Investigation of Biofilms Formed on Steelmaking Slags in Marine Environments for Water Depuration
Abstract
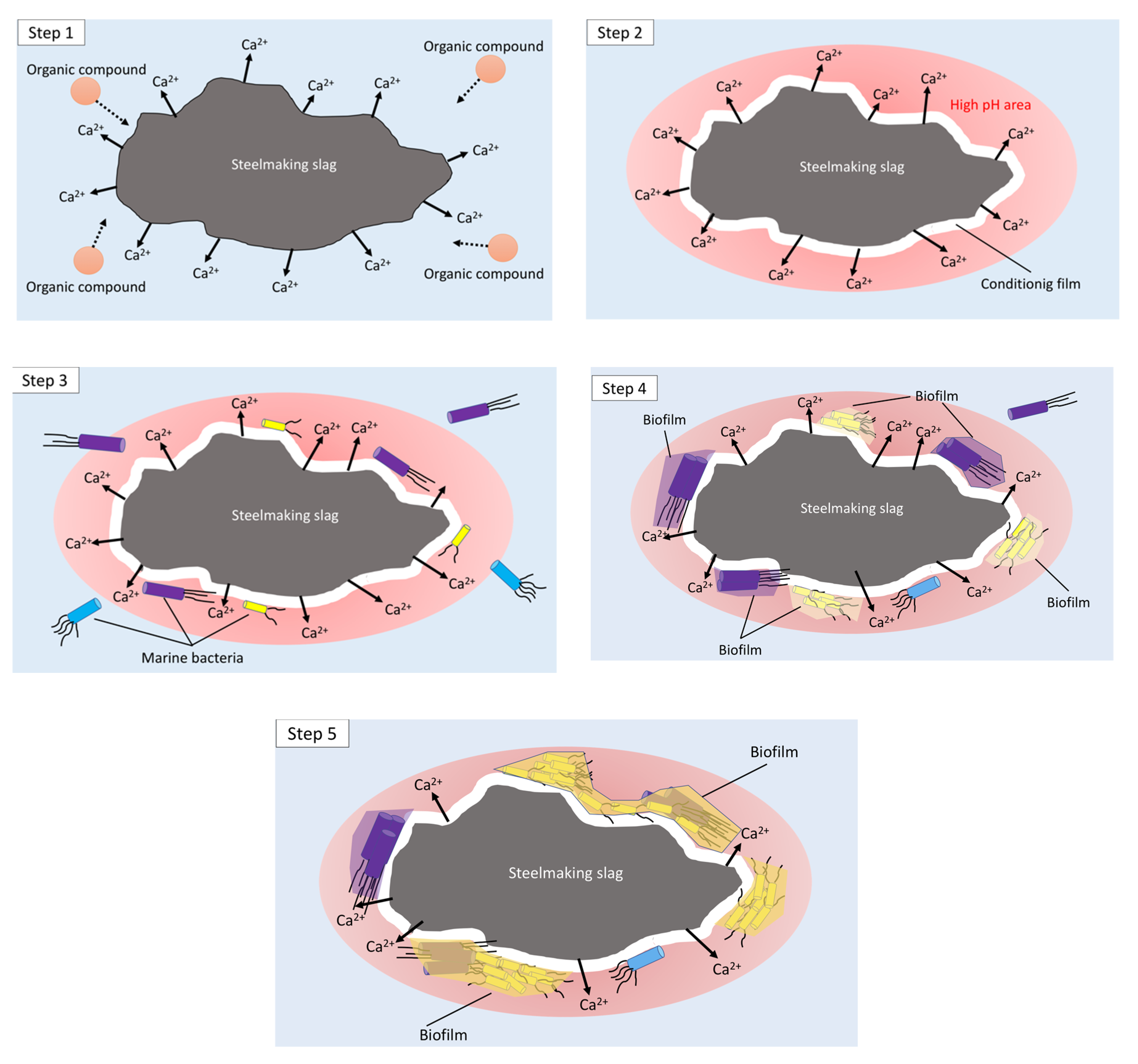
1. Introduction
2. Results and Discussion
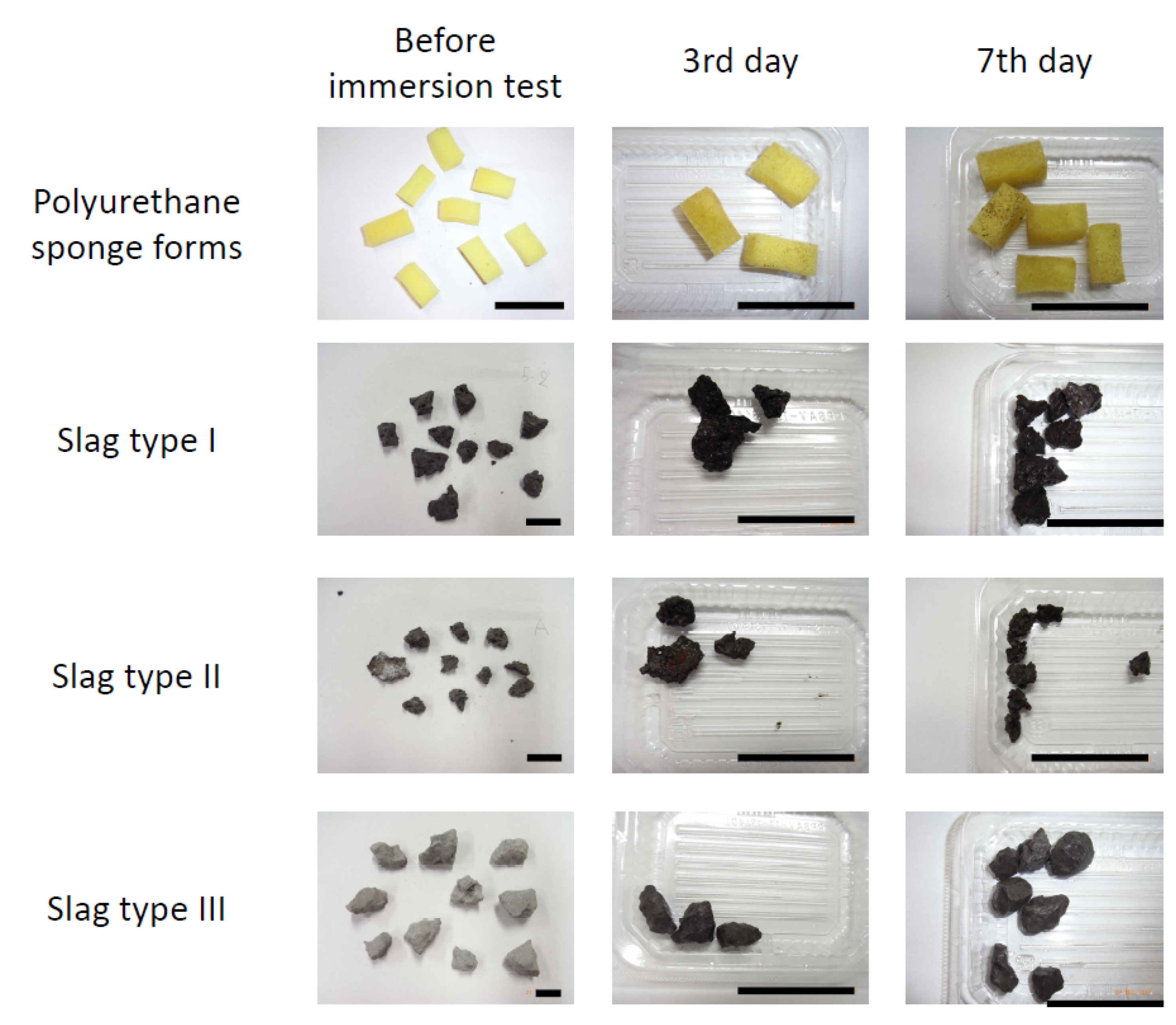
