Sappanone A Prevents Left Ventricular Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model
Abstract
1. Introduction
2. Results
2.1. Experimental Design, Gross Examination, Myocardial Infarct Size, and Serum Cardiac Marker Results
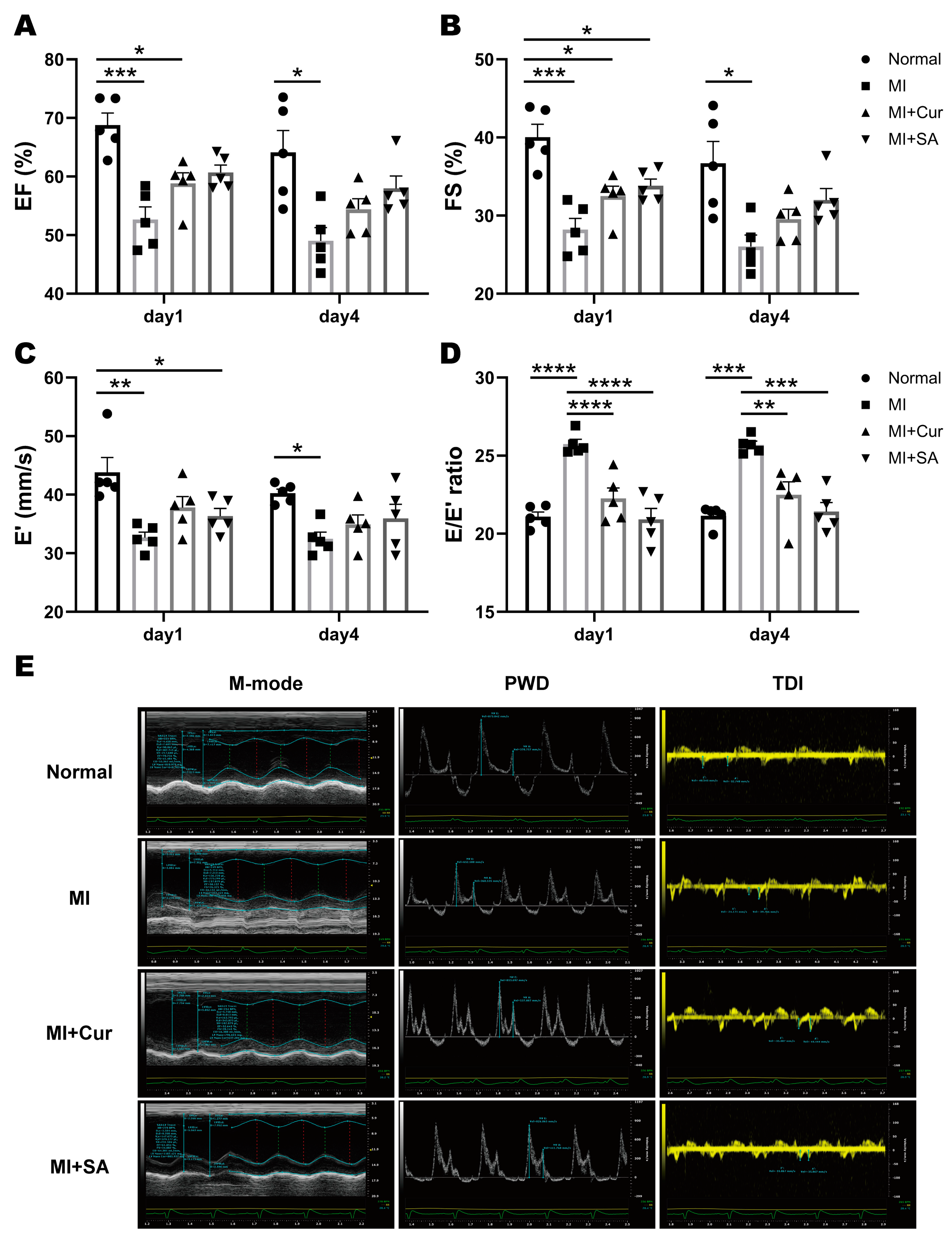
2.2. Echocardiographic Results
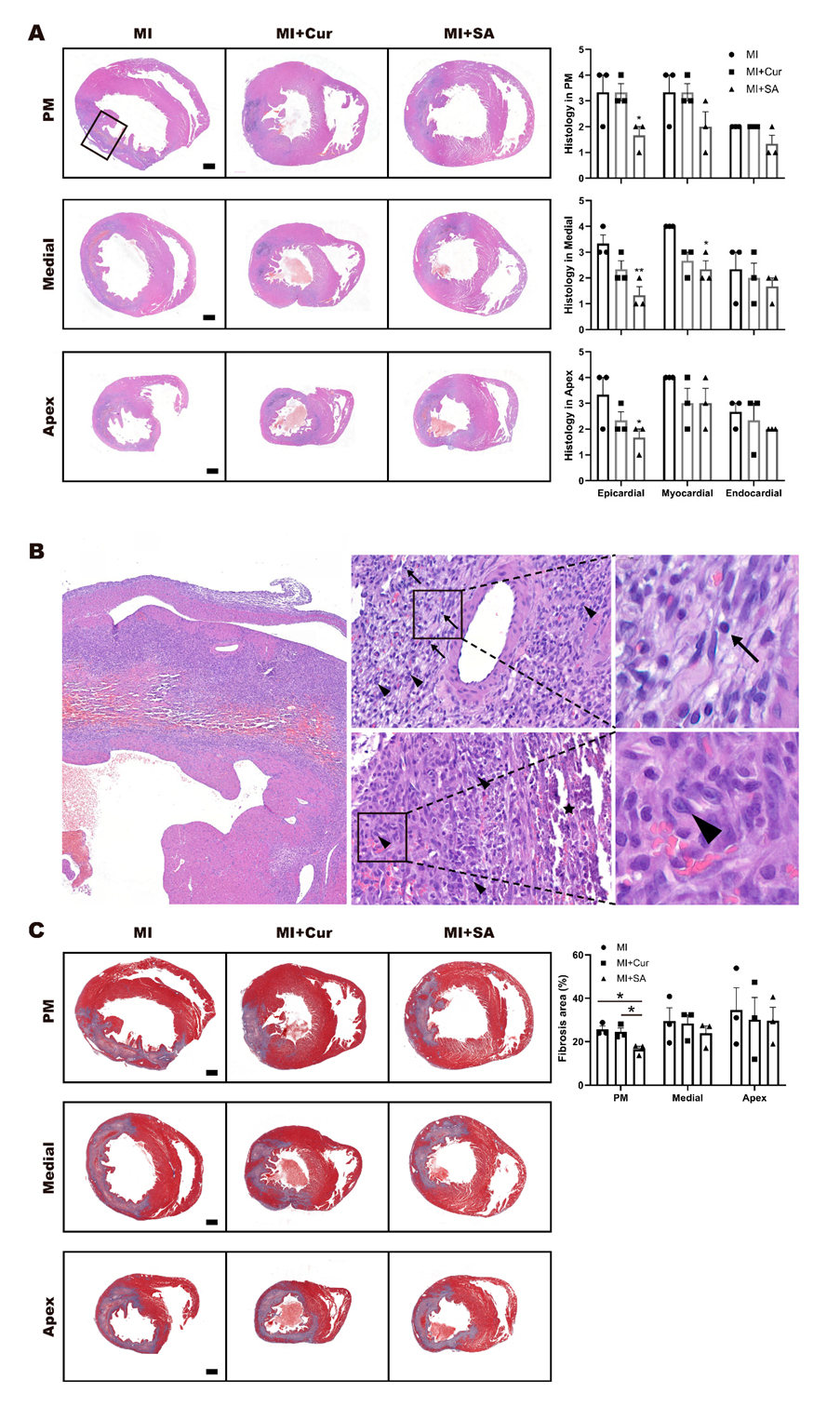
2.3. Histopathological Results
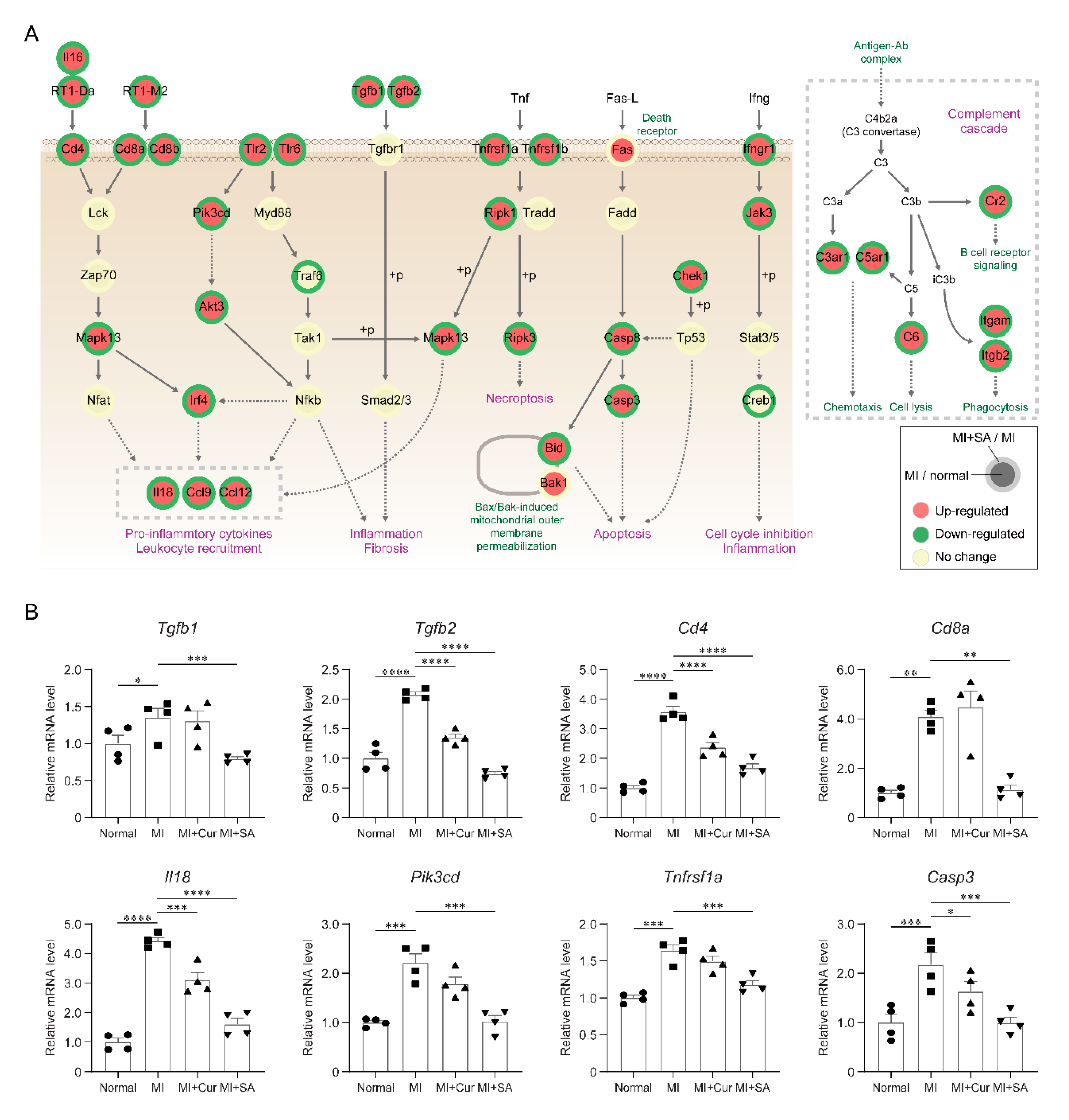
2.4. Sappanone A Treatment Results in Changes of Multiple Cellular Processes in the Rat Myocardial I/R Injury Model
2.5. Inhibitory Effects of Sappanone A on Myocardial Infarction-Related Processes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals, Husbandry, and Experimental Design
4.3. Induction of Myocardial I/R Injury
4.4. Echocardiographic Analysis
4.5. Myocardial Infarct Size
4.6. Serum Chemistry of CK-MB, LDH, and AST
4.7. Histopathological Analysis
4.8. mRNA Sequencing and Data Analysis
4.9. Identification of Differentially Expressed Genes (DEGs)
4.10. RT-PCR
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LAD | left anterior descending |
| LV | left ventricular |
| MI | myocardial infarction |
| EF | ejection fraction |
| FS | fractional shortening |
| PWD | pulse wave doppler |
| TDI | tissue doppler imaging. |
| SV | stroke volume |
| CO | cardiac output |
| LVIDd | left ventricular internal diameter at diastole |
| LVIDs | left ventricular internal diameter at systole |
| IVSd | interventricular septal thickness at diastole |
| IVSs | interventricular septal thickness at systole |
| LVPWd | left ventricular posterior wall thickness at diastole |
| LVPWs | left ventricular posterior wall thickness at systole |
| DEG | differentially expressed gene |
