Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism
Abstract
:1. Introduction
2. Results
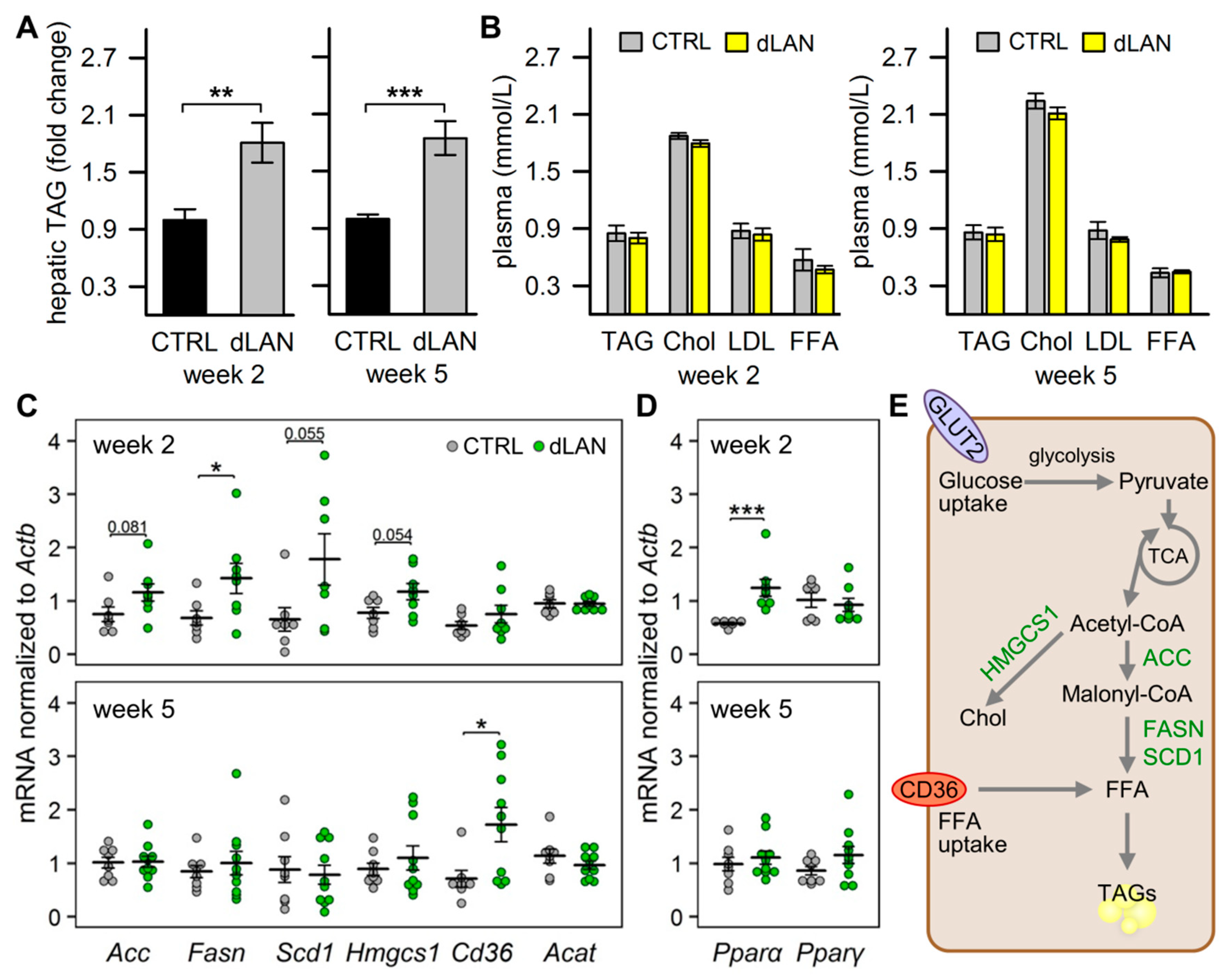
2.1. dLAN Promotes Hepatic Accumulation of Lipids
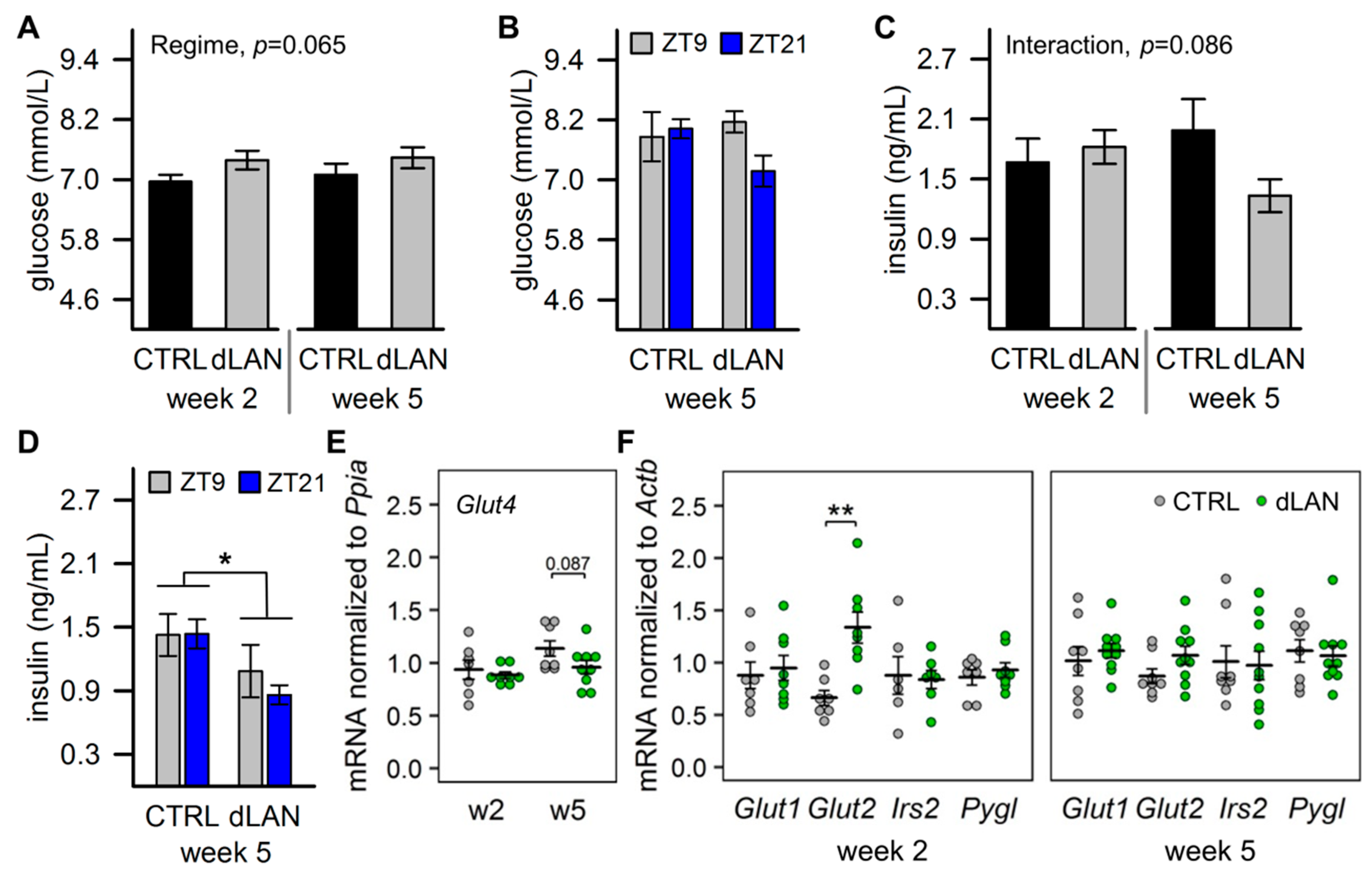
2.2. dLAN Increases Glucose Uptake into the Liver
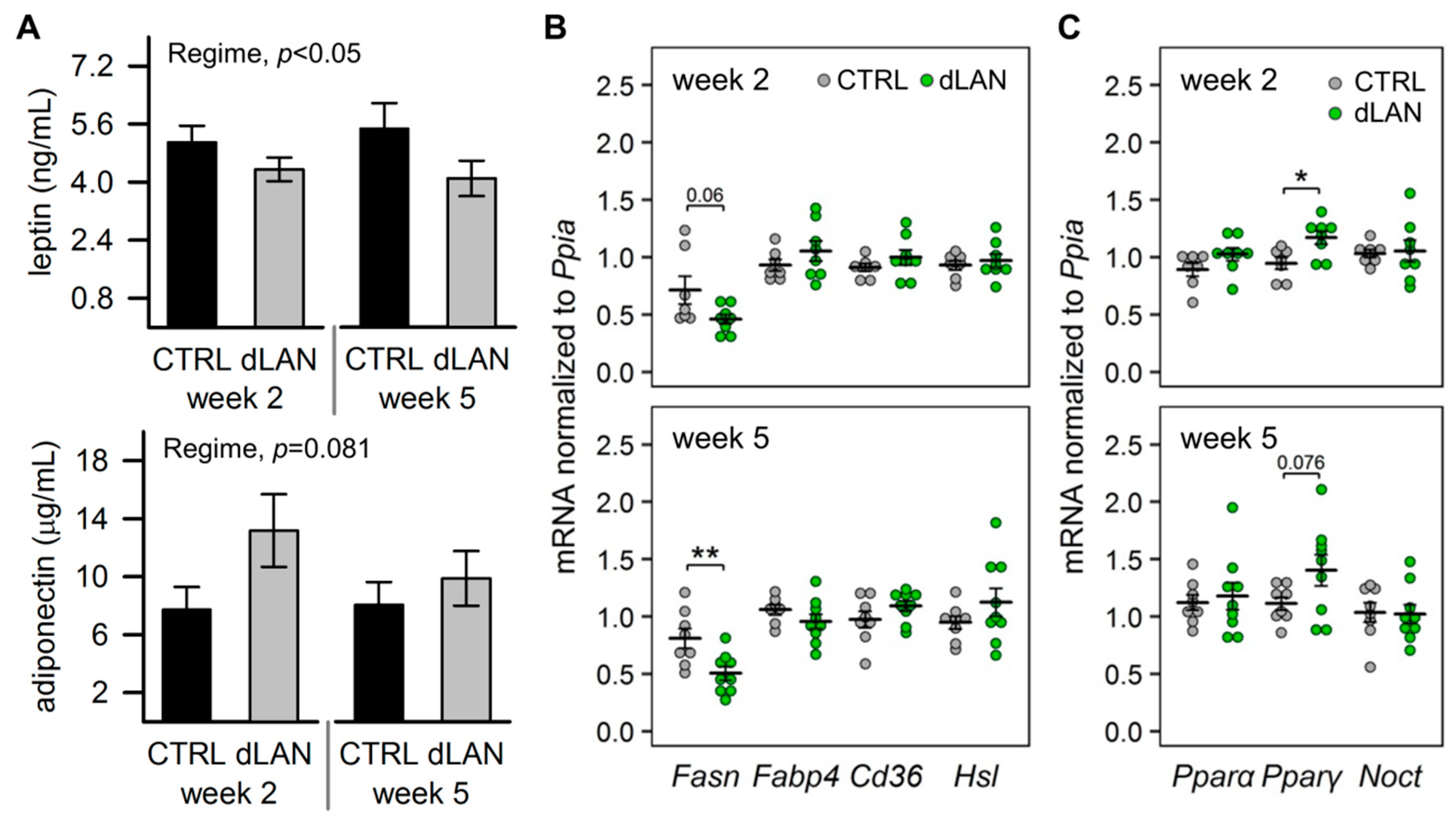
2.3. dLAN Alters Lipid Metabolism in the White Adipose Tissue
2.4. dLAN Does not Affect Plasma Thyroid Hormone Levels
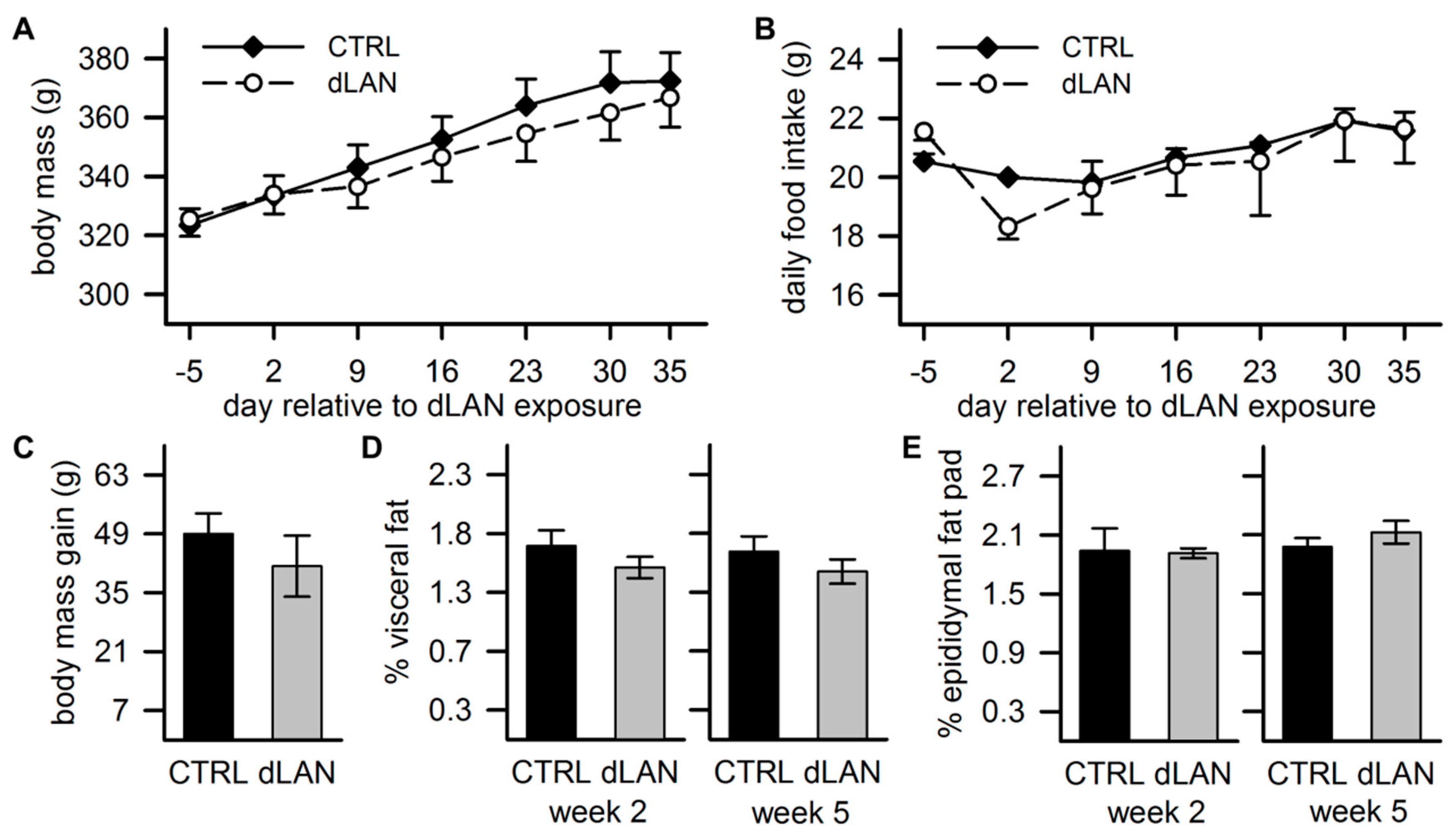
2.5. dLAN Does not Affect Body Mass
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Tissue Collection
4.4. Hormone Analyses
4.5. Biochemical Analyses
4.6. RNA Isolation and Real-Time PCR
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACAT | Acetyl-CoA acetyltransferase |
| ACC | Acetyl-CoA carboxylase |
| ACTB | β-actin |
| CD36 | Fatty acid translocase |
| CHOL | Cholesterol |
| CTRL | Control |
| dLAN | Dim light at night |
| FABP4 | Fatty acid binding protein 4 |
| FASN | Fatty acid synthase |
| FFA | Free fatty acid |
| GLUT | Glucose transporter |
| HMGCS1 | 3-hydroxy-3-methylglutaryl-CoA synthase 1 |
| HSL | Hormone-sensitive lipase |
| IRS2 | Insulin receptor substrate 2 |
| LD | Light–dark |
| LDL | Low-density lipoprotein |
| NAFLD | Nonalcoholic fatty liver disease |
| NOCT | Nocturnin |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPIA | Peptidylprolyl isomerase A |
| PYGL | Glycogen phosphorylase L |
| SCD1 | Stearoyl-CoA desaturase 1 |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TAG | Triacylglycerol |
| ZT | Zeitgeber time |
Appendix A

References
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic fatty liver disease: Pathogenesis and disease spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor srebp-1c. Diabetes. Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Reinke, H.; Asher, G. Circadian clock control of liver metabolic functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008. [Google Scholar] [CrossRef] [Green Version]
