A Hypoxia-Inducible Factor Stabilizer Improves Hematopoiesis and Iron Metabolism Early after Administration to Treat Anemia in Hemodialysis Patients
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Change over Time in EPO Concentration in the Rox and the DA Group
2.3. Changes over Time in Erythrocyte Indices in the Rox Group
2.4. Correlation between Reticulocytes and Red Blood Cell Counts
2.5. Changes over Time in CHr, Hepcidin Levels and Iron-Related Indices in the Rox Group
2.6. Comparison between the Rox 70 mg and 100 mg Groups
2.7. Correlation between s-ft and Hepcidin Levels
2.8. Comparison between the Rox and DA Groups
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Rox Group
4.3. DA Group
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Rox | Roxadustat |
| HIF | Hypoxia-inducible factor |
| HD | Hemodialysis |
| DA | Darbepoietin α |
| EPO | Erythropoietin |
| CHr | Content of reticulocyte hemoglobin |
| QOL | quality of life |
| rHuEPO | Recombinant human erythropoietin |
| ESA | Erythtopoietin stimulating agent |
| PHD | Prolyl hydroxylase domain containing protein |
| RBC | Red blood cells |
| Hb | Hemoglobin |
| Ret | Reticulocyte |
| s-ft | Serum ferritin |
| s-Fe | Serum iron |
| TIBC | Total iron-binding capacity |
| TSAT | Transferrin saturation |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
References