| GOBP | gene ontology biological process |
References
- Jayaraj, J.C.; Davatyan, K.; Subramanian, S.S.; Priya, J. Epidemiology of myocardial infarction. In Myocardial Infarction; Pamukçu, B., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011, 12, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Marwick, T.H.; Cook, S.A.; Go, R.T.; Fix, J.S.; James, K.B.; Sapp, S.K.; MacIntyre, W.J.; Thomas, J.D. Prognosis of patients with left ventricular dysfunction, with and without viable myocardium after myocardial infarction. Relative efficacy of medical therapy and revascularization. Circulation 1994, 90, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Dudley, M.; Groban, L. Diastolic dysfunction, cardiovascular aging, and the anesthesiologist. Anesthesiol. Clin. 2009, 27, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Moller, J.E.; Pellikka, P.A.; Hillis, G.S.; Oh, J.K. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation 2006, 114, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.R.; Xu, H.J.; Wang, L.F.; Liu, C.B.; Yu, F. Mesenchymal stem cell transplantation carried in SVVYGLR modified self-assembling peptide promoted cardiac repair and angiogenesis after myocardial infarction. Biochem. Biophys. Res. Commun. 2017, 491, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Han, B.; Li, J.; Cha, L.; Mu, J. Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem. Biophys. Res. Commun. 2017, 491, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.P.; Wang, Z.F.; Tootle, S.; Philip, T.; Zhao, Z.Q. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [Google Scholar] [CrossRef]
- Cootney, R.W. Ultrasound imaging: Principles and applications in rodent research. ILAR J. 2001, 42, 233–247. [Google Scholar] [CrossRef]
