Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models
Abstract
1. Introduction
2. Results
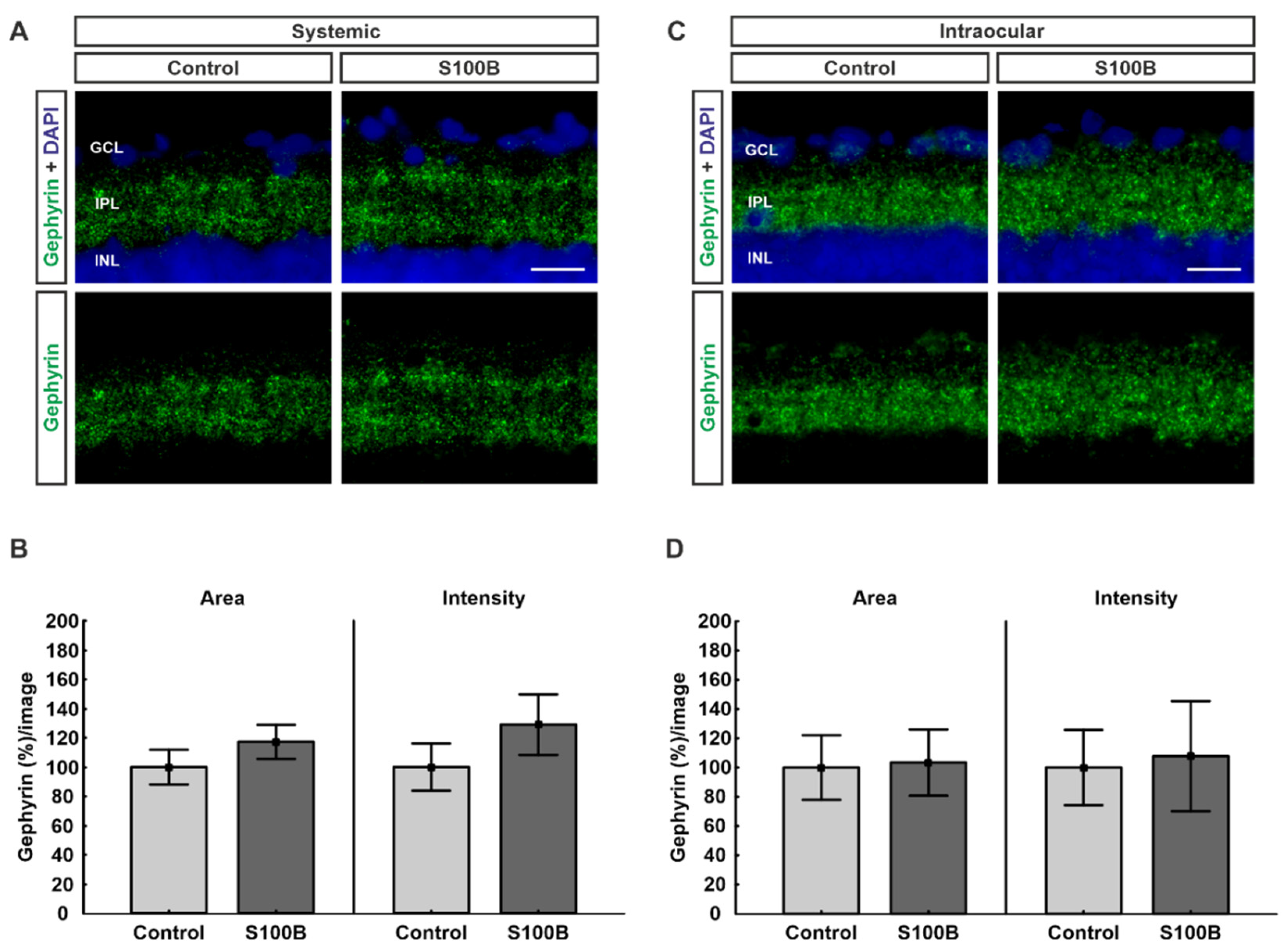
2.1. Inhibitory Post-Synapses Are Not Affected
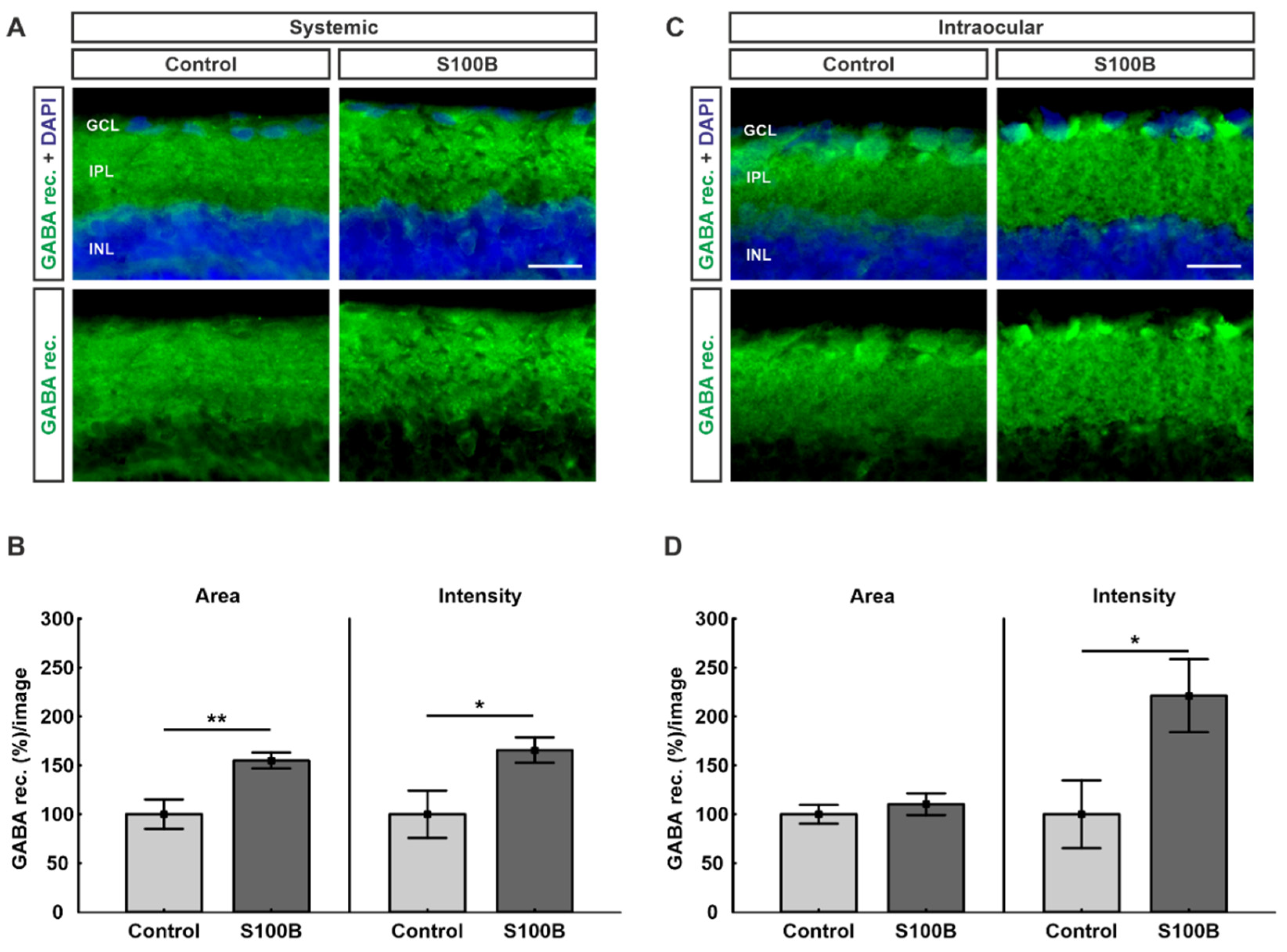
2.2. Upregulation of GABA Receptors in Both Models
2.3. Excitatory Post-Synapses Degenerated in Intraocular-Injected Animals
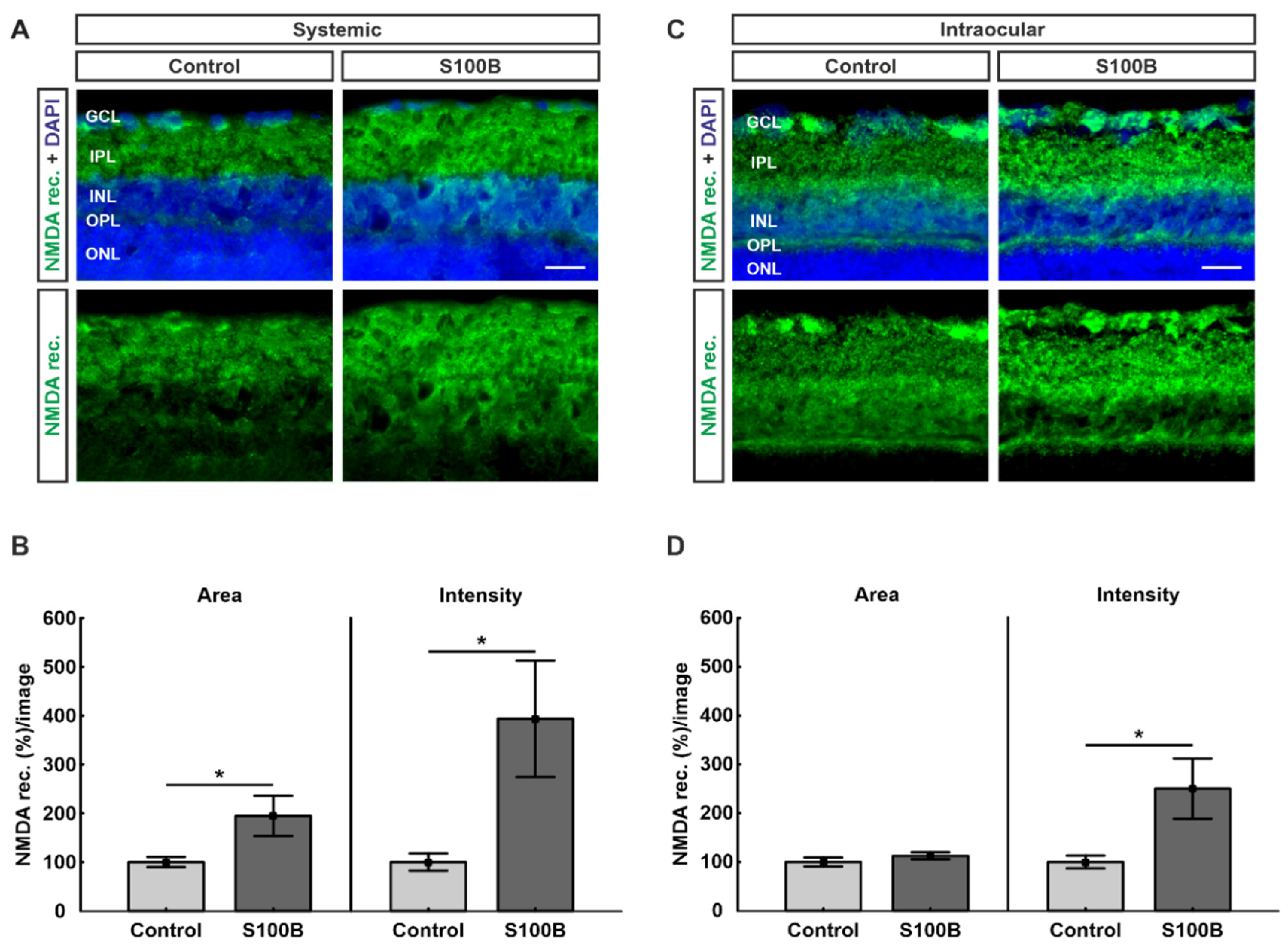
2.4. Upregulation of NMDA Receptors in Both Models
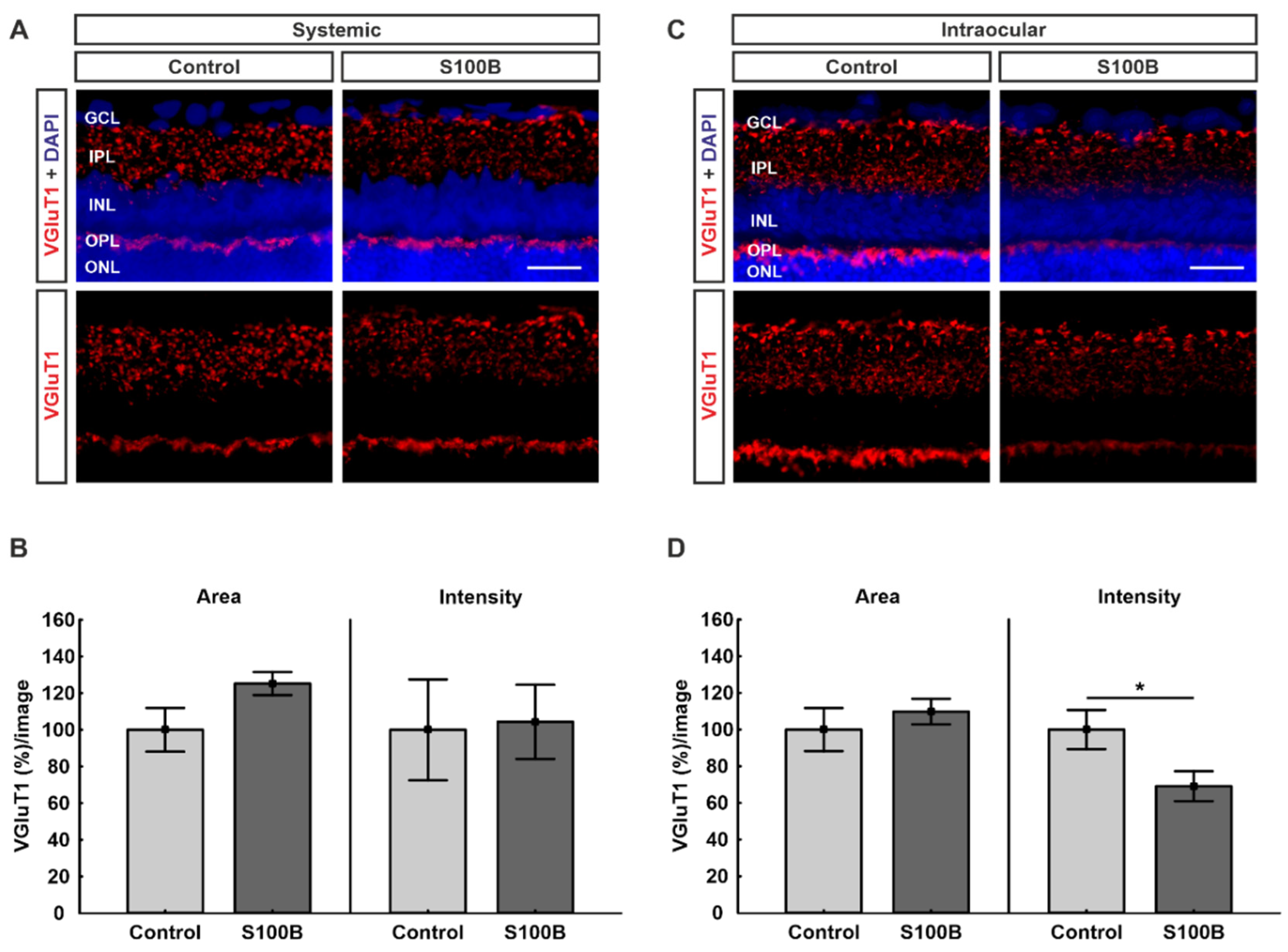
2.5. Excitatory Pre-Synapses Were Remodeled in Intraocular-Injected Animals
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Systemic Immunization
4.3. Intraocular Injection
4.4. Tissue Preparation for Retinal Cross-Sections
4.5. Immunofluorescence Stainings of the Retinas
4.6. Histological Investigation
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Finger, R.P.; Fimmers, R.; Holz, F.G.; Scholl, H.P. Incidence of blindness and severe visual impairment in Germany: Projections for 2030. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4381–4389. [Google Scholar] [CrossRef]
- Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004, 82, 887–888. [Google Scholar]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Le, A.; Mukesh, B.N.; McCarty, C.A.; Taylor, H.R. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3783–3789. [Google Scholar] [CrossRef]
- Chiu, S.L.; Chu, C.L.; Muo, C.H.; Chen, C.L.; Lan, S.J. The Prevalence and the Incidence of Diagnosed Open-Angle Glaucoma and Diagnosed Angle-Closure Glaucoma: Changes from 2001 to 2010. J. Glaucoma 2016, 25, e514–e519. [Google Scholar] [CrossRef]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition—Chapter 2: Classification and terminology Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 Classification and Terminology. Br. J. Ophthalmol. 2017, 101, 73–127. [CrossRef]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.; Singh, K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch. Ophthalmol. 1991, 109, 1090–1095. [Google Scholar] [CrossRef]
- Grus, B.N.; Beck, S.; Beck, S.; Schlich, M.; Lossbrandt, U.; Pfeiffer, N. Autoantibody profiles in tear fluid as a diagnostic tool in glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6110. [Google Scholar]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef]
- Bianchi, R.; Kastrisianaki, E.; Giambanco, I.; Donato, R. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J. Biol. Chem. 2011, 286, 7214–7226. [Google Scholar] [CrossRef]
