Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization
Abstract
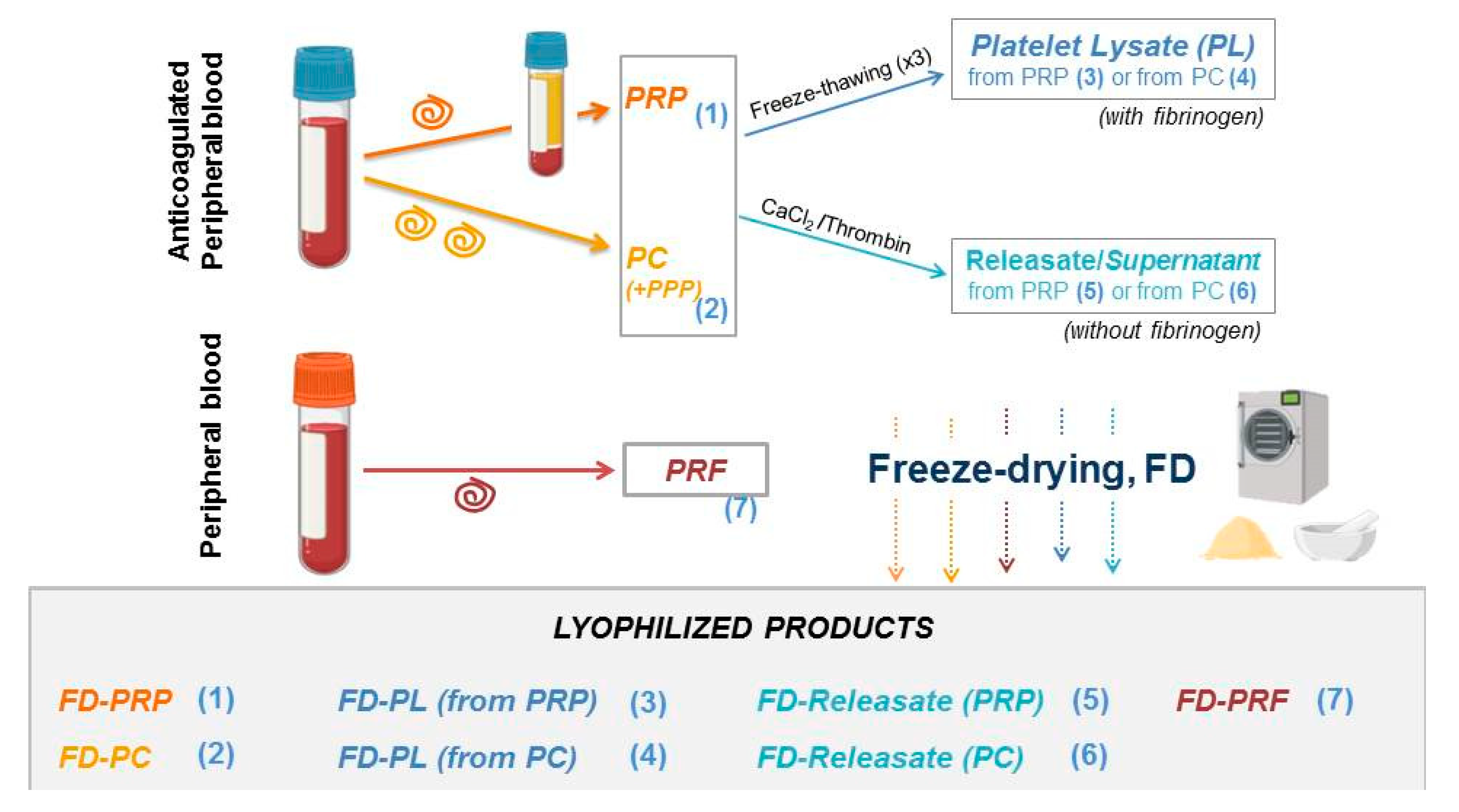
1. Introduction
2. Published Articles on Freeze-Dried PRP
2.1. Lyophilized PRP Preservation
2.2. Skin research
2.2.1. Wound Management
2.2.2. Other Conditions
| Author, Year, (Reference) | Condition | FD-PRP Based Product/Stability | Study Type/Cells/Animal Model | Results |
|---|---|---|---|---|
| Wound healing | ||||
| Horimizu M 2013 [33] | Wounds | Lyophilized collagen sponge coated with PRP Stability at 4 °C: at least 3 months | Human periosteal fibroblasts Diabetic mice model GF antibody microarray Biomechanical characterization Histology: vessel formation, cell number, presence of adipose tissue, steatosis | Stimulation of cell growth in vitro Enhanced wound healing and regenerative potential in vivo |
| Huber CS 2019 [23] | Acute Wounds | Saline vs. fresh PRP vs. FD-PRP | Wistar male rats GF release assessments Histology: coll. deposition, Masson’s trichrome, Wound closure kinetics | EGF, PDGF-AA, TGF-b1 and VEGF levels are conserved in FD-PRP No differences in epithelial thickness, collagen density and wound closure kinetics Enhanced presence of myofibroblasts and vascularization with FD-PRP |
| Lei X 2019 [24] | Acute Full-thickness wounds | Porcine ADM+FD-PRP vs. fresh PRP vs. ADM vs. control | C57 Mouse model Healing evaluation: inflammation, vascularization, epithelialization, collagen deposition | TGF-b1, EGF, PDGF-AA, VEGF levels in ADM+FD-PRP were lower than in PRP Wound closure enhanced with FD-PRP/ADM: it promotes wound healing, neovascularization, collagen deposition and epithelialization |
| Lima AC 2014 [34] | Wounds | FD-PL encapsulated in collagen, hASCs encapsulated in coll+PL beads Fresh PL vs. FD-PL | GF release Beads degradation hASCs activities Chick CAM assay | No changes in VEGF, PDGF-BB release over 72h; sustained GF release. No differences in hASCs proliferation, scratch wound assay and angiogenesis |
| Liu J 2017 [35] | Wounds | FD-(Silk cocoon+PRP) vs. FD-(Silk cocoon+PPP) vs. Mepitel | L929 cell activites Wounds closure in the back of New Zealand white rabbits | FD-(Silk cocoon+PRP) enhanced L929 proliferation and wound |
| Nardini M 2020 [31] | Full-thickness chronic wounds | Alginate/SS vs. FD-PL/Alg/SS vs. Alg/FD-PL | GF release kinetics hBMMSCs, hFB: Cell viability, proliferation and oxidative stress. Western blot: cyclin D1 Mouse model C57/BL6: granulation tissue, early inflammation, collagen deposition, fibroblasts maturation, re-epithelialization, neovascularization | Enhanced GF release over 144h from FD-PL/Alg/SS compared to FD-PL/Alg Increased proliferation and Cylcin D1 expression in FDPL FD-PL/SS rescued the cells from oxidative stress and supported cell proliferation Faster wound healing results with FD-PL/Alg/SS in vivo |
| Notodihardjo SC 2018 [42] | Full-thickness wounds | (PL vs. CL-PL vs. FD-PL vs. FBS) + gelatin Stability at 4 °C: 9 months | GF release hFBs bioactivity Histology in mice: wound area, neovascularization, granulation tissue formation | The levels of PDGF-BB, VEGF and TGF-b1 were reduced in FD conditions Bioactivity of FD is maintained: increased hFBs proliferation in PL conditions vs. FBS No differences in wound healing in vivo |
| Notodihardjo SC 2019 [32] | Full-thickness wounds | FD-PL vs. different concentrations of FD-PL + gelatin Stability at 4 °C: 9 months | C57BL6J/Jcl mice Histology: H&E, Azan and anti-CD31 | Gelatin sheets impregnated with 2- and 3-fold FD-PL concentrations accelerated the healing process by favoring the formation of granulation tissue and capillaries in vivo |
| Pietramaggiori G 2006 [43] | Dorsal Wounds (Diabetic) | FD-PRP, 1.2 × 106 plts/ul vs. fresh-frozen PRP vs. sonicated PRP | Diabetic mouse model Assessment of GFs Histology: cell proliferation, angiogenesis, wound thickness, surface coverage | No differences in PDGF, TGF-b, EGF and VEGF concentrations: preservation maintained Increased tissue formation with FD-PRP and fresh-frozen PRP |
