Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling
Abstract
1. Introduction
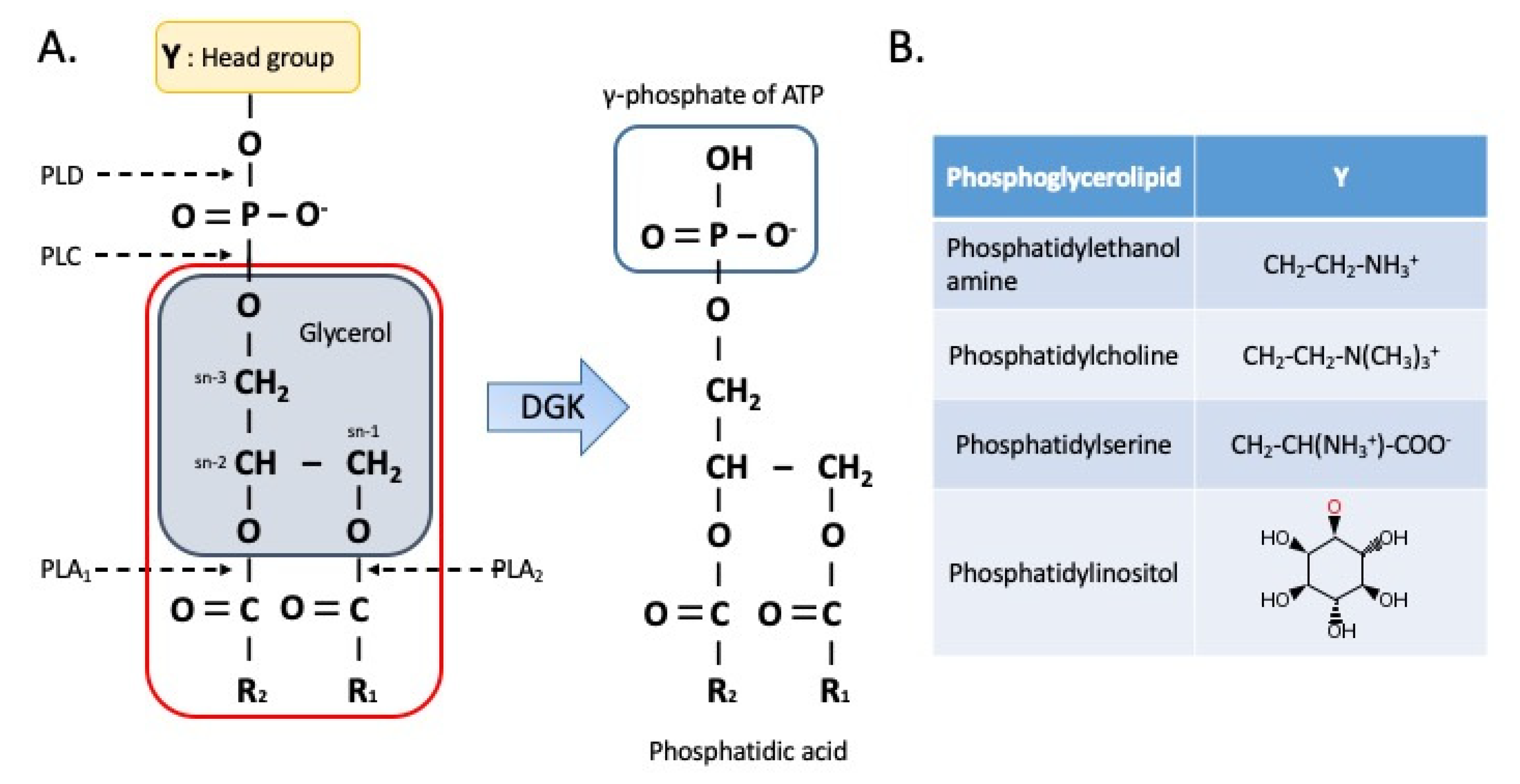
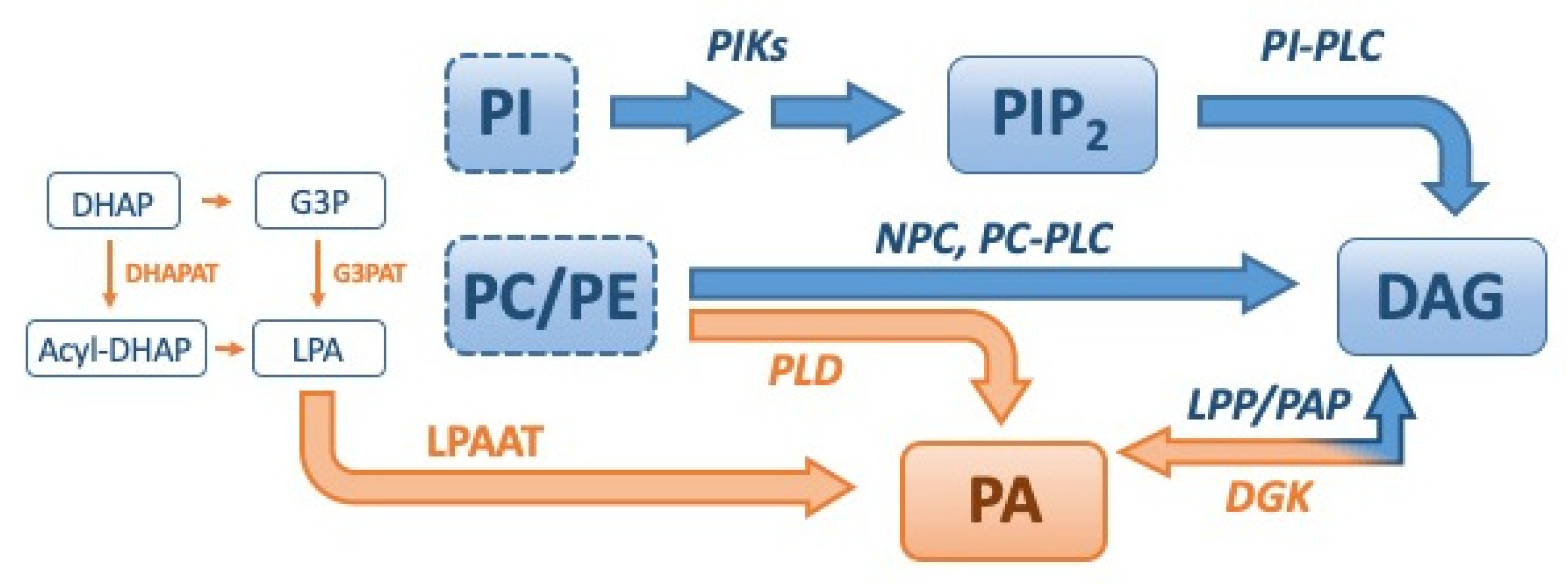
2. Regulation of DAG and PA Levels
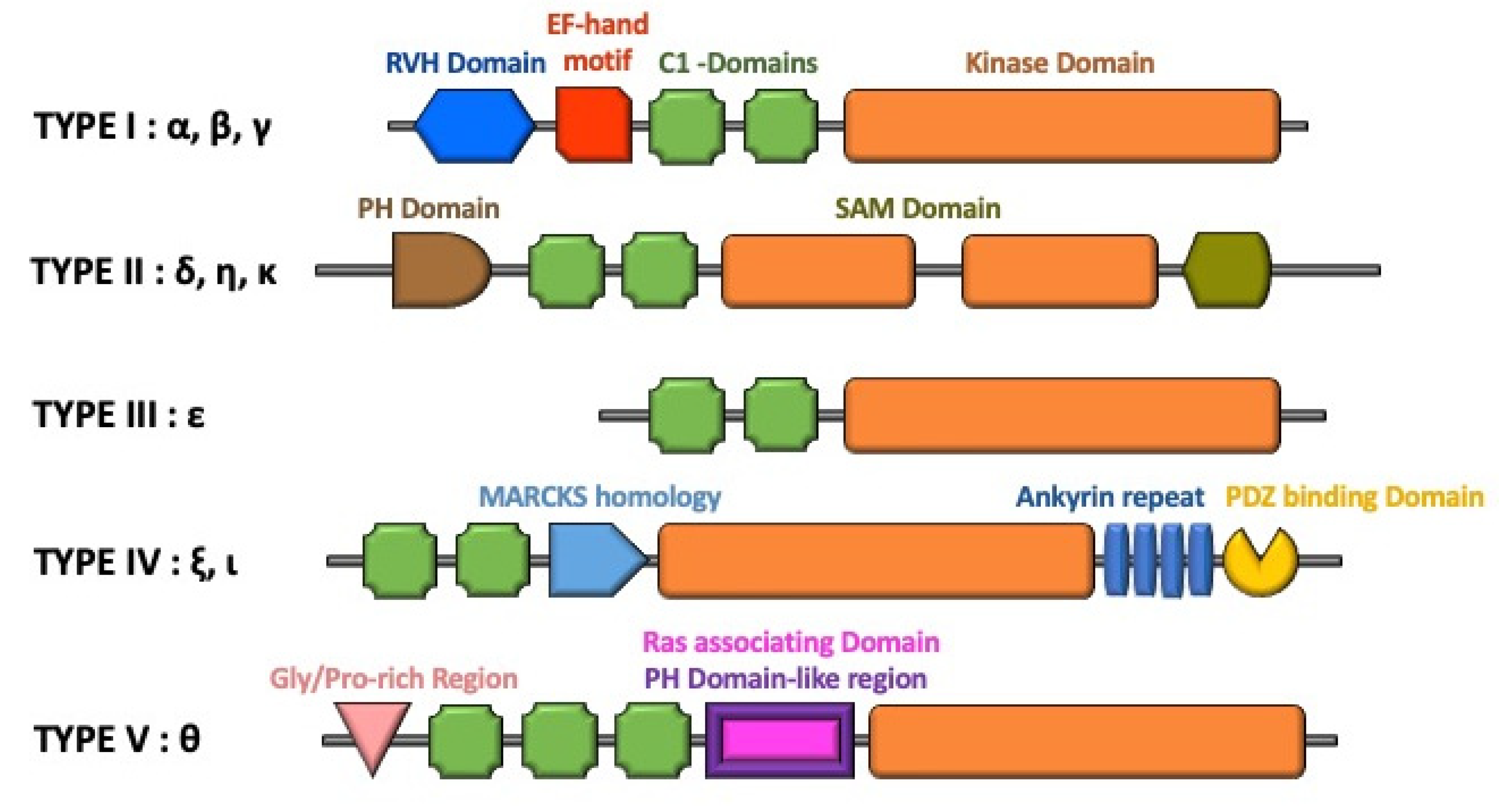
3. Regulation of DGK Isoforms
3.1. Phospholipids
3.2. Subcellular Localization
3.3. Transcriptional Control
3.4. Post-Translational Control
3.5. Inhibitors
4. Signaling Pathways Regulated by DGK Isoforms
4.1. Ras/MEK/ERK Pathway
4.2. NF-κB Signaling
4.3. Insulin Signaling
4.4. Gαq Regulation of PLC
4.5. Hypoxic Responses and Angiogenesis
4.6. Endosomal Trafficking
4.7. mTOR Signaling
4.8. Different Effects of PAs Produced by DGK and PLD
5. Pathological Manifestations
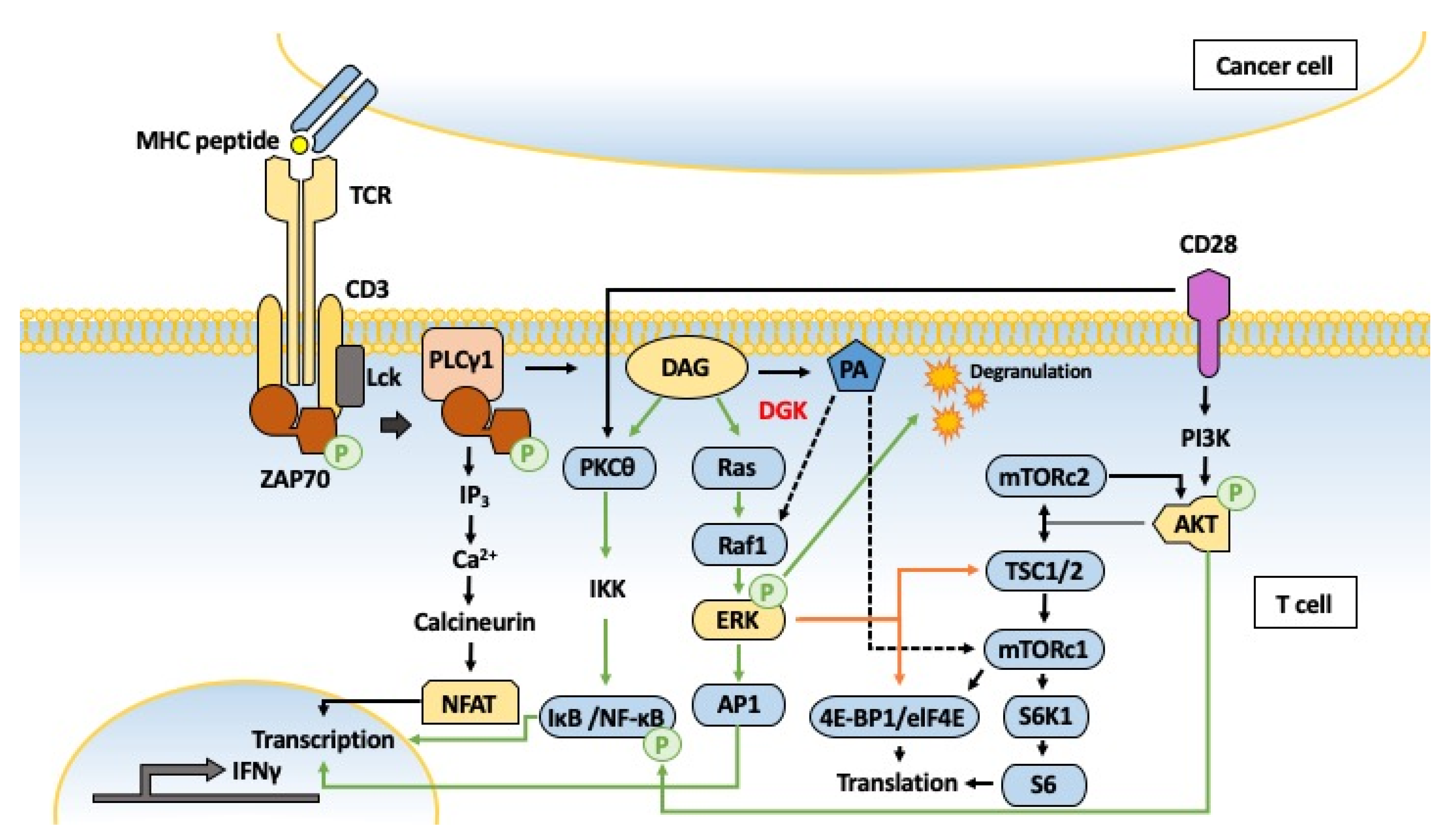
5.1. Cancer
5.2. Diabetes and Its Complications
5.3. Cognition and Mood Impairment
5.4. Inflammation and Immunity
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ER | Endoplasmic reticulum |
| fMLP | N-formylmethionine-leucyl-phenylalanine |
| GPCR | G protein-coupled receptor |
| GSK3 | Glycogen synthase kinase 3 |
| HGF | Hepatocyte growth factor |
| HIF-1 | Hypoxia-inducible factor 1 |
| IκB | Nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor |
| IP3 | Inositol 1,4,5-triphosphate |
| IQGAP | IQ motif-containing GTPase-activating protein |
| PIP2 | Phosphatidylinositol (4,5)-bisphosphate |
| PI3K | Phosphatidylinositide-3-kinase |
| PLs | Phospholipids |
| PLC | Phospholipase C |
| mGluRI | Group I metabotropic glutamate receptors |
| MHC | Major histocompatibility complex |
| MTOC | Microtubule-organizing center |
| mTORC | Mammalian target of rapamycin complex |
| MVB | Multivesicular bodies |
| NFAT | Nuclear factor of activated T cells |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| SF1 | Steroidogenic factor 1 |
| SNX27 | Sorting Nexin 27 |
| SREBP1 | Sterol regulatory element-binding protein 1 |
| TF | Transcription factor |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| TPA | 12-O-tetradecanoyl-phorbol-13-acetate |
| VEGF | Vascular endothelial growth factor |
| YAP/TAZ | Yes-associated protein and transcriptional coactivator with PDZ-binding motif |
References
- Kanoh, H.; Kondoh, H.; Ono, T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J. Biol. Chem. 1983, 258, 1767–1774. [Google Scholar] [PubMed]
- Schaap, D.; de Widt, J.; van der Wal, J.; Vandekerckhove, J.; van Damme, J.; Gussow, D.; Ploegh, H.L.; van Blitterswijk, W.J.; van der Bend, R.L. Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett. 1990, 275, 151–158. [Google Scholar] [CrossRef]
- Hart, T.C.; Champagne, C.; Zhou, J.; Van Dyke, T.E. Assignment of the gene for diacylglycerol kinase (DAGK) to human chromosome 12. Mamm. Genome 1994, 5, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Sakane, F.; Yamada, K.; Kanoh, H.; Yokoyama, C.; Tanabe, T. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 1990, 344, 345–348. [Google Scholar] [CrossRef]
- Goto, K.; Kondo, H. Molecular cloning and expression of a 90-kDa diacylglycerol kinase that predominantly localizes in neurons. Proc. Natl. Acad. Sci. USA 1993, 90, 7598–7602. [Google Scholar] [CrossRef]
- Goto, K.; Funayama, M.; Kondo, H. Cloning and expression of a cytoskeleton-associated diacylglycerol kinase that is dominantly expressed in cerebellum. Proc. Natl. Acad. Sci. USA 1994, 91, 13042–13046. [Google Scholar] [CrossRef]
- Kai, M.; Sakane, F.; Imai, S.; Wada, I.; Kanoh, H. Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J. Biol. Chem. 1994, 269, 18492–18498. [Google Scholar]
- Sakane, F.; Imai, S.; Kai, M.; Wada, I.; Kanoh, H. Molecular cloning of a novel diacylglycerol kinase isozyme with a pleckstrin homology domain and a C-terminal tail similar to those of the EPH family of protein-tyrosine kinases. J. Biol. Chem. 1996, 271, 8394–8401. [Google Scholar] [CrossRef]
- Klauck, T.M.; Xu, X.; Mousseau, B.; Jaken, S. Cloning and characterization of a glucocorticoid-induced diacylglycerol kinase. J. Biol. Chem. 1996, 271, 19781–19788. [Google Scholar] [CrossRef]
- Imai, S.; Kai, M.; Yasuda, S.; Kanoh, H.; Sakane, F. Identification and characterization of a novel human type II diacylglycerol kinase, DGK kappa. J. Biol. Chem. 2005, 280, 39870–39881. [Google Scholar] [CrossRef]
- Tang, W.; Bunting, M.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J. Biol. Chem. 1996, 271, 10237–10241. [Google Scholar] [CrossRef] [PubMed]
- Bunting, M.; Tang, W.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J. Biol. Chem. 1996, 271, 10230–10236. [Google Scholar] [CrossRef]
- Ding, L.; Traer, E.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. The cloning and characterization of a novel human diacylglycerol kinase, DGKiota. J. Biol. Chem. 1998, 273, 32746–32752. [Google Scholar] [CrossRef] [PubMed]
- Houssa, B.; Schaap, D.; van der Wal, J.; Goto, K.; Kondo, H.; Yamakawa, A.; Shibata, M.; Takenawa, T.; van Blitterswijk, W.J. Cloning of a novel human diacylglycerol kinase (DGKtheta) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J. Biol. Chem. 1997, 272, 10422–10428. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.N.; Raben, D.M. Roles of DGKs in neurons: Postsynaptic functions? Adv. Biol. Regul. 2020, 75, 100688. [Google Scholar] [CrossRef]
- Merida, I.; Arranz-Nicolas, J.; Torres-Ayuso, P.; Avila-Flores, A. Diacylglycerol Kinase Malfunction in Human Disease and the Search for Specific Inhibitors. Handb. Exp. Pharmacol. 2020, 259, 133–162. [Google Scholar] [CrossRef]
- Merida, I.; Avila-Flores, A.; Merino, E. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J. 2008, 409, 1–18. [Google Scholar] [CrossRef]
- Krishna, S.; Zhong, X.P. Regulation of Lipid Signaling by Diacylglycerol Kinases during T Cell Development and Function. Front. Immunol. 2013, 4, 178. [Google Scholar] [CrossRef]
- Sakane, F.; Mizuno, S.; Komenoi, S. Diacylglycerol Kinases as Emerging Potential Drug Targets for a Variety of Diseases: An Update. Front. Cell Dev. Biol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Sakane, F.; Yamada, K.; Imai, S.; Kanoh, H. Porcine 80-kDa diacylglycerol kinase is a calcium-binding and calcium/phospholipid-dependent enzyme and undergoes calcium-dependent translocation. J. Biol. Chem. 1991, 266, 7096–7100. [Google Scholar]
- Sanjuan, M.A.; Jones, D.R.; Izquierdo, M.; Merida, I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Epand, R.M. Phylogenetic analysis of the diacylglycerol kinase family of proteins and identification of multiple highly-specific conserved inserts and deletions within the catalytic domain that are distinctive characteristics of different classes of DGK homologs. PLoS ONE 2017, 12, e0182758. [Google Scholar] [CrossRef] [PubMed]