- Holinski, S.; Knebel, F.; Heinze, G.; Konertz, W.; Baumann, G.; Borges, A.C. Noninvasive monitoring of cardiac function in a chronic ischemic heart failure model in the rat: Assessment with tissue Doppler and non-Doppler 2D strain echocardiography. Cardiovasc. Ultrasound 2011, 9, 15. [Google Scholar] [CrossRef]
- Jo, W.; Lee, H.Y.; Kim, S.J.; Son, W.C.; Song, S.; Kim, H. Sustained left ventricular diastolic dysfunction following ischemia reperfusion injury in an acute myocardial infarction rat model. Jpn. J. Vet. Res. 2018, 66, 281–288. [Google Scholar]
- Jo, W.; Kang, K.K.; Chae, S.; Son, W.C. Metformin Alleviates Left Ventricular Diastolic Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model. Int. J. Mol. Sci. 2020, 21, 1489. [Google Scholar] [CrossRef] [PubMed]
- Cuong, T.D.; Hung, T.M.; Kim, J.C.; Kim, E.H.; Woo, M.H.; Choi, J.S.; Lee, J.H.; Min, B.S. Phenolic Compounds from Caesalpinia sappan Heartwood and Their Anti-inflammatory Activity. J. Nat. Prod. 2012, 75, 2069–2075. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.Y.; Choo, Y.Y.; Kim, O.; Tran, P.T.; Dao, C.T.; Min, B.S.; Lee, J.H. Sappanone A exhibits anti-inflammatory effects via modulation of Nrf2 and NF-kappaB. Int. Immunopharmacol. 2015, 28, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.Y.; Tran, P.T.; Min, B.S.; Kim, O.; Nguyen, H.D.; Kwon, S.H.; Lee, J.H. Sappanone A inhibits RANKL-induced osteoclastogenesis in BMMs and prevents inflammation-mediated bone loss. Int. Immunopharmacol. 2017, 52, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Aldous, S.J. Cardiac biomarkers in acute myocardial infarction. Int. J. Cardiol. 2013, 164, 282–294. [Google Scholar] [CrossRef] [PubMed]