2.1. Megascopic Observation of Immerged Samples
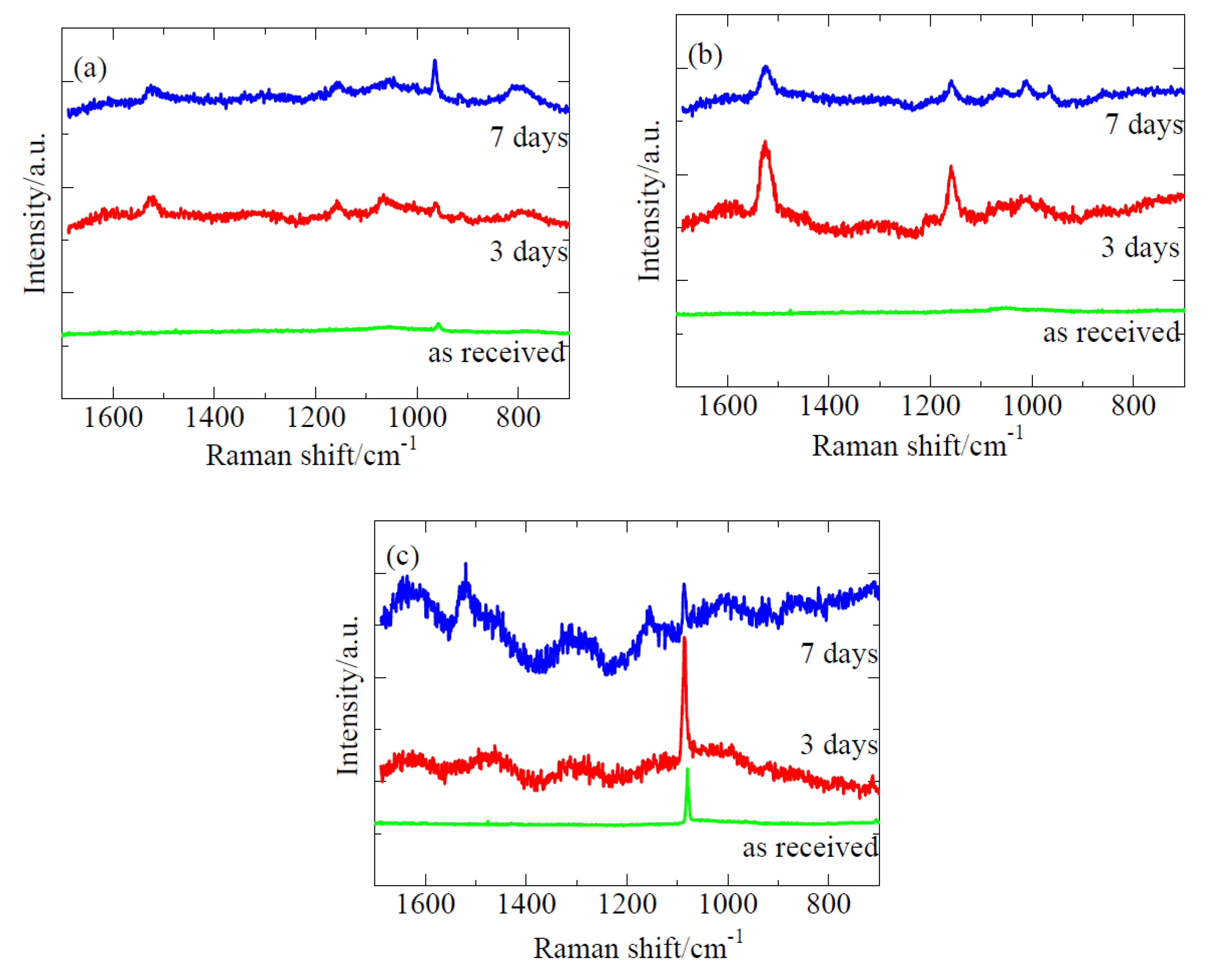
2.2. Identification of Biofilms by Raman Spectroscopy
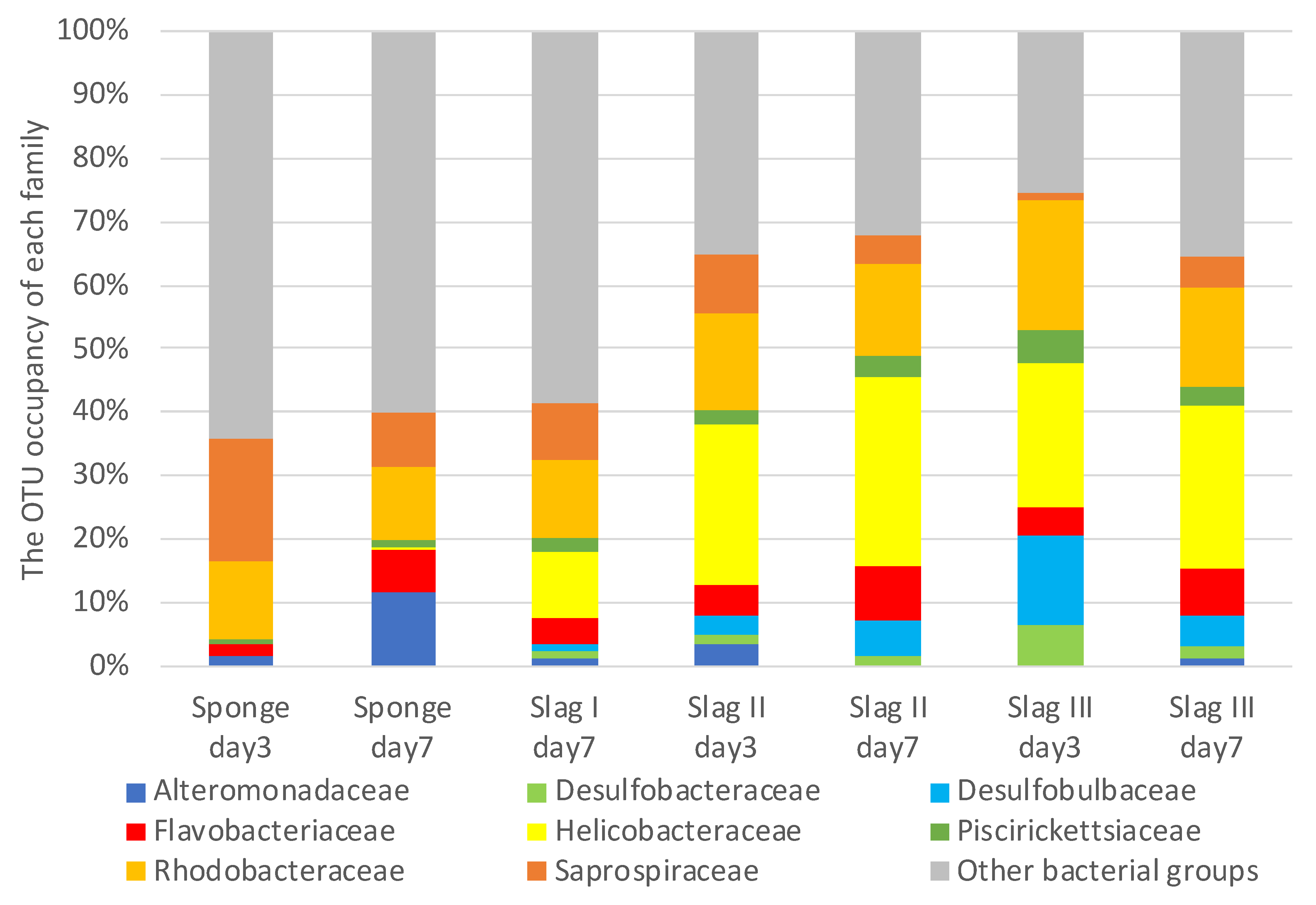
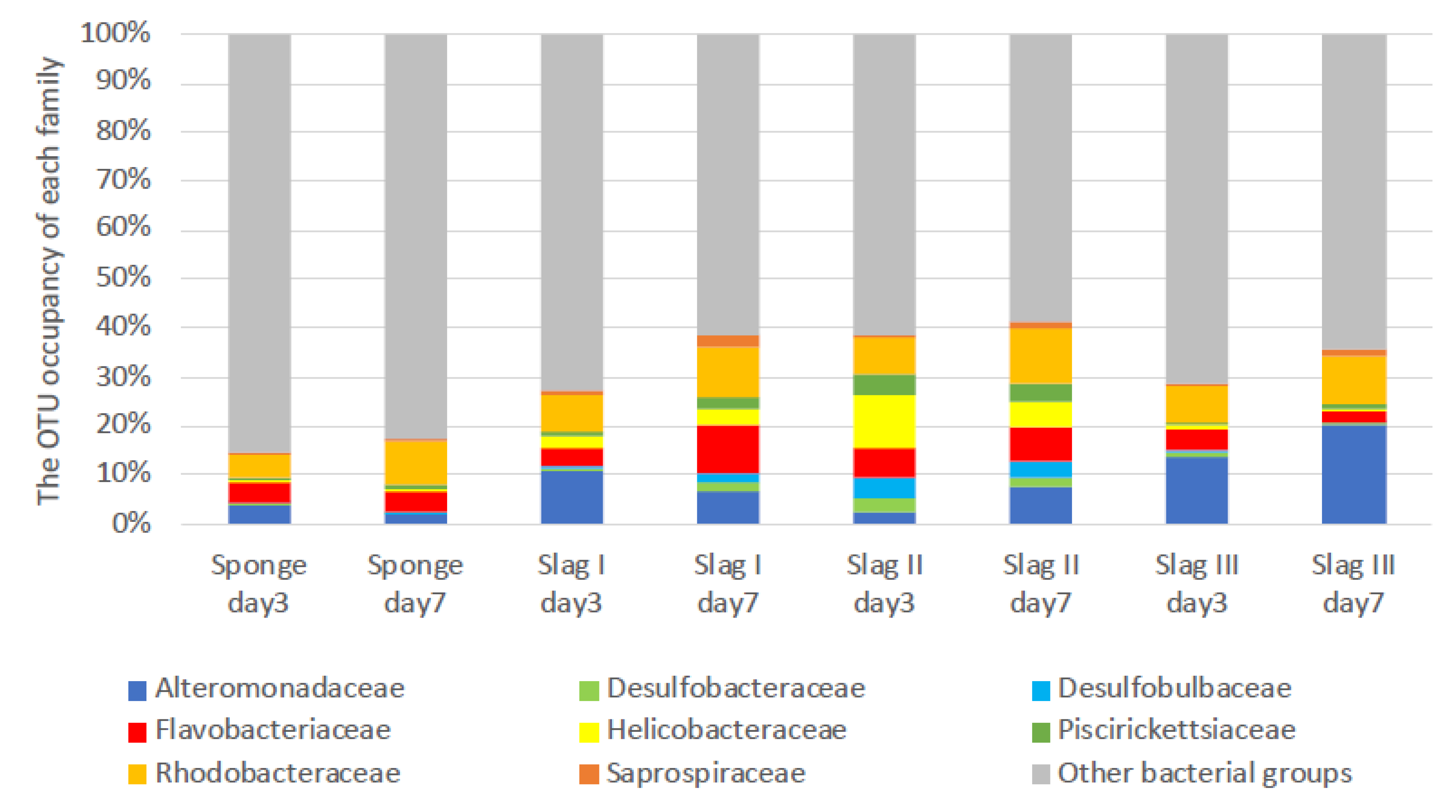