- Griffin, W.S.; Stanley, L.C.; Ling, C.; White, L.; MacLeod, V.; Perrot, L.J.; White, C.L., 3rd; Araoz, C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Funke, S.; Grus, F.H. Autoimmunity and glaucoma. Ophthalmologe 2019, 116, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.; Gramlich, O.W.; Von Thun Und Hohenstein-Blaul, N.; Beck, S.; Funke, S.; Wilding, C.; Pfeiffer, N.; Grus, F.H. Does autoimmunity play a part in the pathogenesis of glaucoma? Prog. Retin. Eye Res. 2013, 36, 199–216. [Google Scholar] [CrossRef]
- Grus, F.H.; Gramlich, O.W. Autoimmunity and glaucoma. Klin. Mon. Augenheilkd. 2011, 228, 439–445. [Google Scholar] [CrossRef]
- Grus, F.H.; Joachim, S.C.; Wuenschig, D.; Rieck, J.; Pfeiffer, N. Autoimmunity and glaucoma. J. Glaucoma 2008, 17, 79–84. [Google Scholar] [CrossRef]
- Rieck, J. The pathogenesis of glaucoma in the interplay with the immune system. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2393–2409. [Google Scholar] [CrossRef]
- Reichelt, J.; Joachim, S.C.; Pfeiffer, N.; Grus, F.H. Analysis of autoantibodies against human retinal antigens in sera of patients with glaucoma and ocular hypertension. Curr. Eye Res. 2008, 33, 253–261. [Google Scholar] [CrossRef]
- Joachim, S.C.; Reichelt, J.; Berneiser, S.; Pfeiffer, N.; Grus, F.H. Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Joachim, S.C.; Pfeiffer, N.; Grus, F.H. Autoantibodies in patients with glaucoma: A comparison of IgG serum antibodies against retinal, optic nerve, and optic nerve head antigens. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Joachim, S.C.; Bruns, K.; Lackner, K.J.; Pfeiffer, N.; Grus, F.H. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr. Eye Res. 2007, 32, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Grus, F.H.; Joachim, S.C.; Hoffmann, E.M.; Pfeiffer, N. Complex autoantibody repertoires in patients with glaucoma. Mol. Vis. 2004, 10, 132–137. [Google Scholar] [PubMed]
- Joachim, S.C.; Reinehr, S.; Kuehn, S.; Laspas, P.; Gramlich, O.W.; Kuehn, M.; Tischoff, I.; von Pein, H.D.; Dick, H.B.; Grus, F.H. Immune response against ocular tissues after immunization with optic nerve antigens in a model of autoimmune glaucoma. Mol. Vis. 2013, 19, 1804–1814. [Google Scholar]
- Laspas, P.; Gramlich, O.W.; Muller, H.D.; Cuny, C.S.; Gottschling, P.F.; Pfeiffer, N.; Dick, H.B.; Joachim, S.C.; Grus, F.H. Autoreactive antibodies and loss of retinal ganglion cells in rats induced by immunization with ocular antigens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8835–8848. [Google Scholar] [CrossRef] [PubMed]
- Casola, C.; Schiwek, J.E.; Reinehr, S.; Kuehn, S.; Grus, F.H.; Kramer, M.; Dick, H.B.; Joachim, S.C. S100 alone has the same destructive effect on retinal ganglion cells as in combination with HSP 27 in an autoimmune glaucoma model. J. Mol. Neurosci. 2015, 56, 228–236. [Google Scholar] [CrossRef]