| Pietramaggiori G 2008 [44] | Wounds (Diabetic) | ADM vs. FD-PRP vs. ADM-FD-PRP | Diabetic mouse model FBs Wound healing kinetics and new tissue formation | The combination of ADM-FD-PRP stimulate fibroblasts proliferation in vitro and revascularization and tissue formation in vivo |
| Sell SA 2012 [45] | Wounds | FD-PRP vs. MH vs. MH-FD-PRP | hFBs, macrophages and endothelial cell activities | FD-PRP and MH-FD-PRP conditions enhance cell activities: proliferation, collagen matrix deposition and migration |
| Wang Q 2019 [29] | Wounds | Chitosan/silk fibroin nanosilver loaded with FD-PRP | BALBc mice Physical and mechanical properties Protein release Biological safety (silver content in organs) Antibacterial properties | Good asymmetric performance, appropriate physical and mechanical properties, slow release of proteins. Wound moisture retention and promotion of healing. |
| Xu F 2018 [46] | Acute full-thickness dorsal skin wounds | Different concentrations of FD-PRF on a PVA hydrogel | Cell activities in L929 and HUVECs Wound healing histologic assesment in mice. | 1% of FD-PRF-PVA hydrogels: - Accelerated wound closure - Enhanced granulation tissue, maturity, collagen deposition and capillary formation |
| Yassin GE 2019 [30] | Wounds | FD-PRP + CMC (wafers) vs. FD-PRP powder Stability at −20 °C: 3 months | Rat wound model Antibacterial activities against Gram-negative bacteria | FD-PRP wafers present greater antimicrobial efficacy and wound size reduction |
| Yeung CY 2018 [37] | Deep second degree burn wounds in the plantar area | FD-PRP (dose: 1 × 107 platelets/cm2), vs. conventional care | Clinical study | Significant reduction in the wound healing rate and bacterial colonization |
| Other Dermal Applications | ||||
| Abdallah M 2020 [41] | Striae distensae (SD) | FD-GF vs. CO2 ablational laser, and combination of both methods | Clinical trial, 20 female patients. Each patient, 3 therapy methods Before treatment and six weeks after: - Assessment of clinical score (reduction % of SD width, appearance, color, size) - Histopathologic examination | The combination of ablational laser and FD-GF was clinically more effective than ablational laser alone |
| Lin YK 2016 [40] | Hair | FBS vs. FD-porcine PRP vs. fresh porcine PRP | hFDPCs activities GF release: elisa, MTT, PCR | Higher GF levels in PRP than FBS and it is stable. No difference in HFDPCs activities in fresh or FD-PRP and FBS |
3. Musculoskeletal Applications
| Author, Year (Reference) | Condition | FD-PRP Based Product/Stability | Study Type/Cells/Animal Model | Results | |
|---|---|---|---|---|---|
| Musculoskeletal Pathology | |||||
| Camargo-Martin L 2019 [51] | Equine OA | Frozen-PRP vs. FD-PRP vs. filtered FD-PRP 1.5-, 3- and 6-fold platelet enrichment | Equine cartilage explants exposed to PRP, FBS and ITS as controls | Better chondroprotective effects with 3-fold PRP products compared to controls No differences between FD-PRP and frozen PRP | |
| Growney EA 2020 [53] | Spine | FD-(PRP biofunctionalized alginate) vs. FD-(PRP encapsulated alginate) vs. alginate control Double spinned PRP | hNPCs viability, adhesion and ECM and GAG secretion in hypoxic and normoxic conditions | Decreased cytotoxicity in the presence of PRP Increased hNPCs adhesion and distribution in PRP-functionalized alginate No differences in cell proliferation Increased GAG content and ECM production in PRP-functionalized alginate in hypoxic conditions | |
| Hahn O 2020 [52] | Cartilage conditions | FD-PRP vs. PRP powder Different PRP stimulation frequency and doses | Chondrocyte cultures for 14 days | Pro-collagen type 1 and -3, GAGs and cell proliferation were time-dependent and increased with FD-PRP concentration | |
| Jain E 2019 [50] | OA | Double spin PRP, comparison of bolus PRP vs. FD-PRP encapsulated in PEG | Kinetics of VEGF, EGF, PDGF-BB and TGF-b1 release until degradation of hydrogel IL-1b treated chondrocytes | VEGF and EGF are released on day 1 while TGF-b and PDGF-BB present a sustained release PRP rescued cell proliferation No effect on NO synthesis Does not rescue changes in gene expression induced by IL-1b Bolus PRP decreased inflammatory NF-kB activation | |
