The Risk of Systemic Diseases in Those with Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic
Abstract
1. Introduction
2. The Risk of Cardiovascular Disease and Metabolic Syndrome in Psoriasis and Psoriatic Arthritis
3. The Risk of Cancer in Psoriasis and Psoriatic Arthritis
4. The Risk of Infection in Psoriasis and Psoriatic Arthritis
5. The Risk of Autoimmune Diseases in Psoriasis and Psoriatic Arthritis
6. The Risk of Psychiatric Diseases in Psoriasis and Psoriatic Arthritis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| AIBD: | autoimmune bullous disease |
| BP: | bullous pemphigoid |
| CAD: | coronary artery disease |
| CD: | Crohn’s disease |
| CI: | confidence interval |
| CPAP: | continuous positive airway pressure |
| CV: | cardiovascular |
| CVD: | cardiovascular disease |
| HBV: | hepatitis B |
| HCV: | hepatitis C |
| HLA: | human leukocyte antigen |
| HR: | hazard ratio |
| IBD: | inflammatory bowel disease |
| IFN: | interferon |
| IHD: | ischemic heart disease |
| IL: | interleukin |
| IRR: | incidence rate ratio |
| MACE: | major cardiovascular events |
| MCP1: | monocyte chemoattractant protein |
| MI: | myocardial infarction |
| NETs: | neutrophil extracellular traps |
| NMSC: | nonmelanoma skin cancer |
| OR: | odds ratio |
| PsA: | psoriatic arthritis |
| PUVA: | psoralen and ultraviolet A |
| RR: | relative risk |
| SCC: | squamous cell carcinoma |
| SMR: | standardized mortality ratio |
| Th: | T helper |
| TNF: | tumor necrosis factor |
| UC: | ulcerative colitis |
References
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Cho, D.H.; Park, H.J. Molecular mechanisms and management of a cutaneous inflammatory disorder: Psoriasis. Int. J. Mol. Sci. 2017, 18, 2684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Krueger, J.; Bowcock, A. Psoriasis: Genetic associations and immune system changes. Genes Immun. 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strauss, H. Zur Lehre von der neurogenen und der thyreogenen Glykosurie. DMW Dtsch. Med. Wochenschr. 1897, 23, 275–278. [Google Scholar]
- Chung, W.S.; Lin, C.L. Increased risks of venous thromboembolism in patients with psoriasis. Thromb. Haemost. 2017, 117, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, C.J.; Calabresi, P. Psoriasis and occlusive vascular disease. Br. J. Dermatol. 1978, 99, 469–475. [Google Scholar] [CrossRef]
- Obasi, O.E.J.I. Psoriasis vulgaris in the Guinea Savanah region of Nigeria. Int. J. Dermatol. 1986, 25, 181–183. [Google Scholar] [CrossRef]
- Ogdie, A.; Kay McGill, N.; Shin, D.B.; Takeshita, J.; Jon Love, T.; Noe, M.H.; Chiesa Fuxench, Z.C.; Choi, H.K.; Mehta, N.N.; Gelfand, J.M. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A general population-based cohort study. Eur. Heart J. 2018, 39, 3608–3614. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of myocardial infarction in patients with psoriasis. JAMA Dermatol. 2006, 296, 1735–1741. [Google Scholar] [CrossRef]
- Parisi, R.; Rutter, M.K.; Lunt, M.; Young, H.S.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Psoriasis and the risk of major cardiovascular events: Cohort study using the clinical practice research datalink. J. Investig. Dermatol. 2015, 135, 2189–2197. [Google Scholar] [CrossRef]
- Brauchli, Y.B.; Jick, S.S.; Miret, M.; Meier, C.R. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: An inception cohort study with a nested case-control analysis. Br. J. Dermatol. 2009, 160, 1048–1056. [Google Scholar] [CrossRef]
- Wakkee, M.; Herings, R.M.; Nijsten, T. Psoriasis may not be an independent risk factor for acute ischemic heart disease hospitalizations: Results of a large population-based Dutch cohort. J. Investig. Dermatol. 2010, 130, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, E.A.; Kavousi, M.; Nijsten, T.; Ikram, M.A.; Hofman, A.; Franco, O.H.; Wakkee, M. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: The Rotterdam Study. J. Investig. Dermatol. 2013, 133, 2347–2354. [Google Scholar] [CrossRef]
- Miller, I.M.; Ellervik, C.; Yazdanyar, S.; Jemec, G.B. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J. Am. Acad. Dermatol. 2013, 69, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Weng, C.L.; Zhao, Y.T.; Liu, Q.H.; Yin, R.X. Psoriasis and risk of cardiovascular disease: A meta-analysis of cohort studies. Int. J. Cardiol. 2013, 168, 4992–4996. [Google Scholar] [CrossRef] [PubMed]
- Horreau, C.; Pouplard, C.; Brenaut, E.; Barnetche, T.; Misery, L.; Cribier, B.; Jullien, D.; Aractingi, S.; Aubin, F.; Joly, P. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Bartosińska, J.; Chodorowska, G.; Szepietowski, J.C.; Paluszkiewicz, P.; Schwartz, R.A. Cardiovascular aspects of psoriasis: An updated review. Int. J. Dermatol. 2013, 52, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Samarasekera, E.J.; Neilson, J.M.; Warren, R.B.; Parnham, J.; Smith, C.H. Incidence of cardiovascular disease in individuals with psoriasis: A systematic review and meta-analysis. J. Investig. Dermatol. 2013, 133, 2340–2346. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Harskamp, C.T.; Armstrong, A.W. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J. Am. Heart Assoc. 2013, 2, e62. [Google Scholar] [CrossRef]
- Gaeta, M.; Castelvecchio, S.; Ricci, C.; Pigatto, P.; Pellissero, G.; Cappato, R. Role of psoriasis as independent predictor of cardiovascular disease: A meta-regression analysis. Int. J. Cardiol. 2013, 168, 2282–2288. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Y.H. Association of psoriasis with stroke and myocardial infarction: Meta-analysis of cohort studies. Br. J. Dermatol. 2012, 167, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Skov, L.; Joshi, A.A.; Mallbris, L.; Gislason, G.H.; Wu, J.J.; Rodante, J.; Lerman, J.B.; Ahlman, M.A.; Gelfand, J.M. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J. Am. Acad. Dermatol. 2017, 77, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Polachek, A.; Touma, Z.; Anderson, M.; Eder, L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: A meta—Analysis of observational studies. Arthritis Care Res. 2017, 69, 67–74. [Google Scholar] [CrossRef]
- Wu, J.J.; Poon, K.Y.T.; Channual, J.C.; Shen, A.Y. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch. Dermatol. 2012, 148, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Jókai, H.; Szakonyi, J.; Kontár, O.; Marschalkó, M.; Szalai, K.; Kárpáti, S.; Holló, P. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: Results of a pilot study. J. Am. Acad. Dermatol. 2013, 69, 523–529. [Google Scholar] [CrossRef]