- Tang, S.; Lai, K.N. Chronic viral hepatitis in hemodialysis patients. Hemodial. Int. 2005, 9, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Taccone-Gallucci, M.; Di Nucci, G.; Meloni, C.; Mariani, G.; Valeri, M.; Piazza, A.; Elia, L.; Torromeo, C.; Mandelli, F.; Casciani, C.U. Risk of iron overload and ‘hemochromatosis allele(s)’ in patients on maintenance hemodialysis. Am. J. Nephrol. 1987, 7, 28–32. [Google Scholar] [CrossRef]
- Friedlaender, M.M.; Kaufman, B.; Rubinger, D.; Moreb, J.; Popovtzer, M.M.; Goredetsky, R. Noninvasive assessment of skin iron content in hemodialysis patients. An index of parenchymal tissue iron content? Am. J. Kidney Dis. 1988, 12, 18–25. [Google Scholar] [CrossRef]
- Lin, F.K.; Suggs, S.; Lin, C.H.; Browne, J.K.; Smalling, R.; Egrie, J.C.; Chen, K.K.; Fox, G.M.; Martin, F.; Stabinsky, Z.; et al. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1985, 82, 7580–7584. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Finkelstein, F.O.; Revicki, D.A.; Evans, C.; Wan, S.; Gitlin, M.; Agodoa, I. Systematic review of the impact of erythropoiesis‒stimulating agents on fatigue in dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Curtis, B.M.; Parfrey, P.S. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2009, 4, 726–733. [Google Scholar] [CrossRef]
- Raine, A.E.; Roger, S.D. Effects of erythropoietin on blood pressure. Am. J. Kidney Dis. 1991, 18 (Suppl. 1), 76–83. [Google Scholar]