- Topham, M.K.; Prescott, S.M. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J. Biol. Chem. 1999, 274, 11447–11450. [Google Scholar] [CrossRef] [PubMed]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 2011, 111, 6186–6208. [Google Scholar] [CrossRef]
- Kazanietz, M.G. Novel “nonkinase” phorbol ester receptors: The C1 domain connection. Mol. Pharmacol. 2002, 61, 759–767. [Google Scholar] [CrossRef]
- Goldschmidt, H.L.; Tu-Sekine, B.; Volk, L.; Anggono, V.; Huganir, R.L.; Raben, D.M. DGKtheta Catalytic Activity Is Required for Efficient Recycling of Presynaptic Vesicles at Excitatory Synapses. Cell Rep. 2016, 14, 200–207. [Google Scholar] [CrossRef]
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108. [Google Scholar] [CrossRef]
- Ganesan, S.; Shabits, B.N.; Zaremberg, V. Tracking Diacylglycerol and Phosphatidic Acid Pools in Budding Yeast. Lipid Insights 2015, 8, 75–85. [Google Scholar] [CrossRef]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef]
- Eichmann, T.O.; Kumari, M.; Haas, J.T.; Farese, R.V., Jr.; Zimmermann, R.; Lass, A.; Zechner, R. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 2012, 287, 41446–41457. [Google Scholar] [CrossRef]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Study of arachidonoyl specificity in two enzymes of the PI cycle. J. Mol. Biol. 2011, 409, 101–112. [Google Scholar] [CrossRef]
- Goto, K.; Hozumi, Y.; Nakano, T.; Saino-Saito, S.; Martelli, A.M. Lipid messenger, diacylglycerol, and its regulator, diacylglycerol kinase, in cells, organs, and animals: History and perspective. Tohoku J. Exp. Med. 2008, 214, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 1992, 258, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Merida, I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol. Biol. Cell 2004, 15, 2932–2942. [Google Scholar] [CrossRef]
- Spitaler, M.; Emslie, E.; Wood, C.D.; Cantrell, D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 2006, 24, 535–546. [Google Scholar] [CrossRef]
- Siliceo, M.; Garcia-Bernal, D.; Carrasco, S.; Diaz-Flores, E.; Coluccio Leskow, F.; Teixido, J.; Kazanietz, M.G.; Merida, I. Beta2-chimaerin provides a diacylglycerol-dependent mechanism for regulation of adhesion and chemotaxis of T cells. J. Cell Sci. 2006, 119, 141–152. [Google Scholar] [CrossRef]
- Song, Y.; Ailenberg, M.; Silverman, M. Human munc13 is a diacylglycerol receptor that induces apoptosis and may contribute to renal cell injury in hyperglycemia. Mol. Biol. Cell 1999, 10, 1609–1619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucas, P.; Ukhanov, K.; Leinders-Zufall, T.; Zufall, F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: Mechanism of pheromone transduction. Neuron 2003, 40, 551–561. [Google Scholar] [CrossRef]
- Hall, C.; Lim, L.; Leung, T. C1, see them all. Trends Biochem. Sci. 2005, 30, 169–171. [Google Scholar] [CrossRef]
- Yang, C.; Kazanietz, M.G. Divergence and complexities in DAG signaling: Looking beyond PKC. Trends Pharmacol. Sci. 2003, 24, 602–608. [Google Scholar] [CrossRef]
- Kassas, N.; Tanguy, E.; Thahouly, T.; Fouillen, L.; Heintz, D.; Chasserot-Golaz, S.; Bader, M.F.; Grant, N.J.; Vitale, N. Comparative Characterization of Phosphatidic Acid Sensors and Their Localization during Frustrated Phagocytosis. J. Biol. Chem. 2017, 292, 4266–4279. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.S.; Hwang, D.; Ryu, S.H. Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners. Prog. Lipid Res. 2012, 51, 71–81. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Martinec, J.; Ruelland, E. The phosphatidic acid paradox: Too many actions for one molecule class? Lessons from plants. Prog. Lipid Res. 2018, 71, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.A. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007, 67, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.H.; Fisette, P.L.; Anderson, R.A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 1994, 269, 11547–11554. [Google Scholar]
- Limatola, C.; Schaap, D.; Moolenaar, W.H.; van Blitterswijk, W.J. Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: Comparison with other protein kinase C isotypes and other acidic lipids. Biochem. J. 1994, 304 Pt 3, 1001–1008. [Google Scholar] [CrossRef]
- Jones, J.A.; Hannun, Y.A. Tight binding inhibition of protein phosphatase-1 by phosphatidic acid. Specificity of inhibition by the phospholipid. J. Biol. Chem. 2002, 277, 15530–15538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, G. Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases. Biochim. Biophys. Acta 2009, 1791, 850–855. [Google Scholar] [CrossRef]
- Consonni, S.V.; Gloerich, M.; Spanjaard, E.; Bos, J.L. cAMP regulates DEP domain-mediated binding of the guanine nucleotide exchange factor Epac1 to phosphatidic acid at the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3814–3819. [Google Scholar] [CrossRef]
- Simons, M.; Gault, W.J.; Gotthardt, D.; Rohatgi, R.; Klein, T.J.; Shao, Y.; Lee, H.J.; Wu, A.L.; Fang, Y.; Satlin, L.M.; et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat. Cell Biol. 2009, 11, 286–294. [Google Scholar] [CrossRef]
- Jones, G.A.; Carpenter, G. The regulation of phospholipase C-gamma 1 by phosphatidic acid. Assessment of kinetic parameters. J. Biol. Chem. 1993, 268, 20845–20850. [Google Scholar] [PubMed]
- Wang, Z.; Zhang, F.; He, J.; Wu, P.; Tay, L.W.R.; Cai, M.; Nian, W.; Weng, Y.; Qin, L.; Chang, J.T.; et al. Binding of PLD2-Generated Phosphatidic Acid to KIF5B Promotes MT1-MMP Surface Trafficking and Lung Metastasis of Mouse Breast Cancer Cells. Dev. Cell 2017, 43, 186–197.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef]
- Thakur, R.; Naik, A.; Panda, A.; Raghu, P. Regulation of Membrane Turnover by Phosphatidic Acid: Cellular Functions and Disease Implications. Front. Cell Dev. Biol. 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Saito, N. Diacylglycerol kinase as a possible therapeutic target for neuronal diseases. J. Biomed. Sci. 2014, 21, 28. [Google Scholar] [CrossRef]