- Dibner, C. The importance of being rhythmic: Living in harmony with your body clocks. Acta Physiol. 2020, 228, e13281. [Google Scholar] [CrossRef]
- Honma, S. The mammalian circadian system: A hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci. 2018, 68, 207–219. [Google Scholar] [CrossRef]
- Stevens, R.G.; Zhu, Y. Electric light, particularly at night, disrupts human circadian rhythmicity: Is that a problem? Philos. Trans. R. Soc. B-Biol. Sci. 2015, 370, 20140120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendová, Z.; Moravcová, S. Erasing day/night differences in light intensity and spectrum affect biodiversity and the health of mammals by confusing the circadian clock. Lnyx New Ser. 2018, 49, 139–161. [Google Scholar]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the national toxicology program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molcan, L.; Sutovska, H.; Okuliarova, M.; Senko, T.; Krskova, L.; Zeman, M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019, 231, 116568. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Dauchy, E.M.; Tirrell, R.P.; Hill, C.R.; Davidson, L.K.; Greene, M.W.; Tirrell, P.C.; Wu, J.; Sauer, L.A.; Blask, D.E. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp. Med. 2010, 60, 348–356. [Google Scholar]
- Stenvers, D.J.; van Dorp, R.; Foppen, E.; Mendoza, J.; Opperhuizen, A.L.; Fliers, E.; Bisschop, P.H.; Meijer, J.H.; Kalsbeek, A.; Deboer, T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016, 6, 35662. [Google Scholar] [CrossRef] [Green Version]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm 2013, 28, 262–271. [Google Scholar] [CrossRef]