- da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Task Force for the Universal Definition of Myocardial Infarction, Third universal definition of myocardial infarction. Nat. Rev. Cardiol. 2012, 9, 620–633. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Khanna, A. Wideband high-frequency echocardiography to evaluate myocardial infarct size. J. Ultrasound Med. 2009, 28, 1527–1534. [Google Scholar] [CrossRef]
- Feigenbaum, H. Journal of the American Society of Echocardiography: 25 Years Old. J. Am. Soc. Echocardiogr. 2012, 25, 1–2. [Google Scholar] [CrossRef]
- Cerisano, G.; Bolognese, L.; Carrabba, N.; Buonamici, P.; Santoro, G.M.; Antoniucci, D.; Santini, A.; Moschi, G.; Fazzini, P.F. Doppler-derived mitral deceleration time: An early strong predictor of left ventricular remodeling after reperfused anterior acute myocardial infarction. Circulation 1999, 99, 230–236. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Lakkis, N.M.; Middleton, K.J.; Spencer, W.H., 3rd; Zoghbi, W.A.; Quinones, M.A. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999, 99, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Mikati, I.; Kopelen, H.A.; Middleton, K.J.; Quinones, M.A.; Zoghbi, W.A. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation 1998, 98, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; van Veen, T.A.B. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol. Sex Differ. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Wang, C.H.; Qiao, Z.Y.; Xu, Y.W. Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm. Biol. 2017, 55, 1144–1148. [Google Scholar] [CrossRef]