- Matsumura, M.; Nomura, H.; Koni, I.; Mabuchi, H. Angiotensin-converting enzyme inhibitors are associated with the need for increased recombinant human erythropoietin maintenance doses in hemodialysis patients. Risks Card. Dis. Dial. Patients Study Group 1997, 77, 164–168. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 11), 39–43. [Google Scholar] [CrossRef]
- Cournoyer, D.; Toffelmire, E.B.; Wells, G.A.; Barber, D.L.; Barrett, B.J.; Delage, R.; Forrest, D.L.; Gagnon, R.F.; Harvey, E.A.; Laneuville, P.; et al. Canadian PRCA Focus Group: Anti‒erythropoietin antibody‒mediated pure red cell aplasia after treatment with recombinant erythropoietin products: Recommendations for minimization of risk. J. Am. Soc. Nephrol. 2004, 15, 2728–2734. [Google Scholar] [CrossRef]
- Howman, R.; Kulkarni, H. Antibody-mediated acquired pure red cell aplasia (PRCA) after treatment with darbepoetin. Nephrol. Dial. Transplant. 2007, 22, 1462–1464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fishbane, S.; Berns, J.S. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005, 68, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Rattanasompattikul, M.; Molnar, M.Z.; Zaritsky, J.J.; Hatamizadeh, P.; Jing, J.; Norris, K.C.; Kovesdy, C.P.; Kalantar-Zadeh, K. Association of malnutrition–inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol. Dial. Transplant. 