| Kinoshita H 2020 [55] | Spine | Fresh PRP vs. FD-PRP Stability: PRP 4 weeks | Osteoblast proliferation and ERK and PDGFR phosphorilation | FD-PRP is functionally (phosphorylation mechanisms) equivalent to fresh PRP | |
| Shiga Y 2016 [54] | Lumbar fusion | FD-PRP (thrombin, CaCl2-activated) + artificial bone vs. fresh PRP + artificial bone vs. BMP + artificial bone vs. autologous bone | Spinal posterolateral fusion in rats Radiography and histology: amount of bone formation, characteristics of trabecular bone Biomechanical strength (3-point bending test) PDGF and TGFb1 determinations | (FD-PRP + artificial bone) accelerated bone union at a rate comparable to (fresh PRP + artificial bone) or (BMP + artificial bone) More trabecular branches and biomechanical rigidity at 8 weeks | |
| Shirata T 2019 [49] | OA | FD-PRP Stability: 6 months | Clinical study Intraarticular injection of FD-PRP (resuspended in normosaline) | Enhanced clinical outcomes (KOOS score) 1, 3, 6 months post treatment | |
| Tendon and Muscle | |||||
| McCarrell T 2009 [57] | Tendon and ligament | BMA vs. PRP vs. FD-PRP | Flexor digitorum superficialis tendon and suspensory ligament explants: TGF-b1 and PDGF release RT-QPCR: COL1A1, COL3A1, COMP, MMP-3 MMP-13 | TGF-b1 and PDGF concentrations higher in PRPs than BMA Correlation between GF concentrations and ECM gene expression PRP and FD-PRP better outcome than BMA Platelet concentration correlated with ECM gene expression | |
| McClure MJ 2018 [59] | Volumetric muscle loss | Aligned electrospun polydioxanone vs. random oriented, loaded with FD-PRP powder | C2C12 murine myoblasts: cell morphology, cell signaling multiplex assay, myogenic gene expression and protein and integrin synthesis and in response to FD-PRP | Compared to random scaffold, fiber alignment + FD-PRP powder favors myogenic differentiation, which is ERK-dependent and dose-dependent | |
| Zheng C 2019 [58] | Tendon bone interface | PRP (double spin, Ca+2-activated) mixed with ICA and lyophilized vs. FD-PRP vs. control | New Zealand rabbits, partial patellectomy At 8 weeks and 16 weeks, microcomputed tomography, histology, biomechanical testing | Sustained release of ICA from FD-PRP+ICA compared to fresh PRP Higher rate of bone formation and remodeling in FD-PRP+ICA Better new bone formation in FD-PRP+ICA Fibrocartilage zone formation in the three groups, better mechanical properties in FD-PRP+ICA | |
4. Dentistry Research
| Author, Year (Reference) | FD-PRP Based Product/Stability | Study Type/Cells/Animal Model | Results |
|---|---|---|---|
| Ansarizadeh M 2019 [71] | PRF (single spin): frozen (−80 °C) vs. FD Mixed with chitosan/collagen | FTIR, SEM, Young’s modulus, hMSCs viability, ALP activity, membrane degradation rate. | Optimized membrane composition based on experimental algorithms: Chitosan: collagen 4:1 + 0.58 mg/mL PRF Increased ALP activity (osteogenic differentiation) with PRF |
| Kardos D 2019 [69] | PRF (single spin) open vs. closed system: fresh, frozen (−20 °C), FD-PRF (−80 °C 30 min, −54 °C o/n) | Tensile strength, surface microstructure, plasmin activity, MSC and human gingival fibroblasts adhesion and proliferation, pro-collagen synthesis | Lower tensile strength in fresh PRF; frozen and thawed PRF lower plasmin activity than fresh and FD-PRF. Improved MSC adhesion in frozen and FD-PRF, no differences in gingival fibroblasts, no differences in pro-collagen synthesis |
| Li J 2017 [66] | PRP (double spin), vs. FD-PRP/PCL vs. traditional PRP (thrombin/Ca2+-activated)/PCL vs. PCL | DPSCs: migration, proliferation, ALP activity, osteogenic genes expression (RUNX2, OCN, OPN) In vivo rat calvarial defect assesment | FD-PRP/PCL better than traditional PRP/PCL and PCL, in terms of osteogenesis (RUNX2, OCN, OPN) and mineralization Faster rate of in vivo bone formation with FD-PRP/PCL |