2.3. Bacterial Biome Analysis of the Formed Biofilms on the Surface of Each Specimen
3. Materials and Methods
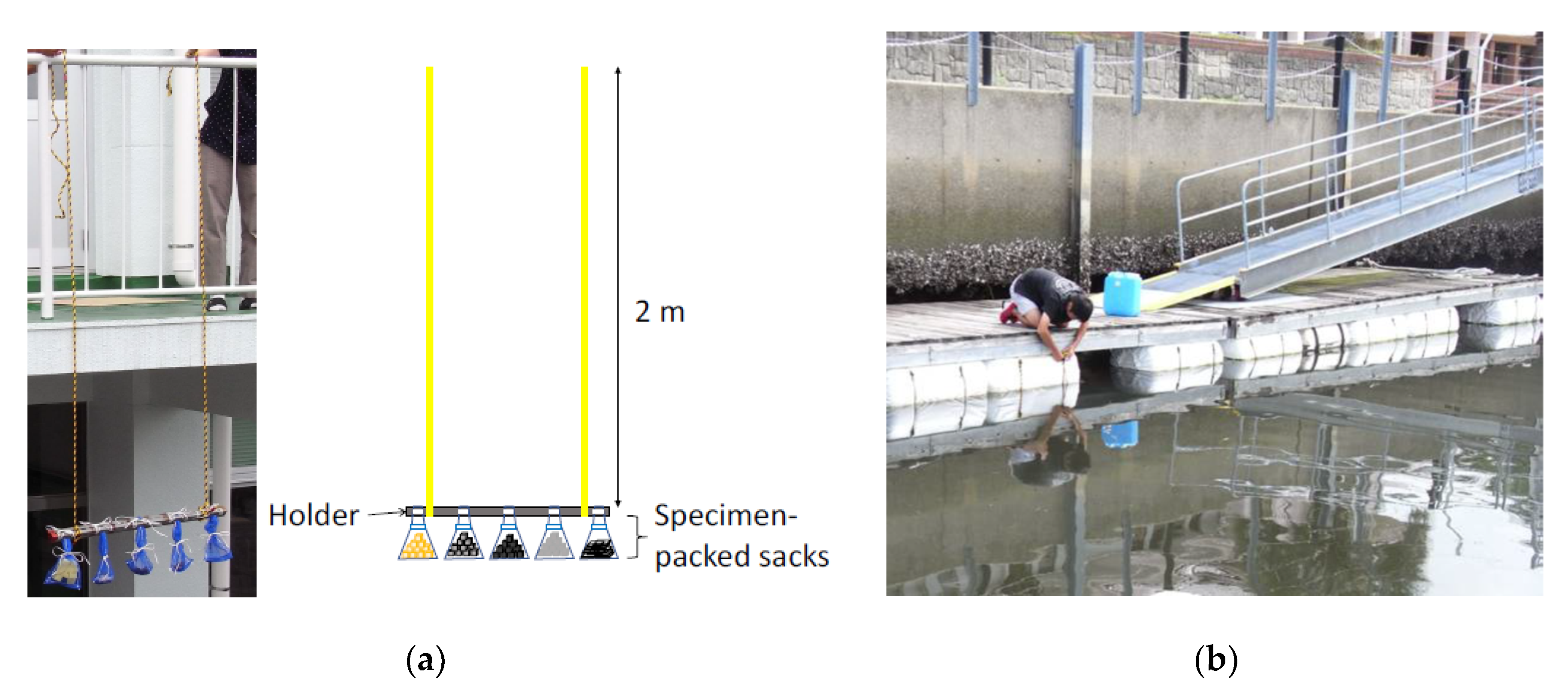
3.1. Specimens
3.2. Marine Immersion Test
3.3. Raman Spectroscopic Analysis of Biofilm
3.4. DNA Extraction
3.5. Microbiome Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iron and Steel Slag Products. Available online: http://www.slg.jp/e/slag/product/index.html (accessed on 17 September 2020).
- Amounts of Steel Slag Produced and Used in FY. 2017. Available online: http://www.slg.jp/pdf/Amounts%20of%20steel%20Slag%202017FY.pdf (accessed on 20 July 2020).
- Chemical Composition of Iron and Steel Slag. Available online: http://www.slg.jp/e/slag/character.html (accessed on 17 September 2020).