2013, 28, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via De Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Peyssonnaux, C.; Zinkernagel, A.S.; Schuepbach, R.A.; Rankin, E.; Vaulont, S.; Haase, V.H.; Nizet, V.; Johnson, R.S. Regulation of Iron Homeostasis by the Hypoxia-Inducible Transcription Factors (HIFs). J. Clin. Investig. 2007, 117, 1926–1932. [Google Scholar] [CrossRef]
- Liu, Q.; Davidoff, O.; Niss, K.; Haase, V.H. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J. Clin. Investig. 2012, 122, 4635–4644. [Google Scholar] [CrossRef]
- Mastrogiannaki, M.; Matak, P.; Mathieu, J.R.; Delga, S.; Mayeux, P.; Vaulont, S.; Peyssonnaux, C. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica 2012, 97, 827–834. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Shoji, S.; Inaba, M.; Tomosugi, N.; Okuno, S.; Ichii, M.; Yamakawa, T.; Kurihara, S. Greater potency of darbepoetin-α than erythropoietin in suppression of serum hepcidin-25 and utilization of iron for erythropoiesis in hemodialysis patients. Eur. J. Haematol. 2013, 90, 237–244. [Google Scholar] [CrossRef]
- Provenzano, R.; Besarab, A.; Wright, S.; Dua, S.; Zeig, S.; Nguyen, P.; Poole, L.; Saikali, K.G.; Saha, G.; Hemmerich, S.; et al. Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study. Am. J. Kidney Dis. 2016, 67, 912–924. [Google Scholar] [CrossRef]