- Grotegut, P.; Kuehn, S.; Meissner, W.; Dick, H.B.; Joachim, S.C. Intravitreal S100B Injection Triggers a Time-Dependent Microglia Response in a Pro-Inflammatory Manner in Retina and Optic Nerve. Mol. Neurobiol. 2020, 57, 1186–1202. [Google Scholar] [CrossRef]
- Kuehn, S.; Meissner, W.; Grotegut, P.; Theiss, C.; Dick, H.B.; Joachim, S.C. Intravitreal S100B Injection Leads to Progressive Glaucoma Like Damage in Retina and Optic Nerve. Front. Cell. Neurosci. 2018, 12, 312. [Google Scholar] [CrossRef]
- Reinehr, S.; Kuehn, S.; Casola, C.; Koch, D.; Stute, G.; Grotegut, P.; Dick, H.B.; Joachim, S.C. HSP27 immunization reinforces AII amacrine cell and synapse damage induced by S100 in an autoimmune glaucoma model. Cell Tissue Res. 2018, 371, 237–249. [Google Scholar] [CrossRef]
- Kornau, H.C.; Schenker, L.T.; Kennedy, M.B.; Seeburg, P.H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 1995, 269, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.L.; Papadakis, M.; Rutter, A.R.; Stephenson, F.A. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J. Neurochem. 2008, 104, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S. NMDA receptors and their interacting proteins in the postsynaptic density. J. Anat. 2001, 76, 185–191. [Google Scholar]
- Tretter, V.; Kerschner, B.; Milenkovic, I.; Ramsden, S.L.; Ramerstorfer, J.; Saiepour, L.; Maric, H.M.; Moss, S.J.; Schindelin, H.; Harvey, R.J.; et al. Molecular basis of the gamma-aminobutyric acid A receptor alpha3 subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 2011, 286, 37702–37711. [Google Scholar] [CrossRef]
- Rosen, A.M.; Stevens, B. The role of the classical complement cascade in synapse loss during development and glaucoma. Adv. Exp. Med. Biol. 2010, 703, 75–93. [Google Scholar]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- DeVries, S.H.; Baylor, D.A. Synaptic circuitry of the retina and olfactory bulb. Cell 1993, 72, 139–149. [Google Scholar] [CrossRef]
- Hunt, C.A.; Schenker, L.J.; Kennedy, M.B. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J. Neurosci. 1996, 16, 1380–1388. [Google Scholar] [CrossRef]
- Beique, J.C.; Andrade, R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J. Physiol. 2003, 546 Pt 3, 859–867. [Google Scholar] [CrossRef]
- El-Husseini, A.E.; Schnell, E.; Chetkovich, D.M.; Nicoll, R.A.; Bredt, D.S. PSD-95 involvement in maturation of excitatory synapses. Science 2000, 290, 1364–1368. [Google Scholar]
- Della Santina, L.; Inman, D.M.; Lupien, C.B.; Horner, P.J.; Wong, R.O. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J. Neurosci. 2013, 33, 17444–17457. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, J.H.; Park, C.K. Alterations of the synapse of the inner retinal layers after chronic intraocular pressure elevation in glaucoma animal model. Mol. Brain 2014, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Incontro, S.; Nicoll, R.A.; Roche, K.W. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc. Natl. Acad. Sci. USA 2016, 113, E4736–E4744. [Google Scholar] [CrossRef]
