Erythropoietin Mimetic Peptide (pHBSP) Corrects Endothelial Dysfunction in a Rat Model of Preeclampsia
Abstract
1. Introduction
2. Results
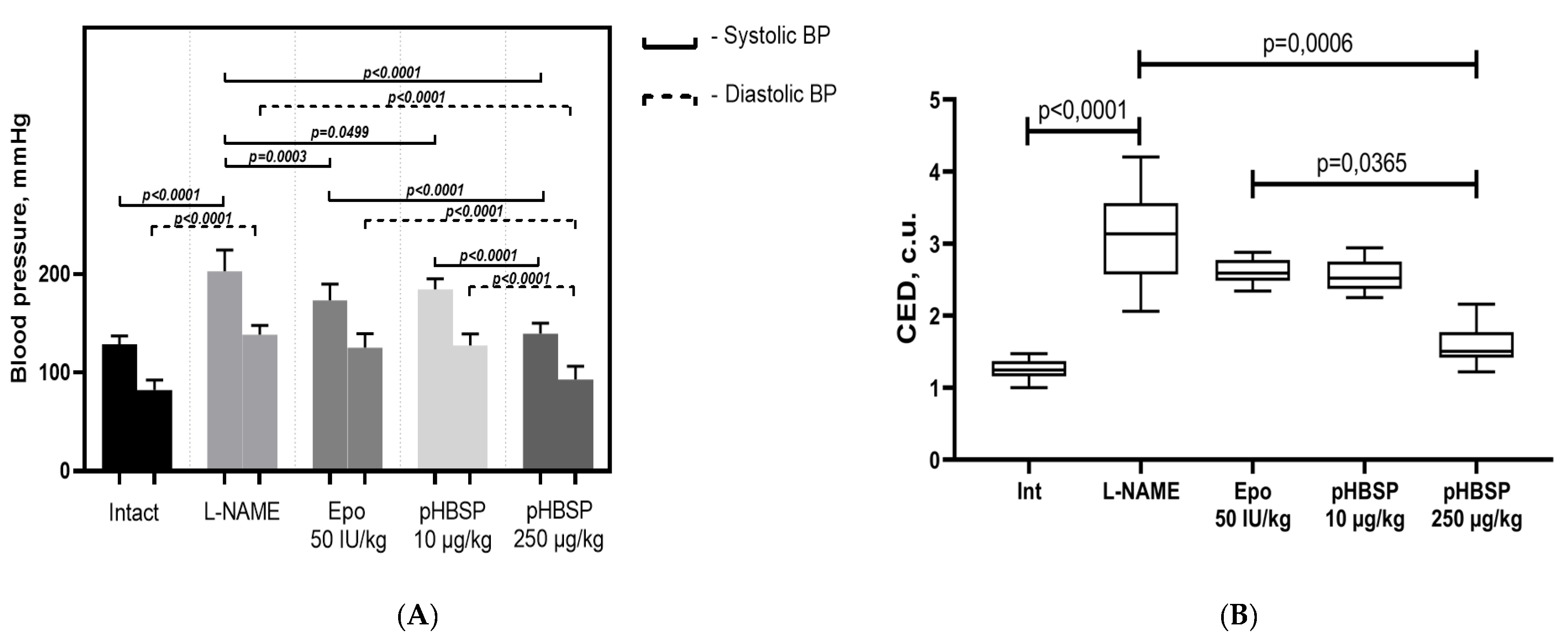
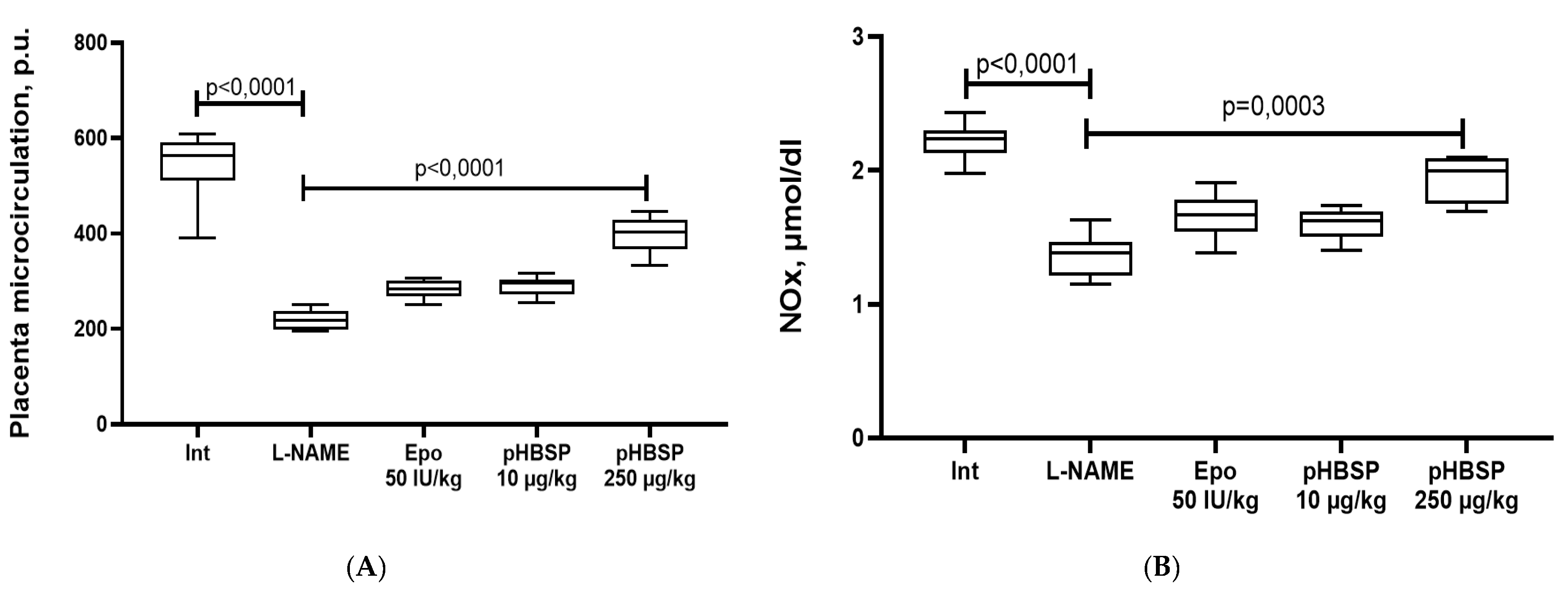
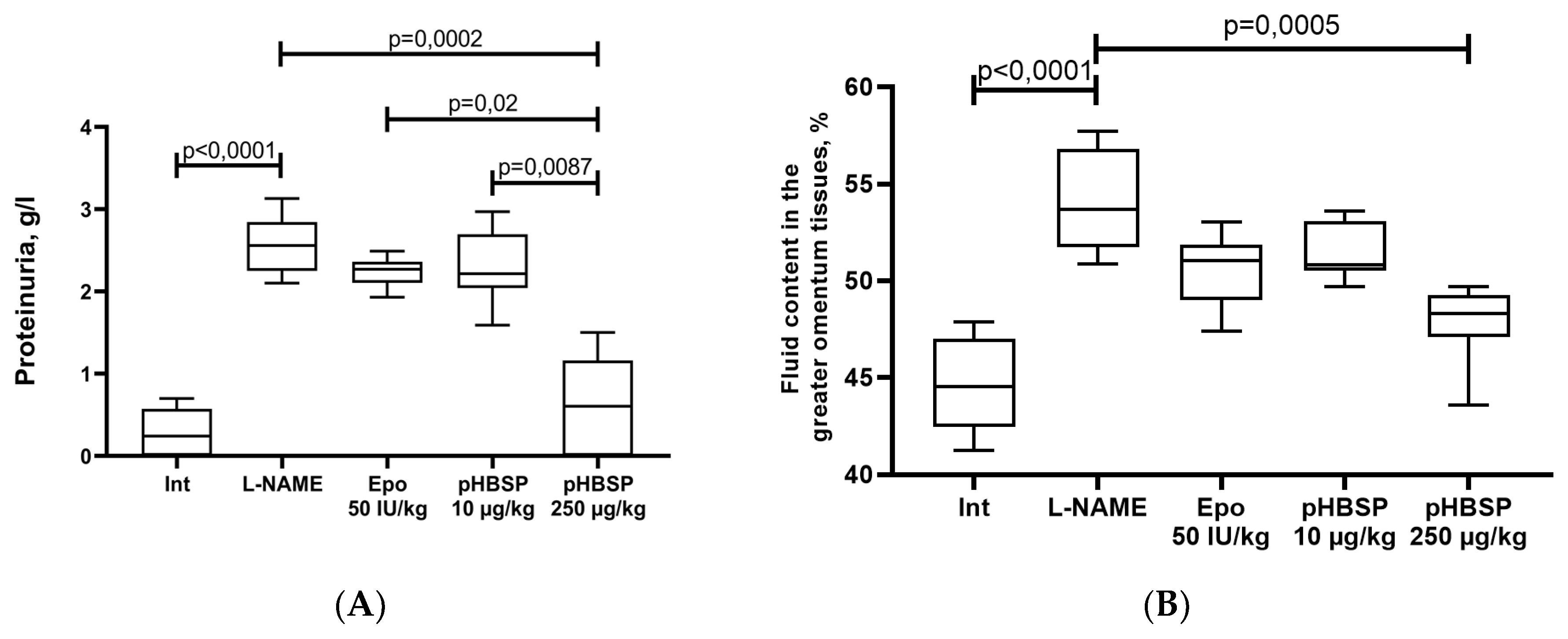
2.1. Results of Function Tests
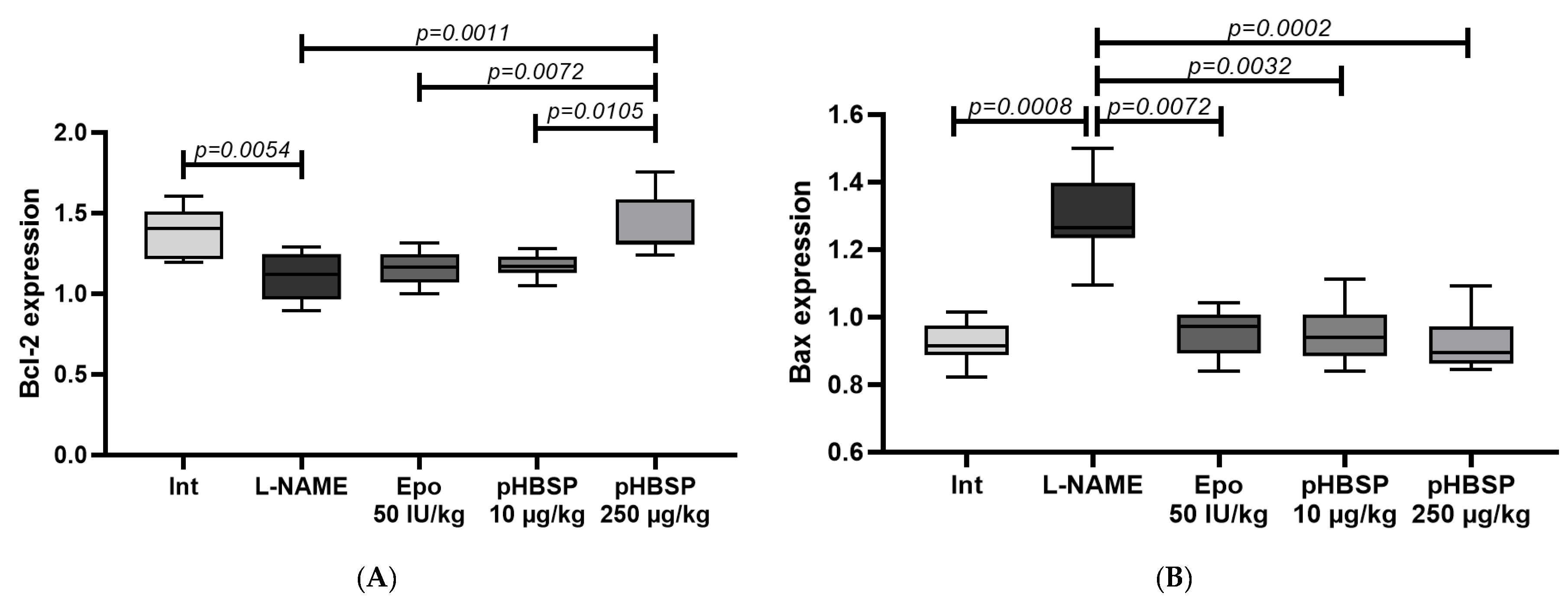
2.2. Results of Histological Examination
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
- 1 group—intact (animals with physiological pregnancy);
- 2 group—control (simulation of ADMA-like preeclampsia in the experimental animals was performed by administration of a non-selective NOS blocker L-NAME 25 mg/kg/day subcutaneously from the 14th to 20th day of pregnancy);
- 3 group—L-NAME + recombinant erythropoietin at the dose of 50 IU/kg/day subcutaneously from the 10th to 20th day of pregnancy;
- 4 group—L-NAME + pHBSP at the dose of 10 µg/kg/day subcutaneously from the 10th to 20th day of pregnancy;
- 5 group—L-NAME + pHBSP at the dose of 250 µg/kg/day subcutaneously from the 10th to 20th day of pregnancy.
