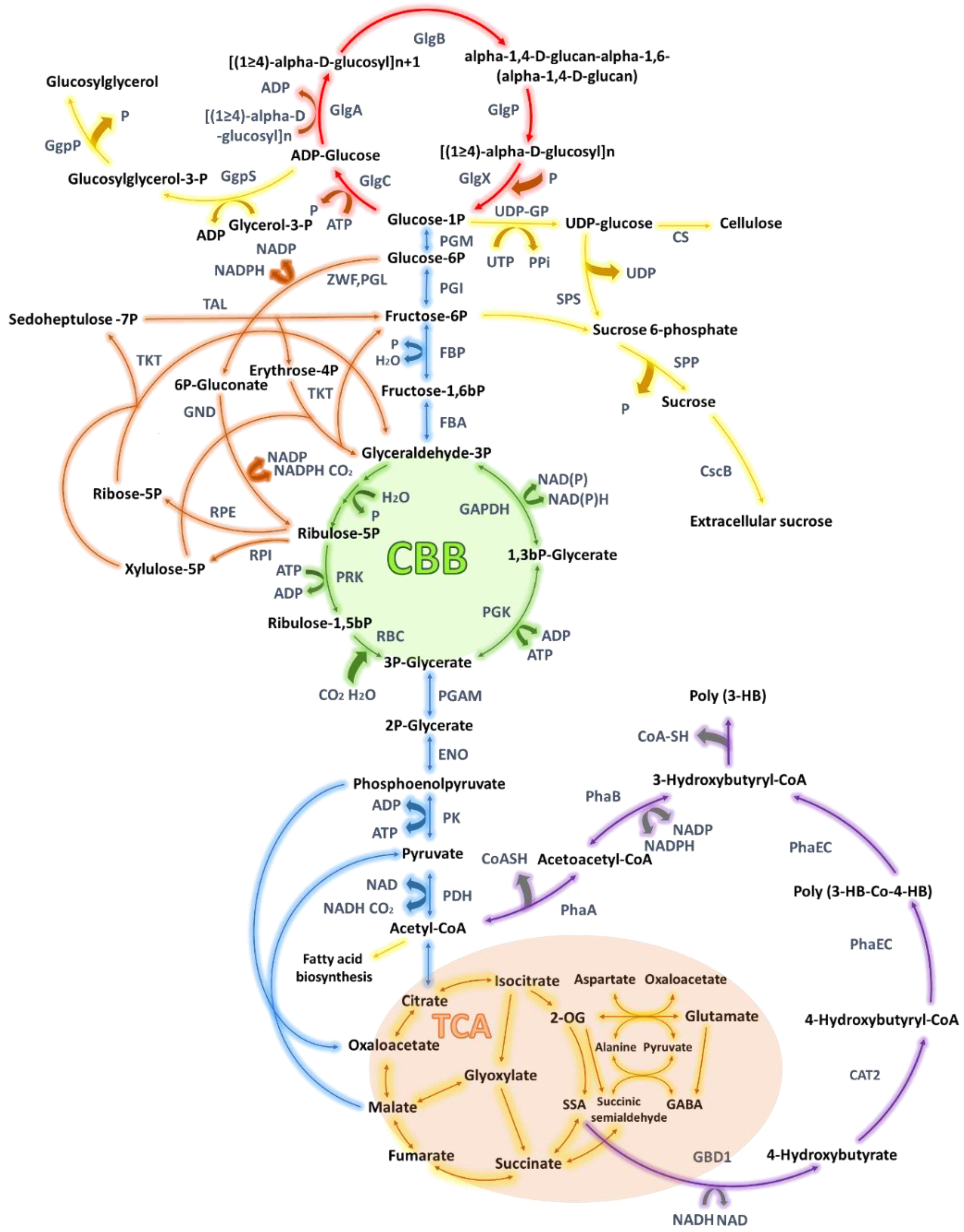

| Species | Modification | Culture Conditions | Change in Glycogen Content | Phenotype | Reference |
|---|---|---|---|---|---|
| S. sp. PCC 6803 | ΔglgA1-ΔglgA2 | BG-11 without nitrogen | Full inhibition of synthesis | Cell division and phycobilisome degradation capacity impairment | [42] |
| S. sp. PCC 6803 | ΔglgA1 or ΔglgA1 | BG-11 without nitrogen | No impact | No impact | [42] |
| S. sp. PCC 7002 | ΔglgAI-ΔglgAII | 38 °C 1% (v/v) CO2 | 4,2% WT | 280% WT of sucrose 172% WT of glucosylglycerate | [43] |
| 38 °C 1% (v/v) CO2, hypersaline | 4,4% WT | 295% WT of sucrose 171% WT of glucosylglycerate | [43] | ||
| 38 °C 1% (v/v) CO2, N-limiting | 7,2% WT | 84% WT of sucrose 60% WT of glucosylglycerate | [43] | ||
| ΔglgAI | 38 °C 1% (v/v) CO2 | 65% WT | 260% WT of sucrose 112% WT of glucosylglycerate | [43] | |
| 38 °C 1% (v/v) CO2, N-limiting | 67% WT | 38% WT of sucrose 55% WT of glucosylglycerate | [43] | ||
| ΔglgAII | 38 °C 1% (v/v) CO2 | 60% WT | 220% WT of sucrose 108% WT of glucosylglycerate | [43] | |
| 38 °C 1% (v/v) CO2, N-limiting | 60% WT | 26% WT of sucrose 21% WT of glucosylglycerate | [43] | ||
| S. sp. PCC 7002 | ΔglgAI-ΔglgAII | 30 °C | <1% WT | Slight reduction in doubling time | [50] |
| S. e. PCC 7942 | ΔglgA | 0.2 M NaCl | Lowering > 90% | Decrease of oxygen secretion by 50% | [51] |
| S. sp. PCC 6803 | ΔglgC | - | Not compatible | Not compatible | [42,52,53] |
| S. sp. PCC 7002 | ΔglgC | - | Not compatible | Not compatible | [44,54] |
| S. e. PCC 7942 | ΔglgC | - | Not compatible | Not compatible | [35,55,56,57] |
| S. sp. PCC 6803 | glgC-knockdown by CRISPRi | 28 °C 1% (v/v) nitrogen starvation | Lowering by 75% | Non-chlorosis | [45] |
| S. e. PCC 7942 | glgC-knockdown by CRISPRi | BG-11 without nitrogen | Lowering by 74.5-95.2% | Non-chlorosis | [46] |
| S. sp. PCC 6803 | glgC-knockdown by small RNA regulatory tools | BG-11, 30 °C, 50 μmol photons m- 2 s- 1 | Lowering by 75% | Not reported | [47] |
