Alternative Options for Skin Cancer Therapy via Regulation of AKT and Related Signaling Pathways
Abstract
1. Introduction
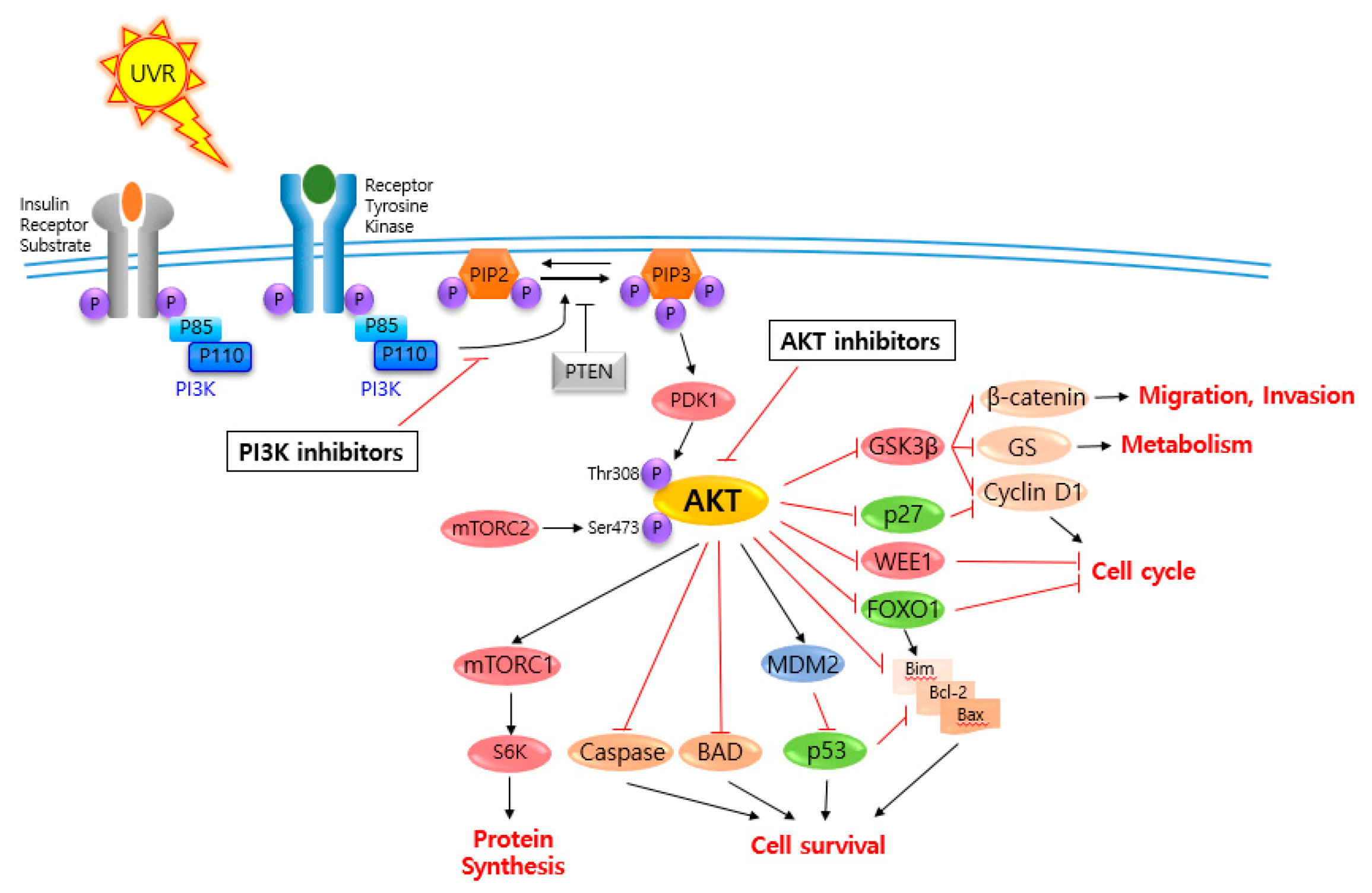
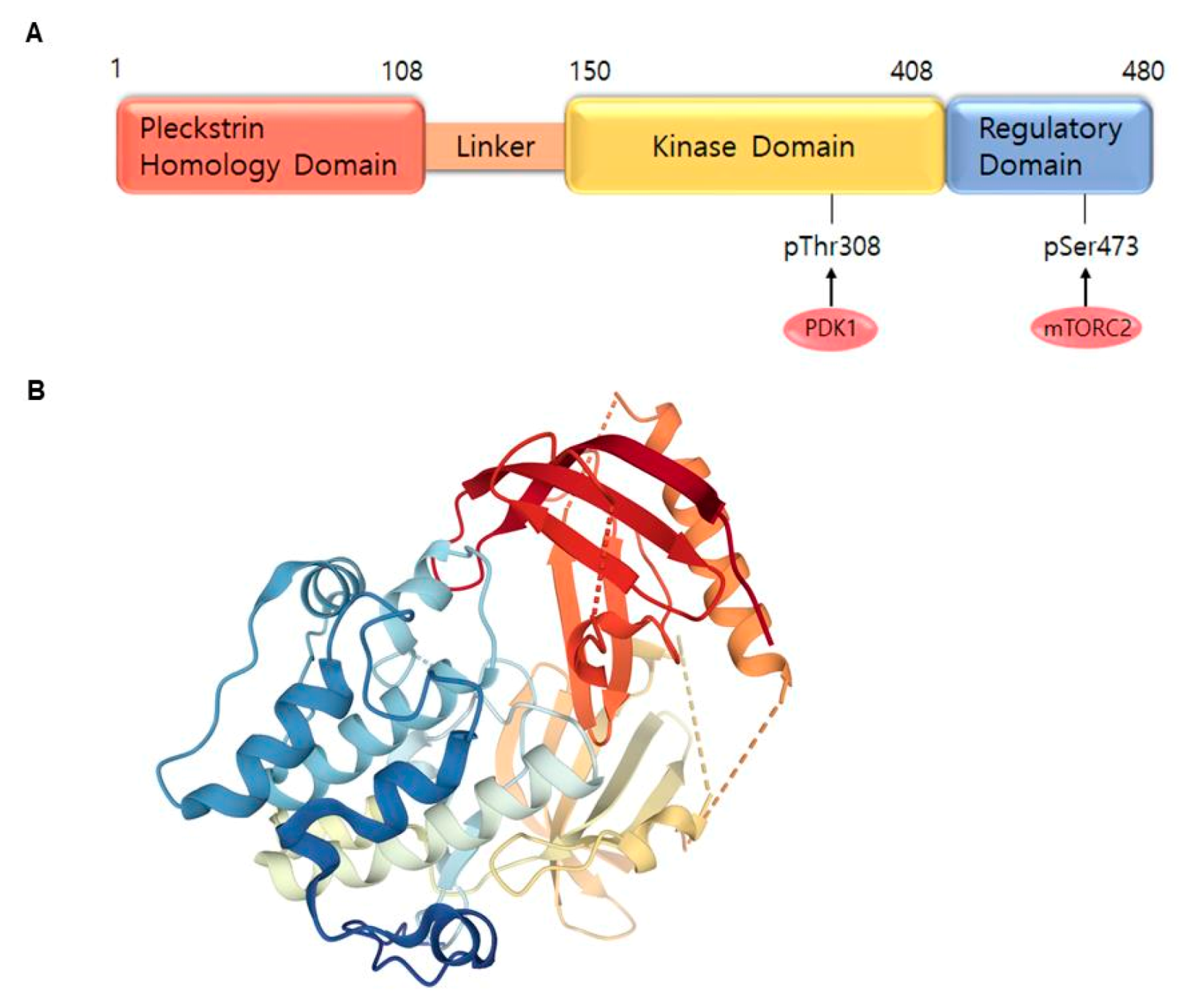
2. AKT and Related Signaling Pathways Are Important in Skin Cancer Regulation
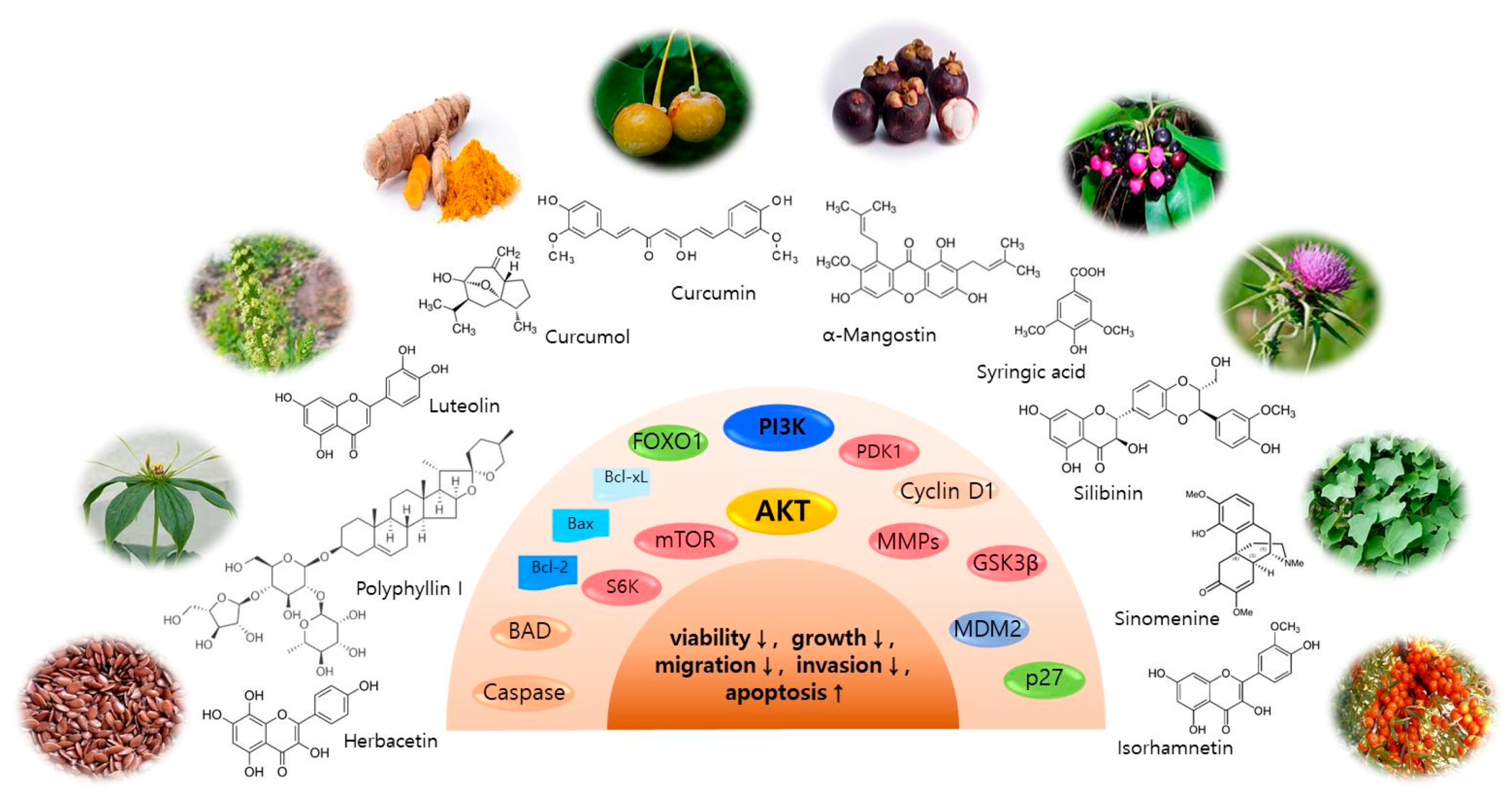
3. AKT and Related Signaling Pathway Inhibitors for Skin Cancer Regulation
3.1. Isorhamnetin
3.2. Curcumol
3.3. Polyphyllin I
3.4. Herbacetin
3.5. Luteolin
3.6. Sinomenine
3.7. Syringic Acid
3.8. Ginkgo Biloba Exocarp Extract
3.9. α-Mangostin
3.10. Silibinin (Silybin)
3.11. Curcumin
3.12. Other Bioactive Components
4. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Search Strategy and Selection Criteria
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Tran, D.A.; Coronado, A.C.; Sarker, S.; Alvi, R. Estimating the health care costs of non-melanoma skin cancer in Saskatchewan using physician billing data. Curr. Oncol. 2019, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsague, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef]
- Wrobel, S.; Przybylo, M.; Stepien, E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Khavari, P.A. Modelling cancer in human skin tissue. Nat. Rev. Cancer 2006, 6, 270–280. [Google Scholar] [CrossRef]
- Roh, E.; Lee, M.H.; Zykova, T.A.; Zhu, F.; Nadas, J.; Kim, H.G.; Bae, K.B.; Li, Y.; Cho, Y.Y.; Curiel-Lewandrowski, C.; et al. Targeting PRPK and TOPK for skin cancer prevention and therapy. Oncogene 2018, 37, 5633–5647. [Google Scholar] [CrossRef]
- Lee, M.H.; Lim, D.Y.; Kim, M.O.; Lee, S.Y.; Shin, S.H.; Kim, J.Y.; Kim, S.H.; Kim, D.J.; Jung, S.K.; Yao, K.; et al. Genetic ablation of caspase-7 promotes solar-simulated light-induced mouse skin carcinogenesis: The involvement of keratin-17. Carcinogenesis 2015, 36, 1372–1380. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, K.W. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Choi, B.Y.; Lee, M.H.; Bode, A.M.; Dong, Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16(INK4a)) in Cancer. EBioMedicine 2016, 8, 30–39. [Google Scholar] [CrossRef]
- Pal, H.C.; Hunt, K.M.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the Management of Melanoma. Mini Rev. Med. Chem. 2016, 16, 953–979. [Google Scholar] [PubMed]
- Lee, M.H.; Huang, Z.; Kim, D.J.; Kim, S.H.; Kim, M.O.; Lee, S.Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K.; et al. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev. Res. 2013, 6, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Paschou, S.A.; Ryu, H.S. The Role of Nicotinamide in Cancer Chemoprevention and Therapy. Biomolecules 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef]
- Kumar, C.C.; Madison, V. AKT crystal structure and AKT-specific inhibitors. Oncogene 2005, 24, 7493–7501. [Google Scholar] [CrossRef]
- Fayard, E.; Tintignac, L.A.; Baudry, A.; Hemmings, B.A. Protein kinase B/Akt at a glance. J. Cell Sci. 2005, 118, 5675–5678. [Google Scholar] [CrossRef]
- Song, M.; Liu, X.; Liu, K.; Zhao, R.; Huang, H.; Shi, Y.; Zhang, M.; Zhou, S.; Xie, H.; Chen, H.; et al. Targeting AKT with Oridonin Inhibits Growth of Esophageal Squamous Cell Carcinoma In Vitro and Patient-Derived Xenografts In Vivo. Mol. Cancer Ther. 2018, 17, 1540–1553. [Google Scholar] [CrossRef]