- Besarab, A.; Provenzano, R.; Hertel, J.; Zabaneh, R.; Klaus, S.J.; Lee, T.; Leong, R.; Hemmerich, S.; Yu, K.-H.P.; Neff, T.B. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol. Dial. Transplant. 2015, 30, 1665–1673. [Google Scholar] [CrossRef]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; Ishikawa, I. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood 2006, 108, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates ypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Erythropoietic regulators of iron metabolism. Free Radic. Biol. Med. 2019, 133, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Galgano, C.; Langley, C.R., Jr.; Canfield, W.; Maesaka, K.J. Reticulocyte Hemoglobin Content in the Evaluation of Iron Status of Hemodialysis Patients. Kidney Int. 1997, 52, 217–222. [Google Scholar] [CrossRef]
- Brugnara, C.; Laufer, M.R.; Friedman, A.J.; Bridges, K.; Platt, O. Reticulocyte hemoglobin content (CHr): Early indicator of iron deficiency and response to therapy. Blood 1994, 83, 3100–3101. [Google Scholar] [CrossRef]
- Rolfs, A.; Kvietikova, I.; Gassmann, M.; Wenger, R.H. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J. Biol. Chem. 1997, 272, 20055–20062. [Google Scholar] [CrossRef]
- Cook, J.D. Clinical evaluation of iron deficiency. Semin. Hematol. 1982, 19, 6–18. [Google Scholar]
- Ogawa, C.; Tsuchiya, K.; Tomosugi, N.; Kanda, F.; Maeda, K.; Maeda, T. Low Levels of Serum Ferritin and Moderate Transferrin Saturation Lead to Adequate Hemoglobin Levels in Hemodialysis Patients, Retrospective Observational Study. PLoS ONE 2017, 12, e0179608. [Google Scholar] [CrossRef]