- Lin, Y.; Skeberdis, V.A.; Francesconi, A.; Bennett, M.V.; Zukin, R.S. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J. Neurosci. 2004, 24, 10138–10148. [Google Scholar] [CrossRef]
- Fan, W.; Xing, Y.; Zhong, Y.; Chen, C.; Shen, Y. Expression of NMDA receptor subunit 1 in the rat retina. Acta Histochem. 2013, 115, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Bai, N.; Aida, T.; Yanagisawa, M.; Katou, S.; Sakimura, K.; Mishina, M.; Tanaka, K. NMDA receptor subunits have different roles in NMDA-induced neurotoxicity in the retina. Mol. Brain 2013, 6, 34. [Google Scholar] [CrossRef]
- Bai, N.; Hayashi, H.; Aida, T.; Namekata, K.; Harada, T.; Mishina, M.; Tanaka, K. Dock3 interaction with a glutamate-receptor NR2D subunit protects neurons from excitotoxicity. Mol. Brain 2013, 6, 22. [Google Scholar] [CrossRef]
- Sucher, N.J.; Lipton, S.A.; Dreyer, E.B. Molecular basis of glutamate toxicity in retinal ganglion cells. Vis. Res. 1997, 37, 3483–3493. [Google Scholar] [CrossRef]
- Casson, R.J. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef]
- Kuehn, S.; Rodust, C.; Stute, G.; Grotegut, P.; Meissner, W.; Reinehr, S.; Dick, H.B.; Joachim, S.C. Concentration-Dependent Inner Retina Layer Damage and Optic Nerve Degeneration in a NMDA Model. J. Mol. Neurosci. 2017, 63, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.; Abler, A.S.; Kwong, J.M.; Tso, M.O. N-Methyl-D-aspartate (NMDA)-induced apoptosis in rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2391–2397. [Google Scholar]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Fan, M.M.; Raymond, L.A. N-Methyl-d-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog. Neurobiol. 2007, 81, 272–293. [Google Scholar] [CrossRef]
- Georgiou, A.L.; Guo, L.; Cordeiro, M.F.; Salt, T.E. Changes in NMDA receptor contribution to synaptic transmission in the brain in a rat model of glaucoma. Neurobiol. Dis. 2010, 39, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tyagarajan, S.K.; Fritschy, J.M. Gephyrin: A master regulator of neuronal function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef]
- Choii, G.; Ko, J. Gephyrin: A central GABAergic synapse organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Reinhard, J.; Wiemann, S.; Hesse, K.; Voss, C.; Gandej, M.; Dick, H.B.; Faissner, A.; Joachim, S.C. Transfer of the Experimental Autoimmune Glaucoma Model from Rats to Mice-New Options to Study Glaucoma Disease. Int. J. Mol. Sci. 2019, 20, 2563. [Google Scholar] [CrossRef]