- Soares, C.D.; Borges, C.F.; Sena-Filho, M.; Almeida, O.P.; Stelini, R.F.; Cintra, M.L.; Graner, E.; Zecchin, K.G.; Jorge, J. Prognostic significance of cyclooxygenase 2 and phosphorylated Akt1 overexpression in primary nonmetastatic and metastatic cutaneous melanomas. Melanoma Res. 2017, 27, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Slipicevic, A.; Holm, R.; Nguyen, M.T.; Bohler, P.J.; Davidson, B.; Florenes, V.A. Expression of activated Akt and PTEN in malignant melanomas: Relationship with clinical outcome. Am. J. Clin. Pathol. 2005, 124, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Robinson, J.P.; Arave, R.A.; Burnett, W.J.; Kircher, D.A.; Chen, G.; Davies, M.A.; Grossmann, A.H.; VanBrocklin, M.W.; McMahon, M.; et al. AKT1 Activation Promotes Development of Melanoma Metastases. Cell Rep. 2015, 13, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Song, W.; Zhou, L.; Li, Q.; Tao, K.; Zhou, J.; Wang, X.; Zheng, Z.; You, N.; et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013, 43, 793–802. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Song, N.R.; Lee, E.; Byun, S.; Kim, J.E.; Mottamal, M.; Park, J.H.; Lim, S.S.; Bode, A.M.; Lee, H.J.; Lee, K.W.; et al. Isoangustone A, a novel licorice compound, inhibits cell proliferation by targeting PI3K, MKK4, and MKK7 in human melanoma. Cancer Prev. Res. 2013, 6, 1293–1303. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, J.E.; Lee, S.Y.; Lee, M.H.; Byun, S.; Kim, Y.A.; Lim, T.G.; Reddy, K.; Huang, Z.; Bode, A.M.; et al. The P110 subunit of PI3-K is a therapeutic target of acacetin in skin cancer. Carcinogenesis 2014, 35, 123–130. [Google Scholar] [CrossRef]
- Antony, J.; Saikia, M.; Vinod, V.; Nath, L.R.; Katiki, M.R.; Murty, M.S.; Paul, A.; Shabna, A.; Chandran, H.; Joseph, S.M.; et al. DW-F5: A novel formulation against malignant melanoma from Wrightia tinctoria. Sci. Rep. 2015, 5, 11107. [Google Scholar] [CrossRef]
- Arcidiacono, P.; Stabile, A.M.; Ragonese, F.; Pistilli, A.; Calvieri, S.; Bottoni, U.; Crisanti, A.; Spaccapelo, R.; Rende, M. Anticarcinogenic activities of sulforaphane are influenced by Nerve Growth Factor in human melanoma A375 cells. Food Chem. Toxicol. 2018, 113, 154–161. [Google Scholar] [CrossRef]
- Olas, B.; Skalski, B.; Ulanowska, K. The Anticancer Activity of Sea Buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front. Pharmacol. 2018, 9, 232. [Google Scholar] [CrossRef]
- Duan, R.; Liang, X.; Chai, B.; Zhou, Y.; Du, H.; Suo, Y.; Chen, Z.; Li, Q.; Huang, X. Isorhamnetin Induces Melanoma Cell Apoptosis via the PI3K/Akt and NF-kappaB Pathways. Biomed. Res. Int. 2020, 2020, 1057943. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Y.W.; Weng, B.X.; Li, X.K.; Yang, S.L.; Ye, F.Q. Synthesis, anti-tumor activity, and structure-activity relationships of curcumol derivatives. J. Asian Nat. Prod. Res. 2014, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ning, N.; Liu, S.; Liu, X.; Tian, Z.; Jiang, Y.; Yu, N.; Tan, B.; Feng, H.; Feng, X.; Zou, L. Curcumol inhibits the proliferation and metastasis of melanoma via the miR-152-3p/PI3K/AKT and ERK/NF-kappaB signaling pathways. J. Cancer 2020, 11, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, J.; Zhang, Z.; Zhou, Y.; Hua, Y. Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expression of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem. Biophys. Res. Commun. 2018, 497, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Li, Y.; Wang, X.; Hu, S.; Wang, H.; Shi, Q.; Wang, Y.; Yang, Y. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci. Rep. 2017, 7, 7605. [Google Scholar] [CrossRef]