- Cipres, A.; Carrasco, S.; Merino, E.; Diaz, E.; Krishna, U.M.; Falck, J.R.; Martinez, A.C.; Merida, I. Regulation of diacylglycerol kinase alpha by phosphoinositide 3-kinase lipid products. J. Biol. Chem. 2003, 278, 35629–35635. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamamoto, T.; Sakai, H.; Sakane, F. Calcium negatively regulates an intramolecular interaction between the N-terminal recoverin homology and EF-hand motif domains and the C-terminal C1 and catalytic domains of diacylglycerol kinase alpha. Biochem. Biophys. Res. Commun. 2012, 423, 571–576. [Google Scholar] [CrossRef]
- Marumo, M.; Nakano, T.; Takeda, Y.; Goto, K.; Wakabayashi, I. Inhibition of thrombin-induced Ca(2)(+) influx in platelets by R59949, an inhibitor of diacylglycerol kinase. J. Pharm. Pharmacol. 2012, 64, 855–861. [Google Scholar] [CrossRef]
- Ostroski, M.; Tu-Sekine, B.; Raben, D.M. Analysis of a novel diacylglycerol kinase from Dictyostelium discoideum: DGKA. Biochemistry 2005, 44, 10199–10207. [Google Scholar] [CrossRef]
- Bregoli, L.; Baldassare, J.J.; Raben, D.M. Nuclear diacylglycerol kinase-theta is activated in response to alpha-thrombin. J. Biol. Chem. 2001, 276, 23288–23295. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Raben, D.M. Dual regulation of diacylglycerol kinase (DGK)-theta: Polybasic proteins promote activation by phospholipids and increase substrate affinity. J. Biol. Chem. 2012, 287, 41619–41627. [Google Scholar] [CrossRef] [PubMed]
- Houssa, B.; de Widt, J.; Kranenburg, O.; Moolenaar, W.H.; van Blitterswijk, W.J. Diacylglycerol kinase theta binds to and is negatively regulated by active RhoA. J. Biol. Chem. 1999, 274, 6820–6822. [Google Scholar] [CrossRef] [PubMed]
- Tu-Sekine, B.; Ostroski, M.; Raben, D.M. Modulation of diacylglycerol kinase theta activity by alpha-thrombin and phospholipids. Biochemistry 2007, 46, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Sakane, F.; Kanoh, H. Phorbol ester-regulated oligomerization of diacylglycerol kinase delta linked to its phosphorylation and translocation. J. Biol. Chem. 2002, 277, 35323–35332. [Google Scholar] [CrossRef]
- Imai, S.; Kai, M.; Yamada, K.; Kanoh, H.; Sakane, F. The plasma membrane translocation of diacylglycerol kinase delta1 is negatively regulated by conventional protein kinase C-dependent phosphorylation at Ser-22 and Ser-26 within the pleckstrin homology domain. Biochem. J. 2004, 382, 957–966. [Google Scholar] [CrossRef]
- Harada, B.T.; Knight, M.J.; Imai, S.; Qiao, F.; Ramachander, R.; Sawaya, M.R.; Gingery, M.; Sakane, F.; Bowie, J.U. Regulation of enzyme localization by polymerization: Polymer formation by the SAM domain of diacylglycerol kinase delta1. Structure 2008, 16, 380–387. [Google Scholar] [CrossRef]
- Shirai, Y.; Segawa, S.; Kuriyama, M.; Goto, K.; Sakai, N.; Saito, N. Subtype-specific translocation of diacylglycerol kinase alpha and gamma and its correlation with protein kinase C. J. Biol. Chem. 2000, 275, 24760–24766. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Shirai, Y.; Matsubara, T.; Sanse, K.; Kuriyama, M.; Oshiro, N.; Yoshino, K.; Yonezawa, K.; Ono, Y.; Saito, N. Phosphorylation and up-regulation of diacylglycerol kinase gamma via its interaction with protein kinase C gamma. J. Biol. Chem. 2006, 281, 31627–31637. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Pradet-Balade, B.; Jones, D.R.; Martinez, A.C.; Stone, J.C.; Garcia-Sanz, J.A.; Merida, I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: A novel mechanism for Ras attenuation. J. Immunol. 2003, 170, 2877–2883. [Google Scholar] [CrossRef]
- Baldanzi, G.; Cutrupi, S.; Chianale, F.; Gnocchi, V.; Rainero, E.; Porporato, P.; Filigheddu, N.; van Blitterswijk, W.J.; Parolini, O.; Bussolino, F.; et al. Diacylglycerol kinase-alpha phosphorylation by Src on Y335 is required for activation, membrane recruitment and Hgf-induced cell motility. Oncogene 2008, 27, 942–956. [Google Scholar] [CrossRef]
- Matsubara, T.; Ikeda, M.; Kiso, Y.; Sakuma, M.; Yoshino, K.; Sakane, F.; Merida, I.; Saito, N.; Shirai, Y. c-Abl tyrosine kinase regulates serum-induced nuclear export of diacylglycerol kinase alpha by phosphorylation at Tyr-218. J. Biol. Chem. 2012, 287, 5507–5517. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga-Takenaka, R.; Shirai, Y.; Yagi, K.; Adachi, N.; Sakai, N.; Merino, E.; Merida, I.; Saito, N. Importance of chroman ring and tyrosine phosphorylation in the subtype-specific translocation and activation of diacylglycerol kinase alpha by D-alpha-tocopherol. Genes Cells 2005, 10, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Yagi, K.; Song, C.; Ueda, S.; Yamanoue, M.; Topham, M.; Suzaki, T.; Saito, N.; Emoto, N.; Shirai, Y. Diacylglycerol Kinase alpha is Involved in the Vitamin E-Induced Amelioration of Diabetic Nephropathy in Mice. Sci Rep. 2017, 7, 2597. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Fiume, R.; Baldanzi, G.; Capello, D.; Ratti, S.; Gesi, M.; Manzoli, L.; Graziani, A.; Suh, P.G.; Cocco, L.; et al. Nuclear Localization of Diacylglycerol Kinase Alpha in K562 Cells Is Involved in Cell Cycle Progression. J. Cell. Physiol. 2017, 232, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ayuso, P.; Daza-Martin, M.; Martin-Perez, J.; Avila-Flores, A.; Merida, I. Diacylglycerol kinase alpha promotes 3D cancer cell growth and limits drug sensitivity through functional interaction with Src. Oncotarget 2014, 5, 9710–9726. [Google Scholar] [CrossRef]
- Wada, I.; Kai, M.; Imai, S.; Sakane, F.; Kanoh, H. Translocation of diacylglycerol kinase alpha to the nuclear matrix of rat thymocytes and peripheral T-lymphocytes. FEBS Lett. 1996, 393, 48–52. [Google Scholar] [CrossRef]
- Matsubara, T.; Shirai, Y.; Miyasaka, K.; Murakami, T.; Yamaguchi, Y.; Ueyama, T.; Kai, M.; Sakane, F.; Kanoh, H.; Hashimoto, T.; et al. Nuclear transportation of diacylglycerol kinase gamma and its possible function in the nucleus. J. Biol. Chem. 2006, 281, 6152–6164. [Google Scholar] [CrossRef]
- Topham, M.K.; Bunting, M.; Zimmerman, G.A.; McIntyre, T.M.; Blackshear, P.J.; Prescott, S.M. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature 1998, 394, 697–700. [Google Scholar] [CrossRef]
- Ito, T.; Hozumi, Y.; Sakane, F.; Saino-Saito, S.; Kanoh, H.; Aoyagi, M.; Kondo, H.; Goto, K. Cloning and characterization of diacylglycerol kinase iota splice variants in rat brain. J. Biol. Chem. 2004, 279, 23317–23326. [Google Scholar] [CrossRef]
- Goto, K.; Watanabe, M.; Kondo, H.; Yuasa, H.; Sakane, F.; Kanoh, H. Gene cloning, sequence, expression and in situ localization of 80 kDa diacylglycerol kinase specific to oligodendrocyte of rat brain. Brain Res. Mol. Brain Res. 1992, 16, 75–87. [Google Scholar] [CrossRef]