| S. sp. PCC 6803 | glgC- riboswitch regulation | Theophylline 0 μM | Lowering by 60% | No change in growth | [49] |
| Theophylline 1100 μM | Increase by 300% | Slightly increased growth | [49] | ||
| S. e. PCC 7942 | glgC-knockdown by riboswitch | 30 °C 150 mM NaCl 1mM IPTG Theophylline 0 μM | Lowering by 90% | Slight decrease in cell growth, decrease in sucrose accumulation | [48] |
| S. sp. PCC 6803 | ΔglgP1-ΔglgP2 | Constant light 2% CO225 °C | 107% WT | No impact on chlorophyll content | [58] |
| S. sp. PCC 6803 | ΔglgP1-ΔglgP2 | In light during 12 h cycles 2% CO2 25 °C | Increase by 236% | Decrease of chlorophyl lcontent | [58] |
| S. sp. PCC 6803 | ΔglgP1-ΔglgP2 | In dark during 12 h cycles 2% CO2 25 °C | Increase by 420% | Decrease of chlorophyl lcontent | [58] |
| S. sp. PCC 7002 | ΔglgP | 1% v/v CO2 38 °C | Not reported | No effect on growth | [59] |
| S. e. PCC 7942 | OE-cscB, OE-sps, OE-glgC | 30 °C 150 mM NaCl 1 mM IPTG | 160% WT | Increase in the amount of sucrose | [48] |
| Name of Species | Modification | Culture Conditions | % PHA (dry cell weight) | Time [d] | Phenotype | Reference |
|---|---|---|---|---|---|---|
| S. sp. PCC 6803 | OE pha AB | Nitrogen deficiency | 26 % | 9 | Slower growth | [75] |
| OE pha AB | Nitrogen deficiency + 0.4% acetate | 35% | Slower growth | [75] | ||
| S. sp. PCC 6803 | WT | Nitrogen deficiency | ∼15% | 14 | 90 µg/108 cells of glycogen | [71] |
| Δ glgP1 | 17% | 90 µg/108 cells of glycogen | [71] | |||
| Δ glgP2 | 2% | 125 µg/108 cells of glycogen | [71] | |||
| Δ glgP1/2 | 2% | 110 µg/108 cells of glycogen | [71] | |||
| Δ pfk1/2 | 2.6% | 110 µg/108 cells of glycogen | [71] | |||
| Δ gnd | 7% | 105 µg/108 cells of glycogen | [71] | |||
| Δ pfk1 | 11% | 105 µg/108 cells of glycogen | [71] | |||
| Δ pfk2 | 12,5% | 110 µg/108 cells of glycogen | [71] | |||
| Δ eda | 17% | 120 µg/108 cells of glycogen | [71] | |||
| Δ glgA1 | 6% | 103 µg/108 cells of glycogen | [71] | |||
| Δ glgA2 | 17% | 110 µg/108 of glycogen cells | [71] | |||