- Gale, J.E.; Cox, H.I.; Qian, J.Y.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythm 2011, 26, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Maury, E.; Hong, H.K.; Bass, J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014, 40, 338–346. [Google Scholar] [CrossRef]
- Inokawa, H.; Umemura, Y.; Shimba, A.; Kawakami, E.; Koike, N.; Tsuchiya, Y.; Ohashi, M.; Minami, Y.; Cui, G.; Asahi, T.; et al. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci. Rep. 2020, 10, 2569. [Google Scholar] [CrossRef]
- McFadden, E.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the breakthrough generations study. Am. J. Epidemiol. 2014, 180, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Y.; Sarkar, C.; Ni, M.Y.; Gallacher, J.; Webster, C. Exposure to light at night (lan) and risk of obesity: A systematic review and meta-analysis of observational studies. Environ. Res. 2020, 187, 109637. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Yamagami, Y.; Kurumatani, N.; Saeki, K. Bedroom lighting environment and incident diabetes mellitus: A longitudinal study of the heijo-kyo cohort. Sleep Med. 2020, 65, 1–3. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Ikada, Y.; Kurumatani, N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol. Int. 2014, 31, 779–786. [Google Scholar] [CrossRef]
- Fonken, L.K.; Bedrosian, T.A.; Zhang, N.; Weil, Z.M.; DeVries, A.C.; Nelson, R.J. Dim light at night impairs recovery from global cerebral ischemia. Exp. Neurol. 2019, 317, 100–109. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumanova, V.S.; Okuliarova, M.; Molcan, L.; Sutovska, H.; Zeman, M. Consequences of low-intensity light at night on cardiovascular and metabolic parameters in spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2019, 97, 863–871. [Google Scholar] [CrossRef]