- Egeberg, A.; Ottosen, M.; Gniadecki, R.; Broesby--Olsen, S.; Dam, T.; Bryld, L.; Rasmussen, M.; Skov, L. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2018, 178, 509–519. [Google Scholar] [CrossRef]
- Tzellos, T.; Kyrgidis, A.; Trigoni, A.; Zouboulis, C.C. Major adverse cardiovascular events and anti-IL 12/23 agents. J. Am. Acad. Dermatol. 2014, 70, 380–381. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and hypertension: A systematic review and meta-analysis of observational studies. J. Hypertens. 2013, 31, 433–442, discussion 442–443. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.; Mu, Y.; Liu, T.; Chen, Y.; Yu, R.; Xiong, X.; Wu, T. A systematic review and meta-analysis of the association between psoriasis and hypertension with adjustment for covariates. Medicine 2020, 99, e19303. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol. 2013, 149, 84–91. [Google Scholar] [CrossRef]
- Coto-Segura, P.; Eiris-Salvado, N.; González-Lara, L.; Queiro-Silva, R.; Martinez-Camblor, P.; Maldonado-Seral, C.; García-García, B.; Palacios-García, L.; Gomez-Bernal, S.; Santos-Juanes, J.; et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 783–793. [Google Scholar] [CrossRef]
- Mamizadeh, M.; Tardeh, Z.; Azami, M. The association between psoriasis and diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 13, 1405–1412. [Google Scholar] [CrossRef]
- Armstrong, A.; Harskamp, C.; Armstrong, E. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef]
- Choudhary, S.; Patel, R.; Pradhan, D.; Deval, R.; Singh, H.; Thomas, G.; Jain, A.K. Psoriasis and cardiovascular disorders: Association or epiphenomenon? Meta-analysis of observational studies. 3 Biotech 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Lee, G.; Fischer, G. Pediatric psoriasis and association with cardiovascular and metabolic comorbidities: Systematic review and meta-analysis. Pediatr. Derm. 2020. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 2013, 68, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zúñiga, M.J.M.; García-Perdomo, H.A. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J. Am. Acad. Dermatol. 2017, 77, 657–666. [Google Scholar] [CrossRef]
- Singh, S.; Young, P.; Armstrong, A.W. An update on psoriasis and metabolic syndrome: A meta-analysis of observational studies. PLoS ONE 2017, 12, e181039. [Google Scholar] [CrossRef]
- Choudhary, S.; Pradhan, D.; Pandey, A.; Khan, M.K.; Lall, R.; Ramesh, V.; Puri, P.; Jain, A.K.; Thomas, G. The association of metabolic syndrome and psoriasis: A systematic review and meta-analysis of observational study. Endocr. Metab. Immune Disord. Drug Targets 2019, 20, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Tam, L.S.; Tomlinson, B.; Chu, T.W.; Li, M.; Leung, Y.Y.; Kwok, L.W.; Li, T.; Yu, T.; Zhu, Y.E.; Wong, K.C.J.R. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls—The role of inflammation. Rheumatology 2008, 47, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Jamnitski, A.; Symmons, D.; Peters, M.J.; Sattar, N.; MciInnes, I.; Nurmohamed, M.T. Cardiovascular comorbidities in patients with psoriatic arthritis: A systematic review. Ann. Rheum. Dis. 2013, 72, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Tobin, A.M.; Veale, D.J.; Fitzgerald, O.; Rogers, S.; Collins, P.; O’Shea, D.; Kirby, B.J. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J. Rheumatol. 2010, 37, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Eng. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J. Derm. Sci. 2011, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davidovici, B.B.; Sattar, N.; Prinz, J.; Puig, L.; Emery, P.; Barker, J.N.; van de Kerkhof, P.; Stahle, M.; Nestle, F.O.; Girolomoni, G.; et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J. Investig. Derm. 2010, 130, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Sajja, A.P.; Joshi, A.A.; Teague, H.L.; Dey, A.K.; Mehta, N.N. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front. Immunol. 2018, 9, 1234. [Google Scholar] [CrossRef]
- Koch, M.; Baurecht, H.; Ried, J.S.; Rodriguez, E.; Schlesinger, S.; Volks, N.; Gieger, C.; Ruckert, I.M.; Heinrich, L.; Willenborg, C.; et al. Psoriasis and cardiometabolic traits: Modest association but distinct genetic architectures. J. Investig. Derm. 2015, 135, 1283–1293. [Google Scholar] [CrossRef]
- Eiris, N.; Gonzalez-Lara, L.; Santos-Juanes, J.; Queiro, R.; Coto, E.; Coto-Segura, P. Genetic variation at IL12B, IL23R and IL23A is associated with psoriasis severity, psoriatic arthritis and type 2 diabetes mellitus. J. Derm. Sci. 2014, 75, 167–172. [Google Scholar] [CrossRef]
- Mehta, N.N.; Li, K.; Szapary, P.; Krueger, J.; Brodmerkel, C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J. Transl. Med. 2013, 11, 194. [Google Scholar] [CrossRef]
- Suarez-Farinas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.; Krueger, J.G. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J. Investig. Derm. 2012, 132, 2552–2564. [Google Scholar] [CrossRef]
- Späh, F. Inflammation in atherosclerosis and psoriasis: Common pathogenic mechanisms and the potential for an integrated treatment approach. Br. J. Dermatol. 2008, 159, 10–17. [Google Scholar] [CrossRef]
- Naik, H.B.; Natarajan, B.; Stansky, E.; Ahlman, M.A.; Teague, H.; Salahuddin, T.; Ng, Q.; Joshi, A.A.; Krishnamoorthy, P.; Dave, J.; et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arter. Thromb. Vasc. Biol. 2015, 35, 2667–2676. [Google Scholar] [CrossRef]
- Quillard, T.; Araujo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Boehncke, S.; Tobin, A.M.; Kirby, B. The ‘psoriatic march’: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Henrichot, E.; Juge-Aubry, C.E.; Pernin, A.; Pache, J.C.; Velebit, V.; Dayer, J.M.; Meda, P.; Chizzolini, C.; Meier, C.A. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arter. Thromb. Vasc. Biol. 2005, 25, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Cerman, A.A.; Bozkurt, S.; Sav, A.; Tulunay, A.; Elbasi, M.O.; Ergun, T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br. J. Dermatol. 2008, 159, 820–826. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85. [Google Scholar] [CrossRef]
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar] [CrossRef]
- Chiesa Fuxench, Z.C.; Shin, D.B.; Ogdie Beatty, A.; Gelfand, J.M. The Risk of Cancer in Patients with Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol. 2016, 152, 282–290. [Google Scholar] [CrossRef]
- Vaengebjerg, S.; Skov, L.; Egeberg, A.; Loft, N.D. Prevalence, Incidence, and Risk of Cancer in Patients with Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 421–429. [Google Scholar] [PubMed]