- Long, J.; Pi, X. Polyphyllin I Promoted Melanoma Cells Autophagy and Apoptosis via PI3K/Akt/mTOR Signaling Pathway. Biomed. Res. Int. 2020, 2020, 5149417. [Google Scholar] [CrossRef]
- Kim, D.J.; Roh, E.; Lee, M.H.; Oi, N.; Lim, D.Y.; Kim, M.O.; Cho, Y.Y.; Pugliese, A.; Shim, J.H.; Chen, H.; et al. Herbacetin Is a Novel Allosteric Inhibitor of Ornithine Decarboxylase with Antitumor Activity. Cancer Res. 2016, 76, 1146–1157. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, M.H.; Liu, K.; Lim, D.Y.; Roh, E.; Chen, H.; Kim, S.H.; Shim, J.H.; Kim, M.O.; Li, W.; et al. Herbacetin suppresses cutaneous squamous cell carcinoma and melanoma cell growth by targeting AKT and ODC. Carcinogenesis 2017, 38, 1136–1146. [Google Scholar] [CrossRef]
- Potocnjak, I.; Simic, L.; Gobin, I.; Vukelic, I.; Domitrovic, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. In Vitro 2020, 66, 104852. [Google Scholar] [CrossRef]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z. Luteolin inhibits protein kinase C(epsilon) and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; May, B.H.; Zhang, A.L.; Guo, X.; Lu, C.; Xue, C.C.; Huang, Q. Chinese Herbal Medicines for Rheumatoid Arthritis: Text-Mining the Classical Literature for Potentially Effective Natural Products. Evid. Based Complement Alternat Med. 2020, 2020, 7531967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Tan, T.; Li, S.; Tang, S.; Chen, X. Sinomenine sensitizes human gastric cancer cells to cisplatin through negative regulation of PI3K/AKT/Wnt signaling pathway. Anticancer Drugs 2019, 30, 983–990. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, L.; Liu, X.; Xing, W.; Liu, X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des. Devel. Ther. 2018, 12, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Periyannan, V.; Annamalai, V.; Veerasamy, V. Syringic acid modulates molecular marker-involved cell proliferation, survival, apoptosis, inflammation, and angiogenesis in DMBA-induced oral squamous cell carcinoma in Syrian hamsters. J. Biochem. Mol. Toxicol. 2020, e22574. [Google Scholar] [CrossRef]
- Cho, H.D.; Kim, J.H.; Won, Y.S.; Moon, K.D.; Seo, K.I. Inhibitory Effects of Pectinase-Treated Prunus Mume Fruit Concentrate on Colorectal Cancer Proliferation and Angiogenesis of Endothelial Cells. J. Food Sci. 2019, 84, 3284–3295. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Song, K.M.; Chang, P.S.; Jeong, C.H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Cao, C.; Han, D.; Su, Y.; Ge, Y.; Chen, H.; Xu, A. Ginkgo biloba exocarp extracts induces autophagy in Lewis lung cancer cells involving AMPK/mTOR/p70S6k signaling pathway. Biomed. Pharmacother. 2017, 93, 1128–1135. [Google Scholar] [CrossRef]
- Han, D.; Cao, C.; Su, Y.; Wang, J.; Sun, J.; Chen, H.; Xu, A. Ginkgo biloba exocarp extracts inhibits angiogenesis and its effects on Wnt/beta-catenin-VEGF signaling pathway in Lewis lung cancer. J. Ethnopharmacol. 2016, 192, 406–412. [Google Scholar] [CrossRef]