- Brady, M.L.; Jacob, T.C. Synaptic localization of alpha5 GABA (A) receptors via gephyrin interaction regulates dendritic outgrowth and spine maturation. Dev. Neurobiol. 2015, 75, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M.; Panzanelli, P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014, 39, 1845–1865. [Google Scholar] [CrossRef] [PubMed]
- Knoflach, F.; Hernandez, M.C.; Bertrand, D. GABAA receptor-mediated neurotransmission: Not so simple after all. Biochem. Pharmacol. 2016, 115, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wassle, H.; Koulen, P.; Brandstatter, J.H.; Fletcher, E.L.; Becker, C.M. Glycine and GABA receptors in the mammalian retina. Vis. Res. 1998, 38, 1411–1430. [Google Scholar] [CrossRef]
- Fuchs, C.; Abitbol, K.; Burden, J.J.; Mercer, A.; Brown, L.; Iball, J.; Anne Stephenson, F.; Thomson, A.M.; Jovanovic, J.N. GABA(A) receptors can initiate the formation of functional inhibitory GABAergic synapses. Eur. J. Neurosci. 2013, 38, 3146–3158. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.L.; Lou, X.T.; Yuan, Y.X.; Chen, L.W.; Ji, P.T.; Li, L.; Zhao, Y.; Zhang, H. The increased expression of GABA receptors within the arcuate nucleus is associated with high intraocular pressure. Mol. Vis. 2018, 24, 574–586. [Google Scholar] [PubMed]
- Okumichi, H.; Mizukami, M.; Kiuchi, Y.; Kanamoto, T. GABA a receptors are associated with retinal ganglion cell death induced by oxidative stress. Exp. Eye Res. 2008, 86, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Siksou, L.; Silm, K.; Biesemann, C.; Nehring, R.B.; Wojcik, S.M.; Triller, A.; El Mestikawy, S.; Marty, S.; Herzog, E. A role for vesicular glutamate transporter 1 in synaptic vesicle clustering and mobility. Eur. J. Neurosci. 2013, 37, 1631–1642. [Google Scholar] [CrossRef]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Nakamura, K.; Quah, H.M.; Okumura, A.; Namekata, K.; Saeki, T.; Aihara, M.; Yoshida, H. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Investig. 2007, 117, 1763–1770. [Google Scholar] [CrossRef]
- Zhang, J.; Tuo, J.; Cao, X.; Shen, D.; Li, W.; Chan, C.C. Early degeneration of photoreceptor synapse in Ccl2/Cx3cr1-deficient mice on Crb1(rd8) background. Synapse 2013, 67, 515–531. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Anderson, J.R.; Kinard, K.; Marshak, D.W.; Wilson, J.H.; Wensel, T.; Lucas, R.J. Neural reprogramming in retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.J.; Otteson, D.C.; Sherry, D.M. Progression of neuronal and synaptic remodeling in the rd10 mouse model of retinitis pigmentosa. J. Comp. Neurol. 2010, 518, 2071–2089. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Greferath, U.; Vessey, K.A.; Grayden, D.B.; Burkitt, A.N.; Fletcher, E.L. Changes in ganglion cells during retinal degeneration. Neuroscience 2016, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Knafo, S.; Alonso-Nanclares, L.; Gonzalez-Soriano, J.; Merino-Serrais, P.; Fernaud-Espinosa, I.; Ferrer, I.; DeFelipe, J. Widespread changes in dendritic spines in a model of Alzheimer’s disease. Cereb. Cortex 2009, 19, 586–592. [Google Scholar] [CrossRef]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Rodriguez-Perdigon, M.; Tordera, R.M.; Gil-Bea, F.J.; Gerenu, G.; Ramirez, M.J.; Solas, M. Down-regulation of glutamatergic terminals (VGLUT1) driven by Abeta in Alzheimer’s disease. Hippocampus 2016, 26, 1303–1312. [Google Scholar] [CrossRef]
- Antes, R.; Ezra-Elia, R.; Weinberger, D.; Solomon, A.; Ofri, R.; Michaelson, D.M. ApoE4 induces synaptic and ERG impairments in the retina of young targeted replacement apoE4 mice. PLoS ONE 2013, 8, e64949. [Google Scholar] [CrossRef]
- Hales, C.M.; Rees, H.; Seyfried, N.T.; Dammer, E.B.; Duong, D.M.; Gearing, M.; Montine, T.J.; Troncoso, J.C.; Thambisetty, M.; Levey, A.I.; et al. Abnormal gephyrin immunoreactivity associated with Alzheimer disease pathologic changes. J. Neuropathol. Exp. Neurol. 2013, 72, 1009–1015. [Google Scholar] [CrossRef]
- Agarwal, S.; Tannenberg, R.K.; Dodd, P.R. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer’s disease. J. Alzheimers Dis. 2008, 14, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lionel, A.C.; Vaags, A.K.; Sato, D.; Gazzellone, M.J.; Mitchell, E.B.; Chen, H.Y.; Costain, G.; Walker, S.; Egger, G.; Thiruvahindrapuram, B.; et al. Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum. Mol. Genet. 2013, 22, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.I.; Harvey, K.; Ward, H.; White, J.H.; Evans, L.; Duguid, I.C.; Hsu, C.C.; Coleman, S.L.; Miller, J.; Baer, K.; et al. Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J. Biol. Chem. 2003, 278, 24688–24696. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Lenz, U.; Aquaviva-Bourdain, C.; Joriot-Chekaf, S.; Mention-Mulliez, K.; Holder-Espinasse, M. A GPHN point mutation leading to molybdenum cofactor deficiency. Clin. Genet. 2011, 80, 598–599. [Google Scholar] [CrossRef]
- Butler, M.H.; Hayashi, A.; Ohkoshi, N.; Villmann, C.; Becker, C.M.; Feng, G.; De Camilli, P.; Solimena, M. Autoimmunity to gephyrin in Stiff-Man syndrome. Neuron 2000, 26, 307–312. [Google Scholar] [CrossRef]
- Feyder, M.; Karlsson, R.M.; Mathur, P.; Lyman, M.; Bock, R.; Momenan, R.; Munasinghe, J.; Scattoni, M.L.; Ihne, J.; Camp, M.; et al. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am. J. Psychiatry 2010, 167, 1508–1517. [Google Scholar] [CrossRef]