- Arita, K.; Umiguchi, Y.; Taniguchi, A. Availability of elements originated from steelmaking slag for phytoplankton enriched simultaneously with treated urban sewage. Tetsu Hagane 2003, 89, 422–429. [Google Scholar] [CrossRef]
- Numata, N.; Miyata, Y.; Yabuta, K.; Takahashi, T.; Yoyota, Y.; Sato, Y. Coastal environment improvement by iron and steelmaking slag. NKK Tech. Rep. 2002, 177, 47–51. [Google Scholar]
- Miyata, Y.; Numata, N.; Takagi, M.; Takahashi, T.; Oyamada, K.; Oda, S. Adhesion of seaweed in steelmaking slag blocks. Proc. Civ. Eng. Ocean 2004, 20, 887–892. [Google Scholar] [CrossRef]
- Miyata, Y.; Takahashi, T.; Yabuta, K.; Tozawa, H.; Sato, Y. Adhesions of plants and animals on steelmaking slag as submerged bank material and steelmaking slag blocks as seaweed reef. Bull. Soc. Sea Water Sci. Jpn. 2006, 60, 152–156. [Google Scholar]
- Miyata, Y.; Honda, H.; Yabuta, K.; Hayashi, A.; Yamamoto, T. Adhesion of plants and animals on steel making slag mound in coastal sea area. J. Jpn. Soc. Civ. Eng. Ser. B3 Ocean Eng. 2011, 67, I_394–I_399. [Google Scholar]
- Miyata, Y.; Matsunaga, H.; Yabuta, K.; Hayashi, A.; Yamamoto, T. Evaluation of long-term effects on facilitation of inhabitability for living things by steel-making slag submerged bank, off Innoshima island, Hiroshima. J. Jpn. Soc. Civ. Eng. Ser. B3 Ocean Eng. 2012, 68, I_564–I_569. [Google Scholar]
- Patent J-GLOBAL ID: 201503007461888434. Available online: https://jglobal.jst.go.jp/detail?JGLOBAL_ID=201503007461888434 (accessed on 20 July 2020).
- Kamei, Y.; Sugino, H.; Satou, J. Evaluation of steel-making slag as algal bed substratum. Bull. Fish. Exp. Stn. Okayama Prefect. 2008, 23, 15–19. [Google Scholar]
- Yamamoto, M.; Ueno, T.; Otsuka, K.; Sugawa, H.; Horiya, S.; Kuwano, K.; Fukushima, M.; Komai, T.; Liu, D. Application of steelmaking slag and humates to a coastal area in Kyusyu for the restoration of seaweed beds. In Proceedings of the Society of Chemical Engineers, Japan 74th Annual Meeting, Yokohama National University, Yokohama, Japan, 18–20 March 2009. [Google Scholar]
- Yamamoto, M. Evaluation of dependence of seaweed bed ecosystem on coastal and terrestrial environment. J. Adv. Mar. Sci. Technol. Soc. 2011, 12, 13–20. [Google Scholar]
- Yamamoto, M.; Fukushima, M.; Liu, D. The effect of humic substances on iron elution in the method of restoration of seaweed beds using steelmaking slag. ISIJ Int. 2012, 52, 1909–1913. [Google Scholar] [CrossRef][Green Version]
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef]
- Kanematsu, H.; Barry, D.M. Chapter 2 Conditioning Films. In Biofilm and Materials Science; Kanematsu, H., Barry, D.M., Eds.; Springer: Heidelberg, Germany, 2015; pp. 9–15. [Google Scholar] [CrossRef]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Ig 2013, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Ogawa, A.; Kanematsu, H.; Sano, K.; Sakai, Y.; Ishida, K.; Beech, I.; Suzuki, O.; Tanaka, T. Effect of silver or copper nanoparticles-dispersed silane coatings on biofilm formation in cooling water systems. Materials 2016, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, H.; Kudara, H.; Kanesaki, S.; Kogo, T.; Ikegai, H.; Ogawa, A.; Hirai, N. Application of a loop-type laboratory biofilm reactor to the evaluation of biofilm for some metallic materials and polymers such as urinary stents and catheters. Materials 2016, 9, 824. [Google Scholar] [CrossRef]
- Sandt, C.; Smith-Palmer, T.; Pink, J.; Brennan, L.; Pink, D. Confocal Raman microspectroscopy as a tool for studying the chemical heterogeneities of biofilms in situ. J. Appl. Microbiol. 2007, 103, 1808. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wagner, M.; Horn, H.; Niessner, R.; Haisch, C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal. Bioanal. Chem. 2009, 393, 197–206. [Google Scholar] [CrossRef]
- Samek, O.; Al-Marashi, J.F.M.; Telle, H.H. The potential of Raman spectroscopy for the identification of biofilm formation by Staphylococcus epidermidis. Laser Phys. Lett. 2010, 7, 378–383. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: From initial attachment to mature biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prakash, A. Combined use of Fourier transform infrared and Raman spectroscopy to study planktonic and biofilm cells of Cronobacter sakazaki. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 310–314. [Google Scholar]
- Sano, K.; Kanematsu, H.; Kogo, T.; Hirai, N.; Tanaka, T. Corrosion and biofilm for a composite coated iron observed by FTIR-ATR and Raman spectroscopy. Trans. IMF 2016, 94, 139–145. [Google Scholar] [CrossRef]