- Wang, X.; Liu, Q.; Wu, L.; Nie, Z.; Mei, Z. Risk of non-melanoma skin cancer in patients with psoriasis: An updated evidence from systematic review with meta-analysis. J. Cancer 2020, 11, 1047–1055. [Google Scholar] [PubMed]
- Pouplard, C.; Brenaut, E.; Horreau, C.; Barnetche, T.; Misery, L.; Richard, M.A.; Aractingi, S.; Aubin, F.; Cribier, B.; Joly, P.; et al. Risk of cancer in psoriasis: A systematic review and meta-analysis of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. Jeadv. 2013, 27 (Suppl. 3), 36–46. [Google Scholar]
- Egeberg, A.; Thyssen, J.P.; Gislason, G.H.; Skov, L. Skin cancer in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. Jeadv. 2016, 30, 1349–1353. [Google Scholar] [PubMed]
- Lee, E.; Koo, J.; Berger, T. UVB phototherapy and skin cancer risk: A review of the literature. Int. J. Dermatol. 2005, 44, 355–360. [Google Scholar]
- Lindelof, B.; Sigurgeirsson, B.; Tegner, E.; Larko, O.; Johannesson, A.; Berne, B.; Ljunggren, B.; Andersson, T.; Molin, L.; Nylander-Lundqvist, E. PUVA and cancer risk: The Swedish follow-up study. Br. J. Dermatol. 1999, 141, 108–112. [Google Scholar] [PubMed]
- Stern, R.S.; Study, P.F.U. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar]
- Brauchli, Y.B.; Jick, S.S.; Miret, M.; Meier, C.R. Psoriasis and risk of incident cancer: An inception cohort study with a nested case–control analysis. J. Investig. Dermatol. 2009, 129, 2604–2612. [Google Scholar]
- Ekström Smedby, K.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martinez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Seniori Costantini, A.; Bracci, P.M. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood J. Am. Soc. Hematol. 2008, 111, 4029–4038. [Google Scholar]
- Ekström, K.; Hjalgrim, H.; Brandt, L.; Baecklund, E.; Klareskog, L.; Ekbom, A.; Askling, J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 963–970. [Google Scholar]
- Koo, J.Y.; Kadonaga, J.N.; Wintroub, B.V.; Lozada-Nur, F.I. The development of B-cell lymphoma in a patient with psoriasis treated with cyclosporine. J. Am. Acad. Dermatol. 1992, 26, 836–840. [Google Scholar] [CrossRef]
- Kamel, O.W. Lymphomas during long-term methotrexate therapy. Arch. Dermatol. 1997, 133, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Peleva, E.; Exton, L.; Kelley, K.; Kleyn, C.; Mason, K.; Smith, C. Risk of cancer in patients with psoriasis on biological therapies: A systematic review. Br. J. Dermatol. 2018, 178, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; Descalzo, M.A.; Mason, K.J.; Cohen, A.D.; Ormerod, A.D.; Gomez-Garcia, F.J.; Cazzaniga, S.; Feldhamer, I.; Ali, H.; Herrera-Acosta, E.; et al. Cumulative exposure to biological therapy and risk of cancer in patients with psoriasis: A meta-analysis of Psonet studies from Israel, Italy, Spain, the U.K. and Republic of Ireland. Br. J. Dermatol. 2018, 179, 863–871. [Google Scholar] [CrossRef]
- Reddy, S.P.; Martires, K.; Wu, J.J. The risk of melanoma and hematologic cancers in patients with psoriasis. J. Am. Acad. Dermatol. 2017, 76, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Trafford, A.M.; Parisi, R.; Kontopantelis, E.; Griffiths, C.E.M.; Ashcroft, D.M. Association of Psoriasis with the Risk of Developing or Dying of Cancer: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Kang, D.; Kang, M.; Shim, S.; Kang, C.K.; Kim, D.W.; Guallar, E.; Cho, J.; Youn, S.W. Risk of acute infections in patients with psoriasis: A nationwide population-based cohort study. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef]
- Takeshita, J.; Shin, D.B.; Ogdie, A.; Gelfand, J.M. Risk of Serious Infection, Opportunistic Infection, and Herpes Zoster among Patients with Psoriasis in the United Kingdom. J. Investig. Derm. 2018, 138, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Tuomanen, E.I.; Wunderink, R.; Kanaya, A.; Newman, A.B.; Harris, T.; De Rekeneire, N.; Kritchevsky, S.B. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am. J. Respir. Crit. Care Med. 2005, 172, 1440–1446. [Google Scholar] [CrossRef]
- Cohen, A.D.; Weitzman, D.; Birkenfeld, S.; Dreiher, J. Psoriasis associated with hepatitis C but not with hepatitis B. Dermatology 2010, 220, 218–222. [Google Scholar] [CrossRef]
- Yang, Y.W.; Keller, J.; Lin, H.C. Medical comorbidity associated with psoriasis in adults: A population--based study. Br. J. Dermatol. 2011, 165, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Chouela, E.; Abeldano, A.; Panetta, J.; Ducard, M.; NEGEIA, V.; Sookoian, S.; Kina, M.; Castano, G.; Vereytou, F.; Frider, B.J.I. Hepatitis C virus antibody (anti--HCV): Prevalence in psoriasis. Int. J. Dermatol. 1996, 35, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, V.; Carugno, A.; Alborghetti, A.; Cananzi, R.; Sangiovanni, L.; Barbarini, G.; De Silvestri, A.; Borroni, R.J.J. Venereology, Hepatitis C infection in Italian psoriatic patients: Prevalence and correlation with patient age and psoriasis severity. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1581–1582. [Google Scholar] [PubMed]
- Imafuku, S.; Naito, R.; Nakayama, J.J. Possible association of hepatitis C virus infection with late-onset psoriasis: A hospital--based observational study. J. Dermatol. 2013, 40, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Afshar, M.; Audish, D.; Kabigting, F.; Paik, A.; Gallo, R.; Hata, T. Hepatitis C may enhance key amplifiers of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 672–678. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef]
- Motaparthi, K.; Stanisic, V.; Van Voorhees, A.S.; Lebwohl, M.G.; Hsu, S. From the Medical Board of the National Psoriasis Foundation: Recommendations for screening for hepatitis B infection prior to initiating anti–tumor necrosis factor-alfa inhibitors or other immunosuppressive agents in patients with psoriasis. J. Am. Acad. Dermatol. 2014, 70, 178–186. [Google Scholar] [CrossRef]
- Cho, Y.T.; Chen, C.H.; Chiu, H.Y.; Tsai, T.F. Use of anti-tumor necrosis factor-α therapy in hepatitis B virus carriers with psoriasis or psoriatic arthritis: A case series in Taiwan. J. Dermatol. 2012, 39, 269–273. [Google Scholar] [CrossRef]
- Cassano, N.; Mastrandrea, V.; Principi, M.; Loconsole, F.; De, N.T.; Di, A.L.; Vena, G. Anti-tumor necrosis factor treatment in occult hepatitis B virus infection: A retrospective analysis of 62 patients with psoriatic disease. J. Biol. Regul. Homeost. Agents 2011, 25, 285–289. [Google Scholar]
- Palazzi, C.; D’Angelo, S.; Leccese, P.; Padula, A.; Olivieri, I. Safety of anti-tumor necrosis factor agents in psoriatic arthritis—An update. Expert Opin. Drug Saf. 2014, 13, 191–196. [Google Scholar] [CrossRef]