- Xing, J.; Kimura, H.; Wang, C.; Ishizuka, K.; Kushima, I.; Arioka, Y.; Yoshimi, A.; Nakamura, Y.; Shiino, T.; Oya-Ito, T.; et al. Resequencing and Association Analysis of Six PSD-95-Related Genes as Possible Susceptibility Genes for Schizophrenia and Autism Spectrum Disorders. Sci. Rep. 2016, 6, 27491. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuropsychopharmacol. Biol. Psychiatr. 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Park, H.L.; Kim, S.W.; Kim, J.H.; Park, C.K. Increased levels of synaptic proteins involved in synaptic plasticity after chronic intraocular pressure elevation and modulation by brain-derived neurotrophic factor in a glaucoma animal model. Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Wu, J. Brimonidine enhances inhibitory postsynaptic activity of OFF- and ON-type retinal ganglion cells in a Wistar rat chronic glaucoma model. Exp. Eye Res. 2019, 189, 107833. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, Y.; Zhang, R.; Zhang, X.; Zhang, S.; Wu, J.; Sun, X. Differential Modulation of GABAA and NMDA Receptors by an α7-nicotinic Acetylcholine Receptor Agonist in Chronic Glaucoma. Front. Mol. Neurosci. 2017, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Said, W.; Fradot, V.; Ivkovic, I.; Sahel, J.A.; Picaud, S.; Froger, N. Taurine Promotes Retinal Ganglion Cell Survival through GABAB Receptor Activation. Adv. Exp. Med. Biol. 2017, 975, 687–701. [Google Scholar] [PubMed]
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 2002, 298, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.H.; Qu, J.; John, S.W.; Howell, G.R.; Jakobs, T.C. Synapse Loss and Dendrite Remodeling in a Mouse Model of Glaucoma. PLoS ONE 2015, 10, e0144341. [Google Scholar] [CrossRef]
- Reinehr, S.; Gomes, S.C.; Gassel, C.J.; Asaad, M.A.; Stute, G.; Schargus, M.; Dick, H.B.; Joachim, S.C. Intravitreal Therapy Against the Complement Factor C5 Prevents Retinal Degeneration in an Experimental Autoimmune Glaucoma Model. Front. Pharmacol. 2019, 10, 1381. [Google Scholar] [CrossRef]
- Reinehr, S.; Reinhard, J.; Wiemann, S.; Stute, G.; Kuehn, S.; Woestmann, J.; Dick, H.B.; Faissner, A.; Joachim, S.C. Early remodelling of the extracellular matrix proteins tenascin-C and phosphacan in retina and optic nerve of an experimental autoimmune glaucoma model. J. Cell. Mol. Med. 2016, 20, 2122–2137. [Google Scholar] [CrossRef]
- Casola, C.; Reinehr, S.; Kuehn, S.; Stute, G.; Spiess, B.M.; Dick, H.B.; Joachim, S.C. Specific Inner Retinal Layer Cell Damage in an Autoimmune Glaucoma Model Is Induced by GDNF With or Without HSP27. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3626–3639. [Google Scholar] [CrossRef]
- Noristani, R.; Kuehn, S.; Stute, G.; Reinehr, S.; Stellbogen, M.; Dick, H.B.; Joachim, S.C. Retinal and Optic Nerve Damage is Associated with Early Glial Responses in an Experimental Autoimmune Glaucoma Model. J. Mol. Neurosci. 2016, 58, 470–482. [Google Scholar] [CrossRef]