| Δ glgC | 18% | Lack of glycogen | [71] | |||
| S. sp. PCC 6803 | OE xfpk | 2% CO2 | 5% | 25 | Accumulation before nitrogen depletion | [76] |
| OE xfpk pta and ach knock out. | 12% | 32 | Accumulation before nitrogen depletion | [76] | ||
| S. sp. PCC 6803 | WT | Light + nitrogen deficiency | 12% | 13 | Not reported | [77] |
| Light limitation + nitrogen deficiency | 17% | [77] | ||||
| Δ zfk | Light + nitrogen deficiency | 13% | [77] | |||
| Light limitation + nitrogen deficiency | 19% | [77] | ||||
| Δ pfk | Light + nitrogen deficiency | 6% | [77] | |||
| Light limitation + nitrogen deficiency | 2% | [77] | ||||
| S. sp. PCC 6803 | WT | Nitrogen deficiency | 9.5% | 7 | 3% fatty acid content under N deprivation | [78] |
| Δ phaB | 0% | 2,8% fatty acid content under N deprivation | [78] | |||
| OV pfabG + ΔphaB | 3% | 2,5% fatty acid content under N deprivation | [78] | |||
| S. sp. PCC 6714 | WT | Nitrogen and phosphorus deficiency | 6% | 7 | [79] | |
| Random mutatons | To 29% | Not compatible | [79] | |||
| S. sp. PCC 7002 | ΔA0171-PHB | Light + CO2 | ∼4.5% | P(3HB-co-4HV) | Increase of total cell dry weight | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciebiada, M.; Kubiak, K.; Daroch, M. Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms. Int. J. Mol. Sci. 2020, 21, 7204. https://doi.org/10.3390/ijms21197204
Ciebiada M, Kubiak K, Daroch M. Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms. International Journal of Molecular Sciences. 2020; 21(19):7204. https://doi.org/10.3390/ijms21197204
Chicago/Turabian StyleCiebiada, Maciej, Katarzyna Kubiak, and Maurycy Daroch. 2020. "Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms" International Journal of Molecular Sciences 21, no. 19: 7204. https://doi.org/10.3390/ijms21197204
APA StyleCiebiada, M., Kubiak, K., & Daroch, M. (2020). Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms. International Journal of Molecular Sciences, 21(19), 7204. https://doi.org/10.3390/ijms21197204