- Kuragano, T.; Shimonaka, Y.; Kida, A.; Furuta, M.; Nanami, M.; Otaki, Y.; Hasuike, Y.; Nonoguchi, H.; Nakanishi, T. Determinants of Hepcidin in Patients on Maintenance Hemodialysis: Role of Inflammation. Am. J. Nephrol. 2010, 31, 534–540. [Google Scholar] [CrossRef]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M. Hypoxia-inducible factor-1 Mediates Transcriptional Activation of the Heme oxygenase-1 Gene in Response to Hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.K.; Mazumder, B.; Fox, P.L. Role of Hypoxia-inducible Factor-1 in Transcriptional Activation of Ceruloplasmin by Iron Deficiency. J. Biol. Chem. 2000, 275, 21048–21054. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Qu, A.; Anderson, E.R.; Matsubara, T.; Martin, A.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 2011, 140, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ponka, P. Identification of a Hypoxia Response Element in the Transferrin Receptor Gene. J. Biol. Chem. 1999, 274, 24147–24152. [Google Scholar] [CrossRef]
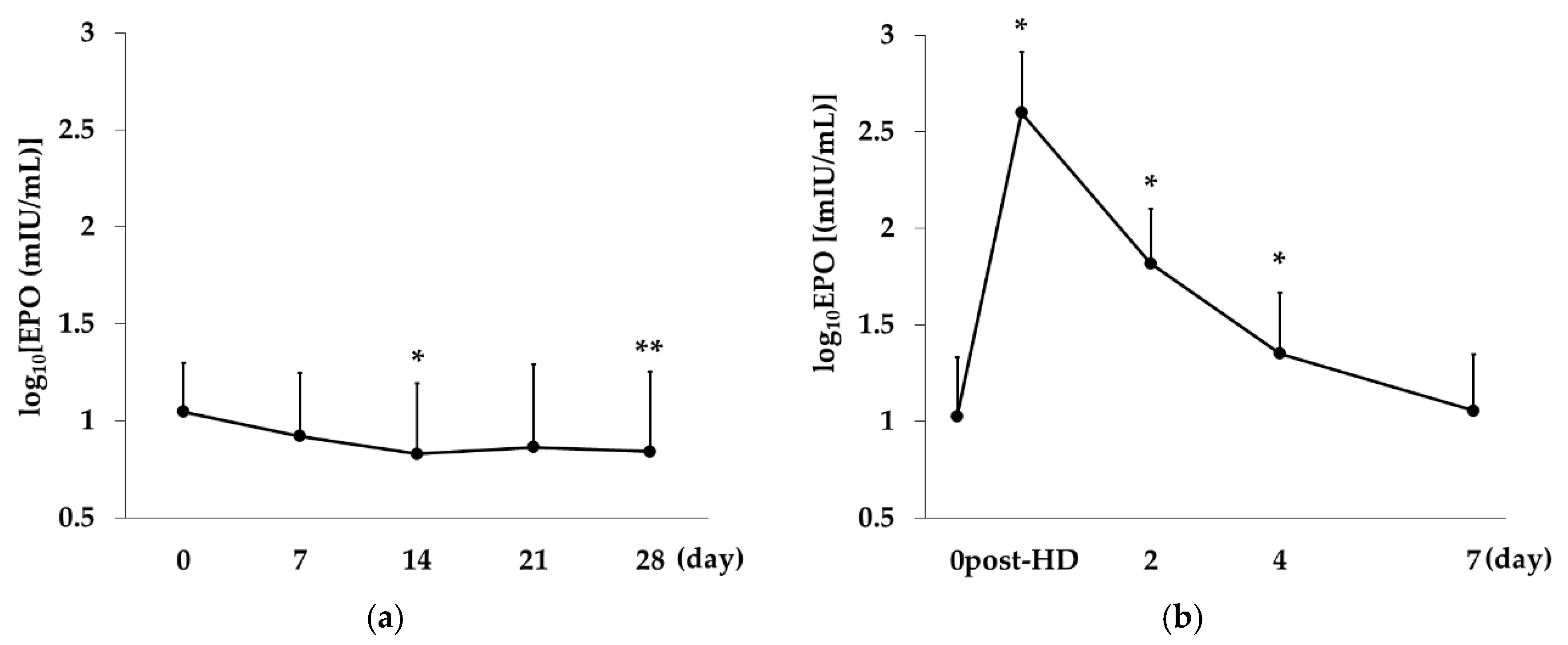
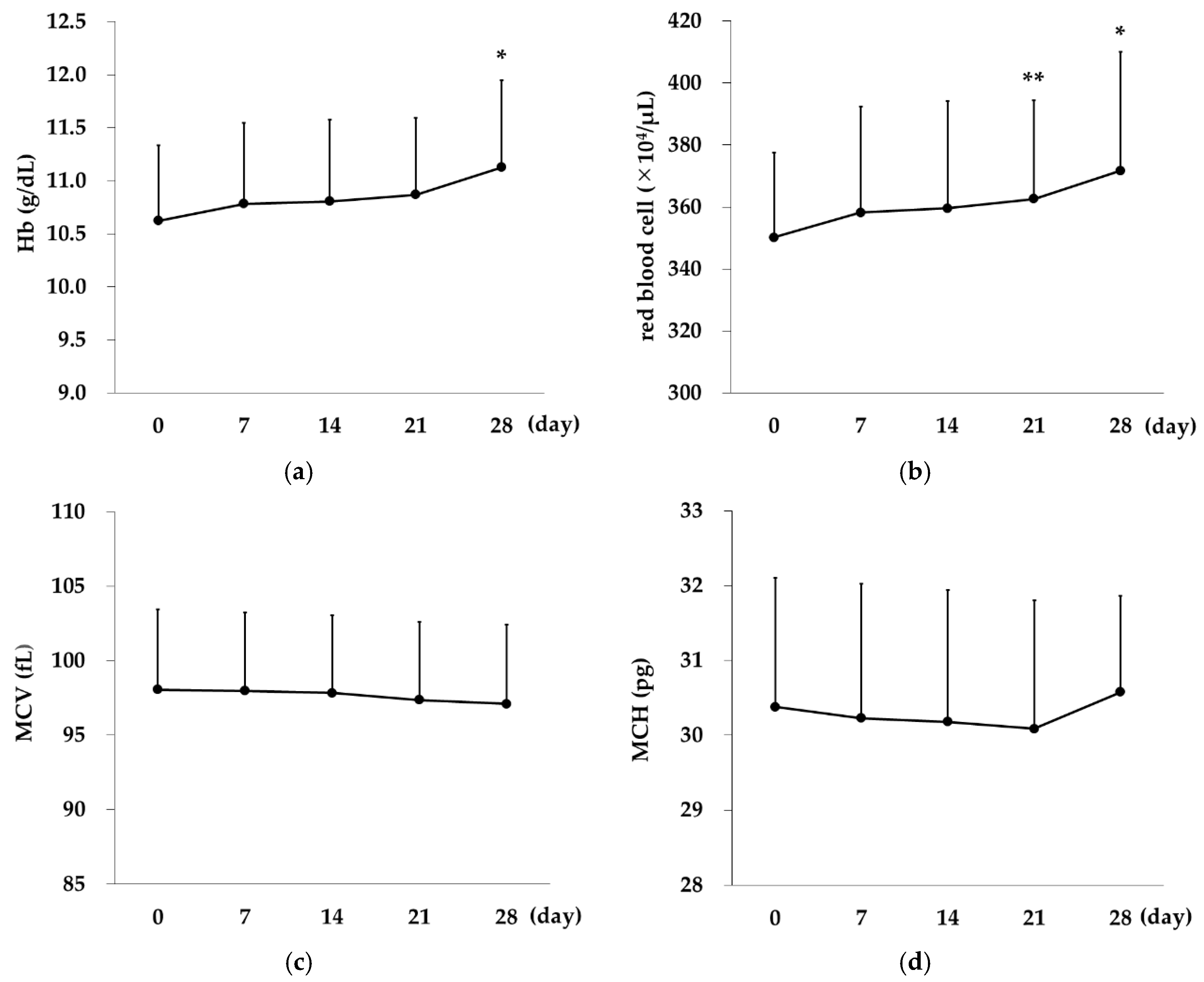
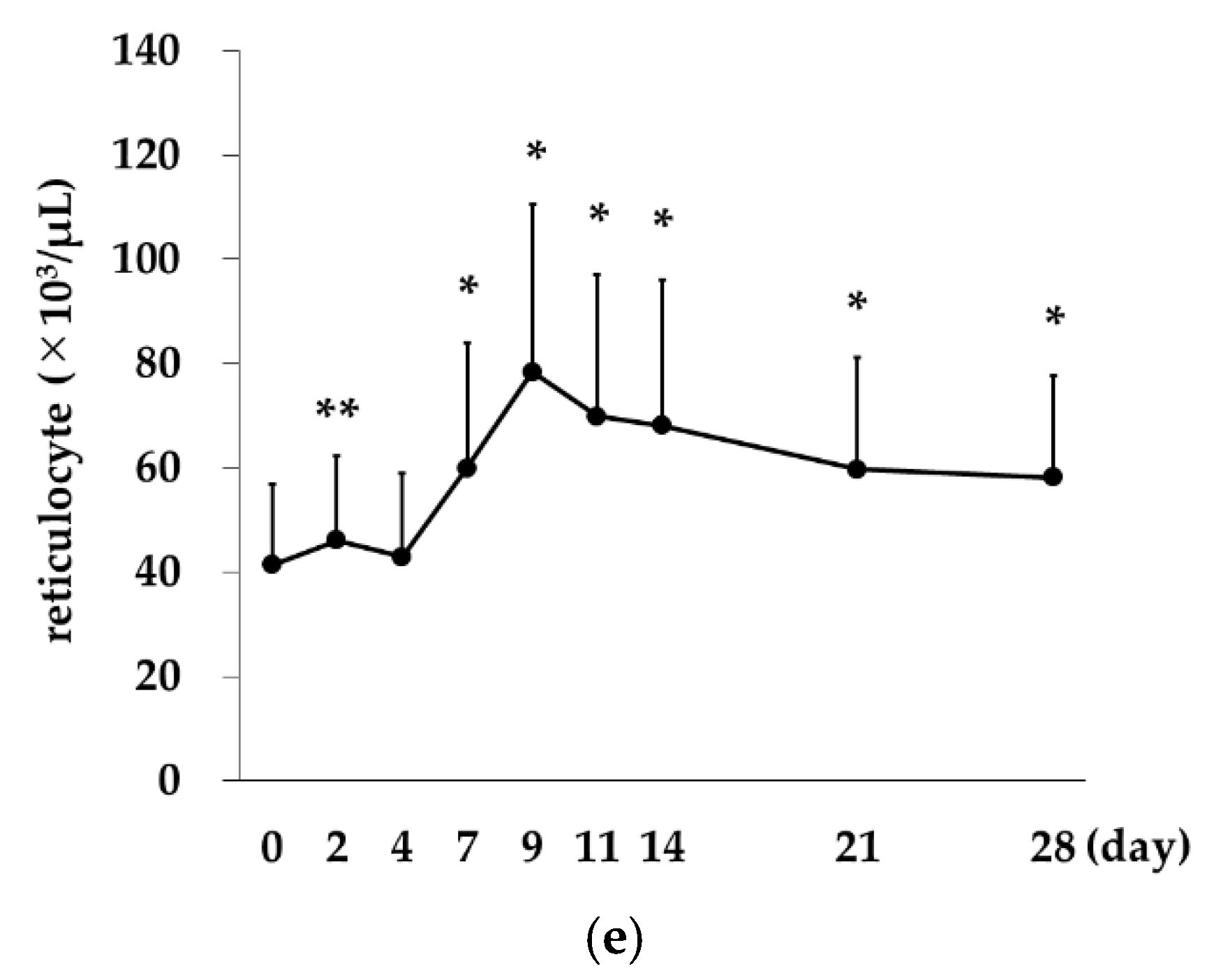

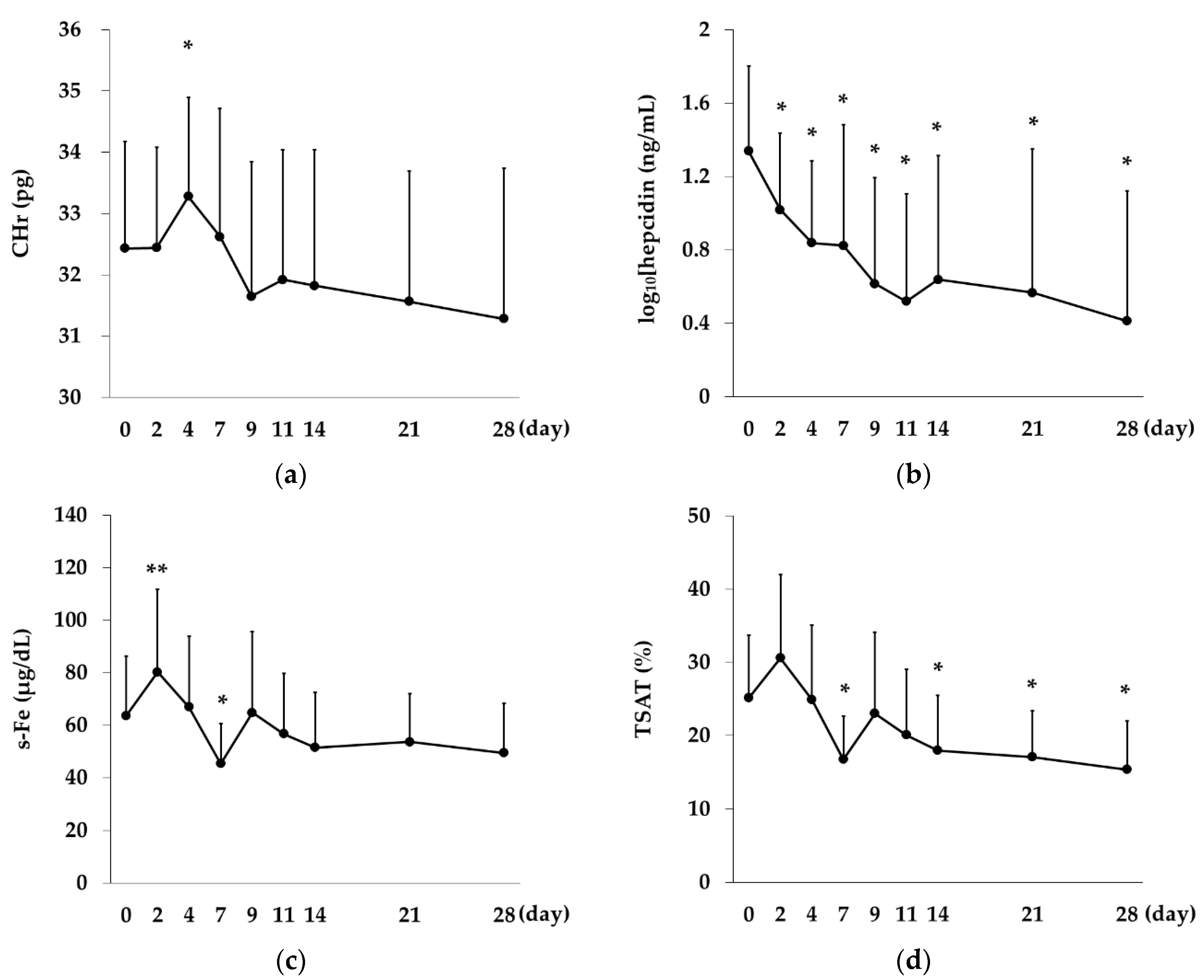
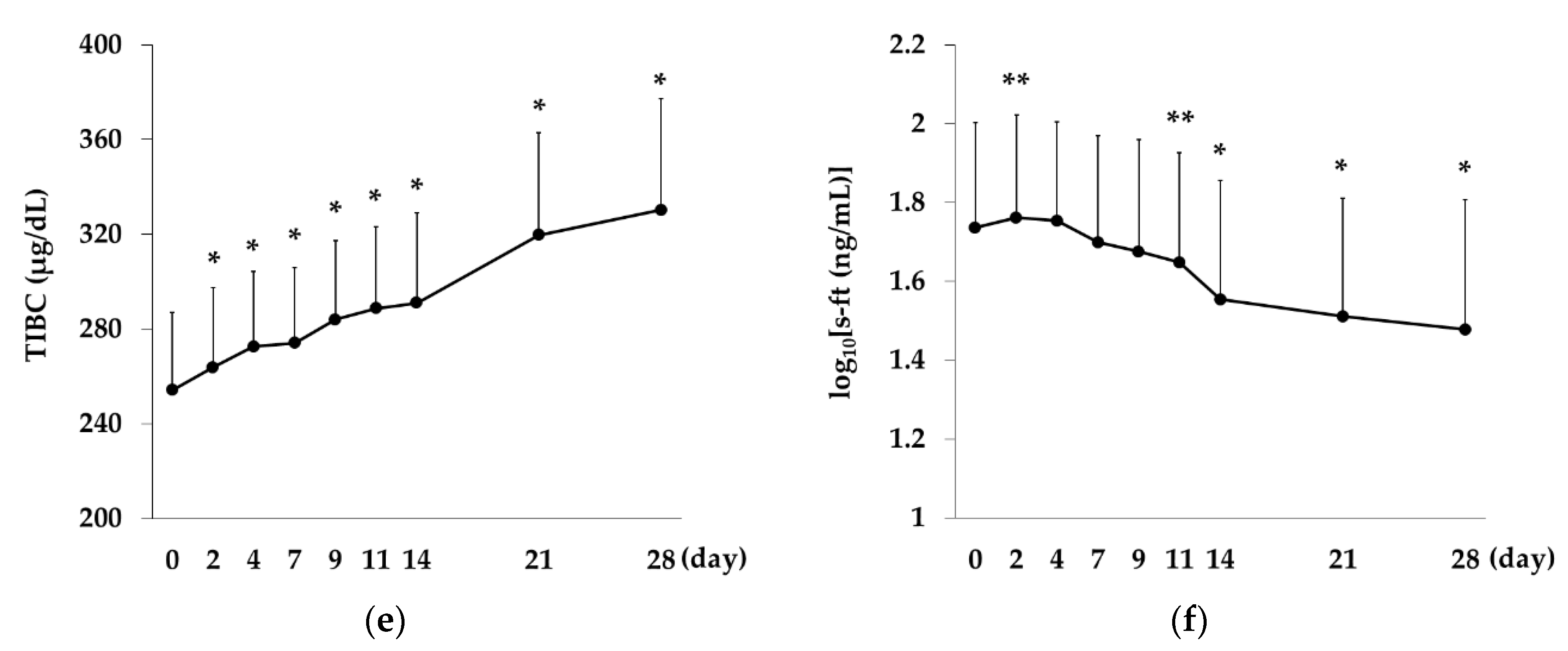
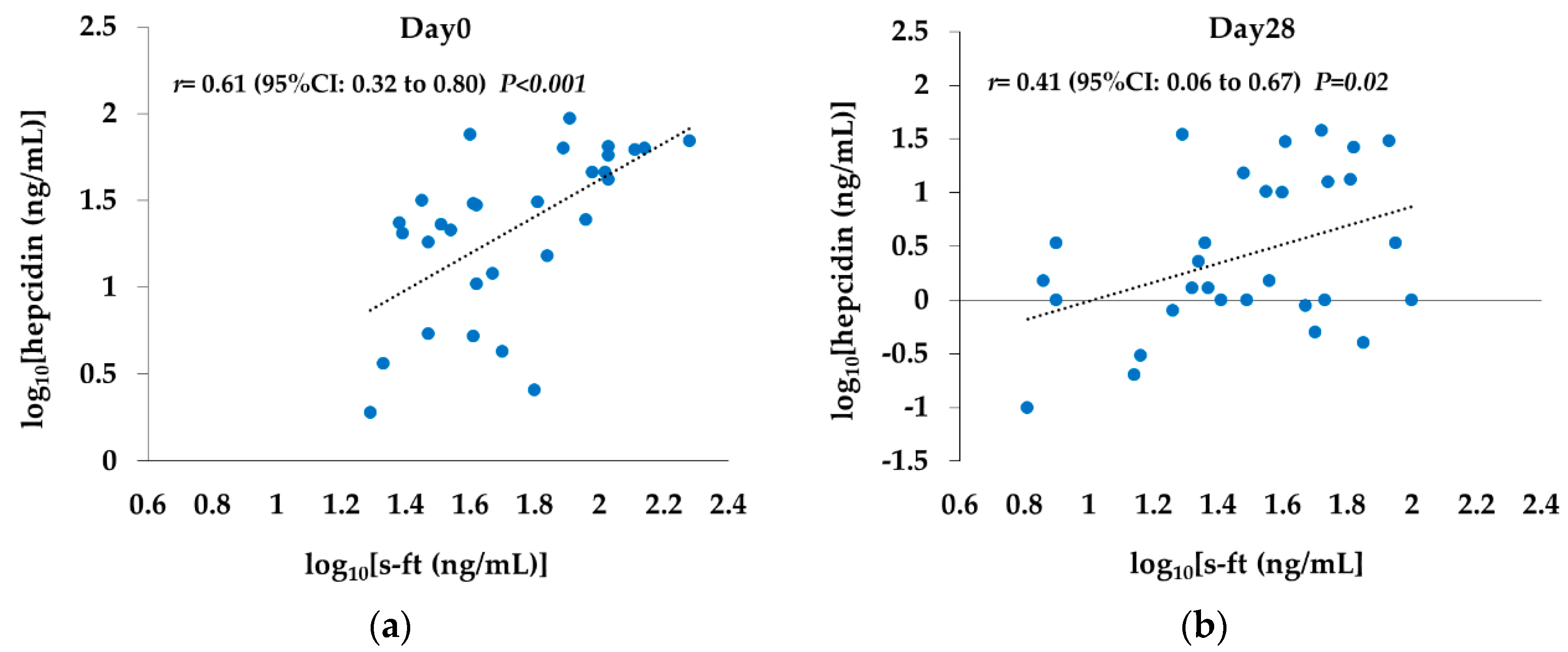
 Rox,
Rox,  DA).