4.3. Assessment of Endothelial Dysfunction
4.4. Registration of Placental Microcirculation
4.5. Proteinuria Estimation
4.6. Determination of Stable Nitric Oxide Metabolites
4.7. Evaluation of Bcl-2 and BAX Expression
4.8. Histological Study
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADMA | Asymmetrical dimethylarginine |
| NOS | Nitric oxide synthase |
| L-NAME | N-nitro-L-arginine-methyl ether |
| sFlt-1 | Soluble FMS-like tyrosine kinase-1 |
| PlGF | Placental growth factor |
| sEng | Soluble endoglin |
| PAPP-A | Pregnancy-associated plasma protein-A |
| EPO | Erythropoietin |
| CED | Coefficient of endothelial dysfunction |
| HCl | Hydrogen chloride |
| NaNo2 | Sodium nitrite |
| ADMA | Asymmetrical dimethylarginine |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| NOx | Nitric oxide metabolites |
| qPCR | quantitative polymerase chain reaction |
| mRNA | messenger RNA |
| JAK2 | Janus kinase 2 |
References
- Hayes-Ryan, D.; Meaney, S.; Hodnett, A.; Geisler, M.; O’Donoghue, K. The maternal and perinatal implications of hypertensive disorders of pregnancy in a multiple pregnancy cohort Acta Obstet. Gynecol. Scand. 2020, 99, 525–536. [Google Scholar] [CrossRef]
- Armaly, Z.; Zaher, M.; Knaneh, S.; Abassi, Z. Preeclampsia: Pathogenesis and mechanisms based therapeutic approaches. Harefuah 2019, 158, 742–747. [Google Scholar]
- Motedayen, M.; Rafiei, M.; Rezaei Tavirani, M.; Sayehmiri, K.; Dousti, M. The relationship between body mass index and preeclampsia: A systematic review and meta-analysis. Int. J. Reprod. Biomed. 2019, 17, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Un Nisa, S.; Shaikh, A.A.; Kumar, R. Maternal and Fetal Outcomes of Pregnancy-related Hypertensive Disorders in a Tertiary Care Hospital in Sukkur, Pakistan. Cureus 2019, 11, e5507. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Raio, L.; Baumann, M.; Rimoldi, S.; Rexhaj, E. Systolic Hypertension, Preeclampsia-Related Mortality, and Stroke in California. Obstet. Gynecol. 2019, 134, 880. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, T.; Mimura, K.; Matsuzaki, S.; Endo, M.; Kumasawa, K.; Kimura, T. Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors. Int. J. Mol. Sci. 2019, 20, 4246. [Google Scholar] [CrossRef]
- Olaoye, T.; Oyerinde, O.O.; Elebuji, O.J.; Ologun, O. Knowledge, Perception and Management of Pre-eclampsia among Health Care Providers in a Maternity Hospital. Int. J. MCH AIDS 2019, 8, 80–88. [Google Scholar] [CrossRef]
- Ponmozhi, G.; Keepanasseril, A.; Mathaiyan, J.; Manikandan, K. Nitric Oxide in the Prevention of Pre-eclampsia (NOPE): A Double-Blind Randomized Placebo-Controlled Trial Assessing the Efficacy of Isosorbide Mononitrate in the Prevention of Pre-eclampsia in High-Risk Women. J. Obstet. Gynaecol. India 2019, 69, 103–110. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhang, G.; Harvey, S.; Nakagawa, K. Temporal Trends of Hospitalization, Mortality, and Financial Impact Related to Preeclampsia with Severe Features in Hawai’i and the United States. Hawai’i J. Health Soc. Welf. 2019, 78, 252–257. [Google Scholar]
- Pankiewicz, K.; Szczerba, E.; Maciejewski, T.; Fijałkowska, A. Non-obstetric complications in preeclampsia. Prz. Menopauzalny Menopause Rev. 2019, 18, 99–109. [Google Scholar] [CrossRef]
- Shaheen, G.; Jahan, S.; Ain, Q.U.; Ullah, A.; Afsar, T.; Almajwal, A.; Alam, I.; Razak, S. Placental endothelial nitric oxide synthase expression and role of oxidative stress in susceptibility to preeclampsia in Pakistani women. Mol. Genet. Genom. Med. 2020, 8, e1019. [Google Scholar] [CrossRef]
- Moodley, J.; Soma-Pillay, P.; Buchmann, E.; Pattinson, R.C. Hypertensive disorders in pregnancy: 2019 National guideline. S. Afr. Med. J. Suid-Afrik. Tydskr. Geneeskd. 2019, 109, 12723. [Google Scholar]
- Meher, S.; Duley, L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2007, 2, CD006490. [Google Scholar] [CrossRef] [PubMed]
- Camarena Pulido, E.E.; García Benavides, L.; Panduro Barón, J.G.; Pascoe Gonzalez, S.; Madrigal Saray, A.J.; García Padilla, F.E.; Totsuka Sutto, S.E. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: A double-blind, randomized, clinical trial. Hypertens. Pregnancy 2016, 35, 217–225. [Google Scholar] [CrossRef]
- Korokin, M.V.; Soldatov, V.O.; Tietze, A.A.; Golubev, M.V.; Belykh, A.E.; Kubekina, M.V.; Puchenkova, O.A.; Denisyuk, T.A.; Gureyev, V.V.; Pokrovskaya, T.G.; et al. 11-amino acid peptide imitating the structure of erythropoietin α-helix b improves endothelial function, but stimulates thrombosis in rats. Pharm. Pharmacol. 2019, 7, 312–320. [Google Scholar] [CrossRef]
- Wolfson, G.H.; Vargas, E.; Browne, V.A.; Moore, L.G.; Julian, C.G. Erythropoietin and Soluble Erythropoietin Receptor: A Role for Maternal Vascular Adaptation to High-Altitude Pregnancy. J. Clin. Endocrinol. Metab. 2019, 102, 242–250. [Google Scholar] [CrossRef]
- Zamudio, S.; Wu, Y.; Ietta, F.; Rolfo, A.; Cross, A.; Wheeler, T.; Post, M.; Illsley, N.P.; Caniggia, I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am. J. Pathol. 2007, 170, 2171–2179. [Google Scholar] [CrossRef]
- Teramo, K.A.; Widness, J.A. Increased fetal plasma and amniotic fluid erythropoietin concentrations: Markers of intrauterine hypoxia. Neonatology 2009, 95, 105–116. [Google Scholar] [CrossRef]
- Fairchild Benyo, D.; Conrad, K.P. Expression of the Erythropoietin Receptor by Trophoblast Cells in the Human Placenta. Biol. Reprod. 1999, 60, 861–870. [Google Scholar] [CrossRef]
- Jain, V.; Lim, M.; Longo, M.; Fisk, N.M. Inhibitory effect of erythropoietin on contractility of human chorionic plate vessels. Am. J. Obstet. Gynecol. 2006, 194, 246. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Breymann, C.; Huch, R.; Huch, A. Hypertension in a pregnancy with renal anemia after recombinant human erythropoietin (rhEPO) therapy. Arch. Gynecol. Obstet. 2002, 267, 54–56. [Google Scholar] [CrossRef]
- Brines, M.; Grasso, G.; Fiordaliso, F.; Sfacteria, A.; Ghezzi, P.; Fratelli, M.; Latini, R.; Xie, Q.W.; Smart, J.; Su-Rick, C.J.; et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. USA 2004, 101, 14907–14912. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Patel, N.S.; Villa, P.; Brines, C.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C.; et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, M.; Zhang, Y.; Yang, K.; Hu, J.; Si, R.; Zhang, G.; Gao, B.; Li, X.; Xu, C.; et al. Helix B surface peptide attenuates diabetic cardiomyopathy via AMPK-dependent autophagy. Biochem. Biophys. Res. Commun. 2017, 482, 665–671. [Google Scholar] [CrossRef]
- Robertson, C.S.; Cherian, L.; Shah, M.; Garcia, R.; Navarro, J.C.; Grill, R.J.; Hand, C.C.; Tian, T.S.; Hannay, H.J. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J. Neurotrauma 2012, 29, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Jiang, J.; Luo, B.; Zhu, W.; Liu, Y.; Wang, Z.; Zhang, Z. Non-erythropoietic erythropoietin-derived peptide protects mice from systemic lupus erythematosus. J. Cell. Mol. Med. 2018, 22, 3330–3339. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.; Gao, S.; Zhu, G.; Zhu, Q.; Gu, Y.; Shao, F. EPO Derivative ARA290 Attenuates Early Renal Allograft Injury in Rats by Targeting NF-κB Pathway. Transplant. Proc. 2018, 50, 1575–1582. [Google Scholar] [CrossRef]
- Collino, M.; Thiemermann, C.; Cerami, A.; Brines, M. Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol. Ther. 2015, 151, 32–40. [Google Scholar] [CrossRef]
- Hache, G.; Garrigue, P.; Bennis, Y.; Stalin, J.; Moyon, A.; Cerami, A.; Brines, M.; Blot-Chabaud, M.; Sabatier, F.; Dignat-George, F.; et al. ARA290, a Specific Agonist of Erythropoietin/CD131 Heteroreceptor, Improves Circulating Endothelial Progenitors’ Angiogenic Potential and Homing Ability. Shock 2016, 46, 390–397. [Google Scholar] [CrossRef]
- Wu, S.; Yang, C.; Xu, N.; Wang, L.; Liu, Y.; Wang, J.; Shen, X. The Protective Effects of Helix B Surface Peptide on Experimental Acute Liver Injury Induced by Carbon Tetrachloride. Dig. Dis. Sci. 2017, 62, 1537–1549. [Google Scholar] [CrossRef]