- Senaldi, G.; Yin, S.; Shaklee, C.L.; Piguet, P.F.; Mak, T.W.; Ulich, T.R. Corynebacterium parvum-and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 1996, 157, 5022–5026. [Google Scholar] [PubMed]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schrelber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Gómez-Reino, J.J.; Carmona, L.; Valverde, V.R.; Mola, E.M.; Montero, M.D. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active--surveillance report. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S. Biologics and infections: Lessons from tumor necrosis factor blocking agents. Infect. Dis. Clin. 2011, 25, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.; Hyrich, K.; Watson, K.; Lunt, M.; Galloway, J.; Ustianowski, A.; Symmons, D.; Consortium, B.C.C. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register (BSRBR). Ann. Rheum. Dis. 2010, 69, 522–528. [Google Scholar] [CrossRef]
- Cho, S.I.; Kang, S.; Kim, Y.E.; Lee, J.Y.; Jo, S.J. Ustekinumab does not increase tuberculosis risk: Results from a national database in South Korea. J. Am. Acad. Dermatol. 2019, 82, 1243–1245. [Google Scholar] [CrossRef]
- Fowler, E.; Ghamrawi, R.; Ghiam, N.; Liao, W.; Wu, J. Risk of tuberculosis reactivation during IL--17 inhibitor therapy for psoriasis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1449–1456. [Google Scholar] [CrossRef]
- Yiu, Z.Z.N.; Smith, C.H.; Ashcroft, D.M.; Lunt, M.; Walton, S.; Murphy, R.; Reynolds, N.J.; Ormerod, A.D.; Griffiths, C.E.M.; Warren, R.B. Risk of Serious Infection in Patients with Psoriasis Receiving Biologic Therapies: A Prospective Cohort Study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J. Investig. Derm. 2018, 138, 534–541. [Google Scholar] [CrossRef]
- Kalb, R.E.; Fiorentino, D.F.; Lebwohl, M.G.; Toole, J.; Poulin, Y.; Cohen, A.D.; Goyal, K.; Fakharzadeh, S.; Calabro, S.; Chevrier, M.; et al. Risk of Serious Infection With Biologic and Systemic Treatment of Psoriasis: Results From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015, 151, 961–969. [Google Scholar] [CrossRef]
- Li, X.; Andersen, K.M.; Chang, H.Y.; Curtis, J.R.; Alexander, G.C. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann. Rheum. Dis. 2019, 79, 285–291. [Google Scholar] [CrossRef]
- Eder, L.; Law, T.; Chandran, V.; Shanmugarajah, S.; Shen, H.; Rosen, C.F.; Cook, R.J.; Gladman, D.D. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis Care Res. 2011, 63, 1091–1097. [Google Scholar]
- Ritchlin, C.T.; Stahle, M.; Poulin, Y.; Bagel, J.; Chakravarty, S.D.; Kafka, S.; Srivastava, B.; Langholff, W.; Gottlieb, A.B. Serious infections in patients with self-reported psoriatic arthritis from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) treated with biologics. BMC Rheumtaol. 2019, 3, 52. [Google Scholar]
- Haddad, A.; Li, S.; Thavaneswaran, A.; Cook, R.J.; Chandran, V.; Gladman, D.D. The incidence and predictors of infection in psoriasis and psoriatic arthritis: Results from longitudinal observational cohorts. J. Rheumatol. 2016, 43, 362–366. [Google Scholar] [PubMed]
- Taglione, E.; Vatteroni, M.; Martini, P.; Galluzzo, E.; Lombardini, F.; Delle, A.S.; Bendinelli, M.; Pasero, G.; Bencivelli, W.; Riente, L.J. Hepatitis C virus infection: Prevalence in psoriasis and psoriatic arthritis. J. Rheumatol. 1999, 26, 370–372. [Google Scholar]
- Palazzi, C.; Olivieri, I.; D’amico, E.; D’agostino, L.; Nicolucci, A.; Pennese, E.; Petricca, A.J. Hepatitis C virus infection in psoriatic arthritis. Arthritis Rheum. 2005, 53, 223–225. [Google Scholar] [PubMed]
- Pattison, E.; Harrison, B.J.; Griffiths, C.E.; Silman, A.J.; Bruce, I.N. Environmental risk factors for the development of psoriatic arthritis: Results from a case-control study. Ann. Rheumat. Dis. 2008, 67, 672–676. [Google Scholar]
- Muramatsu, T.; Yamashina, Y.; Shirai, T.; Ohnishi, T. UVB irradiation reduces the expression of pemphigoid antigens in organ-cultured normal human skin. Arch. Dermatol. Res. 1994, 286, 142–144. [Google Scholar] [PubMed]
- Tsai, T.F.; Wang, T.S.; Hung, S.T.; Phiona, I.; Tsai, C.; Schenkel, B.; Zhang, M.; Tang, C.H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011, 63, 40–46. [Google Scholar] [PubMed]
- Wu, J.J.; Nguyen, T.U.; Poon, K.Y.T.; Herrinton, L.J. The association of psoriasis with autoimmune diseases. J. Am. Acad. Dermatol. 2012, 67, 924–930. [Google Scholar]
- Blegvad, C.; Andersen, A.N.; Groot, J.; Zachariae, C.; Skov, L. Cohort profile: The clinical ‘Psoriasis in Adolescents’ (PIA) cohort in Denmark. BMJ Open 2019, 9, e31448. [Google Scholar]
- Khan, S.R.; Bano, A.; Wakkee, M.; Korevaar, T.I.M.; Franco, O.H.; Nijsten, T.E.C.; Peeters, R.P.; Chaker, L. The association of autoimmune thyroid disease (AITD) with psoriatic disease: A prospective cohort study, systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 177, 347–359. [Google Scholar] [CrossRef]
- Liu, C.Y.; Tung, T.H.; Lee, C.Y.; Chang, K.H.; Wang, S.H.; Chi, C.C. Association of multiple sclerosis with psoriasis: A systematic review and meta-analysis of observational studies. Am. J. Clin. Dermatol. 2019, 20, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Goyal, S.; Murrell, D.F. Association between bullous pemphigoid and psoriasis: Systematic review and meta-analysis of case-control studies. Australas. J. Derm. 2019, 60, 23–28. [Google Scholar] [CrossRef]
- Kridin, K.; Kridin, M.; Shalom, G.; Cohen, A.D. The coexistence of pemphigus and psoriasis: A systematic review and meta-analysis. Immunol. Res. 2019, 67, 134–141. [Google Scholar] [CrossRef]
- Yen, H.; Chi, C.C. Association between psoriasis and vitiligo: A systematic review and meta-analysis. Am. J. Clin. Dermatol. 2019, 20, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of Psoriasis with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A.J.J. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease—A systematic review and meta-analysis. J. Crohns. Colitis. 2020, 14, 351–360. [Google Scholar] [CrossRef]
- Acharya, P.; Mathur, M.J.J. Association between psoriasis and celiac disease: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2020, 82, 1376–1385. [Google Scholar] [CrossRef]
- Bloom, D. Bullous eruption in a psoriatic patient. Arch Derm. 1929, 20, 254–255. [Google Scholar]
- Ohata, C.; Ishii, N.; Koga, H.; Fukuda, S.; Tateishi, C.; Tsuruta, D.; Furumura, M.; Hashimoto, T. Coexistence of autoimmune bullous diseases (AIBDs) and psoriasis: A series of 145 cases. J. Am. Acad. Dermatol. 2015, 73, 50–55. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Lin, M.; Chen, T.; Liao, K.; Chen, Y.; Hwang, C.; Chu, S.; Chen, C.; Lee, D. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br. J. Dermatol. 2011, 165, 593–599. [Google Scholar] [CrossRef]