- Hozumi, Y.; Fukaya, M.; Adachi, N.; Saito, N.; Otani, K.; Kondo, H.; Watanabe, M.; Goto, K. Diacylglycerol kinase beta accumulates on the perisynaptic site of medium spiny neurons in the striatum. Eur. J. Neurosci. 2008, 28, 2409–2422. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sakane, F. Recent progress on type II diacylglycerol kinases: The physiological functions of diacylglycerol kinase delta, eta and kappa and their involvement in disease. J. Biochem. 2012, 152, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Shionoya, T.; Usuki, T.; Komenoi, S.; Isozaki, T.; Sakai, H.; Sakane, F. Distinct expression and localization of the type II diacylglycerol kinase isozymes delta, eta and kappa in the mouse reproductive organs. BMC Dev. Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Manneras-Holm, L.; Kirchner, H.; Bjornholm, M.; Chibalin, A.V.; Zierath, J.R. mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: Effects of obesity and insulin resistance. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Sakane, F.; Imai, S.; Yamada, K.; Murakami, T.; Tsushima, S.; Kanoh, H. Alternative splicing of the human diacylglycerol kinase delta gene generates two isoforms differing in their expression patterns and in regulatory functions. J. Biol. Chem. 2002, 277, 43519–43526. [Google Scholar] [CrossRef]
- Nagaya, H.; Wada, I.; Jia, Y.J.; Kanoh, H. Diacylglycerol kinase delta suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol. Biol. Cell 2002, 13, 302–316. [Google Scholar] [CrossRef]
- Murakami, T.; Sakane, F.; Imai, S.; Houkin, K.; Kanoh, H. Identification and characterization of two splice variants of human diacylglycerol kinase eta. J. Biol. Chem. 2003, 278, 34364–34372. [Google Scholar] [CrossRef]
- Rodriguez de Turco, E.B.; Tang, W.; Topham, M.K.; Sakane, F.; Marcheselli, V.L.; Chen, C.; Taketomi, A.; Prescott, S.M.; Bazan, N.G. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 4740–4745. [Google Scholar] [CrossRef]
- Matsui, H.; Hozumi, Y.; Tanaka, T.; Okada, M.; Nakano, T.; Suzuki, Y.; Iseki, K.; Kakehata, S.; Topham, M.K.; Goto, K. Role of the N-terminal hydrophobic residues of DGKepsilon in targeting the endoplasmic reticulum. Biochim. Biophys. Acta 2014, 1842, 1440–1450. [Google Scholar] [CrossRef]
- Saino-Saito, S.; Hozumi, Y.; Goto, K. Excitotoxicity by kainate-induced seizure causes diacylglycerol kinase zeta to shuttle from the nucleus to the cytoplasm in hippocampal neurons. Neurosci. Lett. 2011, 494, 185–189. [Google Scholar] [CrossRef]
- Hozumi, Y.; Ito, T.; Nakano, T.; Nakagawa, T.; Aoyagi, M.; Kondo, H.; Goto, K. Nuclear localization of diacylglycerol kinase zeta in neurons. Eur. J. Neurosci. 2003, 18, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, Y.; Matsui, H.; Sakane, F.; Watanabe, M.; Goto, K. Distinct expression and localization of diacylglycerol kinase isozymes in rat retina. J. Histochem. Cytochem. 2013, 61, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Draeger, A.; Houssa, B.; van Blitterswijk, W.J.; Ohanian, V.; Ohanian, J. Diacylglycerol kinase theta is translocated and phosphoinositide 3-kinase-dependently activated by noradrenaline but not angiotensin II in intact small arteries. Biochem. J. 2001, 353, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.P.; Koretzky, G.A. Diacylglycerol kinases: Regulated controllers of T cell activation, function, and development. Int. J. Mol. Sci. 2013, 14, 6649–6673. [Google Scholar] [CrossRef]
- Zheng, Y.; Zha, Y.; Driessens, G.; Locke, F.; Gajewski, T.F. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J. Exp. Med. 2012, 209, 2157–2163. [Google Scholar] [CrossRef]
- Zha, Y.; Marks, R.; Ho, A.W.; Peterson, A.C.; Janardhan, S.; Brown, I.; Praveen, K.; Stang, S.; Stone, J.C.; Gajewski, T.F. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 2006, 7, 1166–1173. [Google Scholar] [CrossRef]
- Zheng, Y.; Zha, Y.; Spaapen, R.M.; Mathew, R.; Barr, K.; Bendelac, A.; Gajewski, T.F. Egr2-dependent gene expression profiling and ChIP-Seq reveal novel biologic targets in T cell anergy. Mol. Immunol. 2013, 55, 283–291. [Google Scholar] [CrossRef]
- Luo, B.; Prescott, S.M.; Topham, M.K. Association of diacylglycerol kinase zeta with protein kinase C alpha: Spatial regulation of diacylglycerol signaling. J. Cell Biol. 2003, 160, 929–937. [Google Scholar] [CrossRef]
- Clarke, C.J.; Ohanian, V.; Ohanian, J. Norepinephrine and endothelin activate diacylglycerol kinases in caveolae/rafts of rat mesenteric arteries: Agonist-specific role of PI3-kinase. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2248–H2256. [Google Scholar] [CrossRef]
- Cai, K.; Sewer, M.B. cAMP-stimulated transcription of DGKtheta requires steroidogenic factor 1 and sterol regulatory element binding protein 1. J. Lipid Res. 2013, 54, 2121–2132. [Google Scholar] [CrossRef]
- Boroda, S.; Niccum, M.; Raje, V.; Purow, B.W.; Harris, T.E. Dual activities of ritanserin and R59022 as DGKalpha inhibitors and serotonin receptor antagonists. Biochem. Pharmacol. 2017, 123, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Liu, K.; Sasaki, S.; Kunii, N.; Sakai, H.; Mizuno, H.; Saga, H.; Sakane, F. Evaluations of the selectivities of the diacylglycerol kinase inhibitors R59022 and R59949 among diacylglycerol kinase isozymes using a new non-radioactive assay method. Pharmacology 2013, 92, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kunii, N.; Sakuma, M.; Yamaki, A.; Mizuno, S.; Sato, M.; Sakai, H.; Kado, S.; Kumagai, K.; Kojima, H.; et al. A novel diacylglycerol kinase alpha-selective inhibitor, CU-3, induces cancer cell apoptosis and enhances immune response. J. Lipid Res. 2016, 57, 368–379. [Google Scholar] [CrossRef]
- Suire, S.; Lecureuil, C.; Anderson, K.E.; Damoulakis, G.; Niewczas, I.; Davidson, K.; Guillou, H.; Pan, D.; Jonathan, C.; Phillip, T.H.; et al. GPCR activation of Ras and PI3Kc in neutrophils depends on PLCb2/b3 and the RasGEF RasGRP4. EMBO J. 2012, 31, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Velnati, S.; Ruffo, E.; Massarotti, A.; Talmon, M.; Varma, K.S.S.; Gesu, A.; Fresu, L.G.; Snow, A.L.; Bertoni, A.; Capello, D.; et al. Identification of a novel DGKalpha inhibitor for XLP-1 therapy by virtual screening. Eur. J. Med. Chem. 2019, 164, 378–390. [Google Scholar] [CrossRef]
- Franks, C.E.; Campbell, S.T.; Purow, B.W.; Harris, T.E.; Hsu, K.L. The Ligand Binding Landscape of Diacylglycerol Kinases. Cell Chem. Biol. 2017, 24, 870–880. [Google Scholar] [CrossRef]