- Boarescu, P.M.; Boarescu, I.; Bocsan, I.C.; Pop, R.M.; Gheban, D.; Bulboaca, A.E.; Nicula, C.; Rajnoveanu, R.M.; Bolboaca, S.D. Curcumin Nanoparticles Protect against Isoproterenol Induced Myocardial Infarction by Alleviating Myocardial Tissue Oxidative Stress, Electrocardiogram, and Biological Changes. Molecules 2019, 24, 2802. [Google Scholar] [CrossRef]
- Chang, J.; Nair, V.; Luk, A.; Butany, J. Pathology of myocardial infarction. Diagn. Histopathol. 2013, 19, 7–12. [Google Scholar] [CrossRef]
- Gorcsan, J., 3rd; Tanaka, H. Echocardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011, 58, 1401–1413. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef]
- Christia, P.; Frangogiannis, N.G. Targeting inflammatory pathways in myocardial infarction. Eur. J. Clin. Investig. 2013, 43, 986–995. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Li, J. Inflammation and Inflammatory Cells in Myocardial Infarction and Reperfusion Injury: A Double-Edged Sword. Clin. Med. Insights Cardiol. 2016, 10, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z.; Ding, Z.; Mehta, J.L. Inflammation, Autophagy, and Apoptosis After Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008024. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Chae, S.; Ahn, B.Y.; Byun, K.; Cho, Y.M.; Yu, M.H.; Lee, B.; Hwang, D.; Park, K.S. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal 2013, 6, rs4. [Google Scholar] [CrossRef]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
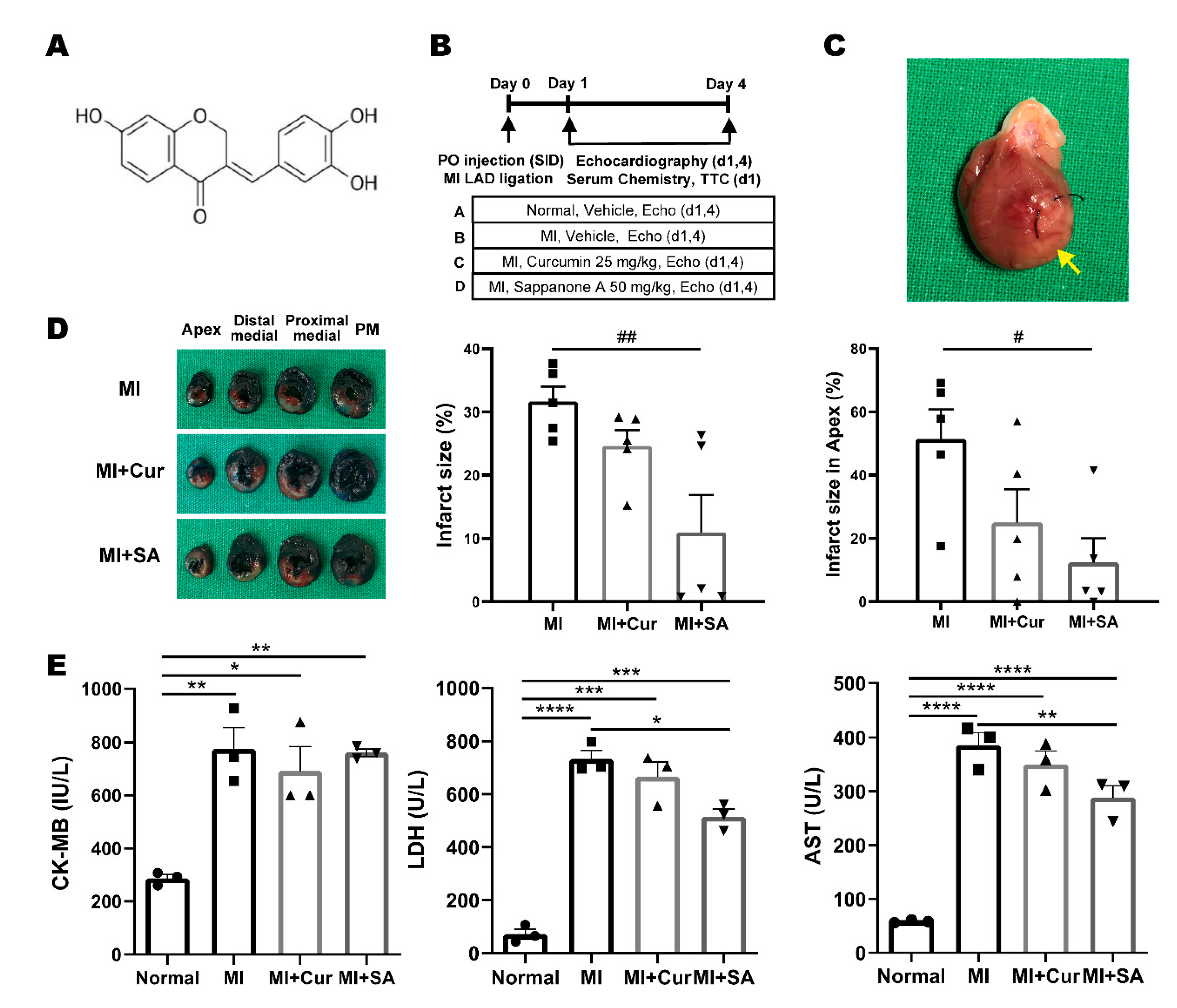

| MI Area Region | MI | MI + Cur | MI + SA |
|---|---|---|---|
| Papillary muscle (PM) | 20.52 ± 11.54 | 25.68 ± 7.45 | 11.53 ± 8.35 |
| Proximal medial | 21.52 ± 2.63 | 23.19 ± 2.38 | 10.19 ± 6.31 |
| Distal medial | 33.09 ± 4.83 | 24.55 ± 3.12 | # 9.64 ± 6.22 |