- Russart, K.L.G.; Chbeir, S.A.; Nelson, R.J.; Magalang, U.J. Light at night exacerbates metabolic dysfunction in a polygenic mouse model of type 2 diabetes mellitus. Life Sci. 2019, 231, 116574. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Zeman, M. Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int. J. Mol. Sci. 2020, 21, 5478. [Google Scholar] [CrossRef]
- Borck, P.C.; Batista, T.M.; Vettorazzi, J.F.; Soares, G.M.; Lubaczeuski, C.; Guan, D.; Boschero, A.C.; Vieira, E.; Lazar, M.A.; Carneiro, E.M. Nighttime light exposure enhances rev-erbα-targeting micrornas and contributes to hepatic steatosis. Metabolism 2018, 85, 250–258. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, B.L.; Hebbachi, A.; Hauton, D.; Brown, A.-M.; Wiggins, D.; Patel, D.D.; Gibbons, G.F. A role for pparα in the control of srebp activity and lipid synthesis in the liver. Biochem. J. 2005, 389, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Stubblefield, J.J.; Terrien, J.; Green, C.B. Nocturnin: At the crossroads of clocks and metabolism. Trends Endocrinol. Metab. 2012, 23, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Maroni, M.J.; Capri, K.M.; Cushman, A.V.; Monteiro De Pina, I.K.; Chasse, M.H.; Seggio, J.A. Constant light alters serum hormone levels related to thyroid function in male cd-1 mice. Chronobiol. Int. 2018, 35, 1456–1463. [Google Scholar] [CrossRef]
- Fleury, G.; Masís-Vargas, A.; Kalsbeek, A. Metabolic implications of exposure to light at night: Lessons from animal and human studies. Obesity 2020, 28, S18–S28. [Google Scholar] [CrossRef]
- Christie, S.; Vincent, A.D.; Li, H.; Frisby, C.L.; Kentish, S.J.; O’Rielly, R.; Wittert, G.A.; Page, A.J. A rotating light cycle promotes weight gain and hepatic lipid storage in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G932–G942. [Google Scholar] [CrossRef] [Green Version]