- Kridin, K.; Bergman, R. Association between bullous pemphigoid and psoriasis: A case-control study. J. Am. Acad. Dermatol. 2017, 77, 370–372. [Google Scholar] [CrossRef]
- Vaccaro, M.; Magaudda, L.; Cutroneo, G.; Trimarchi, F.; Barbuzza, O.; Guarneri, F.; Guarneri, B. Changes in the distribution of laminin α1 chain in psoriatic skin: Immunohistochemical study using confocal laser scanning microscopy. Br. J. Dermatol. 2002, 146, 392–398. [Google Scholar] [CrossRef]
- McFadden, J.; Powles, A.; Kimber, I.; Fry, L. Psoriasis and basement-membrane laminin. Br. J. Dermatol. 1951 2013, 169, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Banno, T.; Walsh, R.; Blumenberg, M. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J. Cell. Physiol. 2008, 214, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ameglio, F.; D’auria, L.; Bonifati, C.; Ferraro, C.; Mastroianni, A.; Giacalone, B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: Relationships with disease intensity. Br. J. Dermatol. 1998, 138, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Danno, K.; Takigawa, M.; Horio, T. The alterations of keratinocyte surface and basement membrane markers by treatment with 8-methoxypsoralen plus long-wave ultraviolet light. J. Investig. Dermatol. 1983, 80, 172–174. [Google Scholar] [CrossRef]
- Zhu, K.J.; Lv, Y.M.; Yin, X.Y.; Wang, Z.X.; Sun, L.D.; He, S.M.; Cheng, H.; Hu, D.Y.; Zhang, Z.; Li, Y. Psoriasis regression analysis of MHC loci identifies shared genetic variants with vitiligo. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Seçkin, D.; Durusoy, Ç.; Şahin, S. Concomitant vitiligo and psoriasis in a patient treated with interferon alfa-2a for chronic hepatitis B infection. Pediatric Dermatol. 2004, 21, 577–579. [Google Scholar] [CrossRef]
- Afshar, M.; Martinez, A.; Gallo, R.; Hata, T. Induction and exacerbation of psoriasis with Interferon--alpha therapy for hepatitis C: A review and analysis of 36 cases. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 771–778. [Google Scholar] [CrossRef]
- Simsek, H.; Savas, C.; Akkiz, H.; Telatar, H. Interferon-lnduced Vitiligo in a Patient with Chronic Viral Hepatitis C Infection. Dermatology 1996, 193, 65–66. [Google Scholar]
- Damsky, W.; King, B.A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar]
- Kuo, C.M.; Tung, T.H.; Wang, S.H.; Chi, C.C. Efficacy and safety of tofacitinib for moderate--to--severe plaque psoriasis: A systematic review and meta-analysis of randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 355–362. [Google Scholar]
- Malerba, M.; Damiani, G.; Radaeli, A.; Ragnoli, B.; Olivini, A.; Calzavara-Pinton, P. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br. J. Dermatol. 2015, 173, 1544–1545. [Google Scholar] [PubMed]
- Kim, M.; Choi, K.H.; Hwang, S.W.; Lee, Y.B.; Park, H.J.; Bae, J.M. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: A population-based cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 40–48. [Google Scholar] [PubMed]
- Cohen, A.; Dreiher, J.; Birkenfeld, S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 561–565. [Google Scholar] [PubMed]
- Yang, S.K.; Park, M.; Lim, J.; Park, S.H.; Ye, B.D.; Lee, I.; Song, K. Contribution of IL23R but not ATG16L1 to Crohn’s disease susceptibility in Koreans. Inflamm. Bowel Dis. 2009, 15, 1385–1390. [Google Scholar]
- Nair, R.P.; Ruether, A.; Stuart, P.E.; Jenisch, S.; Tejasvi, T.; Hiremagalore, R.; Schreiber, S.; Kabelitz, D.; Lim, H.W.; Voorhees, J.J. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J. Investig. Dermatol. 2008, 128, 1653–1661. [Google Scholar]
- Duerr, R.H.; Taylor, K.D.; Brant, S.R.; Rioux, J.D.; Silverberg, M.S.; Daly, M.J.; Steinhart, A.H.; Abraham, C.; Regueiro, M.; Griffiths, A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006, 314, 1461–1463. [Google Scholar]
- Fieldhouse, K.A.; Ukaibe, S.; Crowley, E.L.; Khanna, R.; O’Toole, A.; Gooderham, M.J. Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context 2020, 9. [Google Scholar]
- Vassilatou, E.; Papadavid, E.; Papastamatakis, P.; Alexakos, D.; Koumaki, D.; Katsimbri, P.; Hadjidakis, D.; Dimitriadis, G.; Rigopoulos, D. No association of psoriasis with autoimmune thyroiditis. J. Eur. Acad. Dermatol. Venereol. Jeadv. 2017, 31, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Gul, U.; Gonul, M.; Kaya, I.; Aslan, E. Autoimmune thyroid disorders in patients with psoriasis. Eur. J. Derm. 2009, 19, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Kiguradze, T.; Bruins, F.M.; Guido, N.; Bhattacharya, T.; Rademaker, A.; Florek, A.G.; Posligua, A.; Amin, S.; Laumann, A.E.; West, D.P. Evidence for the association of Hashimoto’s thyroiditis with psoriasis: A cross-sectional retrospective study. Int. J. Dermatol. 2017, 56, 553–556. [Google Scholar] [PubMed]
- Chiriac, A.; Foia, L.; Chiriac, A.E.; Gorduza, V.E.; Solovan, C.; Brzezinski, P.J. Research, A case of subacute thyroiditis in a patient on adalimumab for treatment of refractory palmo-plantar psoriasis. Muller. J. Med. Sci. Res. 2014, 5, 70. [Google Scholar]
- Nakamura, K.; Kamata, M.; Sato, S.; Asano, Y.J. Subacute thyroiditis in psoriasis patients treated with biologics targeting tumor necrosis factor-α and interleukin-17A, a report of two cases. J. Cut. Immunol. Allergy. 2020, 3, 33–34. [Google Scholar] [CrossRef]
- Antonelli, A.; Delle Sedie, A.; Fallahi, P.; Ferrari, S.M.; Maccheroni, M.; Ferrannini, E.; Bombardieri, S.; Riente, L. High prevalence of thyroid autoimmunity and hypothyroidism in patients with psoriatic arthritis. J. Rheumatol. 2006, 33, 2026–2028. [Google Scholar]
- Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Elia, G.; Miccoli, M.; Delle Sedie, A.; Riente, L.; Antonelli, A. Increased incidence of autoimmune thyroid disorders in patients with psoriatic arthritis: A longitudinal follow-up study. Immunol. Res. 2017, 65, 681–686. [Google Scholar] [CrossRef]
- Antonelli, A.; Fallahi, P.; Sedie, A.D.; Ferrari, S.M.; Maccheroni, M.; Bombardieri, S.; Riente, L.; Ferrannini, E. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity 2008, 41, 537–542. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Feldman, S.R.; Stern, R.S.; Thomas, J.; Rolstad, T.; Margolis, D.J. Determinants of quality of life in patients with psoriasis: A study from the US population. J. Am. Acad. Dermatol. 2004, 51, 704–708. [Google Scholar] [CrossRef]
- Helmick, C.G.; Lee-Han, H.; Hirsch, S.C.; Baird, T.L.; Bartlett, C.L. Prevalence of psoriasis among adults in the US: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am. J. Prev. Med. 2014, 47, 37–45. [Google Scholar] [CrossRef]