| Li Q 2014 [63] | FD-PRF vs. traditional PRF (porcine) | ABs, PDLs and DFs: proliferation, migration, differentiation/mineralization, steogenic genes expression (RUNX2, MGP) In vivo, nude mice, calvarial defect: histology—bone formation, collagen synthesis; CT scans—bone regeneration | FD-PRF promotes RUNX2 expression in alveolar bone, not in dental follicle, partially in periodontal progenitors Histology reveals enhanced bone formation with FD-PRP (nodules after 14d) compared with fresh PRF |
| Liu Z 2019 [67] | FD-PRF vs. FD-PRF supplementing fresh PRF vs. fresh PRF (prepared from New Zealand rabbits) | PDGF-AB, TGF-b1 and VEGF quantification SEM hBMMSCs: proliferation (MTT), differentiation, mineralization nodules In vivo rabbit calvarial defect: histomorphometric analyses, CT scan | Sustained factor release in fresh+FD-PRF No differences in hBMMSCs proliferation Higher differentiation characteristics in FD-PRF Higher bone formation area at 12 weeks in fresh PRF, FD-PRF group, fresh+FD-PRF FD-PRF maintains the ability to promote bone proliferation and chemotaxis in osteoblasts |
| Nakatani Y 2016 [64] | FD-PRP vs. fresh PRP | PDGF-BB, TGF-b and VEGF release immunocompromised mice BULB: Bone formation histology and immunohistochemistry | Equivalent GFs release in fresh vs. FD-PRP Maintained bone regeneration at 4 and 8 weeks |
| Wang L 2019 [68] | (FD-PRP vs. fresh PRP) mixed with chitosan and alginate | TGF-b1, PDGF-AB, IGF-1, VEGF and TSP-1 release during 28d MC3T3-E1 murine osteoblast precursor cell line: Cytotoxicity, proliferation mineralization, osteogenic gene expression (OPN, OPG, Runx2, bone sialoprotein, osteocalcin) | More rapid GF release from FD-PRP composites versus sustained release from PRP composites Better osteogenic performance in FD-PRP in early stages Better osteogenic mineralization in fresh PRP at later stages |
| Xie Y 2020 [65] | CaCl2-activated fresh PRP vs. FD-PRP | PDGF-AB, TGF-b and VEGF quantification SEM Rabbit BMMSC: proliferation and differentiation (ALP activity, OCN, BMP-2 gene expression) | Higher PDGF, TGF and VEGF release in fresh PRP Enhanced osteogenic differentiation with fresh PRP at 1, 3, 6 and 9 days. |
| Zhang J 2017 [70] | Autologous fresh PRF (single spin) vs. autologous FD-PRF | Randomized clinical trial in guided bone regeneration (alveolar bone). Healing mucosa score (color, shape and quality), clinical outcomes (pain, color, swelling) at 24h, 3 and 7 days; computed tomography at 4 months | No statistical differences in soft-tissue healing or bone formation. No bone infection. Similar ratios of bone and soft connective tissues in the histological sections |
5. The Way Forward
Author Contributions
Funding
Conflicts of Interest
References
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; The Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Pusateri, A.E.; Butler, F.K.; Shackelford, S.A.; Sperry, J.L.; Moore, E.E.; Cap, A.P.; Taylor, A.L.; Homer, M.J.; Hoots, W.K.; Weiskopf, R.B.; et al. The need for dried plasma—A national issue. Transfusion 2019, 59, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Daban, J.; Clapson, P.; Ausset, S.; Deshayes, A.V.; Sailliol, A. Freeze dried plasma: A French army specialty. Crit. Care 2010, 14, 412. [Google Scholar] [CrossRef]
- Feuerstein, S.J.; Skovmand, K.; Møller, A.M.; Wildgaard, K. Freeze-dried plasma in major haemorrhage: A systematic review. Vox Sang. 2020, 115, 263–274. [Google Scholar] [CrossRef]
- Read, M.S.; Reddick, R.L.; Bode, A.P.; Bellinger, D.A.; Nichols, T.C.; Taylor, K.; Smith, S.V.; McMahon, D.K.; Griggs, T.R.; Brinkhous, K.M. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: Potential for long-term storage of dried platelets for transfusion. Proc. Natl. Acad. Sci. USA 1995, 92, 397–401. [Google Scholar] [CrossRef]
- Valeri, C.R.; Macgregor, H.; Barnard, M.R.; Summaria, L.; Michelson, A.D.; Ragno, G. In vitro testing of fresh and lyophilized reconstituted human and baboon platelets. Transfusion 2004, 44, 1505–1512. [Google Scholar] [CrossRef]