- Cao, C.; Su, Y.; Gao, Y.; Luo, C.; Yin, L.; Zhao, Y.; Chen, H.; Xu, A. Ginkgo biloba Exocarp Extract Inhibits the Metastasis of B16-F10 Melanoma Involving PI3K/Akt/NF-kappaB/MMP-9 Signaling Pathway. Evid. Based Complement Alternat Med. 2018, 2018, 4969028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, Y.P.; Zhao, L.; Wang, L.; Fu, N.J.; Zheng, S.P.; Shen, X.F. Anticancer activity of dietary xanthone alpha-mangostin against hepatocellular carcinoma by inhibition of STAT3 signaling via stabilization of SHP1. Cell Death Dis. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.J.; Ying, T.H.; Hsieh, S.C.; Lin, C.L.; Yu, Y.L.; Kao, S.H.; Hsieh, Y.H. alpha-Mangostin attenuates stemness and enhances cisplatin-induced cell death in cervical cancer stem-like cells through induction of mitochondrial-mediated apoptosis. J. Cell. Physiol. 2020, 235, 5590–5601. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, H.; Liu, Z.; Huang, W.; Xu, X.; Zhang, X. alpha-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed. Pharmacother. 2017, 92, 672–680. [Google Scholar] [CrossRef]
- Sellam, L.S.; Zappasodi, R.; Chettibi, F.; Djennaoui, D.; Yahi-Ait Mesbah, N.; Amir-Tidadini, Z.C.; Touil-Boukoffa, C.; Ouahioune, W.; Merghoub, T.; Bourouba, M. Silibinin down-regulates PD-L1 expression in nasopharyngeal carcinoma by interfering with tumor cell glycolytic metabolism. Arch. Biochem. Biophys. 2020, 690, 108479. [Google Scholar] [CrossRef]
- Tilley, C.; Deep, G.; Agarwal, C.; Wempe, M.F.; Biedermann, D.; Valentova, K.; Kren, V.; Agarwal, R. Silibinin and its 2,3-dehydro-derivative inhibit basal cell carcinoma growth via suppression of mitogenic signaling and transcription factors activation. Mol. Carcinog. 2016, 55, 3–14. [Google Scholar] [CrossRef]
- Dheeraj, A.; Rigby, C.M.; O’Bryant, C.L.; Agarwal, C.; Singh, R.P.; Deep, G.; Agarwal, R. Silibinin Treatment Inhibits the Growth of Hedgehog Inhibitor-Resistant Basal Cell Carcinoma Cells via Targeting EGFR-MAPK-Akt and Hedgehog Signaling. Photochem. Photobiol. 2017, 93, 999–1007. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Mottaghi, R.; Razavi, Z.S.; Shafiee, A.; Hajighadimi, S.; Mirzaei, H. Therapeutic Applications of Curcumin and its Novel Formulations in the Treatment of Bladder Cancer: A Review of Current Evidence. Anticancer Agents Med. Chem. 2020. [Google Scholar] [CrossRef]
- Avila-Galvez, M.A.; Gimenez-Bastida, J.A.; Espin, J.C.; Gonzalez-Sarrias, A. Dietary Phenolics against Breast Cancer. A Critical Evidence-Based Review and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 5718. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Yan, F.; Liao, R.; Silva, M.; Li, S.; Jiang, Y.; Peng, T.; Lazarovici, P.; Zheng, W. Pristimerin-induced uveal melanoma cell death via inhibiting PI3K/Akt/FoxO3a signalling pathway. J. Cell. Mol. Med. 2020, 24, 6208–6219. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, Q.; Wang, X.M.; Li, G.X.; Shen, S.; Wei, X.L. Gambogic acid exhibits anti-metastatic activity on malignant melanoma mainly through inhibition of PI3K/Akt and ERK signaling pathways. Eur. J. Pharmacol. 2019, 864, 172719. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.N.; Baek, S.B.; Jung, H.J. Bee Venom and Its Peptide Component Melittin Suppress Growth and Migration of Melanoma Cells via Inhibition of PI3K/AKT/mTOR and MAPK Pathways. Molecules 2019, 24, 929. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, P.; Sun, J.; Guo, L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J. BUON 2018, 23, 218–223. [Google Scholar]