- Kimball, A.B.; Wu, E.Q.; Guérin, A.; Andrew, P.Y.; Tsaneva, M.; Gupta, S.R.; Bao, Y.; Mulani, P.M. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J. Am. Acad. Dermatol. 2012, 67, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef]
- Tsintsadze, N.; Beridze, L.; Krichun, Y.; Tsivadze, N.; Tsintsadze, M. Psychosomatic aspects in patients with dermatologic diseases. Georgian Med. News 2015, 243, 70–75. [Google Scholar]
- Dowlatshahi, E.A.; Wakkee, M.; Arends, L.R.; Nijsten, T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: A systematic review and meta-analysis. J. Investig. Dermatol. 2014, 134, 1542–1551. [Google Scholar] [CrossRef]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar]
- Schmitt, J.; Ford, D.E. Psoriasis is independently associated with psychiatric morbidity and adverse cardiovascular risk factors, but not with cardiovascular events in a population-based sample. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Martires, K.J.; Ho, R.S. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009–2012. JAMA Dermatol. 2016, 152, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Ahlehoff, O.; Egeberg, A.; Gislason, G.; Hansen, P.R.; Skov, L. Psoriasis and new-onset depression: A Danish nationwide cohort study. Acta Derm. Venereol. 2016, 96, 39–42. [Google Scholar] [CrossRef]
- Wu, J.; Penfold, R.; Primatesta, P.; Fox, T.; Stewart, C.; Reddy, S.; Egeberg, A.; Liu, J.; Simon, G. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Tzur Bitan, D.; Krieger, I.; Comaneshter, D.; Cohen, A.D.; Feingold, D. The association between the socioeconomic status and anxiety-depression comorbidity in patients with psoriasis: A nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. Jeadv. 2019, 33, 1555–1561. [Google Scholar] [CrossRef]
- Tu, H.P.; Yu, C.L.; Lan, C.C.E.; Yu, S. Prevalence of schizophrenia in patients with psoriasis: A nationwide study. Dermatol. Sin. 2017, 35, 1–6. [Google Scholar]
- Egeberg, A.; Khalid, U.; Gislason, G.H.; Mallbris, L.; Skov, L.; Hansen, P.R. Psoriasis and Sleep Apnea: A Danish Nationwide Cohort Study. J. Clin. Sleep Med. 2016, 12, 663–671. [Google Scholar] [PubMed]
- Yu, S.; Yu, C.L.; Huang, Y.C.; Tu, H.P.; Lan, C.C. Risk of developing psoriasis in patients with schizophrenia: A nationwide retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1497–1504. [Google Scholar] [PubMed]
- Chi, C.C.; Chen, T.H.; Wang, S.H.; Tung, T.H. Risk of Suicidality in People with Psoriasis: A Systematic Review and Meta-Analysis of Cohort Studies. Am. J. Clin. Dermatol. 2017, 18, 621–627. [Google Scholar] [PubMed]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440. [Google Scholar]
- Zhao, S.S.; Miller, N.; Harrison, N.; Duffield, S.J.; Dey, M.; Goodson, N.J. Systematic review of mental health comorbidities in psoriatic arthritis. Clin. Rheumatol. 2020, 39, 217–225. [Google Scholar]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Fardan, A.S.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Attia, S.M. IL-17A causes depression-like symptoms via NFκB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 2017, 97, 14–24. [Google Scholar]
- Lindqvist, D.; Janelidze, S.; Hagell, P.; Erhardt, S.; Samuelsson, M.; Minthon, L.; Hansson, O.; Björkqvist, M.; Träskman-Bendz, L.; Brundin, L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 2009, 66, 287–292. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Fareed, J.; Hoppensteadt, D.A.; Roberts, R.C.; Conley, R.R.; Dwivedi, Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res. 2012, 46, 57–63. [Google Scholar] [CrossRef]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar]
- Debnath, M.; Berk, M. Th17 pathway–mediated immunopathogenesis of schizophrenia: Mechanisms and implications. Schizophr. Bull. 2014, 40, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Reich, K.; Paul, C.; Blauvelt, A.; Baran, W.; Bolduc, C.; Toth, D.; Langley, R.; Cather, J.; Gottlieb, A. A prospective phase III, randomized, double—Blind, placebo—Controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2016, 175, 273–286. [Google Scholar]
- Thaci, D.; Kimball, A.; Foley, P.; Poulin, Y.; Levi, E.; Chen, R.; Feldman, S. Apremilast, an oral phosphodiesterase 4 inhibitor, improves patient-reported outcomes in the treatment of moderate to severe psoriasis: Results of two phase III randomized, controlled trials. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 498–506. [Google Scholar] [CrossRef]
- Langley, R.G.; Feldman, S.R.; Han, C.; Schenkel, B.; Szapary, P.; Hsu, M.C.; Ortonne, J.P.; Gordon, K.B.; Kimball, A.B. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J. Am. Acad. Dermatol. 2010, 63, 457–465. [Google Scholar]
- Crowley, J.; Thaçi, D.; Joly, P.; Peris, K.; Papp, K.A.; Goncalves, J.; Day, R.M.; Chen, R.; Shah, K.; Ferrándiz, C. Long-term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J. Am. Acad. Dermatol. 2017, 77, 310–317. [Google Scholar]
- Gupta, M.A.; Simpson, F.C.; Gupta, A.K. Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2016, 29, 63–75. [Google Scholar] [CrossRef]
- Minoguchi, K.; Tazaki, T.; Yokoe, T.; Minoguchi, H.; Watanabe, Y.; Yamamoto, M.; Adachi, M. Elevated production of tumor necrosis factor-α by monocytes in patients with obstructive sleep apnea syndrome. Chest 2004, 126, 1473–1479. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef]
- Yokoe, T.; Minoguchi, K.; Matsuo, H.; Oda, N.; Minoguchi, H.; Yoshino, G.; Hirano, T.; Adachi, M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003, 107, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
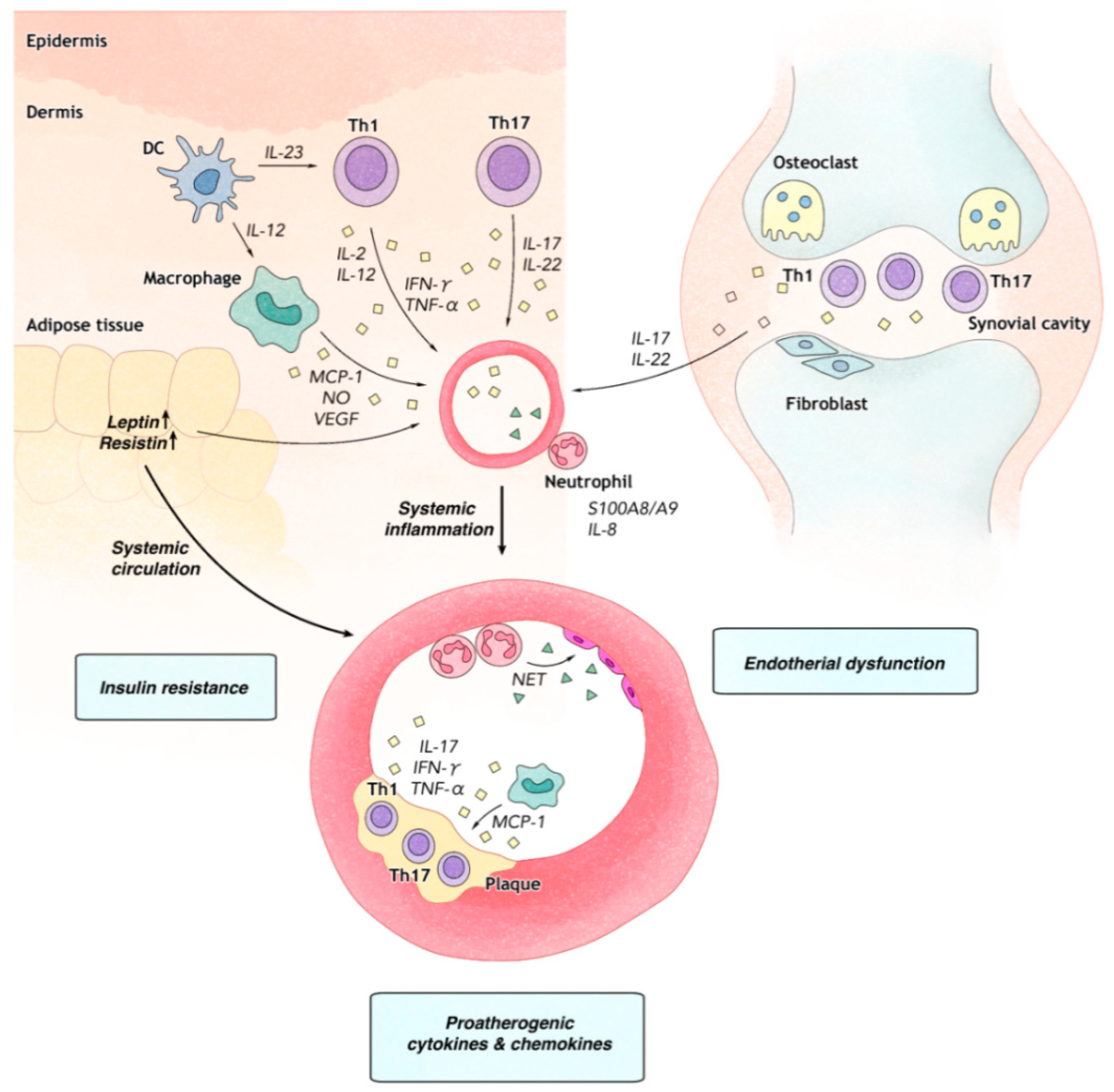
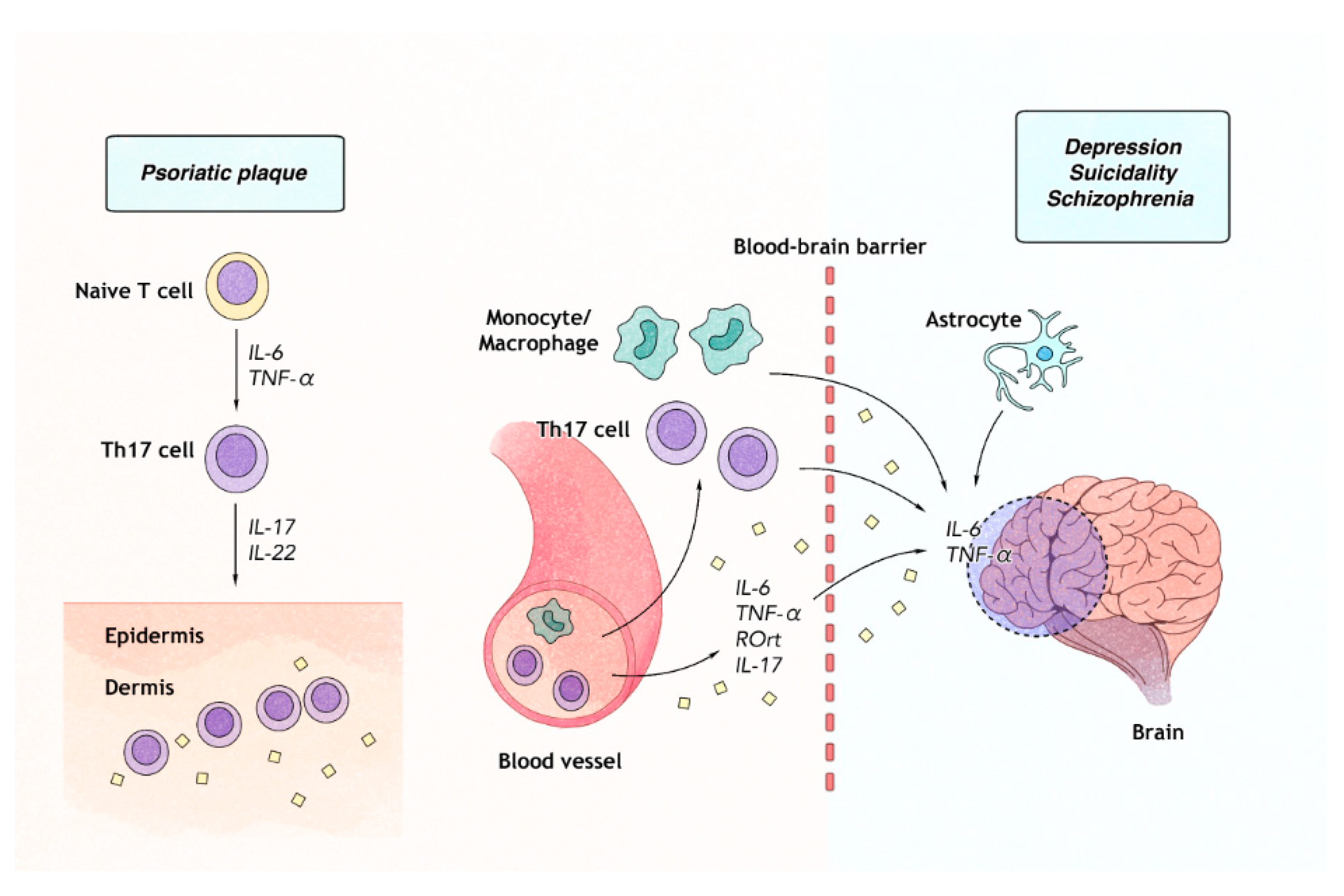
| Study Number | Study Population | Identified Risk Among CV Conditions | The Relative Risk of Measures |
|---|---|---|---|
| Psoriasis | |||
| 1 [14] | Psoriasis patients: 503,686 Controls: 29,686,694 | CVD, IHD, cerebrovascular disease, and CV mortality | CVD in total OR 1.4 (1.2–1.7) IHD OR 1.5 (1.2–1.9) Cerebrovascular disease OR 1.1 (0.9–1.3) CV mortality OR 0.9 (0.4–2.2) |
| 2 [15] | Psoriasis patients + controls: 6,230,774 | Stroke, MI, CVD, and CV mortality | Stroke RR 1.26 (1.12–1.41) MI RR 1.32 (1.13–1.55) CVD RR 1.47 (1.30–1.60) CV mortality RR 1.33 (1.00–1.77) |
| 3 [16] | Psoriasis patients: 324,650 Controls: 5,309,087 | MI, CAD, and stroke | Cohort studies: MI OR 1.25 (1.03–1.52), CAD OR 1.20 (1.13–1.27), stroke OR 1.02 (0.92–1.14) Cross-sectional studies: MI OR 1.57 (1.08–2.27), CAD OR 1.84 (1.09 –3.09), stroke OR 1.14 (1.08–1.19) |