- Kumar Jha, P.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leturque, A.; Brot-Laroche, E.; Le Gall, M. Glut2 mutations, translocation, and receptor function in diet sugar managing. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E985–E992. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Lieberman, R.A.; Weil, Z.M.; Nelson, R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 2013, 154, 3817–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borniger, J.C.; Maurya, S.K.; Periasamy, M.; Nelson, R.J. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol. Int. 2014, 31, 917–925. [Google Scholar] [CrossRef]
- Ranganathan, G.; Unal, R.; Pokrovskaya, I.; Yao-Borengasser, A.; Phanavanh, B.; Lecka-Czernik, B.; Rasouli, N.; Kern, P.A. The lipogenic enzymes dgat1, fas, and lpl in adipose tissue: Effects of obesity, insulin resistance, and tzd treatment. J. Lipid Res. 2006, 47, 2444–2450. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Leptin in nonalcoholic fatty liver disease: A narrative review. Metabolism 2015, 64, 60–78. [Google Scholar] [CrossRef]
- Rodríguez, A.; Moreno, N.R.; Balaguer, I.; Méndez-Giménez, L.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar]
- D’Incao, R.B.; Tovo, C.V.; Mattevi, V.S.; Borges, D.O.; Ulbrich, J.M.; Coral, G.P.; Ramos, M.J.; Meinhardt, N.G. Adipokine levels versus hepatic histopathology in bariatric surgery patients. Obes. Surg. 2017, 27, 2151–2158. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of pparα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canaple, L.; Rambaud, J.; Dkhissi-Benyahya, O.; Rayet, B.A.; Tan, N.S.; Michalik, L.; Delaunay, F.; Wahli, W.; Laudet, V. Reciprocal regulation of brain and muscle arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol. Endocrinol. 2006, 20, 1715–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.D.; Knight, B.L.; Wiggins, D.; Humphreys, S.M.; Gibbons, G.F. Disturbances in the normal regulation of srebp-sensitive genes in ppar alpha-deficient mice. J. Lipid Res. 2001, 42, 328–337. [Google Scholar] [PubMed]