- Carpi, S.; Polini, B.; Poli, G.; Alcantara Barata, G.; Fogli, S.; Romanini, A.; Tuccinardi, T.; Guella, G.; Frontini, F.P.; Nieri, P.; et al. Anticancer Activity of Euplotin C, Isolated from the Marine Ciliate Euplotes crassus, Against Human Melanoma Cells. Mar. Drugs 2018, 16, 166. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Liu, W.B. Lycorine inhibits melanoma A375 cell growth and metastasis through the inactivation of the PI3K/AKT signaling pathway. Med. Sci. 2018, 34, 33–38. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Z.; Liu, Y.; Peng, X.; Liu, Y.; Zhu, S.; Zhang, Z.; Qiu, Y.; Jin, M.; Wang, R.; et al. Oxyfadichalcone C inhibits melanoma A375 cell proliferation and metastasis via suppressing PI3K/Akt and MAPK/ERK pathways. Life Sci. 2018, 206, 35–44. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, D.F.; Han, J.C.; Wang, B.; Dong, Z.P.; Yu, L.N.; Pan, Z.H.; Qu, C.J.; Chen, Y.; Sun, S.G.; et al. Reprogramming induced by isoliquiritigenin diminishes melanoma cachexia through mTORC2-AKT-GSK3beta signaling. Oncotarget 2017, 8, 34565–34575. [Google Scholar] [CrossRef]
- Zou, N.; Wei, Y.; Li, F.; Yang, Y.; Cheng, X.; Wang, C. The inhibitory effects of compound Muniziqi granule against B16 cells and harmine induced autophagy and apoptosis by inhibiting Akt/mTOR pathway. BMC Complement Altern Med. 2017, 17, 517. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef]
- Shih, Y.L.; Chou, H.M.; Chou, H.C.; Lu, H.F.; Chu, Y.L.; Shang, H.S.; Chung, J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-kappaB signaling pathways. Environ. Toxicol. 2017, 32, 2097–2112. [Google Scholar] [CrossRef]
- Chen, H.Y.; Jiang, Y.W.; Kuo, C.L.; Way, T.D.; Chou, Y.C.; Chang, Y.S.; Chung, J.G. Chrysin inhibit human melanoma A375.S2 cell migration and invasion via affecting MAPK signaling and NF-kappaB signaling pathway in vitro. Environ. Toxicol. 2019, 34, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Lai, K.C.; Peng, S.F.; Maraming, P.; Huang, Y.P.; Huang, A.C.; Chueh, F.S.; Huang, W.W.; Chung, J.G. Berberine Inhibits Human Melanoma A375.S2 Cell Migration and Invasion via Affecting the FAK, uPA, and NF-kappaB Signaling Pathways and Inhibits PLX4032 Resistant A375.S2 Cell Migration In Vitro. Molecules 2018, 23, 2019. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Hongbo, T.; Xu, Y.; Wu, M.; Wu, Y. Anticancer activity of caffeic acid nbutyl ester against A431 skin carcinoma cell line occurs via induction of apoptosis and inhibition of the mTOR/PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 17, 5652–5657. [Google Scholar] [PubMed]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
| Compounds | Plants | Cancer Types | Cell Lines | Mechanisms | Ref. |
|---|---|---|---|---|---|
| Isorhamnetin | Persicaria thunbergii H., Elaeagnus rhamnoides (L.) | melanoma | B16F10 | proliferation↓, migration↓ pAKT↓, PFKFB4↓ | [31] |
| Curcumol | Curcuma wenyujin | melanoma | B16F10 | viability↓, colony formation↓ migration↓, pAKT↓, c-MET↓, miR-152-3p↓ | [33] |