| 4 [17] | Psoriasis patients: 367,358 Controls: 9,199,656 | CV events: MI, IHD, cerebral ischemic stroke, and sudden cardiac death | CV events OR 1.28 (1.18–1.38) |
| 5 [18] | Psoriasis patients: 488,315 (mild: 327,418; severe: 12,854) Controls: 10,024,815 | CVD mortality, MI, and Stroke | All psoriasis: MI HR 1.40 (1.03–1.89) and stroke HR 1.13 (1.01–1.26) Mild psoriasis: CVD mortality SMR 1.03 (0.86–1.25), MI HR 1.34 (1.07– 1.68), and stroke HR 1.15 (0.98–1.35) Severe psoriasis: CVD mortality SMR 1.37 (1.17–1.60), CVD mortality HR 1.57 (1.26–1.96), MI HR 3.04 (0.65–14.35) and stroke HR 1.59 (1.34– 1.89) |
| 6 [19] | Psoriasis patients: 218,654 (mild: 201,239; severe: 17,415) Controls: 9,914,799 | MACE: CV mortality, MI, and stroke | Mild Psoriasis: MI RR 1.29 (1.02–1.63), stroke RR 1.12 (1.08–1.16) Severe Psoriasis: MI RR 1.70 (1.32–2.18), stroke RR 1.56 (1.32–1.84), CV mortality RR 1.39 (1.11–1.74) |
| 7 [20] | Psoriasis patients: 1,862,297 Controls: 43,407,300 | MI, CVD, and CV death | Overall CV RR 1.24 (1.18–1.31), MI RR 1.24 (1.11–1.39) Vascular disease RR 1.27 (1.12–1.43), CV Mortality RR 1.41 (0.97–2.04) |
| 8 [21] | Psoriasis patients: 326,598 Controls: 5,230,048 | MI, and stroke | MI and stroke RR 1.20 (1.10–1.31) MI RR 1.22 (1.05–1.42) Stroke RR 1.21 (1.04–1.40) |
| Psoriatic arthritis | |||
| 1 [23] | Psoriatic arthritis patients: 32,973 Controls: 4,568,723 | CVD, CV events Morbidity risks for MI, cerebrovascular events, and heart failure | CVD OR 1.43 (1.24–1.66) CV events 1.55 (1.22–1.96) Morbidity risk for MI OR 1.68 (1.31–2.15) Morbidity risk for cerebrovascular events OR 1.22 (1.05–1.41) Morbidity risk for heart failure OR 1.32 (1.11–1.57) |
| Study | Population | Identified Cardiovascular Risk Factors with Relative Risk of Measures |
|---|---|---|
| Psoriasis | ||
| Armstrong (2013) [28] | Psoriasis: 309,469 Control: 2,088,197 | Hypertension in all psoriasis OR 1.58 (1.42–1.76); in mild psoriasis OR 1.30 (1.15–1.47); in severe psoriasis OR 1.49 (1.20–1.86) |
| Duan (2020) [29] | Psoriasis: 255,132 Control: 814,631 | Hypertension OR 1.43 (1.25–1.64) |
| Armstrong (2013) [30] | Psoriasis: 314,036 Control: 3,717,217 | Diabetes OR 1.59 (1.38–1.83); in mild psoriasis pooled OR 1.53 (1.16–2.04); in severe psoriasis pooled OR 1.97 (1.48–2.62) |
| Coto-Segura (2013) [31] | Psoriasis: 557,697 Control: 5,186,485 | Type 2 diabetes pooled OR 1.76 (1.59–1.96) |
| Mamizadeh (2019) [32] | Psoriasis: 922,870 Control: 12,808,071 | Diabetes OR 1.69 (1.51–1.89) |
| Armstrong (2012) [33] | Psoriasis: 201,831 Control: 1,898,169 | Obesity OR 1.66 (1.46-1.89); in mild psoriasis OR 1.46 (1.17–1.82); in severe psoriasis OR 2.23 (1.63–3.05) |
| Miller (2013) [14] | Psoriasis: 503,686 Control: 27,686,694 | Diabetes OR 1.9 (1.5–2.5); hypertension OR 1.8 (1.6–2.0), dyslipidemia OR 1.5 (1.4–1.7); obesity OR 1.8 (1.4–2.2); metabolic syndrome OR 1.8 (1.2–2.8) |
| Choudhary (2020) [34] | Psoriasis: 17,672 Control: 66,407 | Increased systolic blood pressure OR 2.31 (1.12–4.74); diastolic blood pressure OR 2.31 (1.58–3.38); abdominal obesity OR 1.90 (1.45–2.50); Triglycerides OR 1.80 (1.29–2.51) |
| Phan (2020) [35] | Pediatric psoriasis: 43,808 Control: 5,384,057 | Obesity OR 2.45 (1.73–3.48); diabetes OR 2.32 (1.34–4.03); hypertension OR 2.19 (1.62–2.95); hyperlipidemia OR 2.01 (1.66–2.42); metabolic syndrome OR 1.75 (1.75–7.14) |
| Armstrong (2013) [36] | Psoriasis: 41,853 Control: 1,358,147 | Metabolic syndrome OR 2.26 (1.70–3.01) |
| Rodríguez-Zúniga (2017) [37] | Psoriasis: 25,042 Control: 131,609 | Metabolic syndrome pooled OR 1.42 (1.28–1.65) |
| Singh (2017) [38] | Psoriasis: 46,714 Control: 1,403,474 | Metabolic syndrome pooled OR 2.14 (1.84–2.48) |
| Choudhary (2019) [39] | Psoriasis: 15,939 Control: 103,984 | Metabolic syndrome OR 2.077 (1.84–2.34) |
| Psoriatic arthritis | ||
| Coto-Segura (2013) [31] | Psoriatic arthritis: 3568 Control: 13,346 | Type II diabetes mellitus OR 2.18 (1.36–3.50) |
| Number | Study | Main Outcomes | |||
|---|---|---|---|---|---|
| Overall Cancer | Solid Organ Cancer | Hematologic Cancer | Skin Cancer | ||
| Psoriasis | |||||
| 1 [63] | Pouplard (2013) | Overall cancer SIR 1.16 (1.07–1.25) | Respiratory tract cancer SIR 1.52 (1.35–1.71); upper aerodigestive tract cancer SIR 3.05 (1.74–5.32); urinary tract cancer SIR 1.31 (1.11–1.55); liver cancer SIR 1.90 (1.48–2.44) | Non-Hodgkin lymphoma SIR 1.40 (1.06–1.86); | BCC SIR 2.00 (1.83–2.20) |
| 2 [76] | Trafford (2019) | Overall cancer in all severities of psoriasis RR 1.18 (1.06–1.31); severe psoriasis RR 1.22 (1.08–1.39) | Colon cancer RR 1.18 (1.03–1.35); colorectal RR 1.34 (1.06–1.70); kidney RR 1.58 (1.11–2.24); laryngeal RR 1.79 (1.06–3.01); liver RR 1.83 (1.28–2.61); pancreatic cancer RR 1.41 (1.16–1.73) | Lymphoma RR 1.40 (1.24–1.57); non-Hodgkin lymphoma RR 1.28 (1.15–1.43) | Keratinocyte cancer RR 1.71 (1.08–2.71); SCC RR 2.15 (1.32–3.50) |
| 3 [61] | Vaengebjerg (2020) | Overall cancer RR 1.21 (1.11–1.33); cancer excluding keratinocyte cancer RR 1.14 (1.04–1.25) | Lung cancer RR 1.26 (1.13–1.40); bladder cancer RR 1.12 (1.04-1.19) | Lymphoma overall RR 1.56 (1.37–1.78); non-Hodgkin lymphoma RR 1.48 (1.30–1.69) | Keratinocyte cancer RR 2.28 (1.73–3.01) |
| 4 [62] | Wang (2020) | N/A | N/A | N/A | NMSC RR 1.72 (1.46–2.02); risk of NMSC in moderate to severe psoriasis RR 1.82 (1.38–2.41); risk of NMSC in mild psoriasis RR 1.61 (1.25–2.09); SCC RR 2.08 (1.53–2.83); BCC RR 1.28 (0.81–2.00) |
| Psoriatic arthritis | |||||
| 1 [61] | Vaengebjerg (2020) | Overall cancer RR 1.02 (0.97–1.08) | Breast cancer RR 1.73 (1.15–2.59) | ||
| Study | Country | Subjects | Outcomes |
|---|---|---|---|
| Risk of general infections in psoriasis | |||
| Takeshita (2018) [78] | UK | Psoriasis: 199,700 Control: 954,315 | HR of serious infection in overall patients with psoriasis 1.21 (1.18–1.23); in mild psoriasis 1.18 (1.16–1.21); in moderate to severe psoriasis 1.63 (1.52–1.75); HR of opportunistic infection in moderate to severe psoriasis 1.57 (1.06–2.34); HR of herpes zoster in mild psoriasis 1.07 (1.05–1.10); in moderate to severe psoriasis 1.17 (1.06–1.30) |
| Kim (2019) [77] | Korean | Psoriasis: 16,383 Control: 613,144 | RR of all infections 1.89 (1.83–1.94); RR of all infections except skin and soft tissue 1.58 (1.53–1.63) |
| Risk of general infections in PsA | |||
| Pattison (2008) [106] | UK | PsA: 98 Control: 163 | OR of recurrent oral ulceration 4.20 (1.96–9.0) |
| Eder (2011) [101] | Canada | PsA: 159 Control: 159 | OR of infection that required antibiotics 1.7 (1.00–2.77) |