- Takahashi, D.; Suzuki, K.; Sakamoto, T.; Iwamoto, T.; Murata, T.; Sakane, F. Crystal structure and calcium-induced conformational changes of diacylglycerol kinase alpha EF-hand domains. Protein Sci. A Publ. Protein Soc. 2019, 28, 694–706. [Google Scholar] [CrossRef]
- Zhong, X.P.; Hainey, E.A.; Olenchock, B.A.; Jordan, M.S.; Maltzman, J.S.; Nichols, K.E.; Shen, H.; Koretzky, G.A. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 2003, 4, 882–890. [Google Scholar] [CrossRef]
- Guo, R.; Wan, C.K.; Carpenter, J.H.; Mousallem, T.; Boustany, R.M.; Kuan, C.T.; Burks, A.W.; Zhong, X.P. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc. Natl. Acad. Sci. USA 2008, 105, 11909–11914. [Google Scholar] [CrossRef]
- Yang, E.; Singh, B.K.; Paustian, A.M.; Kambayashi, T. Diacylglycerol Kinase zeta Is a Target To Enhance NK Cell Function. J. Immunol. 2016, 197, 934–941. [Google Scholar] [CrossRef]
- Macian, F.; Garcia-Cozar, F.; Im, S.H.; Horton, H.F.; Byrne, M.C.; Rao, A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell 2002, 109, 719–731. [Google Scholar] [CrossRef]
- Merida, I.; Andrada, E.; Gharbi, S.I.; Avila-Flores, A. Redundant and specialized roles for diacylglycerol kinases alpha and zeta in the control of T cell functions. Sci. Signal. 2015, 8, re6. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moreno, M.; Garcia-Lievana, J.; Soutar, D.; Torres-Ayuso, P.; Andrada, E.; Zhong, X.P.; Koretzky, G.A.; Merida, I.; Avila-Flores, A. FoxO-dependent regulation of diacylglycerol kinase alpha gene expression. Mol. Cell. Biol. 2012, 32, 4168–4180. [Google Scholar] [CrossRef] [PubMed]
- Quann, E.J.; Merino, E.; Furuta, T.; Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 2009, 10, 627–635. [Google Scholar] [CrossRef]
- Takeishi, K.; Taketomi, A.; Shirabe, K.; Toshima, T.; Motomura, T.; Ikegami, T.; Yoshizumi, T.; Sakane, F.; Maehara, Y. Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEK-ERK pathway. J. Hepatol. 2012, 57, 77–83. [Google Scholar] [CrossRef]
- Yasuda, S.; Kai, M.; Imai, S.; Takeishi, K.; Taketomi, A.; Toyota, M.; Kanoh, H.; Sakane, F. Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J. Biol. Chem. 2009, 284, 29559–29570. [Google Scholar] [CrossRef]
- Crotty, T.; Cai, J.; Sakane, F.; Taketomi, A.; Prescott, S.M.; Topham, M.K. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 15485–15490. [Google Scholar] [CrossRef]
- Cai, J.; Crotty, T.M.; Reichert, E.; Carraway, K.L., 3rd; Stafforini, D.M.; Topham, M.K. Diacylglycerol kinase delta and protein kinase C(alpha) modulate epidermal growth factor receptor abundance and degradation through ubiquitin-specific protease 8. J. Biol. Chem. 2010, 285, 6952–6959. [Google Scholar] [CrossRef]
- Regier, D.S.; Higbee, J.; Lund, K.M.; Sakane, F.; Prescott, S.M.; Topham, M.K. Diacylglycerol kinase iota regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 7595–7600. [Google Scholar] [CrossRef]
- Tsushima, S.; Kai, M.; Yamada, K.; Imai, S.; Houkin, K.; Kanoh, H.; Sakane, F. Diacylglycerol kinase gamma serves as an upstream suppressor of Rac1 and lamellipodium formation. J. Biol. Chem. 2004, 279, 28603–28613. [Google Scholar] [CrossRef]
- Kim, J. Regulation of Immune Cell Functions by Metabolic Reprogramming. J. Immunol. Res. 2018, 2018, 8605471. [Google Scholar] [CrossRef] [PubMed]
- Noessner, E. DGK-alpha: A Checkpoint in Cancer-Mediated Immuno-Inhibition and Target for Immunotherapy. Front. Cell Dev. Biol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.U.; Mendler, A.N.; Masouris, I.; Durner, L.; Oberneder, R.; Noessner, E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J. Immunol. 2012, 188, 5990–6000. [Google Scholar] [CrossRef]
- Singh, B.K.; Kambayashi, T. The Immunomodulatory Functions of Diacylglycerol Kinase zeta. Front. Cell Dev. Biol. 2016, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Okada, M.; Hozumi, Y.; Tachibana, K.; Kitanaka, C.; Hamamoto, Y.; Martelli, A.M.; Topham, M.K.; Iino, M.; Goto, K. Cytoplasmic localization of DGKzeta exerts a protective effect against p53-mediated cytotoxicity. J. Cell Sci. 2013, 126, 2785–2797. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Tanaka, T.; Hozumi, Y.; Nakano, T.; Okada, M.; Topham, M.K.; Iino, M.; Goto, K. Downregulation of diacylglycerol kinase zeta enhances activation of cytokine-induced NF-kappaB signaling pathway. Biochim. Biophys. Acta 2015, 1853, 361–369. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsuchiya, R.; Hozumi, Y.; Nakano, T.; Okada, M.; Goto, K. Reciprocal regulation of p53 and NF-kappaB by diacylglycerol kinase zeta. Adv. Biol. Regul. 2016, 60, 15–21. [Google Scholar] [CrossRef]
- Sano, H.; Kane, S.; Sano, E.; Miinea, C.P.; Asara, J.M.; Lane, W.S.; Garner, C.W.; Lienhard, G.E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003, 278, 14599–14602. [Google Scholar] [CrossRef]
- Nagle, C.A.; An, J.; Shiota, M.; Torres, T.P.; Cline, G.W.; Liu, Z.X.; Wang, S.; Catlin, R.L.; Shulman, G.I.; Newgard, C.B.; et al. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J. Biol. Chem. 2007, 282, 14807–14815. [Google Scholar] [CrossRef]
- Turinsky, J.; O’Sullivan, D.M.; Bayly, B.P. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J. Biol. Chem. 1990, 265, 16880–16885. [Google Scholar]
- Monetti, M.; Levin, M.C.; Watt, M.J.; Sajan, M.P.; Marmor, S.; Hubbard, B.K.; Stevens, R.D.; Bain, J.R.; Newgard, C.B.; Farese, R.V., Sr.; et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kumashiro, N.; Erion, D.M.; Zhang, D.; Kahn, M.; Beddow, S.A.; Chu, X.; Still, C.D.; Gerhard, G.S.; Han, X.; Dziura, J.; et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2011, 108, 16381–16385. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Yerges-Armstrong, L.M.; Horenstein, R.B.; Pollin, T.I.; Sreenivasan, U.T.; Chai, S.; Blaner, W.S.; Snitker, S.; O’Connell, J.R.; Gong, D.W.; et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N. Engl. J. Med. 2014, 370, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Duncan, R.E.; Varady, K.A.; Frasson, D.; Hellerstein, M.K.; Birkenfeld, A.L.; Samuel, V.T.; Shulman, G.I.; Wang, Y.; Kang, C.; et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 2009, 58, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Miele, C.; Paturzo, F.; Teperino, R.; Sakane, F.; Fiory, F.; Oriente, F.; Ungaro, P.; Valentino, R.; Beguinot, F.; Formisano, P. Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J. Biol. Chem. 2007, 282, 31835–31843. [Google Scholar] [CrossRef]
- Litosch, I. Decoding Galphaq signaling. Life Sci 2016, 152, 99–106. [Google Scholar] [CrossRef]