| Polyphyllin I | Paris polyphylla | melanoma | A375 | cells growth↓, migration↓, invasion↓, cell cycle progression↓, apoptosis↑, Bax↑, cleaved caspases-3↑, Bcl-2↓ autophagy↑, Beclin 1↑, LC3II ↑, p62 ↓ pPI3K↓, pAKT↓, pmTOR↓ | [36] |
| Herbacetin | Flaxseed, ramose scouring rush herb | SCC, melanoma | JB6, A431, SK-MEL-5, SK-MEL-28 | AKT1/2 activity↓, ODC activity↓, growth↓, neoplastic transformation↓, pGSK3β↓, ODC activity↓, AP1 activity↓, NF-κB activity↓, pERK1/2↓, p65↓ | [38] |
| melanoma | A375, Hs294T | tumor growth↓, angiogenesis↓, pEGFR↓, pAKT↓, pERK ↓ pGSK3β↓, ODC activity↓, AP1 activity↓, NF-κB activity↓ | |||
| Luteolin | Reseda luteola | melanoma | A375.S2 | proliferation↓, migration↓, invasion↓, apoptosis↑, MMP-2↓, MMP-9↓, TIMP-1↑, TIMP-2↑, pAKT1↓, pPI3K↓ | [41] |
| Sinomenine | Sinomenium acutum | melanoma | B16F10 | cell viability↓, apoptosis↑, Bax↑, Bcl-2↓, caspase-3 activity↑, autophagy↑, Beclin-1↑, LC3II/LC3I ratio↑, pp62/SQSTM1↓, pAKT↓, pmTOR↓ | [45] |
| Syringic acid | Euterpe oleracea, Rhus javanica | non-melanoma | HaCaT | UVB-induced COX-2↓, UVB-induced MMP-1↓, UVB-induced PGE2 generation↓, UVB-induced AP-1 activity↓, pERK1/2↓, pJNK1/2↓, pp38↓, pMEK1/2↓, p-MKK4/7↓, pMKK3/6↓, pB-Raf↓, pAKT↓, pSrc↓, EGFR↓, UVB-induced cyclooxygenase-2↓, matrix metalloproteinase-1↓, prostaglandin E2↓ | [48] |
| Ginkgo biloba Exocarp Extract | Ginkgo biloba L. | melanoma | B16F10 | proliferation↓, migration↓, heterogeneous adhesion↓, pPI3K↓, pAKT↓, NF-κB↓, MMP-9↓ | [51] |
| Silibinin | Milk thistle plant (Silybum marianum) | BCC | ASZ001, Sant-1, GDC-0449 resistance ASZ001 | growth↓, colony formation ↓, pEGFR↓, pAKT↓, cyclin D1↓, Gli-1↓, SMO↓, SUFU↓, apoptosis↑, caspase-3↑, Bcl-2↓ | [56] |
| Silybum marianum (L.) Gaertn., Asteraceae | BCC | ASZ, BSZ | cell growth↓, clonogenicity↓, apoptosis↑, pEGFR↓, pERK1/2↓, pAKT↓, pSTAT3↓ | [57] | |
| Curcumin | rhizome of Curcuma longa | melanoma | A375 and C8161 | proliferation↓, invasion↓, G2/M phase cell-cycle arrest↑, autophagy↑, pAKT↓, pmTORC1↓, pp70S6K↓ | [60] |
| Pristimerin | Celastraceae, Hippocrateacea | uveal melanoma | UM-1 | apoptosis↑, viability↓, colony formation↓, mitochondrial membrane potential↓, ROS level↑, G0/G1 phase arrest↑ migration↓, invasion↓ pAKT↓, pFoxO3a ↓, Bim↑, p27Kip1↑, cleaved caspase-3↑, PARP↑, Bax↑, Cyclin D1↓, Bcl-2↓ | [61] |
| Gambogic acid | resin of Garciania hanburyi | melanoma | A375, B16F10, | proliferation↓, migration↓, invasion↓, adhesion↓, EMT↓, angiogenesis processes↓ MMP-2 and MMP-9 activities↓ PI3K–AKT–mTOR signaling pathway↓ | [62] |
| Melittin/Bee Venom | honey bees (Apis mellifera) | melanoma | B16F10, A375SM, SK-MEL-28 | growth↓, colony-forming ability↓, migration↓, invasion↓, apoptosis↑, cleaved caspase-3 and -9↑, pPI3K↓, pAKT↓, mTOR↓, ERK↓, p38↓ | [63] |
| Kaempferol | piper | melanoma | A375 | proliferation↓, migration↓, colony formation↓, apoptosis↑, G2/M cell cycle arrest↑, pmTOR↓, pPI3K↓, pAKT↓ | [64] |
| Euplotin C | Euplotes crassus | melanoma | A375, 501Mel, MeWo, HDFa | viability↓, apoptosis↑, migration↓, B-Raf↓, pERK 1/2↓, pAKT↓ | [65] |
| Lycorine | Lycoris radiate spider lilies (Lycoris), daffodils (Narcissus) and snowdrops (Galanthus) | malignant melanoma | HEMa, A375 | proliferation↓, cell migration↓, invasion↓, apoptosis↑, caspase-3↑, Bax↑, Bcl-2↓, pAKT↓, pmTOR↓, 4EBP1↓ | [66] |