- Schmid, H.; Renner, M.; Dick, H.B.; Joachim, S.C. Loss of inner retinal neurons after retinal ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2777–2787. [Google Scholar] [CrossRef]
- Horstmann, L.; Schmid, H.; Heinen, A.P.; Kurschus, F.C.; Dick, H.B.; Joachim, S.C. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2013, 10, 120. [Google Scholar] [CrossRef]
- Reinhard, J.; Renner, M.; Wiemann, S.; Shakoor, D.A.; Stute, G.; Dick, H.B.; Faissner, A.; Joachim, S.C. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017, 7, 43470. [Google Scholar] [CrossRef] [PubMed]

| Primary Antibodies | Secondary Antibodies | ||||
|---|---|---|---|---|---|
| Antibody | Company | Dilution | Antibody | Company | Dilution |
| Anti-GABA-A receptor α3 | Synaptic Systems | 1:200 | Donkey anti-guinea pig Alexa Fluor 488 | Jackson ImmunoResearch | 1:500 |
| Anti-gephyrin | Synaptic Systems | 1:700 | Donkey anti-rabbit Alexa Fluor 488 | Invitrogen | 1:500 |
| Anti-NMDA receptor 1 (GluN1) | Synaptic Systems | 1:200 | Donkey anti-rabbit Alexa Fluor 488 | Invitrogen | 1:500 |
| Anti-PSD-95 | Millipore | 1:300 | Donkey anti-mouse Alexa Fluor 555 | Invitrogen | 1:500 |
| Anti-VGluT1 | Synaptic Systems | 1:500 | Donkey anti-chicken Cy3 | Millipore | 1:500 |
| Staining | Background | Lower Threshold | Upper Threshold |
|---|---|---|---|
| GABA receptor | 95 | 1.11 | 63.11 |
| Gephyrin | 50 | 5.45 | 79.83 |
| NMDA receptor | 80 | 1.24 | 61.47 |
| PSD-95 (IPL) | 50 | 3.14 | 96.69 |
| PSD-95 (OPL) | 50 | 9.09 | 71.58 |
| VGluT1 | 50 | 4.55 | 81.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benning, L.; Reinehr, S.; Grotegut, P.; Kuehn, S.; Stute, G.; Dick, H.B.; Joachim, S.C. Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models. Int. J. Mol. Sci. 2020, 21, 6998. https://doi.org/10.3390/ijms21196998
Benning L, Reinehr S, Grotegut P, Kuehn S, Stute G, Dick HB, Joachim SC. Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models. International Journal of Molecular Sciences. 2020; 21(19):6998. https://doi.org/10.3390/ijms21196998
Chicago/Turabian StyleBenning, Lara, Sabrina Reinehr, Pia Grotegut, Sandra Kuehn, Gesa Stute, H. Burkhard Dick, and Stephanie C. Joachim. 2020. "Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models" International Journal of Molecular Sciences 21, no. 19: 6998. https://doi.org/10.3390/ijms21196998
APA StyleBenning, L., Reinehr, S., Grotegut, P., Kuehn, S., Stute, G., Dick, H. B., & Joachim, S. C. (2020). Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models. International Journal of Molecular Sciences, 21(19), 6998. https://doi.org/10.3390/ijms21196998