- Nurden, A.T. The biology of the platelet with special reference to inflammation wound healing and immunity. Front. Biosci. 2018, 23, 726–751. [Google Scholar] [CrossRef]
- Anitua, E.; de la Fuente, M.; Muruzábal, F.; Merayo-Lloves, J. Stability of freeze-dried plasma rich in growth factors eye drops stored for 3 months at different temperature conditions. Eur. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Walker, N.J.; Tamari, Y.; Tablin, F.; Crowe, J.H. Towards a Clinical Application of Freeze-Dried Human Platelets. Cell Preserv. Technol. 2002, 1, 175–188. [Google Scholar] [CrossRef]
- Brogna, R.; Oldenhof, H.; Sieme, H.; Figueiredo, C.; Kerrinnes, T.; Wolkers, W.F. Increasing storage stability of freeze-dried plasma using trehalose. PLoS ONE 2020, 15, e0234502. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yong, Z.; Yuk, K.S.; Hoon, K.Y.; Yuedong, S.; Xu, J. Growth Factor Release from Lyophilized Porcine Platelet-Rich Plasma: Quantitative Analysis and Implications for Clinical Applications. Aesth. Plast. Surg. 2016, 40, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.M.; Heijnen, H.F.G.; Horne, M.K.; White, J.G.; Gahl, W.A. Proteomic analysis of platelet α-granules using mass spectrometry: Platelet α-granule proteomics. J. Thromb. Haemost. 2007, 5, 1945–1955. [Google Scholar] [CrossRef]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef]
- Wijten, P.; van Holten, T.; Woo, L.L.; Bleijerveld, O.B.; Roest, M.; Heck, A.J.R.; Scholten, A. High Precision Platelet Releasate Definition by Quantitative Reversed Protein Profiling—Brief Report. Arter. Thromb. Vasc. Biol. 2013, 33, 1635–1638. [Google Scholar] [CrossRef]
- Shiga, Y.; Kubota, G.; Orita, S.; Inage, K.; Kamoda, H.; Yamashita, M.; Iseki, T.; Ito, M.; Yamauchi, K.; Eguchi, Y.; et al. Freeze-Dried Human Platelet-Rich Plasma Retains Activation and Growth Factor Expression after an Eight-Week Preservation Period. Asian Spine J. 2017, 11, 329–336. [Google Scholar] [CrossRef]
- Da Silva, L.Q.; Montalvão, S.A.d.L.; Justo-Junior, A.d.S.; Cunha Júnior, J.L.R.; Huber, S.C.; Oliveira, C.C.; Annichino-Bizzacchi, J.M. Platelet-rich plasma lyophilization enables growth factor preservation and functionality when compared with fresh platelet-rich plasma. Regen. Med. 2018, 13, 775–784. [Google Scholar] [CrossRef]
- Kieb, M.; Sander, F.; Prinz, C.; Adam, S.; Mau-Möller, A.; Bader, R.; Peters, K.; Tischer, T. Platelet-Rich Plasma Powder: A New Preparation Method for the Standardization of Growth Factor Concentrations. Am. J. Sports Med. 2017, 45, 954–960. [Google Scholar] [CrossRef]
- Muraglia, A.; Ottonello, C.; Spanò, R.; Dozin, B.; Strada, P.; Grandizio, M.; Cancedda, R.; Mastrogiacomo, M. Biological activity of a standardized freeze-dried platelet derivative to be used as cell culture medium supplement. Platelets 2014, 25, 211–220. [Google Scholar] [CrossRef]
- Perez-Zabala, E.; Basterretxea, A.; Larrazabal, A.; Perez-del-Pecho, K.; Rubio-Azpeitia, E.; Andia, I. Biological approach for the management of non-healing diabetic foot ulcers. J. Tissue Viability 2016, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Alonso, N.; Lobato, I.; Hernández, I.; Sebastian, K.; Rodríguez, B.; Grandes, G.; Andia, I. Adjuvant Biological Therapies in Chronic Leg Ulcers. Int. J. Mol. Sci. 2017, 18, 2561. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.C.; Junior, J.L.R.C.; Silva, L.Q.; Montalvão, S.A.L.; Annichino-Bizzacchi, J.M. Freeze-dried versus fresh platelet-rich plasma in acute wound healing of an animal model. Regen. Med. 2019, 14, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Yang, Y.; Shan, G.; Pan, Y.; Cheng, B. Preparation of ADM/PRP freeze-dried dressing and effect of mice full-thickness skin defect model. Biomed. Mater. 2019, 14, 035004. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chou, M.-L.; Wu, Y.-W.; Su, C.-Y.; Lee, L.-W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria: ANTIMICROBIAL ACTIVITY OF PLASMA AND PLT GEL. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef]