- Culver, D.A.; Dahan, A.; Bajorunas, D.; Jeziorska, M.; van Velzen, M.; Aarts, L.; Tavee, J.; Tannemaat, M.R.; Dunne, A.N.; Kirk, R.I.; et al. Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO52–BIO60. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; You, W.; Lin, L.; Lin, Y.; Tang, X.; Liu, Y.; Miao, F. Helix B Surface Peptide Protects against Acute Myocardial Ischemia-Reperfusion Injury via the RISK and SAFE Pathways in a Mouse Model. Cardiology 2016, 134, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Mannino, F.; Vaccaro, M.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; Colonna, M.R.; et al. Activation of the EPOR-β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, C.; Zhu, T. From Erythropoietin to Its Peptide Derivatives: Smaller but Stronger. Curr. Protein Pept. Sci. 2017, 18, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Wu, Y.; Yang, B. Renoprotection and Mechanisms of Erythropoietin and Its Derivatives Helix B Surface Peptide in Kidney Injuries. Curr. Protein Pept. Sci. 2017, 18, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Bischoff, P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin. Ther. Pat. 2010, 20, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Tian, H.; Yang, B.; Zhang, B.; Dai, C.; Han, Z.; Wang, M.; Li, Y.; Wei, L.; Chen, D.; et al. Autophagy and Akt in the protective effect of erythropoietin helix B surface peptide against hepatic ischaemia/reperfusion injury in mice. Sci. Rep. 2018, 8, 14703. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, O.E.; Canning, P.; Reid, E.; Bertelli, P.M.; McKeown, S.; Brines, M.; Cerami, A.; Du, X.; Xu, H.; Chen, M.; et al. The vasoreparative potential of endothelial colony-forming cells in the ischemic retina is enhanced by cibinetide, a non-hematopoietic erythropoietin mimetic. Exp. Eye Res. 2019, 182, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Haschka, D.; Dichtl, S.; Sonnweber, T.; Schroll, A.; Aßhoff, M.; Mindur, J.E.; Moser, P.L.; Wolf, D.; Swirski, F.K.; et al. Cibinetide dampens innate immune cell functions thus ameliorating the course of experimental colitis. Sci. Rep. 2017, 7, 13012. [Google Scholar] [CrossRef]
- Devalliere, J.; Dooley, K.; Hu, Y.; Kelangi, S.S.; Uygun, B.E.; Yarmush, M.L. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017, 141, 149–160. [Google Scholar] [CrossRef]
- Yao, M.; Watanabe, M.; Sun, S.; Tokodai, K.; Cerami, A.; Brines, M.; Östenson, C.G.; Ericzon, B.G.; Lundgren, T.; Kumagai-Braesch, M. Improvement of Islet Allograft Function Using Cibinetide, an Innate Repair Receptor Ligand. Transplantation 2020. [Google Scholar] [CrossRef]
- Atkins, C.; Wilson, A.M. Managing fatigue in sarcoidosis—A systematic review of the evidence. Chronic Respir. Dis. 2017, 14, 161–173. [Google Scholar] [CrossRef]
- Stupakova, E.G.; Lazareva, G.A.; Gureev, V.V. Correction of morphofunctional disturbances arising when modelling Preeclampsia with resveratrol and nicorandil. Res. Results Pharmacol. 2018, 4, 59–71. [Google Scholar] [CrossRef][Green Version]
- Sgarbossa, F.; Barbisan, L.F.; Brasil, M.A.; Costa, E.; Calderon, I.M.; Gonçalves, C.R.; Bevilacqua, E.; Rudge, M.V. Changes in apoptosis and Bcl-2 expression in human hyperglycemic, term placental trophoblast. Diabetes Res. Clin. Pract. 2006, 73, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Gokalp-Ozkorkmaz, E.; Asir, F.; Basaran, S.O.; Agacayak, E.; Sahin, F.; Kaya, S.; Erdogan, G.; Deveci, E. Examination of Bcl-2 and Bax Protein Levels for Determining the Apoptotic Changes in Placentas with Gestational Diabetes and Preeclampsia. Proceedings 2018, 2, 1548. [Google Scholar] [CrossRef]
- Pytela, R.; Pierschbacher, M.D.; Ginsberg, M.H.; Plow, E.F.; Ruoslahti, E. Platelet membrane glycoprotein IIb/IIIa: Member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986, 231, 1559–1562. [Google Scholar] [CrossRef]
- Sheu, J.R.; Yen, M.H.; Peng, H.C.; Chang, M.C.; Huang, T.F. Triflavin, an Arg-Gly-Asp-containing peptide, prevents platelet plug formation in in vivo experiments. Eur. J. Pharmacol. 1995, 294, 231–238. [Google Scholar] [CrossRef]
- Hung, Y.C.; Kuo, Y.J.; Huang, S.S.; Huang, T.F. Trimucrin, an Arg-Gly-Asp containing disintegrin, attenuates myocardial ischemia-reperfusion injury in murine by inhibiting platelet function. Eur. J. Pharmacol. 2017, 813, 24–32. [Google Scholar] [CrossRef]
- Shevchenko, K.V.; Nagaev, I.Y.; Andreeva, L.A.; Shevchenko, V.P.; Myasoedov, N.F. Stability of prolin-containing peptides in biological media. Biomed. Khimiya 2019, 65, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Jin, H.; Wang, W.; Huo, J.; Zhou, L.; Wang, Y.; Feng, F.; Zhang, L. Angiotensin-I-converting enzyme inhibitory peptides: Chemical feature based pharmacophore generation. Eur. J. Med. Chem. 2011, 46, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin II and vascular injury. Curr. Hypertens Rep. 2014, 16, 431. [Google Scholar] [CrossRef]
- Pastorova, V.E.; Liapina, L.A.; Alshmarin, I.P.; Ostrovskaia, P.U.; Gudasheva, T.A.; Lugovskoĭ, E.V. Fibrin-depolymerization activity and the antiplatelet effect of small cyclic and linear proline-containing peptides. Izv. Akad. Nauk Ser. Biol. 2001, 5, 593. [Google Scholar]
- Lyapina, L.A.; Pastorova, V.E.; Obergan, T.Y. Changes in hemostatic parameters after intranasal administration of peptide Pro-Gly-Pro. Bull. Exp. Biol. Med. 2007, 144, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Liapina, L.A.; Grigor'eva, M.E.; Andreeva, L.A.; Miasoedov, N.F. Protective antithrombotic effects of proline-containing peptides in the animal body subjected to stress. Izv. Akad. Nauk Ser. Biol. 2010, 4, 462–467. [Google Scholar]
- Severinova, O.V.; Gureev, V.V.; Zhilinkova, L.A.; Lazareva, G.A.; Gureeva, A.V.; Lazareva, S.S. Study of the effect of selective inhibitor of Arginase II KUD 975 and of low doses of Acetylsalicylic acid on the functional parameters of the cardiovascular system In experimental preeclampsia. Res. Results Pharmacol. 2019, 5, 47–56. [Google Scholar] [CrossRef][Green Version]
- Lokteva, T.I.; Rozhkov, S.; Gureev, V.V.; Gureeva, A.V.; Zatolokina, M.A.; Avdeeva, E.V.; Zhilinkova, L.A.; Prohoda, E.E.; Yarceva, E.O. Correction of morphofunctional disorders of the cardiovascular system with asialized erythropoietin and arginase II selective inhibitors KUD 974 and KUD 259 in experimental preeclampsia. Res. Results Pharmacol. 2020, 6, 29–40. [Google Scholar] [CrossRef][Green Version]
- Gureev, V.V.; Pokrovskii, M.V.; Korokin, M.V.; Gudyrev, O.S.; Philippova, O.V.; Dolzhikov, A.A.; Lazareva, G.A. Correction of ADMA-induced preeclampsia with use of tetrahydrobiopterin and selective inhibitor of arginase II ZB49-0010. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1538–1541. [Google Scholar] [CrossRef]
- Korokin, M.V.; Pokrovskii, M.V.; Gudyrev, O.S.; Korokina, L.V.; Pokrovskaia, T.G.; Lazarev, A.I.; Philippenko, N.G.; Gureev, V.V. Pharmacological correction of endothelial dysfunction in rats using e-NOS cofactors. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1548–1552. [Google Scholar]
- Korokin, M.; Gudyrev, O.; Gureev, V.; Korokina, L.; Peresypkina, A.; Pokrovskaia, T.; Lazareva, G.; Soldatov, V.; Zatolokina, M.; Pokrovskii, M. Studies to Elucidate the Effects of Furostanol Glycosides from Dioscorea deltoidea Cell Culture in a Rat Model of Endothelial Dysfunction. Molecules 2020, 25, 169. [Google Scholar] [CrossRef]
- Yalamati, P.; Bhongir, A.V.; Karra, M.; Beedu, S.R. Comparative Analysis of Urinary Total Proteins by Bicinchoninic Acid and Pyrogallol Red Molybdate Methods. J. Clin. Diagn. Res. JCDR 2015, 9, BC1–BC4. [Google Scholar] [CrossRef]
- Metel’skaia, V.A.; Gumanova, N.G. Screening as a method for determining the serum level of nitric oxide metabolites. Klin. Lab. Diagn. 2005, 6, 15–18. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16078527 (accessed on 28 June 2005).