- Kanematsu, H.; Sato, M.; Shindo, K.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kogo, T.; Utsumi, D.Y.; Yamaguchi, A.; Ikegai, H.; et al. Biofilm formation behaviors on graphene by E. coli and S. epidermidis. ECS Trans. 2017, 80, 1167–1175. [Google Scholar] [CrossRef]
- Ogawa, A.; Kiyohara, T.; Kobayashi, Y.; Sano, K.; Kanematsu, H. Nickel, molybdenum, and tungsten nanoparticle-dispersed alkylalkoxysilane polymer for biomaterial coating: Evaluation of effects on bacterial biofilm formation and biosafety. Biomed. Res. Clin. Pract. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Larkin, P. Chapter 7—General outline and strategies for IR and Raman spectral interpretation. In Infrared and Raman Spectroscopy; Larkin, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 117–133. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Hirai, N.; Tanaka, T. Preparation and its anti-biofouling effect observation of organic metal dispersed silane based composite coating. J. Surf. Finish. Soc. Jpn. 2016, 67, 268–273. [Google Scholar] [CrossRef]
- Nakagawa, S.; Saito, H.; Tame, A.; Hirai, M.; Yamaguchi, H.; Sunata, T.; Aida, M.; Muto, H.; Sawayama, S.; Takaki, Y. Microbiota in the coelomic fluid of two common coastal starfish species and characterization of an abundant Helicobacter-related taxon. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Grote, J.; Labrenz, M.; Pfeiffer, B.; Jost, G.; Jűrgens, K. Quantitative distributions of Epsilonproteobacteria and a Sulfurimonas subgroup in pelagic redoxclines of the gentral baltic sea. Appl. Environ. Microbiol. 2007, 73, 7155–7161. [Google Scholar] [CrossRef]
- Carlström, C.I.; Lucas, L.N.; Rohde, R.A.; Haratian, A.; Engelbrektson, A.L.; Coates, J.D. Characterization of an anaerobic marine microbial community exposed to combined fluxes of perchlorate and salinity. Appl. Microbiol. Biotechnol. 2016, 100, 9719–9732. [Google Scholar] [CrossRef]
- Takai, K.; Suzuki, M.; Nakagawa, S.; Miyazaki, M.; Suzuki, Y.; Inagaki, F.; Horikoshi, K. Sulfurimonas paralvinellae sp. nov., a novel mesophilic, hydrogen- and sulfur-oxidizing chemolithoautotroph within the Epsilonproteobacteria isolated from a deep-sea hydrothermal vent polychaete nest, reclassification of Thiomicrospira denitrificans as Sulfurimonas denitrificans comb. nov. and emended description of the genus Sulfurimonas. Int. J. Syst. Evol. Microbiol. 2006, 56, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G. Bacterial power cords. Nature 2012, 491, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Burdorf, L.D.W.; Tramper, A.; Seitaj, D.; Meire, L.; Hidalgo-Martinez, S.; Zetsche, E.; Boschker, H.T.S.; Meysman, F.J.R. Long-distance electron transport occurs globally in marine sediments. Biogeosciences 2017, 14, 683–701. [Google Scholar] [CrossRef]
- Boden, R.; Hutt, L.P.; Rae, A.W. Reclassification of Thiobacillus aquaesulis (Wood & Kelly, 1995) as Annwoodia aquaesulis gen. nov., comb. nov., transfer of Thiobacillus (Beijerinck, 1904) from the Hydrogenophilales to the Nitrosomonadales, proposal of Hydrogenophilalia class. nov. within the ‘Proteobacteria’, and four new families within the orders Nitrosomonadales and Rhodocyclales. Int. J. Syst. Evol. Microbiol. 2017, 67, 1191–1205. [Google Scholar] [CrossRef]
- Yoon, J.; Matsuo, Y.; Kasai, H.; Yokota, A. Portibacter lacus gen. nov., sp.nov., a new member of the family Saprospiraceae isolated from a saline lake. J. Gen. Appl. Microbiol. 2012, 58, 191–197. [Google Scholar] [CrossRef]
- Patwardhan, S.; Foustoukos, D.I.; Giovannelli, D.; Yücel, M.; Vetriani, C. Ecological succession of sulfur-oxidizing Epsilon- and Gammaproteobacteria during colonization of a shallow-water gas vent. Front. Microbiol. 2018, 9, 2970. [Google Scholar] [CrossRef]
- Khan, S.T.; Fukunaga, Y.; Nakagawa, Y.; Harayama, S. Emended descriptions of the genus Lewinella and of Lewinella cohaerens, Lewinella nigricans and Lewinella persica, and description of Lewinella lutea sp. nov. and Lewinella marina sp. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2946–2951. [Google Scholar] [CrossRef]
- Lee, S.D. Lewinella agarilytica sp. nov., a novel marine bacterium of the phylum Bacteroidetes, isolated from beach sediment. Int. J. Syst. Evol. Microbiol. 2007, 57, 2814–2818. [Google Scholar] [CrossRef]
- Iijima, S.; Okahara, R.; Washio, K.; Morikawa, M. Microbiological characteristics of Pseudoalteromonas spp. that form biofilms in the sea water environment~Their possible contribution to water purification and fish farming. Bull. Soc. Sea Water Sci. Jpn. 2012, 66, 186–190. [Google Scholar]
- The Result of Neritic Fixed Line Observation of August of 2017. Available online: https://www.pref.mie.lg.jp/common/content/000745495.pdf (accessed on 20 July 2020).