- Litosch, I. Negative feedback regulation of Gq signaling by protein kinase C is disrupted by diacylglycerol kinase zeta in COS-7 cells. Biochem. Biophys. Res. Commun. 2012, 417, 956–960. [Google Scholar] [CrossRef]
- Temes, E.; Martin-Puig, S.; Aragones, J.; Jones, D.R.; Olmos, G.; Merida, I.; Landazuri, M.O. Role of diacylglycerol induced by hypoxia in the regulation of HIF-1alpha activity. Biochem. Biophys. Res. Commun. 2004, 315, 44–50. [Google Scholar] [CrossRef]
- Temes, E.; Martin-Puig, S.; Acosta-Iborra, B.; Castellanos, M.C.; Feijoo-Cuaresma, M.; Olmos, G.; Aragones, J.; Landazuri, M.O. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J. Biol. Chem. 2005, 280, 24238–24244. [Google Scholar] [CrossRef]
- Baldanzi, G.; Mitola, S.; Cutrupi, S.; Filigheddu, N.; van Blitterswijk, W.J.; Sinigaglia, F.; Bussolino, F.; Graziani, A. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene 2004, 23, 4828–4838. [Google Scholar] [CrossRef]
- Shimomura, T.; Nakano, T.; Goto, K.; Wakabayashi, I. R59949, a diacylglycerol kinase inhibitor, inhibits inducible nitric oxide production through decreasing transplasmalemmal L-arginine uptake in vascular smooth muscle cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, Y.; Li, W.; Yang, B.; Yu, S.; Zhou, H.; Yang, C.; Xu, F.; Wang, J.; Gao, Y.; et al. Diacylglycerol kinase (DGK) inhibitor II (R59949) could suppress retinal neovascularization and protect retinal astrocytes in an oxygen-induced retinopathy model. J. Mol. Neurosci. 2015, 56, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodnia, L.; Aghadavod, E.; Beigrezaei, S.; Rafieian-Kopaei, M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J. Renal Inj. Prev. 2017, 6, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Naslavsky, N.; Caplan, S. Diacylglycerol kinases in membrane trafficking. Cell. Logist. 2015, 5, e1078431. [Google Scholar] [CrossRef]
- Alonso, R.; Mazzeo, C.; Rodriguez, M.C.; Marsh, M.; Fraile-Ramos, A.; Calvo, V.; Avila-Flores, A.; Merida, I.; Izquierdo, M. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011, 18, 1161–1173. [Google Scholar] [CrossRef]
- Xie, S.; Naslavsky, N.; Caplan, S. Diacylglycerol kinase alpha regulates tubular recycling endosome biogenesis and major histocompatibility complex class I recycling. J. Biol. Chem. 2014, 289, 31914–31926. [Google Scholar] [CrossRef]
- Rainero, E.; Caswell, P.T.; Muller, P.A.; Grindlay, J.; McCaffrey, M.W.; Zhang, Q.; Wakelam, M.J.; Vousden, K.H.; Graziani, A.; Norman, J.C. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 2012, 196, 277–295. [Google Scholar] [CrossRef]
- Alonso, R.; Rodriguez, M.C.; Pindado, J.; Merino, E.; Merida, I.; Izquierdo, M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J. Biol. Chem. 2005, 280, 28439–28450. [Google Scholar] [CrossRef]
- Diaz Anel, A.M.; Malhotra, V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 2005, 169, 83–91. [Google Scholar] [CrossRef]
- Wang, Q.J. PKD at the crossroads of DAG and PKC signaling. Trends Pharm. Sci. 2006, 27, 317–323. [Google Scholar] [CrossRef]
- Lopez, C.I.; Pelletan, L.E.; Suhaiman, L.; De Blas, G.A.; Vitale, N.; Mayorga, L.S.; Belmonte, S.A. Diacylglycerol stimulates acrosomal exocytosis by feeding into a PKC- and PLD1-dependent positive loop that continuously supplies phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2012, 1821, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yang, D.M.; Lin, C.C.; Kao, L.S. Involvement of Rab3A in vesicle priming during exocytosis: Interaction with Munc13-1 and Munc18-1. Traffic 2011, 12, 1356–1370. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, T.; Luini, A.; Malhotra, V.; Burger, K.N.; Kozlov, M.M. Prefission constriction of Golgi tubular carriers driven by local lipid metabolism: A theoretical model. Biophys. J. 2003, 85, 3813–3827. [Google Scholar] [CrossRef]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Merida, I.; Arranz-Nicolas, J.; Rodriguez-Rodriguez, C.; Avila-Flores, A. Diacylglycerol kinase control of protein kinase C. Biochem. J. 2019, 476, 1205–1219. [Google Scholar] [CrossRef]
- Okada, M.; Hozumi, Y.; Iwazaki, K.; Misaki, K.; Yanagida, M.; Araki, Y.; Watanabe, T.; Yagisawa, H.; Topham, M.K.; Kaibuchi, K.; et al. DGKzeta is involved in LPS-activated phagocytosis through IQGAP1/Rac1 pathway. Biochem. Biophys. Res. Commun. 2012, 420, 479–484. [Google Scholar] [CrossRef]
- You, J.S.; Lincoln, H.C.; Kim, C.R.; Frey, J.W.; Goodman, C.A.; Zhong, X.P.; Hornberger, T.A. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J. Biol. Chem. 2014, 289, 1551–1563. [Google Scholar] [CrossRef]
- Winter, J.N.; Fox, T.E.; Kester, M.; Jefferson, L.S.; Kimball, S.R. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am. J. Physiol. Cell Physiol. 2010, 299, C335–C344. [Google Scholar] [CrossRef]
- Yoon, M.S.; Sun, Y.; Arauz, E.; Jiang, Y.; Chen, J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J. Biol. Chem. 2011, 286, 29568–29574. [Google Scholar] [CrossRef]
- Gorentla, B.K.; Wan, C.K.; Zhong, X.P. Negative regulation of mTOR activation by diacylglycerol kinases. Blood 2011, 117, 4022–4031. [Google Scholar] [CrossRef]
- Pettitt, T.R.; McDermott, M.; Saqib, K.M.; Shimwell, N.; Wakelam, M.J. Phospholipase D1b and D2a generate structurally identical phosphatidic acid species in mammalian cells. Biochem. J. 2001, 360, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Pettitt, T.R.; Powell, W.; Grove, J.; Savage, C.O.; Wakelam, M.J. Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J. Am. Soc. Nephrol. 2007, 18, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Holden, N.J.; Savage, C.O.; Young, S.P.; Wakelam, M.J.; Harper, L.; Williams, J.M. A dual role for diacylglycerol kinase generated phosphatidic acid in autoantibody-induced neutrophil exocytosis. Mol. Med. 2011, 17, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Qi, R.; Zhou, J.J.; Ta, A.P.; Yang, B.; Nakaoka, H.J.; Seo, G.; Guan, K.L.; Luo, R.; Wang, W. Regulation of the Hippo Pathway by Phosphatidic Acid-Mediated Lipid-Protein Interaction. Mol. Cell 2018, 72, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Hong, A.W.; Plouffe, S.W.; Zhao, B.; Liu, G.; Yu, F.X.; Xu, Y.; Guan, K.L. Opposing roles of conventional and novel PKC isoforms in Hippo-YAP pathway regulation. Cell Res. 2015, 25, 985–988. [Google Scholar] [CrossRef]
- Goto, K.; Nakano, T.; Hozumi, Y. Diacylglycerol kinase and animal models: The pathophysiological roles in the brain and heart. Adv. Enzym. Regul. 2006, 46, 192–202. [Google Scholar] [CrossRef]