- Peresypkina, A.; Pazhinsky, A.; Pokrovskii, M.; Beskhmelnitsyna, E.; Pobeda, A.; Korokin, M. Correction of Experimental Retinal Ischemia by l-Isomer of Ethylmethylhydroxypyridine Malate. Antioxidants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
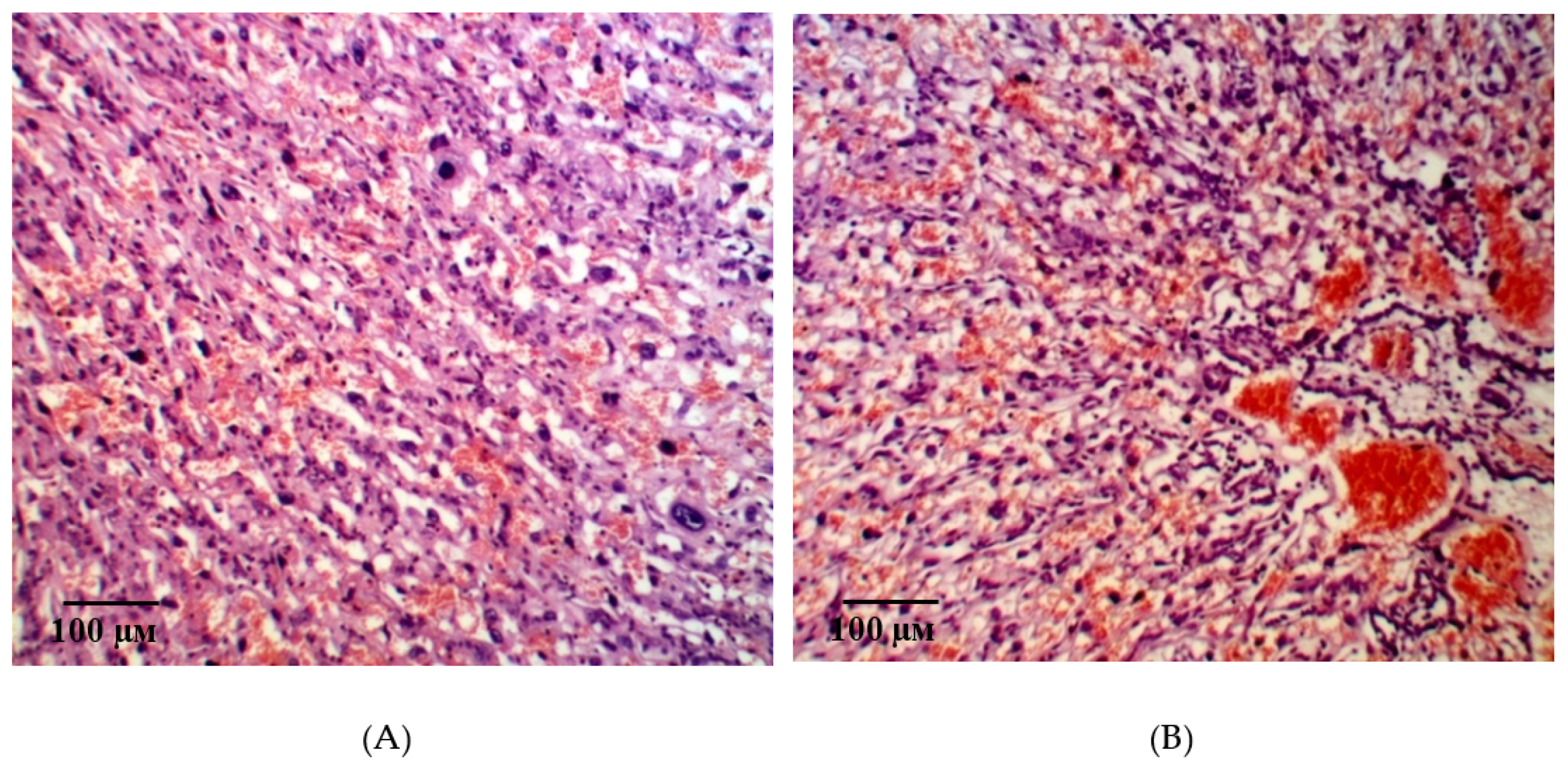
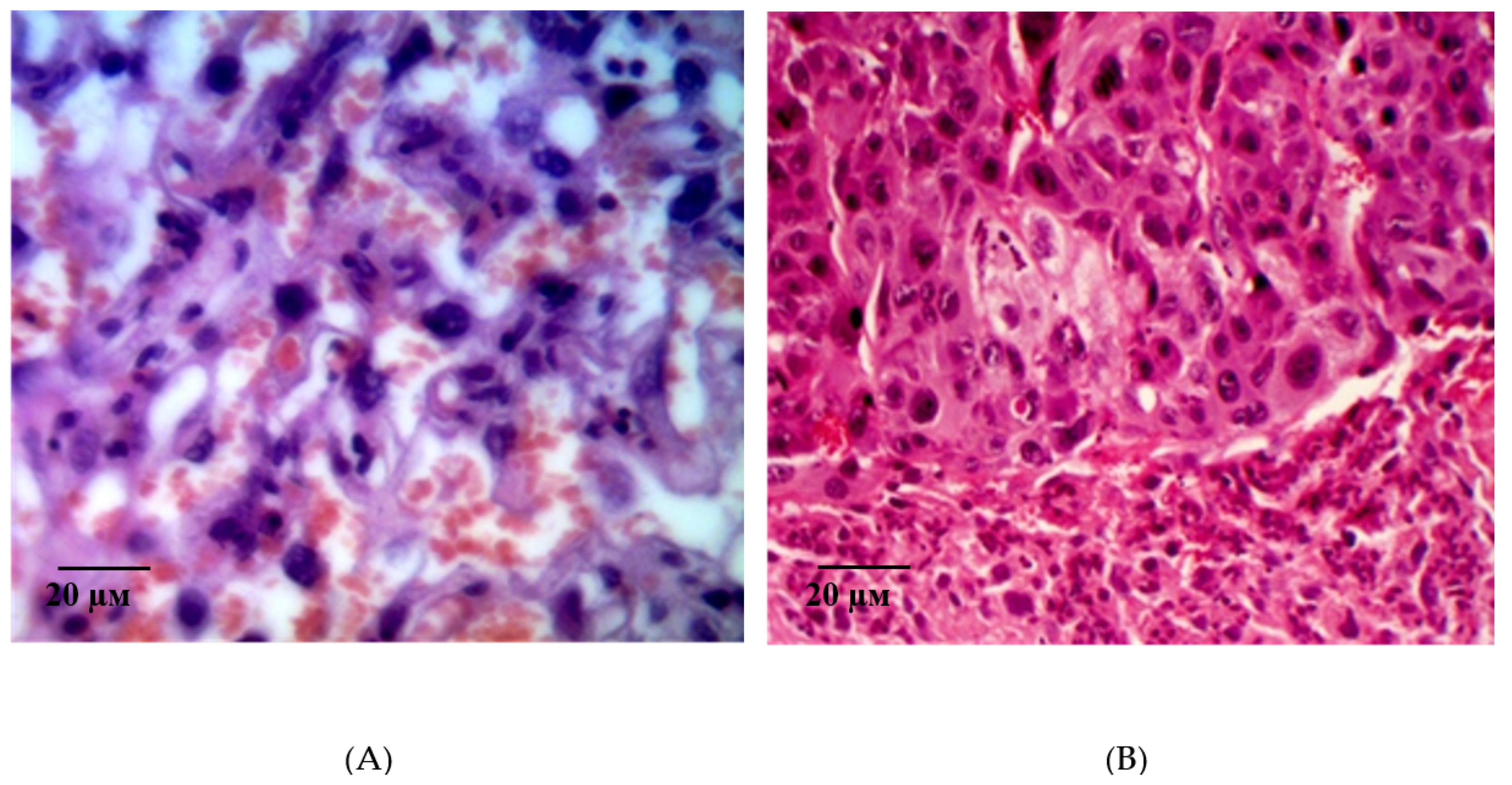
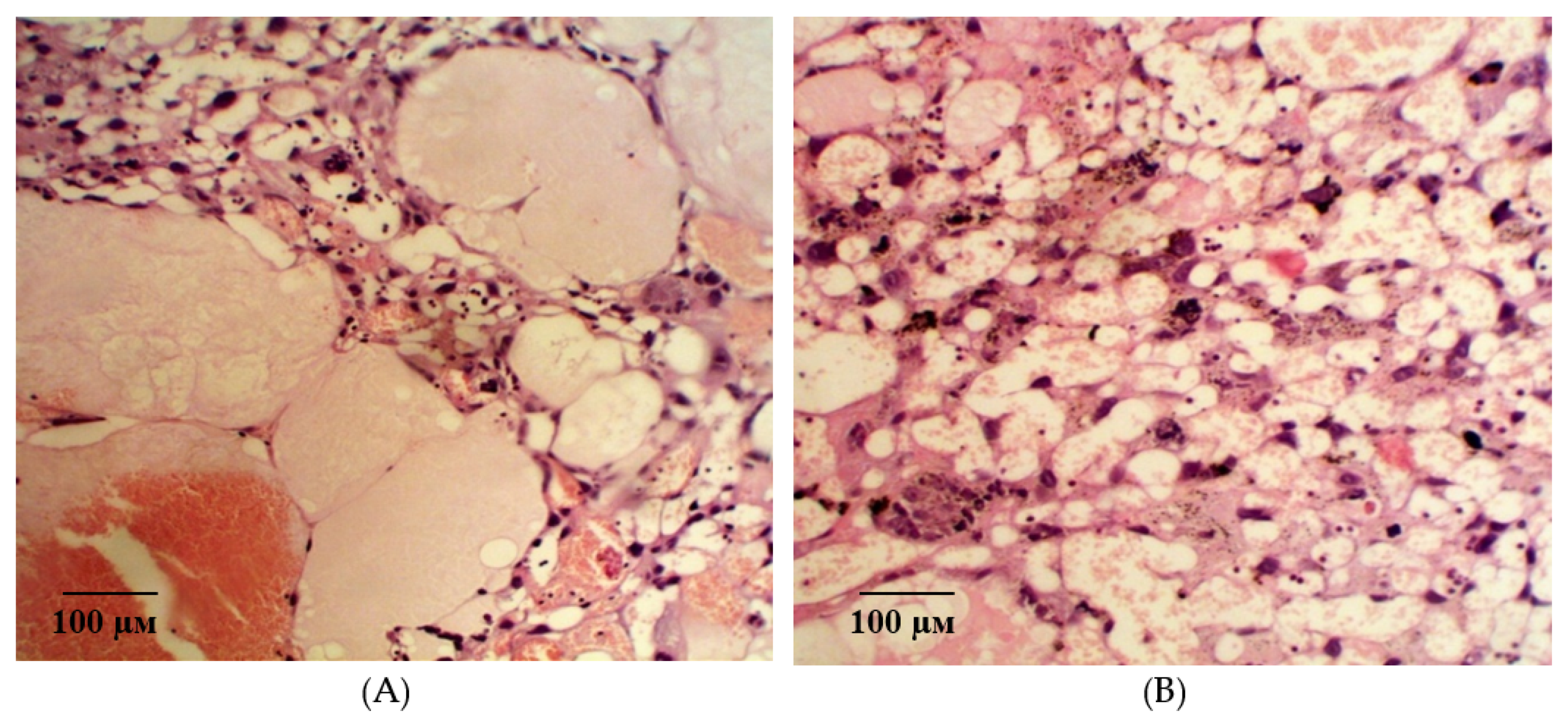
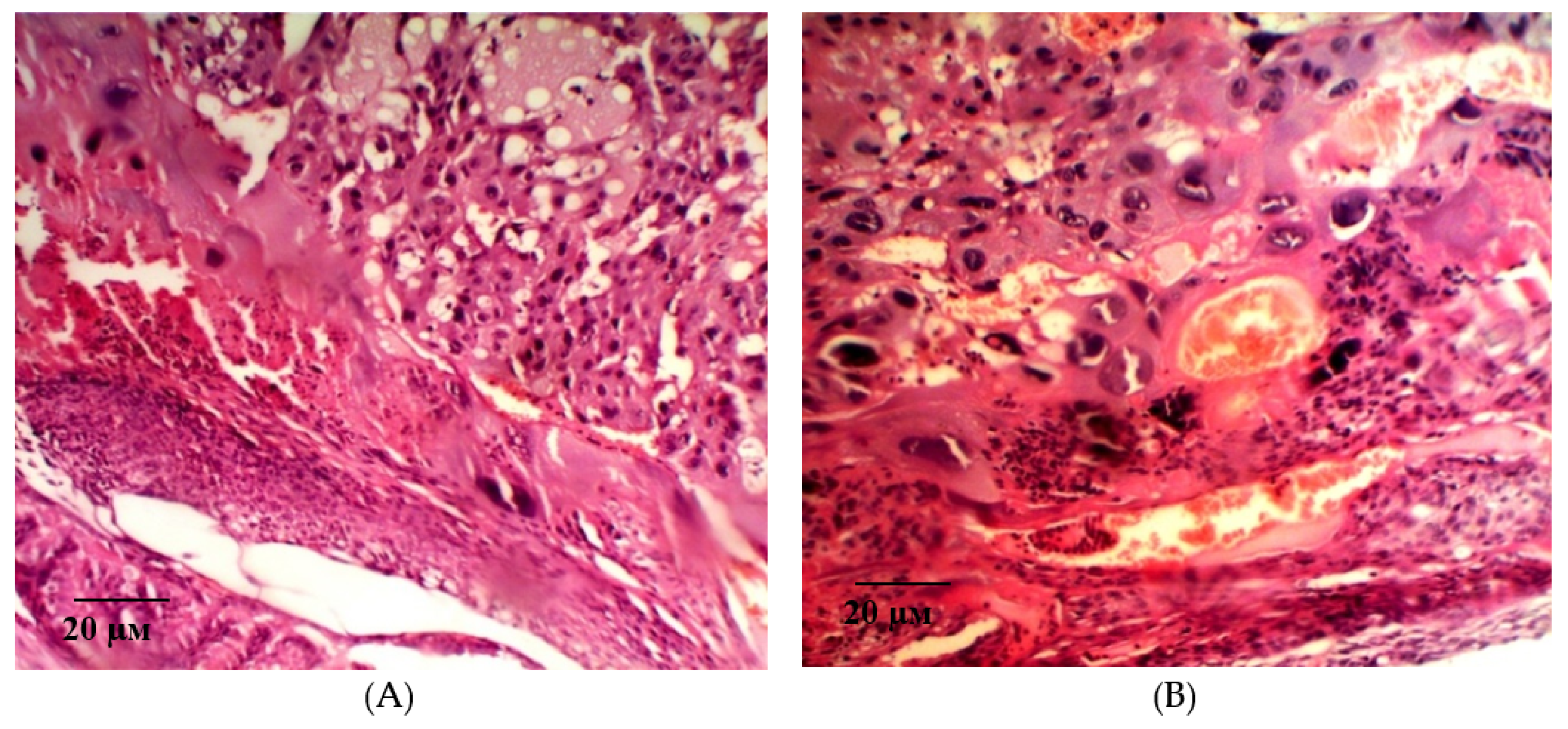
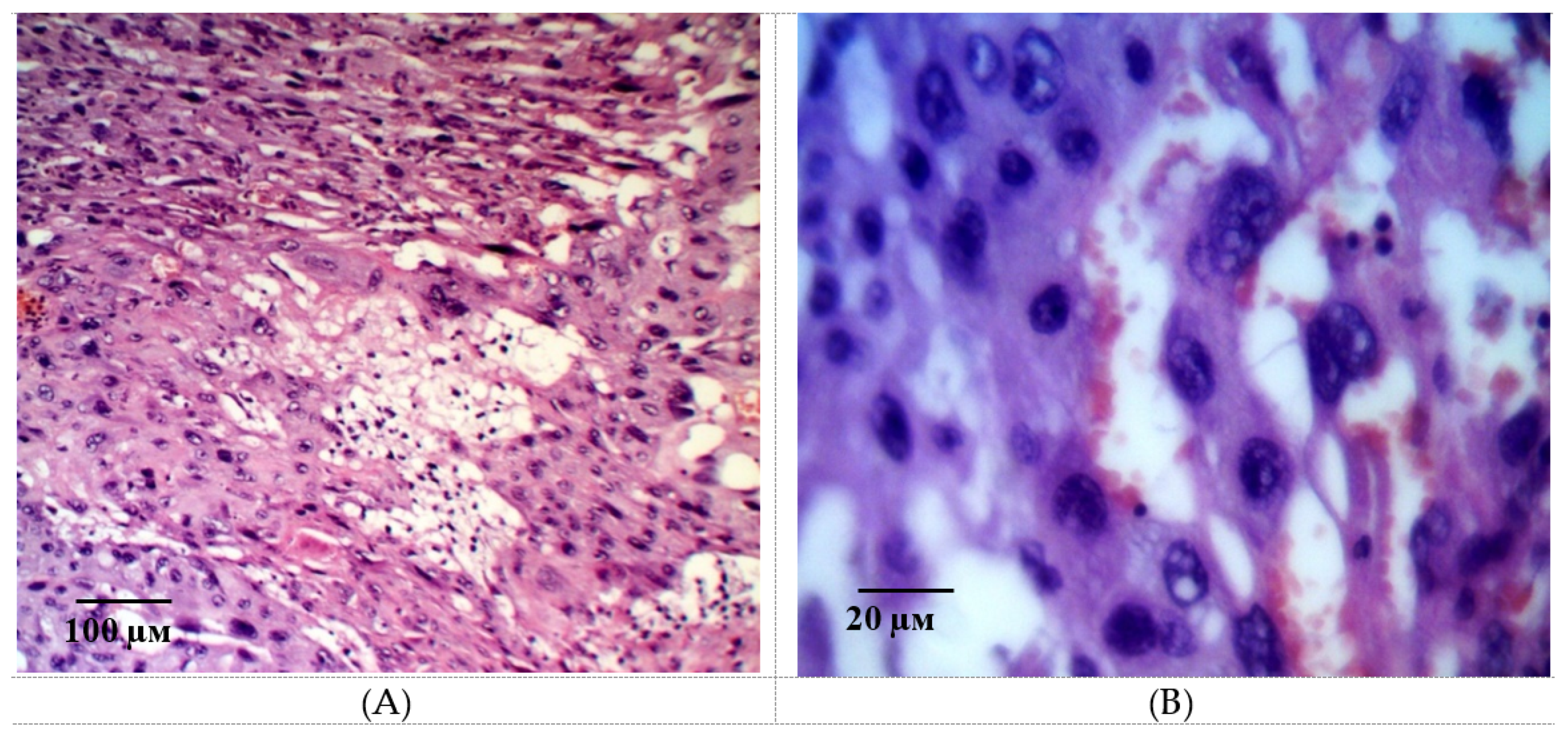
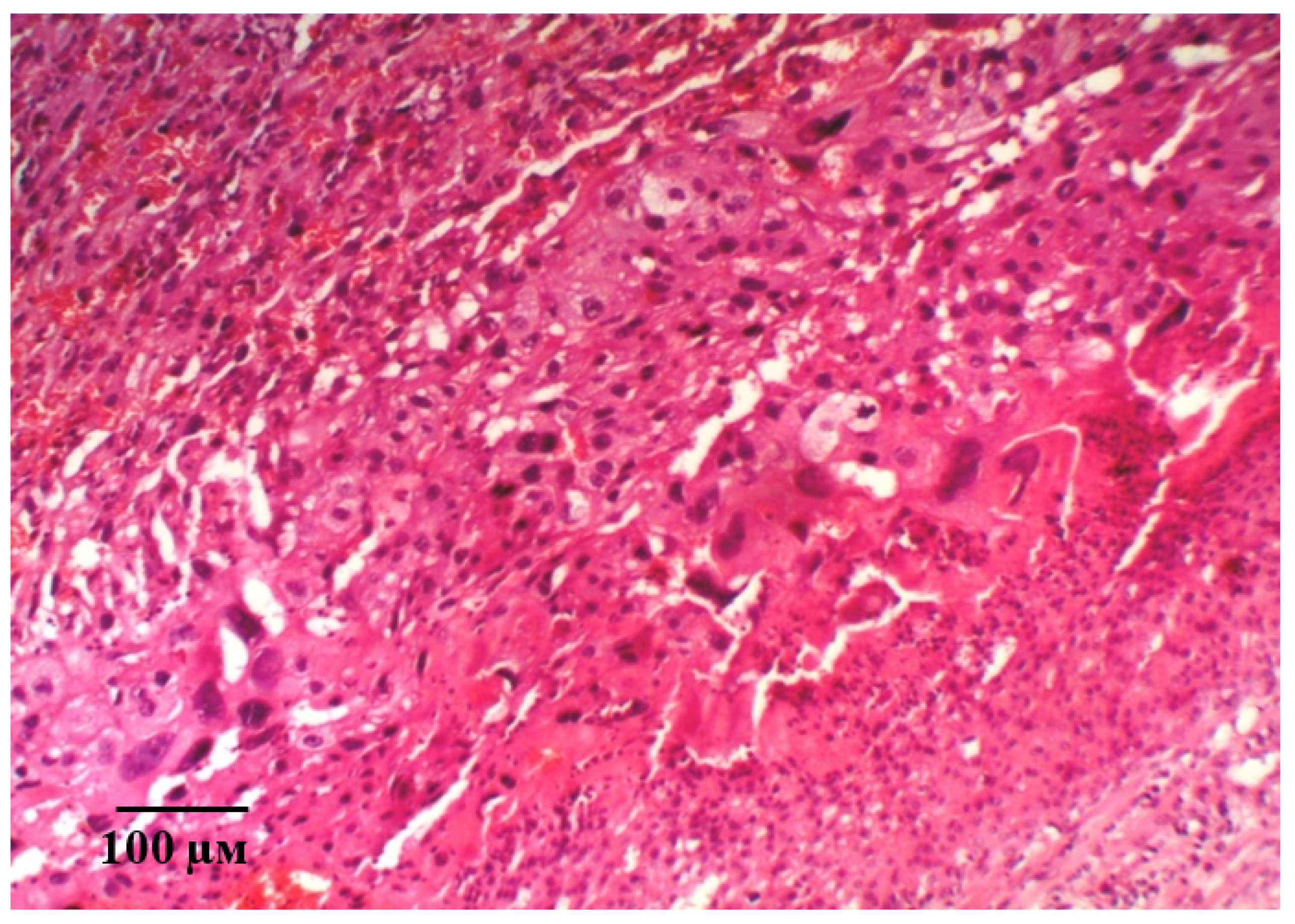

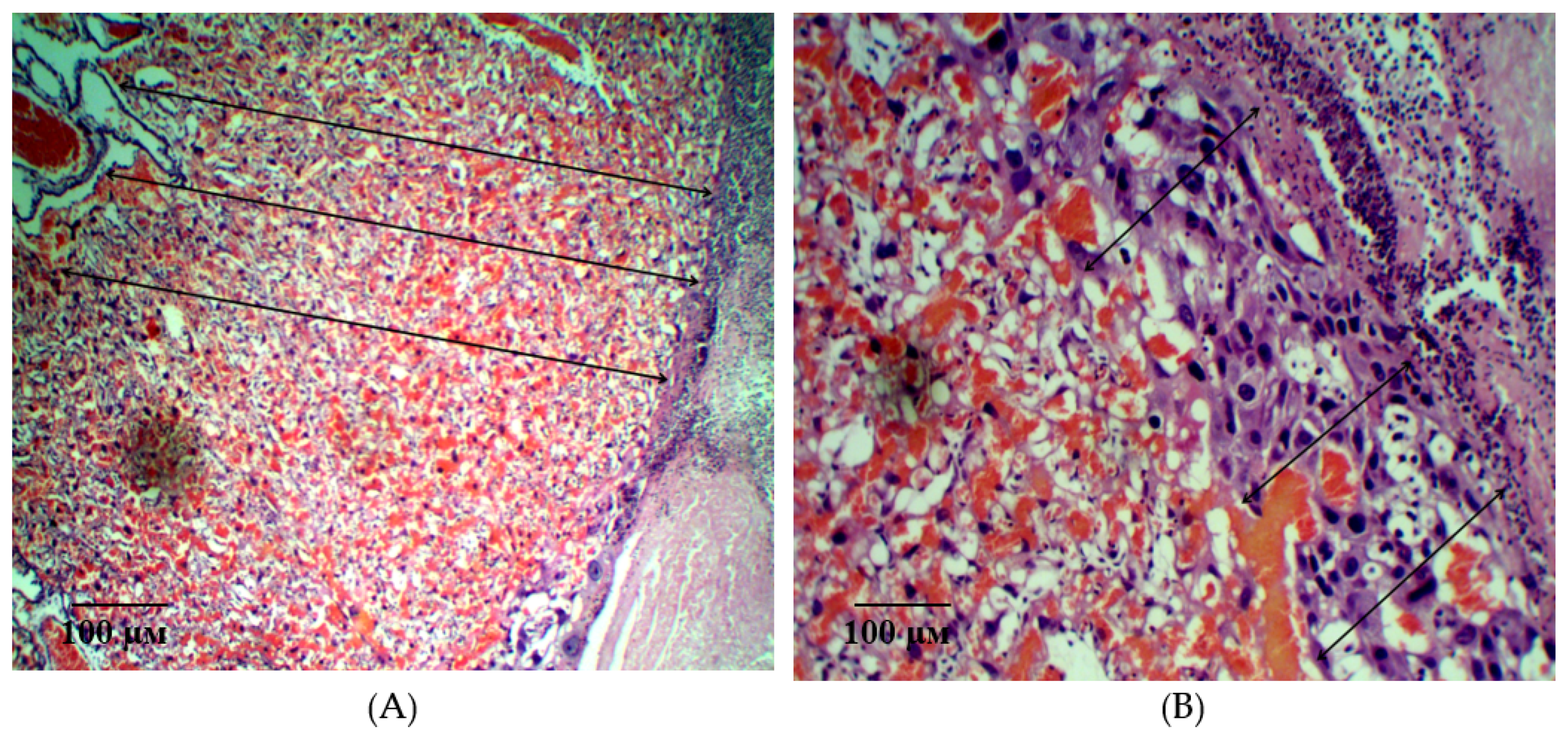


| Group | Bax/Bcl-2 Ratio |
|---|---|
| Int | 0.68 ± 0.1 y |
| L-NAME | 1.2 ± 0.21 * |
| Epo (50 IU/kg) | 0.83 ± 0.08 *,y |
| pHBSP (10 μg/kg) | 0.81 ± 0.08 *,y |
| pHBSP (250 μg/kg) | 0.66 ± 0.1 *,y |