- Maghsoudi, O.; Ranjbar, R.; Mirjalili, S.H.; Fasihi Ramandi, M. Inhibitory activities of platelet-rich and platelet-poor plasma on the growth of pathogenic bacteria. Iran J. Pathol. 2017, 12, 79–87. [Google Scholar] [CrossRef]
- Kapur, R.; Zufferey, A.; Boilard, E.; Semple, J.W. Nouvelle Cuisine: Platelets Served with Inflammation. J. Immunol. 2015, 194, 5579–5587. [Google Scholar] [CrossRef]
- Watson, C.N.; Kerrigan, S.W.; Cox, D.; Henderson, I.R.; Watson, S.P.; Arman, M. Human platelet activation by Escherichia coli: Roles for FcγRIIA and integrin αIIbβ3. Platelets 2016, 27, 535–540. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, Z.; Liu, B.; Liu, J.; Zhang, L.; Xu, J. In vitro and in vivo evaluation of new PRP antibacterial moisturizing dressings for infectious wound repair. J. Biomater. Sci. Polym. Ed. 2019, 30, 462–485. [Google Scholar] [CrossRef]
- Yassin, G.E.; Dawoud, M.H.S.; Wasfi, R.; Maher, A.; Fayez, A.M. Comparative lyophilized platelet-rich plasma wafer and powder for wound-healing enhancement: Formulation, in vitro and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 1379–1387. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Notodihardjo, S.C.; Morimoto, N.; Kakudo, N.; Mitsui, T.; Le, T.M.; Tabata, Y.; Kusumoto, K. Efficacy of Gelatin Hydrogel Impregnated with Concentrated Platelet Lysate in Murine Wound Healing. J. Surg. Res. 2019, 234, 190–201. [Google Scholar] [CrossRef]
- Horimizu, M.; Kawase, T.; Nakajima, Y.; Okuda, K.; Nagata, M.; Wolff, L.F.; Yoshie, H. An improved freeze-dried PRP-coated biodegradable material suitable for connective tissue regenerative therapy. Cryobiology 2013, 66, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Mano, J.F.; Concheiro, A.; Alvarez-Lorenzo, C. Fast and Mild Strategy, Using Superhydrophobic Surfaces, to Produce Collagen/Platelet Lysate Gel Beads for Skin Regeneration. Stem Cell Rev. Rep. 2015, 11, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, F.; Chen, H.; Bao, R.; Li, Z.; Lu, B.; Yu, K.; Dai, F.; Wu, D.; Lan, G. Healing of skin wounds using a new cocoon scaffold loaded with platelet-rich or platelet-poor plasma. RSC Adv. 2017, 7, 6474–6485. [Google Scholar] [CrossRef]
- del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Andia, I.; Aragón-Sánchez, J.; Herrera-Ramos, E.; Iruzubieta Barragán, F.J.; Serrano-Aguilar, P. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis: Platelet-rich plasma for diabetic foot ulcers. Wound Repair Regen. 2019, 27, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.-Y.; Hsieh, P.-S.; Wei, L.-G.; Hsia, L.-C.; Dai, L.-G.; Fu, K.-Y.; Dai, N.-T. Efficacy of Lyophilised Platelet-Rich Plasma Powder on Healing Rate in Patients With Deep Second Degree Burn Injury: A Prospective Double-Blind Randomized Clinical Trial. Ann. Plast. Surg. 2018, 80 (2S Suppl. 1), S66–S69. [Google Scholar] [CrossRef]
- Evans, A.G.; Mwangi, J.M.; Pope, R.W.; Ivanic, M.G.; Botros, M.A.; Glassman, G.E.; Pearce, F.B.; Kassis, S. Platelet-rich plasma as a therapy for androgenic alopecia: A systematic review and meta-analysis. J. Dermatol. Treat. 2020, 1–14. [Google Scholar] [CrossRef]
- Bruce, A.J.; Pincelli, T.P.; Heckman, M.G.; Desmond, C.M.; Arthurs, J.R.; Diehl, N.N.; Douglass, E.J.; Bruce, C.J.; Shapiro, S.A. A Randomized, Controlled Pilot Trial Comparing Platelet-Rich Plasma to Topical Minoxidil Foam for Treatment of Androgenic Alopecia in Women. Dermatol. Surg. 2020, 46, 826–832. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Sugiri, F.; Ma, H.; Chiu, Y.-H.; Yao, C.-L. Industrial-scale processing of activated platelet-rich plasma from specific pathogen-free pigs and its effect on promoting human hair follicle dermal papilla cell cultivation. J. Taiwan Inst. Chem. Eng. 2017, 71, 28–37. [Google Scholar] [CrossRef]