| Haddad (2016) [103] | Canada | PsA: 695 Psoriasis: 509 | HR of infection for PsA versus psoriasis 1.56 (1.18–2.06) |
| Risk of viral hepatitis in psoriasis | |||
| Taglione (1999) [107] | Italy | Psoriasis: 50 Control: 76 | Prevalence of HCV was not higher in patients with psoriasis than controls. |
| Cohen (2010) [80] | Israel | Psoriasis: 12,502 Control: 24,287 | OR of HCV in smokers 1.93 (1.30–2.67); OR of HCV in nonsmokers 2.22 (1.63–3.04) OR of HBV 1.22 (0.93–1.60) |
| Tsai (2011) [108] | Taiwan | Psoriasis: 51,800 Control: 207,200 | OR of HCV 2.02 (1.67–2.44 OR of HBV 1.73 (1.47–2.04) |
| Yang (2011) [81] | Taiwan | Psoriasis: 1,685 Control: 5,055 | OR of HBV or HCV in overall psoriasis 1.34 (1.04–1.73); OR of HBV or HCV in moderate to severe psoriasis 1.39 (1.06–1.83) |
| Imafuku (2013) [84] | Japan | Psoriasis: 717 Control: 38,057 | OR of HCV 2.42 (1.82–3.21) |
| Risk of viral hepatitis in PsA | |||
| Palazzi (2005) [105] | Italy | PsA: 100 Control: 100 | No significant difference in HCV prevalence between PsA and control (p = 0.68) |
| Taglione (1999) [107] | Italy | PsA: 50 Control: 76 | Prevalence of HCV in PsA was higher than controls. |
| Study | Subjects | Outcomes |
|---|---|---|
| Psoriasis | ||
| Khan (2017) [111] | Autoimmune thyroid disease: 3,535 Control: 416,649 | OR of psoriasis 1.25 (1.14–1.37) |
| Liu (2019) [112] | Multiple sclerosis: 25,187; Control: 227,225 | HR of psoriasis 1.92 (1.32–2.80) |
| Phan (2019) [113] | Bullous pemphigoid: 4,035; Control: 19,215 | OR of psoriasis 2.5 (1.4–4.6) |
| Kridin (2019) [114] | Pemphigus: 12,238; Control: 87,051,168 | OR of psoriasis 3.5 (1.6–7.6) |
| Yen (2019) [115] | Psoriasis: 118,178; Control: 3,262,337; Vitiligo: 79,907 | OR of vitiligo in psoriasis 2.29 (1.56–3.37) OR of psoriasis in vitiligo 3.43 (1.86–6.33) |
| Fu (2018) [116] | Psoriasis: 212,544; Control: 7,581,541 | OR of CD 1.7 (1.20–2.40); OR of UC 1.75 (1.49–2.05); RR of CD 2.74 (1.41–5.32); RR of UC 1.74 (0.72–4.17) |
| Alinaghi (2020) [117] | Prevalence of psoriasis in 199,672 patients with IBD; prevalence of IBD in 481,853 patients with psoriasis | Prevalence of psoriasis in CD 3.6% (3.1–4.6); in UC 2.8% (2.0–3.8) OR of psoriasis in IBD 1.8 (1.5–2.2); OR of psoriasis in CD 2.0 (1.4–2.9); OR of psoriasis in UC 1.5 (1.2–2.0); OR of CD in psoriasis 2.2 (1.6–3.1); OR of UC in psoriasis 1.6 (1.3–2.0). |
| Acharya (2020) [118] | Psoriasis: 136,536; Control: 5,716,558 Celiac disease: 48,803; Control: 1,833,038 | OR of celiac disease in psoriasis 2.03 (1.49–2.75); OR of psoriasis in celiac disease 1.8 (1.36–2.38) |
| PsA | ||
| Fu (2018) [116] | PsA:10,956; Control: 262,649 | Case-control study: CD OR 2.20 (1.59–3.03); UC OR 1.91 (1.21–3.00); Cohort study: CD RR 2.74 (1.41–5.32); UC RR 1.74 (0.72–4.17) |
| Alinaghi (2020) [117] | Prevalence of IBD in 13,788 patients with PsA; prevalence of PsA in 47,740 patients with IBD | Prevalence of IBD 1.8% (1.0–2.9); prevalence of CD 1.2% (0.4–2.3); prevalence of UC 0.7% (0.3–1.2); prevalence of PsA in IBD 1.0% (0.4–1.9) |
| Acharya (2020) [118] | Celiac disease: 48,803; Control: 1,833,038 | OR of PsA in celiac disease 2.31 (1.7–3.14) |
| Psychiatric Disorder | Study | Study Population | Relative Risk of Measures |
|---|---|---|---|
| Psoriasis | |||
| Depression and/or suicidality | Kurd (2010) [155] | Psoriasis: 149,998 (mild: 146,042; severe: 3,956) Control: 766,950 | Depression HR in all psoriasis 1.39 (1.37–1.41); in mild psoriasis 1.38 (1.35–1.40); in severe psoriasis 1.72 (1.57–1.88) Suicidality HR in all psoriasis 1.44(1.32–1.57); in mild psoriasis 1.44 (1.32–1.57) |
| Schmitt (2010) [156] | Psoriasis: 3,147 Control: 3,147 | Depression OR 1.49 (1.20–1.86) | |
| Tsai (2011) [108] | Psoriasis: 51,800 Control: 207,200 | Depression RR 1.50 (1.39–1.61) | |
| Kimball (2012) [151] | Pediatric patients with psoriasis: 7,404 Control: 37,020 | Depression HR 1.23 (1.06–1.43) | |
| Parisi (2015) [10] | Psoriasis: 48,523 Control: 208,187 | Depression HR 1.16 (1.01–1.34) | |
| Cohen (2016) [157] | History of psoriasis: 351 No history of psoriasis: 12,031 | Major depression OR 2.09 (1.41–43.11); Symptoms of depression OR 1.44 (1.03–2.00) | |
| Jensen (2016) [158] | Mild psoriasis: 35,001, severe psoriasis: 7,510 | Depression IRR in mild psoriasis 1.08 (1.04–1.12); in severe psoriasis 1.36 (1.27–1.46) | |
| Wu (2017) [159] | Psoriasis: 36,214 Control: 156,093 | Depression IRR in psoriasis 1.14 (1.11–1.17) | |
| Tzur Bitan (2019) [160] | Psoriasis: 127,931 Control: 127,931 | Depression OR 1.17 (1.08–1.26) | |
| Anxiety | Kurd (2010) [155] | Psoriasis: 149,998 Control: 766,950 | Anxiety HR in all psoriasis 1.31 (1.29–1.34); in mild psoriasis 1.29 (1.15–1.43); in severe psoriasis 1.29 (1.15–1.43) |
| Kimball (2012) [151] | Pediatric patients with psoriasis: 7,404 Control: 37,020 | Anxiety HR 1.32 (1.09–1.61) | |
| Tzur Bitan (2019) [160] | Psoriasis: 127,931 Control: 127,931 | Anxiety OR 1.11 (1.01–1.23) | |
| Schizophrenia | Tu (2017) [161] | Psoriasis: 10,796 Control: 10,796 | Schizophrenia OR 1.44 (1.08– 1.92) |
| Sleep disorder | Tsai (2011) [108] | Psoriasis: 51,800 Control: 207,200 | Sleep disorder RR 3.89 (2.26–6.71) |
| Egeberg (2016) [162] | Psoriasis: 60,175 (mild: 53,290; severe: 6,885) Control: 5,393,040 | Sleep apnea in mild psoriasis IRR 1.30 (1.17–1.44); severe psoriasis IRR 1.65 (1.23-2.22) | |
| PsA | |||
| Depression and/or suicidality | Wu (2017) [159] | PsA: 5,138 Control: 156,093 | Depression IRR 1.22 (1.16–1.29) |
| Sleep disorder | Egeberg (2016) [162] | PsA: 6,348 Control: 5,393,040 | Sleep apnea IRR 1.75 (1.35–2.26) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, Y.R.; Park, C.J.; Kang, H.; Kim, J.E. The Risk of Systemic Diseases in Those with Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic. Int. J. Mol. Sci. 2020, 21, 7041. https://doi.org/10.3390/ijms21197041
Woo YR, Park CJ, Kang H, Kim JE. The Risk of Systemic Diseases in Those with Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic. International Journal of Molecular Sciences. 2020; 21(19):7041. https://doi.org/10.3390/ijms21197041
Chicago/Turabian StyleWoo, Yu Ri, Chul Jong Park, Hoon Kang, and Jung Eun Kim. 2020. "The Risk of Systemic Diseases in Those with Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic" International Journal of Molecular Sciences 21, no. 19: 7041. https://doi.org/10.3390/ijms21197041
APA StyleWoo, Y. R., Park, C. J., Kang, H., & Kim, J. E. (2020). The Risk of Systemic Diseases in Those with Psoriasis and Psoriatic Arthritis: From Mechanisms to Clinic. International Journal of Molecular Sciences, 21(19), 7041. https://doi.org/10.3390/ijms21197041