- Yang, Z.-H.; Miyahara, H.; Iwasaki, Y.; Takeo, J.; Katayama, M. Dietary supplementation with long-chain monounsaturated fatty acids attenuates obesity-related metabolic dysfunction and increases expression of ppar gamma in adipose tissue in type 2 diabetic kk-ay mice. Nutr. Metab. (Lond) 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Anghel, S.I.; Wahli, W. Fat poetry: A kingdom for pparγ. Cell Res. 2007, 17, 486–511. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.M.; Staels, B. Peroxisome proliferator-activated receptor γ and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 386–395. [Google Scholar] [CrossRef]
- Asher, G.; Schibler, U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011, 13, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Caimari, A.; Oliver, P.; Palou, A. Impairment of nutritional regulation of adipose triglyceride lipase expression with age. Int. J. Obes. 2008, 32, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuliarova, M.; Rumanova, V.S.; Stebelova, K.; Zeman, M. Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6919. https://doi.org/10.3390/ijms21186919
Okuliarova M, Rumanova VS, Stebelova K, Zeman M. Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism. International Journal of Molecular Sciences. 2020; 21(18):6919. https://doi.org/10.3390/ijms21186919
Chicago/Turabian StyleOkuliarova, Monika, Valentina Sophia Rumanova, Katarina Stebelova, and Michal Zeman. 2020. "Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism" International Journal of Molecular Sciences 21, no. 18: 6919. https://doi.org/10.3390/ijms21186919
APA StyleOkuliarova, M., Rumanova, V. S., Stebelova, K., & Zeman, M. (2020). Dim Light at Night Disturbs Molecular Pathways of Lipid Metabolism. International Journal of Molecular Sciences, 21(18), 6919. https://doi.org/10.3390/ijms21186919