- Abdallah, M.; Fahmy, H.; Abdel Hameed, S.; Mostafa, A.E. Ablative fractional CO2 laser vs lyophilized growth factor intralesional injection vs combination of both modalities for striae distensae treatment. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Notodihardjo, S.C.; Morimoto, N.; Kakudo, N.; Mitsui, T.; Le, T.M.; Tabata, Y.; Kusumoto, K. Comparison of the efficacy of cryopreserved human platelet lysate and refrigerated lyophilized human platelet lysate for wound healing. Regen. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Kaipainen, A.; Czeczuga, J.M.; Wagner, C.T.; Orgill, D.P. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006, 14, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Pietramaggiori, G.; Scherer, S.S.; Mathews, J.C.; Alperovich, M.; Yang, H.-J.; Neuwalder, J.; Czeczuga, J.M.; Chan, R.K.; Wagner, C.T.; Orgill, D.P. Healing modulation induced by freeze-dried platelet-rich plasma and micronized allogenic dermis in a diabetic wound model. Wound Repair Regen. 2008, 16, 218–225. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Spence, A.J.; Rodriguez, I.A.; McCool, J.M.; Petrella, R.L.; Garg, K.; Ericksen, J.J.; Bowlin, G.L. A Preliminary Study on the Potential of Manuka Honey and Platelet-Rich Plasma in Wound Healing. Int. J. Biomater. 2012, 2012, 313781. [Google Scholar] [CrossRef]
- Xu, F.; Zou, D.; Dai, T.; Xu, H.; An, R.; Liu, Y.; Liu, B. Effects of incorporation of granule-lyophilised platelet-rich fibrin into polyvinyl alcohol hydrogel on wound healing. Sci. Rep. 2018, 8, 14042. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; de Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef]
- Tan, J.; Chen, H.; Zhao, L.; Huang, W. Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-Analysis of 26 Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020. [Google Scholar] [CrossRef]
- Shirata, T.; Kato, Y. Can intra-articular injection of freeze-dried platelet-derived factor concentrate regenerate articular cartilage in the knee joint? Regen. Ther. 2019, 11, 5–7. [Google Scholar] [CrossRef]
- Jain, E.; Chinzei, N.; Blanco, A.; Case, N.; Sandell, L.J.; Sell, S.; Rai, M.F.; Zustiak, S.P. Platelet-Rich Plasma Released from Polyethylene Glycol Hydrogels Exerts Beneficial Effects on Human Chondrocytes. J. Orthop. Res. 2019, 37, 2401–2410. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; McIlwraith, C.W.; Frisbie, D.D. Evaluation of allogeneic freeze-dried platelet lysate in cartilage exposed to interleukin 1-β in vitro. BMC Vet. Res. 2019, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Kieb, M.; Jonitz-Heincke, A.; Bader, R.; Peters, K.; Tischer, T. Dose-Dependent Effects of Platelet-Rich Plasma Powder on Chondrocytes In Vitro. Am. J. Sports Med. 2020, 48, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Growney, E.A.; Linder, H.R.; Garg, K.; Bledsoe, J.G.; Sell, S.A. Bio-conjugation of platelet-rich plasma and alginate through carbodiimide chemistry for injectable hydrogel therapies. J. Biomed. Mater. Res. 2020, 108, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Orita, S.; Kubota, G.; Kamoda, H.; Yamashita, M.; Matsuura, Y.; Yamauchi, K.; Eguchi, Y.; Suzuki, M.; Inage, K.; et al. Freeze-Dried Platelet-Rich Plasma Accelerates Bone Union with Adequate Rigidity in Posterolateral Lumbar Fusion Surgery Model in Rats. Sci. Rep. 2016, 6, 36715. [Google Scholar] [CrossRef]
- Kinoshita, H.; Orita, S.; Inage, K.; Fujimoto, K.; Shiga, Y.; Abe, K.; Inoue, M.; Norimoto, M.; Umimura, T.; Ishii, T.; et al. Freeze-Dried Platelet-Rich Plasma Induces Osteoblast Proliferation via Platelet-Derived Growth Factor Receptor-Mediated Signal Transduction. Asian Spine J. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Biological Therapies in Regenerative Sports Medicine. Sports Med. 2017, 47, 807–828. [Google Scholar] [CrossRef]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 2009, 27, 1033–1042. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, H.; Tang, Y.; Wang, Z.; Ma, H.; Li, H.; Chen, H.; Chen, Y.; Chen, C. Autologous Freeze-Dried, Platelet-Rich Plasma Carrying Icariin Enhances Bone-Tendon Healing in a Rabbit Model. Am. J. Sports Med. 2019, 47, 1964–1974. [Google Scholar] [CrossRef]