- Chibalin, A.V.; Leng, Y.; Vieira, E.; Krook, A.; Bjornholm, M.; Long, Y.C.; Kotova, O.; Zhong, Z.; Sakane, F.; Steiler, T.; et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008, 132, 375–386. [Google Scholar] [CrossRef]
- Tabet, R.; Moutin, E.; Becker, J.A.; Heintz, D.; Fouillen, L.; Flatter, E.; Krezel, W.; Alunni, V.; Koebel, P.; Dembele, D.; et al. Fragile X Mental Retardation Protein (FMRP) controls diacylglycerol kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E3619–E3628. [Google Scholar] [CrossRef]
- Bisio, H.; Lunghi, M.; Brochet, M.; Soldati-Favre, D. Phosphatidic acid governs natural egress in Toxoplasma gondii via a guanylate cyclase receptor platform. Nat. Microbiol. 2019, 4, 420–428. [Google Scholar] [CrossRef]
- Van der Zanden, L.F.; van Rooij, I.A.; Feitz, W.F.; Knight, J.; Donders, A.R.; Renkema, K.Y.; Bongers, E.M.; Vermeulen, S.H.; Kiemeney, L.A.; Veltman, J.A.; et al. Common variants in DGKK are strongly associated with risk of hypospadias. Nat. Genet. 2011, 43, 48–50. [Google Scholar] [CrossRef]
- Lemaire, M.; Fremeaux-Bacchi, V.; Schaefer, F.; Choi, M.; Tang, W.H.; Le Quintrec, M.; Fakhouri, F.; Taque, S.; Nobili, F.; Martinez, F.; et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 2013, 45, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chaki, M.; Lu, D.; Ren, C.; Wang, S.S.; Rauhauser, A.; Li, B.; Zimmerman, S.; Jun, B.; Du, Y.; et al. Loss of diacylglycerol kinase epsilon in mice causes endothelial distress and impairs glomerular Cox-2 and PGE2 production. Am. J. Physiol. Ren. Physiol. 2016, 310, F895–F908. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.L.; Floyd, D.H.; Xiao, A.; Mullins, G.R.; Kefas, B.A.; Xin, W.; Yacur, M.N.; Abounader, R.; Lee, J.K.; Wilson, G.M.; et al. Diacylglycerol kinase alpha is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 2013, 3, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Purow, B. Molecular Pathways: Targeting Diacylglycerol Kinase Alpha in Cancer. Clin. Cancer Res. 2015, 21, 5008–5012. [Google Scholar] [CrossRef] [PubMed]
- Weigel, C.; Veldwijk, M.R.; Oakes, C.C.; Seibold, P.; Slynko, A.; Liesenfeld, D.B.; Rabionet, M.; Hanke, S.A.; Wenz, F.; Sperk, E.; et al. Epigenetic regulation of diacylglycerol kinase alpha promotes radiation-induced fibrosis. Nat. Commun. 2016, 7, 10893. [Google Scholar] [CrossRef]
- McMurray, H.R.; Sampson, E.R.; Compitello, G.; Kinsey, C.; Newman, L.; Smith, B.; Chen, S.R.; Klebanov, L.; Salzman, P.; Yakovlev, A.; et al. Synergistic response to oncogenic mutations defines gene class critical to cancer phenotype. Nature 2008, 453, 1112–1116. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.; Jia, Y.; Li, P.; Wang, Y. Decreased LIPF expression is correlated with DGKA and predicts poor outcome of gastric cancer. Oncol. Rep. 2016, 36, 1852–1860. [Google Scholar] [CrossRef]
- Berrar, D.; Sturgeon, B.; Bradbury, I.; Downes, C.S.; Dubitzky, W. Survival trees for analyzing clinical outcome in lung adenocarcinomas based on gene expression profiles: Identification of neogenin and diacylglycerol kinase alpha expression as critical factors. J. Comput. Biol. 2005, 12, 534–544. [Google Scholar] [CrossRef]
- Merida, I.; Torres-Ayuso, P.; Avila-Flores, A.; Arranz-Nicolas, J.; Andrada, E.; Tello-Lafoz, M.; Liebana, R.; Arcos, R. Diacylglycerol kinases in cancer. Adv. Biol. Regul. 2017, 63, 22–31. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Yasuda, S.; Kai, M.; Imai, S.; Yamada, K.; Yamashita, T.; Jimbow, K.; Kanoh, H.; Sakane, F. Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim. Biophys. Acta 2007, 1771, 462–474. [Google Scholar] [CrossRef]
- Kai, M.; Yamamoto, E.; Sato, A.; Yamano, H.O.; Niinuma, T.; Kitajima, H.; Harada, T.; Aoki, H.; Maruyama, R.; Toyota, M.; et al. Epigenetic silencing of diacylglycerol kinase gamma in colorectal cancer. Mol. Carcinog. 2017, 56, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Baldanzi, G.; Pietronave, S.; Locarno, D.; Merlin, S.; Porporato, P.; Chianale, F.; Filigheddu, N.; Cantelmo, A.R.; Albini, A.; Graziani, A.; et al. Diacylglycerol kinases are essential for hepatocyte growth factor-dependent proliferation and motility of Kaposi’s sarcoma cells. Cancer Sci. 2011, 102, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Wu, C.; Zhang, J.; Liu, J.; Zhang, X.; Hao, P.; Zhao, S.; Zhang, Z. Loss of Diacylglycerol Kinase-Zeta Inhibits Cell Proliferation and Survival in Human Gliomas. Mol. Neurobiol. 2016, 53, 5425–5435. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Mulatz, K.; Ard, R.; Nguyen, T.; Gee, S.H. Increased diacylglycerol kinase zeta expression in human metastatic colon cancer cells augments Rho GTPase activity and contributes to enhanced invasion. BMC Cancer 2014, 14, 208. [Google Scholar] [CrossRef]
- Torres-Ayuso, P.; Tello-Lafoz, M.; Merida, I.; Avila-Flores, A. Diacylglycerol kinase-zeta regulates mTORC1 and lipogenic metabolism in cancer cells through SREBP-1. Oncogenesis 2015, 4, e164. [Google Scholar] [CrossRef]
- Park, S.; Kang, H.J.; Jeon, J.H.; Kim, M.J.; Lee, I.K. Recent advances in the pathogenesis of microvascular complications in diabetes. Arch. Pharm. Res. 2019, 42, 252–262. [Google Scholar] [CrossRef]
- Yang, D.; Kim, J. Mitochondrial Retrograde Signalling and Metabolic Alterations in the Tumour Microenvironment. Cells 2019, 8, 275. [Google Scholar] [CrossRef]
- Nascimento, E.B.M.; Manneras-Holm, L.; Chibalin, A.V.; Bjornholm, M.; Zierath, J.R. Diacylglycerol kinase alpha deficiency alters inflammation markers in adipose tissue in response to a high-fat diet. J. Lipid Res. 2018, 59, 273–282. [Google Scholar] [CrossRef]
- Kurohane Kaneko, Y.; Kobayashi, Y.; Motoki, K.; Nakata, K.; Miyagawa, S.; Yamamoto, M.; Hayashi, D.; Shirai, Y.; Sakane, F.; Ishikawa, T. Depression of type I diacylglycerol kinases in pancreatic beta-cells from male mice results in impaired insulin secretion. Endocrinology 2013, 154, 4089–4098. [Google Scholar] [CrossRef]
- Manneras-Holm, L.; Schonke, M.; Brozinick, J.T.; Vetterli, L.; Bui, H.H.; Sanders, P.; Nascimento, E.B.M.; Bjornholm, M.; Chibalin, A.V.; Zierath, J.R. Diacylglycerol kinase epsilon deficiency preserves glucose tolerance and modulates lipid metabolism in obese mice. J. Lipid Res. 2017, 58, 907–915. [Google Scholar] [CrossRef]