| Group | Decidual Cell Density, /0.008 mm2 | Cell Dencity in the Fetal Placenta, /0.008 mm2 | Villi Diameter, ×10−3μm |
|---|---|---|---|
| Int | 114.2 ± 2.03 y | 236.9 ± 2.75 y | 32.18 ± 0.39 y |
| L-NAME | 22.3 ± 0.28 * | 78.5 ± 2.51 * | 16.79 ± 0.24 * |
| Epo (50 IU/kg) | 74.3 ± 0.73 *,y | 137.0 ± 4.17 *,y | 22.33 ± 0.16 *,y |
| pHBSP (10 μg/kg) | 67.7 ± 0.59 *,y | 92.3 ± 1.24 *,y | 21.01 ± 0.16 *,y |
| pHBSP (250 μg/kg) | 80.2 ± 0.75 *,y | 160.0 ± 3.22 *,y | 30.91 ± 0.17 *,y |
| Name of Primers | Nucleotide Sequence 5′- > 3′ | Length |
|---|---|---|
| Bcl2 F | GGCCTTTTTGCTACAGGGTTTC | 105 |
| Bcl2 R | TTCTTGGTGGATGCGTCCTG | |
| Bax F | GTGGACAACATCGCTCTGTG | 95 |
| Bax R | AGTTCCACAAAGGCATCCCAG | |
| Actb F | CGCCACCAGTTCGCCAT | 96 |
| Actb R | GGGAGCATCGTCGCCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korokin, M.; Gureev, V.; Gudyrev, O.; Golubev, I.; Korokina, L.; Peresypkina, A.; Pokrovskaia, T.; Lazareva, G.; Soldatov, V.; Zatolokina, M.; et al. Erythropoietin Mimetic Peptide (pHBSP) Corrects Endothelial Dysfunction in a Rat Model of Preeclampsia. Int. J. Mol. Sci. 2020, 21, 6759. https://doi.org/10.3390/ijms21186759
Korokin M, Gureev V, Gudyrev O, Golubev I, Korokina L, Peresypkina A, Pokrovskaia T, Lazareva G, Soldatov V, Zatolokina M, et al. Erythropoietin Mimetic Peptide (pHBSP) Corrects Endothelial Dysfunction in a Rat Model of Preeclampsia. International Journal of Molecular Sciences. 2020; 21(18):6759. https://doi.org/10.3390/ijms21186759
Chicago/Turabian StyleKorokin, Mikhail, Vladimir Gureev, Oleg Gudyrev, Ivan Golubev, Liliya Korokina, Anna Peresypkina, Tatiana Pokrovskaia, Galina Lazareva, Vladislav Soldatov, Mariya Zatolokina, and et al. 2020. "Erythropoietin Mimetic Peptide (pHBSP) Corrects Endothelial Dysfunction in a Rat Model of Preeclampsia" International Journal of Molecular Sciences 21, no. 18: 6759. https://doi.org/10.3390/ijms21186759
APA StyleKorokin, M., Gureev, V., Gudyrev, O., Golubev, I., Korokina, L., Peresypkina, A., Pokrovskaia, T., Lazareva, G., Soldatov, V., Zatolokina, M., Pobeda, A., Avdeeva, E., Beskhmelnitsyna, E., Denisyuk, T., Avdeeva, N., Bushueva, O., & Pokrovskii, M. (2020). Erythropoietin Mimetic Peptide (pHBSP) Corrects Endothelial Dysfunction in a Rat Model of Preeclampsia. International Journal of Molecular Sciences, 21(18), 6759. https://doi.org/10.3390/ijms21186759