- McClure, M.J.; Clark, N.M.; Schwartz, Z.; Boyan, B.D. Platelet-rich plasma and alignment enhance myogenin via ERK mitogen activated protein kinase signaling. Biomed. Mater. 2018, 13, 055009. [Google Scholar] [CrossRef]
- Kandil, M.I.; Tabl, E.A.; Elhammady, A.S. Prospective Randomized Evaluation of Local Injection of Allogeneic Growth Factors in Plantar Fasciitis. Foot Ankle Int. 2020. [Google Scholar] [CrossRef]
- Peterson, L.J. Oral and Maxillofacial Surgery. Oral Surg. Oral Med. Oral Pathol. 1998, 85, 9. [Google Scholar]
- Ehrenfest, D.M.D.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef]
- Li, Q.; Reed, D.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.; Li, L.; Geng, Y.; Galang, M.-T.; Gajendrareddy, P.; et al. Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Agata, H.; Sumita, Y.; Koga, T.; Asahina, I. Efficacy of freeze-dried platelet-rich plasma in bone engineering. Arch. Oral Biol. 2017, 73, 172–178. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, M.; Chen, Y.; Xu, Y.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. Effects of PRP and LyPRP on osteogenic differentiation of MSCs. J. Biomed. Mater. Res. 2020, 108, 116–126. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, H.; Xie, Q.; Jiang, Z.; Guo, S.; Li, Y.; Zhang, B. Controlled Release Strategies for the Combination of Fresh and Lyophilized Platelet-Rich Fibrin on Bone Tissue Regeneration. BioMed Res. Int. 2019, 2019, 4923767. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, X.; Luo, X.; Li, D.; Wang, H.; Li, T. Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin. Implant Dent. Relat. Res. 2017, 19, 466–477. [Google Scholar] [CrossRef]
- Ansarizadeh, M.; Mashayekhan, S.; Saadatmand, M. Fabrication, modeling and optimization of lyophilized advanced platelet rich fibrin in combination with collagen-chitosan as a guided bone regeneration membrane. Int. J. Biol. Macromol. 2019, 125, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kawase, T.; Kobayashi, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. Bioactivity of freeze-dried platelet-rich plasma in an adsorbed form on a biodegradable polymer material. Platelets 2012, 23, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Perez-Valle, A.; Del Amo, C.; Andia, I. Overview of Current Advances in Extrusion Bioprinting for Skin Applications. Int. J. Mol. Sci. 2020, 21, 6679. [Google Scholar] [CrossRef] [PubMed]

| Advantage | Weaknesses |
|---|---|
| Preserve PRP bioactivity Allows standardization of platelet number and growth factor levels, doses can be adjusted Avoids interdonor variability (if allogeneic) Easy to handle and mix with other biomaterials Quick reconstitution process by rehydration at the point of care, no need of specific equipment Stability at room temperature for several months No need of venipuncture at the point of care Saves time of preparation Immediate availability (off-the-shelf product) Timely use in case of emergency Ease of shipping and transport | Costs of the research needed to fulfill regulatory requirements Costs of fabrication Minor risks of contamination and disease transmission Needs optimization and standardization of freeze-drying procedures |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andia, I.; Perez-Valle, A.; Del Amo, C.; Maffulli, N. Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. Int. J. Mol. Sci. 2020, 21, 6904. https://doi.org/10.3390/ijms21186904
Andia I, Perez-Valle A, Del Amo C, Maffulli N. Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. International Journal of Molecular Sciences. 2020; 21(18):6904. https://doi.org/10.3390/ijms21186904
Chicago/Turabian StyleAndia, Isabel, Arantza Perez-Valle, Cristina Del Amo, and Nicola Maffulli. 2020. "Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization" International Journal of Molecular Sciences 21, no. 18: 6904. https://doi.org/10.3390/ijms21186904
APA StyleAndia, I., Perez-Valle, A., Del Amo, C., & Maffulli, N. (2020). Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. International Journal of Molecular Sciences, 21(18), 6904. https://doi.org/10.3390/ijms21186904