| Oxyfadichalcone C | Oxytropis falcate | melanoma | A375 | proliferation↓, G1 phase arrest↑, apoptosis↑, migration↓, invasion↓, p27↑, cyclin D1↓, ppRb↓, pIntegrin β1↓, MMP-2/9↓, metastasis↓, pPDK1↓, pAKT↓, pGSK-3β↓, pmTOR↓, pp70s6k↓, pERK↓ | [67] |
| Isoliquiritigenin | Glycyrrhizae Radix | melanoma | A375 | proliferation↓, G2/M cell cycle arrest↑, mTOR↓, RICTOR↓, pAKT↓, pGSK-3β↓ | [68] |
| Muniziqi granule/harmine | Peganum harmala, Cichorium intybus, Dracocephalum moldavica, Ocimum basilicum, Althaea rosea, and Nigella glandulifera | melanoma | B16F10 | proliferation↓, autophagy, autophagosome formation↑, LC3-II↑, P62↓, apoptosis↑, G1 cell cycle arrest↑, pAKT↓, pmTOR↓, pERK1/2↓ | [69] |
| Apigenin | Various fruits and vegetables | A375, C8161 | proliferation↓, migration↓, invasion↓, apoptosis↑, G2/M cell cycle arrest↑, cleaved caspase-3↑, cleaved PARP↑, pERK1/2↓, pAKT↓, pmTOR↓ | [70] | |
| Casticin | Fructus viticis | melanoma | B16F10 | migration↓, invasion↓, MMP-9↓, MMP-2↓, MMP-1↓, FAK↓, 14-3-3↓, GRB2↓, AKT↓, NF-κB↓, p65↓, SOS-1↓, p-EGFR↓, p-JNK 1/2↓, uPA↓, Rho A↓ | [71] |
| Chrysin | passionflower, silver linden, honey, propolis | melanoma | A375.S2 | mobility↓, migration↓, invasion↓, MMP-2 activity↓, GRB2↓, SOS-1↓, PKC↓, pAKT (Thr308)↓, NF-κBp65↓, NF-κBp50↓ uPA↓, N-cadherin↓, MMP-1↓, MMP-2↓, VEGF↓, E-cadherin↑, NF-κBp65↓ | [72] |
| Berberine | the roots and bark of Berberis genus | melanoma | A375.S2 | morphological changes↑, viability↓, mobility↓, migration↓, invasion↓, MMP-9 activity↓, MMP-1↓, MMP-13↓, E-cadherin↑, N-cadherin↓, RhoA↓, ROCK1↓, SOS-1↓, GRB2↓, Ras↓, pERK1/2↓, pc-Jun↓, p-FAK↓, pAKT↓, NF-κB↓, uPA↓, PKC↓, PI3K↓ | [73] |
| caffeic acid n-butyl ester | skin carcinoma | A431 | Apoptosis↑, Bax↑, Bcl-2↓, ROS↑, MMP↓, G2 phase arrest↑, migration↓, pmTOR↓, pPI3K, pAKT↓ | [74] |
| Compounds | Plants | Cancer Types | Model | Treatment | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Isorhamnetin | Hippophae rhamnoides L. | melanoma | C57BL/6 mice injected with B16F10 cells, 1 × 105 | 20 mg/kg per day; for 7 days | Proliferation↓, Ki67↓ | [31] |
| Curcumol | Curcuma wenyujin | melanoma | C57BL/6 mice injected (s.c. into the right lower paw and i.v. into the tail vein) with B16 cells, 2 × 106 | 20 mg/kg, i.p.; 3 times per week; for 30 days | proliferation↓, growth↓ invasion↓, metastasis↓ | [33] |
| Polyphyllin I | Paris polyphylla | melanoma | male BALB/c -nude mice with A375 cells, 2 × 106 | Polyphyllin I 5 mg/kg; i.p.; once a day for 35 days | tumor weight↓, tumor size↓ apoptosis↑, TUNEL positive cells↑, Ki67↓ | [36] |
| Herbacetin | Flaxseed, ramose scouring rush herb | SCC | -DMBA/TPA model; Hairless SKH:HR-1-hrBr (SKH-1) (8–9 weeks old), initiation with DMBA (200 nmol), and promotion with 17 nmol of TPA in acetone, topically applied twice weekly for 20 weeks -solar–UV induced-skin carcinogenesis model; exposed to solar–UV (48 kJ/UVA/2.9 kJ/UVB) three times weekly for 12 weeks -xenograft model; Athymic mice (Cr:NIH(S), NIH Swiss nude, 6–9-wk-old) with SK-MEL-5 cells, 3 × 106 | -DMBA/TPA model; 100 or 500 nmol of herbacetin applied to dorsal mouse skin at 30 min before TPA treatment. -solar–UV induced-skin tumor mouse model; after 20 weeks later, herbacetin 100 or 500 nmol for an additional 7 weeks -xenograft model; herbacetin 0.2 and 1 mg/kg; i.p. injected three times per week for 15 days | skin papillomas↓, tumor volume↓, Ki67↓, pAKT↓, pGSK3β↓, pRSK↓, ODC↓ | [38] |