| Apex | 51.46 ± 9.33 | # 25.01 ± 10.50 | ### 12.39 ± 7.64 |
| Total | 31.65 ± 2.37 | 24.61 ± 2.53 | ## 10.94 ± 5.95 |
| Cardiac Function | Day 1 | Day 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | MI | MI+Cur | MI+SA | Normal | MI | MI+Cur | MI+SA | |
| EF, % | 68.79 ± 4.59 | *** 52.63 ± 4.85 | * 58.81 ± 4.12 | 60.68 ± 2.82 | 64.13 ± 8.30 | * 49.01 ± 5.06 | 54.39 ± 4.07 | 57.97 ± 4.70 |
| FS, % | 40.05 ± 3.66 | *** 28.21 ± 3.18 | * 32.48 ± 2.85 | * 33.80 ± 1.98 | 36.69 ± 6.24 | * 26.03 ± 3.28 | 29.55 ± 2.80 | 31.99 ± 3.30 |
| HR, BPM | 256.16 ± 21.28 | 302.19 ± 48.56 | 303.10 ± 20.50 | 259.16 ± 49.83 | 244.41 ± 30.13 | 263.63 ± 15.35 | 256.91 ± 38.13 | 244.75 ± 11.91 |
| SV, µL | 219.43 ± 27.86 | 182.83 ± 10.82 | 210.01 ± 23.71 | 216.28 ± 21.84 | 233.88 ± 23.15 | 206.55 ± 22.86 | 224.25 ± 28.84 | 228.74 ± 38.15 |
| CO, mL/min | 55.97 ± 6.27 | 54.97 ± 6.07 | 63.39 ± 8.80 | 62.82 ± 11.05 | 56.93 ± 7.21 | 54.23 ± 5.61 | 56.93 ± 2.95 | 55.90 ± 9.42 |
| LVIDd, mm | 7.73 ± 0.53 | 7.90 ± 0.27 | 7.96 ± 0.46 | 8.15 ± 0.52 | 8.18 ± 0.75 | 8.73 ± 0.15 | 8.63 ± 0.26 | 8.49 ± 0.71 |
| LVIDs, mm | 4.66 ± 0.53 | * 5.66 ± 0.41 | 5.37 ± 0.41 | 5.43 ± 0.52 | 5.19 ± 0.89 | 6.36 ± 0.41 | 6.13 ± 0.20 | 5.88 ± 0.73 |
| IVSd, mm | 1.52 ± 0.05 | 1.53 ± 0.16 | 1.59 ± 0.16 | 1.54 ± 0.23 | 1.48 ± 0.20 | 1.36 ± 0.07 | 1.40 ± 0.16 | 1.41 ± 0.10 |
| IVSs, mm | 2.57 ± 0.11 | 2.24 ± 0.20 | 2.55 ± 0.22 | 2.65 ± 0.27 | 2.52 ± 0.24 | 2.19 ± 0.20 | 2.22 ± 0.21 | 2.34 ± 0.23 |
| LVPWd, mm | 1.66 ± 0.15 | 1.57 ± 0.12 | 1.70 ± 0.09 | 1.98 ± 0.55 | 1.57 ± 0.17 | 1.62 ± 0.27 | 1.57 ± 0.16 | 1.66 ± 0.12 |
| LVPWs, mm | 2.65 ± 0.23 | 2.36 ± 0.28 | 2.53 ± 0.07 | 2.69 ± 0.37 | 2.42 ± 0.26 | 2.28 ± 0.26 | 2.38 ± 0.24 | 2.58 ± 0.23 |