- Benziane, B.; Borg, M.L.; Tom, R.Z.; Riedl, I.; Massart, J.; Bjornholm, M.; Gilbert, M.; Chibalin, A.V.; Zierath, J.R. DGKzeta deficiency protects against peripheral insulin resistance and improves energy metabolism. J. Lipid Res. 2017, 58, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Song, W.; Huang, R.; Chen, K.; Zhao, B.; Li, J.; Yang, Y.; Shang, H.F. GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson’s disease in a Chinese population. J. Clin. Neurosci. 2013, 20, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.; Guerreiro, R.; Hardy, J. SnapShot: Genetics of Parkinson’s disease. Cell 2015, 160. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, E.; Tanaka-Yamamoto, K. Diacylglycerol Kinases in the Coordination of Synaptic Plasticity. Front. Cell Dev. Biol. 2016, 4, 92. [Google Scholar] [CrossRef]
- Hansell, N.K.; Halford, G.S.; Andrews, G.; Shum, D.H.; Harris, S.E.; Davies, G.; Franic, S.; Christoforou, A.; Zietsch, B.; Painter, J.; et al. Genetic basis of a cognitive complexity metric. PLoS ONE 2015, 10, e0123886. [Google Scholar] [CrossRef]
- Shirai, Y.; Kouzuki, T.; Kakefuda, K.; Moriguchi, S.; Oyagi, A.; Horie, K.; Morita, S.Y.; Shimazawa, M.; Fukunaga, K.; Takeda, J.; et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS ONE 2010, 5, e11602. [Google Scholar] [CrossRef]
- Ishisaka, M.; Hara, H. The roles of diacylglycerol kinases in the central nervous system: Review of genetic studies in mice. J. Pharmacol. Sci. 2014, 124, 336–343. [Google Scholar] [CrossRef]
- Kakefuda, K.; Oyagi, A.; Ishisaka, M.; Tsuruma, K.; Shimazawa, M.; Yokota, K.; Shirai, Y.; Horie, K.; Saito, N.; Takeda, J.; et al. Diacylglycerol kinase beta knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS ONE 2010, 5, e13447. [Google Scholar] [CrossRef]
- Usuki, T.; Takato, T.; Lu, Q.; Sakai, H.; Bando, K.; Kiyonari, H.; Sakane, F. Behavioral and pharmacological phenotypes of brain-specific diacylglycerol kinase delta-knockout mice. Brain Res. 2016, 1648, 193–201. [Google Scholar] [CrossRef]
- Isozaki, T.; Komenoi, S.; Lu, Q.; Usuki, T.; Tomokata, S.; Matsutomo, D.; Sakai, H.; Bando, K.; Kiyonari, H.; Sakane, F. Deficiency of diacylglycerol kinase eta induces lithium-sensitive mania-like behavior. J. Neurochem. 2016, 138, 448–456. [Google Scholar] [CrossRef]
- Zhang, N.; Li, B.; Al-Ramahi, I.; Cong, X.; Held, J.M.; Kim, E.; Botas, J.; Gibson, B.W.; Ellerby, L.M. Inhibition of lipid signaling enzyme diacylglycerol kinase epsilon attenuates mutant huntingtin toxicity. J. Biol. Chem. 2012, 287, 21204–21213. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yang, J.; Zhong, X.P.; Kim, M.H.; Kim, Y.S.; Lee, H.W.; Han, S.; Choi, J.; Han, K.; Seo, J.; et al. Synaptic removal of diacylglycerol by DGKzeta and PSD-95 regulates dendritic spine maintenance. EMBO J. 2009, 28, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, P.; Uyemura, B.; Malarkannan, S.; Riese, M.J. Beyond the Cell Surface: Targeting Intracellular Negative Regulators to Enhance T cell Anti-Tumor Activity. Int. J. Mol. Sci. 2019, 20, 5821. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Lu, W.; Schmidt Paustian, A.M.; Ge, M.Q.; Koziol-White, C.J.; Flayer, C.H.; Killingbeck, S.S.; Wang, N.; Dong, X.; Riese, M.J.; et al. Diacylglycerol kinase zeta promotes allergic airway inflammation and airway hyperresponsiveness through distinct mechanisms. Sci Signal. 2019, 12. [Google Scholar] [CrossRef]
- Mahajan, S.; Mellins, E.D.; Faccio, R. Diacylglycerol Kinase zeta Regulates Macrophage Responses in Juvenile Arthritis and Cytokine Storm Syndrome Mouse Models. J. Immunol. 2020, 204, 137–146. [Google Scholar] [CrossRef]
- Olenchock, B.A.; Guo, R.; Silverman, M.A.; Wu, J.N.; Carpenter, J.H.; Koretzky, G.A.; Zhong, X.P. Impaired degranulation but enhanced cytokine production after Fc epsilonRI stimulation of diacylglycerol kinase zeta-deficient mast cells. J. Exp. Med. 2006, 203, 1471–1480. [Google Scholar] [CrossRef]
- Sakuma, M.; Shirai, Y.; Ueyama, T.; Saito, N. Diacylglycerol kinase gamma regulates antigen-induced mast cell degranulation by mediating Ca(2+) influxes. Biochem. Biophys. Res. Commun. 2014, 445, 340–345. [Google Scholar] [CrossRef]
- Gray, R.D.; Lucas, C.D.; MacKellar, A.; Li, F.; Hiersemenzel, K.; Haslett, C.; Davidson, D.J.; Rossi, A.G. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J. Inflamm. 2013, 10, 12. [Google Scholar] [CrossRef]
- Baldanzi, G.; Malerba, M. DGKalpha in Neutrophil Biology and Its Implications for Respiratory Diseases. Int. J. Mol. Sci. 2019, 20, 5673. [Google Scholar] [CrossRef]
- Muid, R.E.; Penfield, A.; Dale, M.M. The diacylglycerol kinase inhibitor, R59022, enhances the superoxide generation from human neutrophils induced by stimulation of fMet-Leu-Phe, IgG and C3b receptors. Biochem. Biophys. Res. Commun. 1987, 143, 630–637. [Google Scholar] [CrossRef]
| Isoform | Main tissue Expression | Subcellular Localization |
|---|---|---|
| α | T cell [2], brain [4,80] | Plasma membrane and nucleus [24,76], cytoplasm [4,76] |
| β | Brain [5,81] | Cytoskeleton [6,24], perisynaptic membrane [81] |
| γ | Brain [6,7] | Golgi and nucleus [24,77] |
| δ | Ubiquitous [8,82,83,84] | Cytoplasm [8,24,60,85], endoplasmic reticulum [24], endosomes [86], nucleus [60] |
| η | Ubiquitous [9,83,84,87] | Cytoplasm [9,87], endosomes [24] |
| κ | Reproductive organs [10,83,84] | Plasma membrane [10,24], |
| ε | Ubiquitous [11,84,88] | Plasma membrane and endoplasmic reticulum [24,89] |
| ξ | Ubiquitous [12,84] | Cytoplasm [90], plasma membrane and nucleus [24,78,91] |
| ι | Brain [13,79], retina [13,92] | Cytoplasm [90], nucleus [24,79] |
| θ | Brain [14], smooth muscle [93] and endothelial cells [93] | Plasma membrane and nucleus [24,60], presynaptic vesicles [26] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.A.; Kim, J.; Yang, D. Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling. Int. J. Mol. Sci. 2020, 21, 6861. https://doi.org/10.3390/ijms21186861
Sim JA, Kim J, Yang D. Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling. International Journal of Molecular Sciences. 2020; 21(18):6861. https://doi.org/10.3390/ijms21186861
Chicago/Turabian StyleSim, Jae Ang, Jaehong Kim, and Dongki Yang. 2020. "Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling" International Journal of Molecular Sciences 21, no. 18: 6861. https://doi.org/10.3390/ijms21186861
APA StyleSim, J. A., Kim, J., & Yang, D. (2020). Beyond Lipid Signaling: Pleiotropic Effects of Diacylglycerol Kinases in Cellular Signaling. International Journal of Molecular Sciences, 21(18), 6861. https://doi.org/10.3390/ijms21186861