| Luteolin | Reseda luteola | Melanoma | Female BALB/c -nude mice with A375 cells, 1 × 107 | 100 mg/kg/day, i.p. for 22 days | tumor growth↓ PI3K/AKT↓, MMP-2↓, MMP-9↓ | [41] |
| Sinomenine | Sinomenium acutum | Melanoma | xenograft model; BALB/c nude mice (6-week-old) by subcutaneously injection with B16-F10 cells | 100 mg/kg/day; s.c., daily for 35 days. | tumor weight↓, tumor volume↓, Ki67↓, PCNA↓ | [45] |
| Syringic acid | Euterpe oleracea, Rhus javanica | non-melanoma | SKH-1 hairless mouse, UVB (0.2 J/cm2) exposure (three times per week for 22 weeks) | 0.2 or 1 mM per mouse in 200 μL acetone on the dorsal surface 1 h before UVB irradiation | UVB-induced skin tumor↓, COX-2↓, MMP-13↓, | [48] |
| Ginkgo biloba Exocarp Extract | C57BL/6J female mice (6-week-old) by subcutaneously injection with B16-F10, 2.0 × 106 cells | 50, 100, 200 mg/kg by intragestic gavage, once a day for 17 days | tumor growth↓, lung metastasis↓, MMP-9↓ | [51] | ||
| α-Mangostin | pericarp of mangosteen | Skin cancer | DMBA (60 μg)/TPA (4 μg) induced skin carcinogenesis model in ICR female mice, once a week for 20 weeks | 5 and 20 mg/kg, (dissolved in 0.2 mL olive oil) once a day, starting from the day after TPA was topically applied, i.p. for 20 weeks | Skin papilloma↓, growth↓, LC3↑, LC3-II↑, Beclin1↑, LC3-I↓, p62↓, Bax↑, cleaved caspase-3↑, cleaved PARP↑, Bad↑, Bcl-2↓, Bcl-xl↓, apoptosis↑, p-PI3K↓, p-AKT↓, p-mTOR↓ | [54] |
| Silibinin and its 2,3-dehydro-derivative | Silybum marianum (L.) Gaertn., Asteraceae | BCC | ectopic allograft model; five weeks old nude mice (Foxn1nu/nu) by subcutaneously injection with 1 × 106 ASZ cells | silibinin (200 mg/kg in 0.5% CMC) or DHS (200 mg/kg); oral administration, 6 days per week for a total of 7 weeks | tumor growth↓, PCNA↓, cyclin D1↓, proliferation↓, NF-κB↓, AP-1↓, c-Fos↓ | [56] |
| Curcumin | rhizome of Curcuma longa | Melanoma | BALB/c nude female mice (6-week-old) by subcutaneously injection with A375 cells (1 × 107/mL) | 25 mg/kg by i.p. injections, every day for 3 weeks | growth↓ | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-Y.; Chae, J.-I.; Kwak, A.-W.; Lee, M.-H.; Shim, J.-H. Alternative Options for Skin Cancer Therapy via Regulation of AKT and Related Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 6869. https://doi.org/10.3390/ijms21186869
Hwang S-Y, Chae J-I, Kwak A-W, Lee M-H, Shim J-H. Alternative Options for Skin Cancer Therapy via Regulation of AKT and Related Signaling Pathways. International Journal of Molecular Sciences. 2020; 21(18):6869. https://doi.org/10.3390/ijms21186869
Chicago/Turabian StyleHwang, Sun-Young, Jung-Il Chae, Ah-Won Kwak, Mee-Hyun Lee, and Jung-Hyun Shim. 2020. "Alternative Options for Skin Cancer Therapy via Regulation of AKT and Related Signaling Pathways" International Journal of Molecular Sciences 21, no. 18: 6869. https://doi.org/10.3390/ijms21186869
APA StyleHwang, S.-Y., Chae, J.-I., Kwak, A.-W., Lee, M.-H., & Shim, J.-H. (2020). Alternative Options for Skin Cancer Therapy via Regulation of AKT and Related Signaling Pathways. International Journal of Molecular Sciences, 21(18), 6869. https://doi.org/10.3390/ijms21186869