DA).
 Rox,
Rox,  DA).
DA).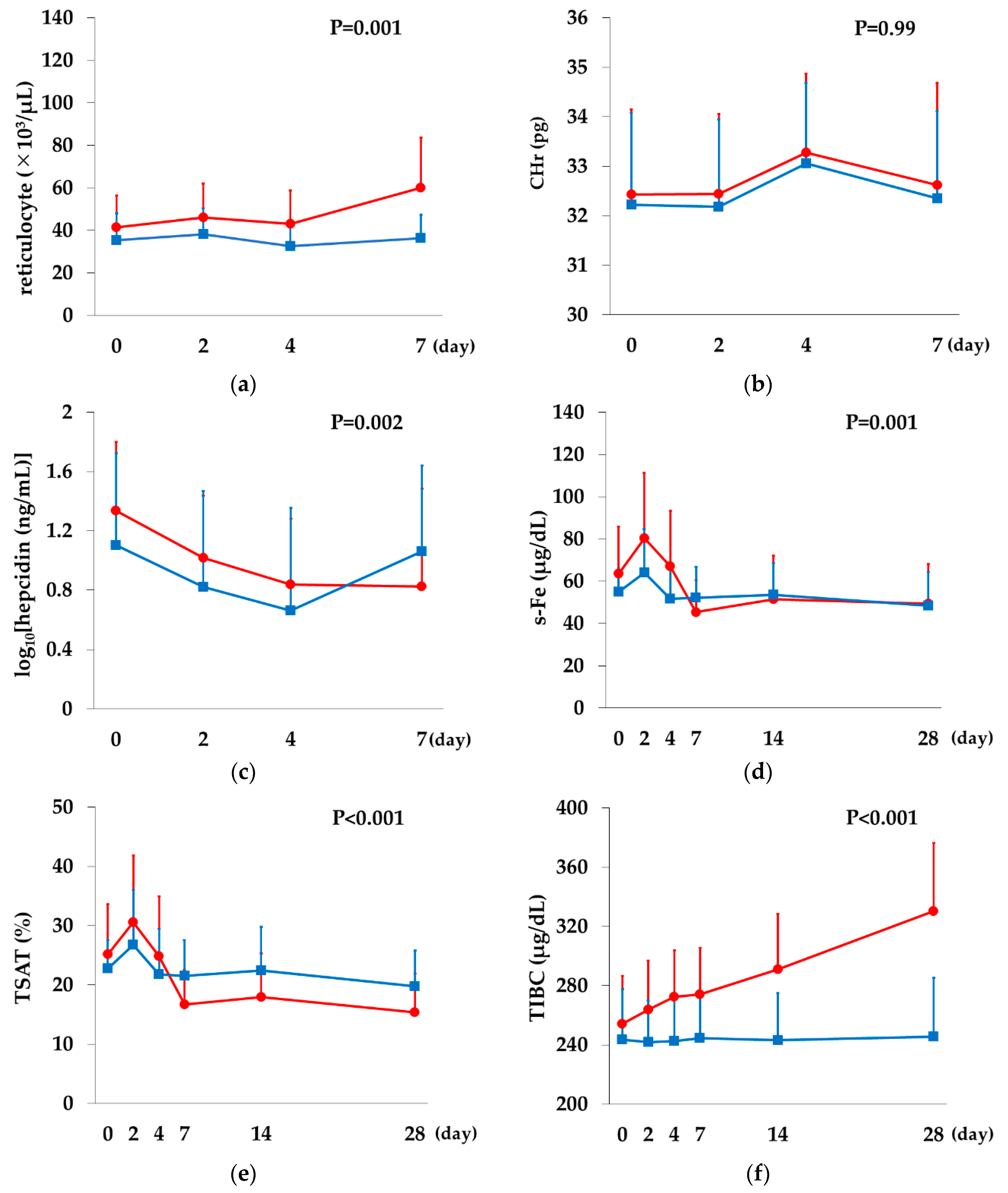

| Variables | Rox | DA | p Value * | ||
|---|---|---|---|---|---|
| All | (70 mg) | (100 mg) | |||
| N | 30 | 19 | 11 | 15 | |
| Age (years) | 71.3 ± 12.5 | 69.4 ± 13.1 | 77.0 ± 10.2 | 73.2 ± 10.5 | 0.95 |
| Gender | 1 | ||||
| Men | 17 | 13 | 4 | 8 | |
| Women | 13 | 6 | 7 | 7 | |
| Duration of dialysis (years) † | 7.2 (2.8–16.6) | 9.2 (2.8–18.5) | 3.3 (3.0–10.5) | 18.3 (2.4–26.8) | 0.29 |
| Primary diagnosis | 0.44 | ||||
| Chronic glomerulonephritis | 7 | 5 | 2 | 6 | |
| Diabetes nephropathy | 14 | 10 | 4 | 3 | |
| Renal sclerosis | 8 | 4 | 4 | 5 | |
| Other | 1 | - | 1 | 1 | |
| Kt/V | 1.50 ± 0.20 | 1.58 ± 0.17 | 1.46 ± 0.14 | 1.50 ± 0.23 | 0.93 |
| Red blood cells (×104/μL) | 350.2 ± 26.9 | 354.6 ± 27.5 | 342.5 ± 22.1 | 345.0 ± 42.1 | 0.63 |
| Hemoglobin (g/dL) | 10.6 ± 0.7 | 10.8 ± 0.6 | 10.3 ± 0.8 | 10.4 ± 0.7 | 0.37 |
| MCV (fL) | 98.1 ± 5.4 | 98.1 ± 4.4 | 98.0 ± 6.0 | 97.2 ± 5.8 | 0.62 |
| MCH (pg) | 30.4 ± 1.7 | 30.6 ± 1.6 | 30.1 ± 1.8 | 30.4 ± 2.1 | 0.92 |
| Reticulocytes (×103/μL) | 41.4 ± 15.1 | 35.0 ± 8.7 | 52.4 ± 17.6 | 35.3 ± 12.8 | 0.34 |
| CHr (pg) | 32.4 ± 1.7 | 32.8 ± 1.7 | 31.8 ± 1.5 | 32.2 ± 1.9 | 0.72 |
| serum-Ferritin (ng/mL) † | 48.2 (31.4–97.8) | 68.4 (40.2–99.9) | 41.0 (26.3–64.2) | 57.6 (34.3–96.8) | 0.97 |
| Iron (μg/dL) | 63.5 ± 22.4 | 67.8 ± 23.3 | 56.1 ± 16.0 | 54.9 ± 11.2 | 0.20 |
| TIBC (μg/dL) | 254.2 ± 32.3 | 246.5 ± 32.3 | 267.5 ± 27.5 | 243.5 ± 34.2 | 0.32 |
| Transferrin saturation (%) | 25.2 ± 8.4 | 27.3 ± 7.9 | 21.4 ± 7.8 | 22.8 ± 4.8 | 0.38 |
| Hepcidin (ng/mL) † | 26.8 (12.8–54.4) | 29.9 (18.2–62.6) | 20.2 (4.7–31.2) | 15.4 (5.2–40.2) | 0.28 |