- The Result of Neritic Fixed Line Observation of December of 2017. Available online: https://www.pref.mie.lg.jp/common/content/000759385.pdf (accessed on 20 July 2020).
- FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 20 July 2020).
- omicX. Available online: https://omictools.com/sickle-tool (accessed on 24 June 2020).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]

| Family | Genus | Sponge Day 3 (%) | Sponge Day 7 (%) | Slag I Day 3 (%) | Slag I Day 7 (%) | Slag II Day 3 (%) | Slag II Day 7 (%) | Slag III Day 3 (%) | Slag III Day 7 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Helicobacteraceae | Sulfurimonas | 0 | 0 | - | 5 | 8 | 4 | 5 | 4 |
| unassigned | 100 | 100 | - | 95 | 92 | 96 | 95 | 96 | |
| Desulfobulbaceae | unassigned | 100 | 100 | - | 100 | 100 | 100 | 100 | 100 |
| Saprospiraceae | Lewinella | 92 | 78 | - | 93 | 92 | 94 | 89 | 91 |
| unassigned | 8 | 22 | - | 7 | 8 | 6 | 11 | 9 |
| Family | Genus | Sponge Day 3 (%) | Sponge Day 7 (%) | Slag I Day 3 (%) | Slag I Day 7 (%) | Slag II Day 3 (%) | Slag II Day 7 (%) | Slag III Day 3 (%) | Slag III Day 7 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Helicobacteraceae | unassigned | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Desulfobulbaceae | unassigned | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Saprospiraceae | Lewinella | 0 | 0 | 0 | 0 | 0 | 7 | 17 | 0 |
| unassigned | 100 | 100 | 100 | 100 | 100 | 93 | 83 | 100 |
| Slag | Total CaO | f-CaO | SiO2 | Al2O3 | MgO | Total Fe | MnO | P2O5 | S | C/S |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 37.6 | 22.6 | 4.2 | 6.5 | 4.7 | 12.0 | 5.4 | 0.02 | 1.67 | |
| II | 55.3 | 18.7 | 3.0 | 1.9 | 4.3 | 5.6 | 4.6 | 0.29 | 2.91 | |
| III | 52.2 | 15.9 | 14.1 | 3.0 | 2.8 | 12.2 | 4.3 | 2.9 | 3.71 |
| Parameter | Summer (1) | Winter (2) |
|---|---|---|
| Temperature (°C) | 27.98 | 14.83 |
| Salinity (PSU) (3) | 26.167 | 20.903 |
| COD (ppm) | 3.49 | 0.38 |
| NH4-N (μg-at./L) | 0.60 | 0.42 |
| NO2, 3-N (μg-at./L) | 1.15 | 3.23 |
| DIN (μg-at./L) | 1.76 | 3.65 |
| PO4-P (μg-at./L) | 0.07 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, A.; Tanaka, R.; Hirai, N.; Ochiai, T.; Ohashi, R.; Fujimoto, K.; Akatsuka, Y.; Suzuki, M. Investigation of Biofilms Formed on Steelmaking Slags in Marine Environments for Water Depuration. Int. J. Mol. Sci. 2020, 21, 6945. https://doi.org/10.3390/ijms21186945
Ogawa A, Tanaka R, Hirai N, Ochiai T, Ohashi R, Fujimoto K, Akatsuka Y, Suzuki M. Investigation of Biofilms Formed on Steelmaking Slags in Marine Environments for Water Depuration. International Journal of Molecular Sciences. 2020; 21(18):6945. https://doi.org/10.3390/ijms21186945
Chicago/Turabian StyleOgawa, Akiko, Reiji Tanaka, Nobumitsu Hirai, Tatsuki Ochiai, Ruu Ohashi, Karin Fujimoto, Yuka Akatsuka, and Masanori Suzuki. 2020. "Investigation of Biofilms Formed on Steelmaking Slags in Marine Environments for Water Depuration" International Journal of Molecular Sciences 21, no. 18: 6945. https://doi.org/10.3390/ijms21186945
APA StyleOgawa, A., Tanaka, R., Hirai, N., Ochiai, T., Ohashi, R., Fujimoto, K., Akatsuka, Y., & Suzuki, M. (2020). Investigation of Biofilms Formed on Steelmaking Slags in Marine Environments for Water Depuration. International Journal of Molecular Sciences, 21(18), 6945. https://doi.org/10.3390/ijms21186945