| E′, mm/s | 43.82 ± 5.66 | ** 32.67 ± 2.14 | * 37.82 ± 4.16 | 36.33 ± 2.95 | 40.23 ± 1.62 | * 32.44 ± 2.62 | 34.93 ± 3.63 | 35.94 ± 5.40 |
| E/A ratio | 1.66 ± 0.32 | 2.10 ± 0.40 | 1.91 ± 0.37 | 1.75 ± 0.34 | 1.82 ± 0.28 | 1.95 ± 0.17 | 2.04 ± 0.50 | 2.16 ± 0.64 |
| E/E′ ratio | 21.09 ± 0.68 | **** 25.74 ± 0.68 | **** 22.26 ± 1.50 | **** 20.92 ± 1.58 | 21.14 ± 0.68 | *** 25.69 ± 0.54 | ** 22.50 ± 1.83 | *** 21.42 ± 1.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, W.; Min, B.S.; Yang, H.-Y.; Park, N.-H.; Kang, K.-K.; Lee, S.; Chae, S.; Ma, E.S.; Son, W.-C. Sappanone A Prevents Left Ventricular Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model. Int. J. Mol. Sci. 2020, 21, 6935. https://doi.org/10.3390/ijms21186935
Jo W, Min BS, Yang H-Y, Park N-H, Kang K-K, Lee S, Chae S, Ma ES, Son W-C. Sappanone A Prevents Left Ventricular Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model. International Journal of Molecular Sciences. 2020; 21(18):6935. https://doi.org/10.3390/ijms21186935
Chicago/Turabian StyleJo, Woori, Byung Sun Min, Hee-Young Yang, Na-Hye Park, Kyung-Ku Kang, Sijoon Lee, Sehyun Chae, Eun Sook Ma, and Woo-Chan Son. 2020. "Sappanone A Prevents Left Ventricular Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model" International Journal of Molecular Sciences 21, no. 18: 6935. https://doi.org/10.3390/ijms21186935
APA StyleJo, W., Min, B. S., Yang, H.-Y., Park, N.-H., Kang, K.-K., Lee, S., Chae, S., Ma, E. S., & Son, W.-C. (2020). Sappanone A Prevents Left Ventricular Dysfunction in a Rat Myocardial Ischemia Reperfusion Injury Model. International Journal of Molecular Sciences, 21(18), 6935. https://doi.org/10.3390/ijms21186935