| Albumin (g/dL) | 3.5 ± 0.3 | 3.5 ± 0.3 | 3.6 ± 0.4 | 3.4 ± 0.3 | 0.46 |
| C-reactiveprotein (mg/dL) † | 0.12 (0.07–0.60) | 0.10 (0.08–0.33) | 0.19 (0.07–0.97) | 0.19 (0.08–0.33) | 0.91 |
| erythropoietin (mIU/mL) † | 11.6 (6.9–17.6) | 9.7 (6.7–11.6) | 18.5 (12.9–28.7) | 9.2 (7.0–13.8) | 0.39 |
| Darbepoetin α (IU/week) | 18.3 ± 14.7 | 9.5 ± 3.0 | 33.6 ± 15.0 | 17.0 ± 13.8 | 0.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, C.; Tsuchiya, K.; Tomosugi, N.; Maeda, K. A Hypoxia-Inducible Factor Stabilizer Improves Hematopoiesis and Iron Metabolism Early after Administration to Treat Anemia in Hemodialysis Patients. Int. J. Mol. Sci. 2020, 21, 7153. https://doi.org/10.3390/ijms21197153
Ogawa C, Tsuchiya K, Tomosugi N, Maeda K. A Hypoxia-Inducible Factor Stabilizer Improves Hematopoiesis and Iron Metabolism Early after Administration to Treat Anemia in Hemodialysis Patients. International Journal of Molecular Sciences. 2020; 21(19):7153. https://doi.org/10.3390/ijms21197153
Chicago/Turabian StyleOgawa, Chie, Ken Tsuchiya, Naohisa Tomosugi, and Kunimi Maeda. 2020. "A Hypoxia-Inducible Factor Stabilizer Improves Hematopoiesis and Iron Metabolism Early after Administration to Treat Anemia in Hemodialysis Patients" International Journal of Molecular Sciences 21, no. 19: 7153. https://doi.org/10.3390/ijms21197153
APA StyleOgawa, C., Tsuchiya, K., Tomosugi, N., & Maeda, K. (2020). A Hypoxia-Inducible Factor Stabilizer Improves Hematopoiesis and Iron Metabolism Early after Administration to Treat Anemia in Hemodialysis Patients. International Journal of Molecular Sciences, 21(19), 7153. https://doi.org/10.3390/ijms21197153





