Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus)
Abstract
:1. Introduction
2. Results
2.1. Conformational Study
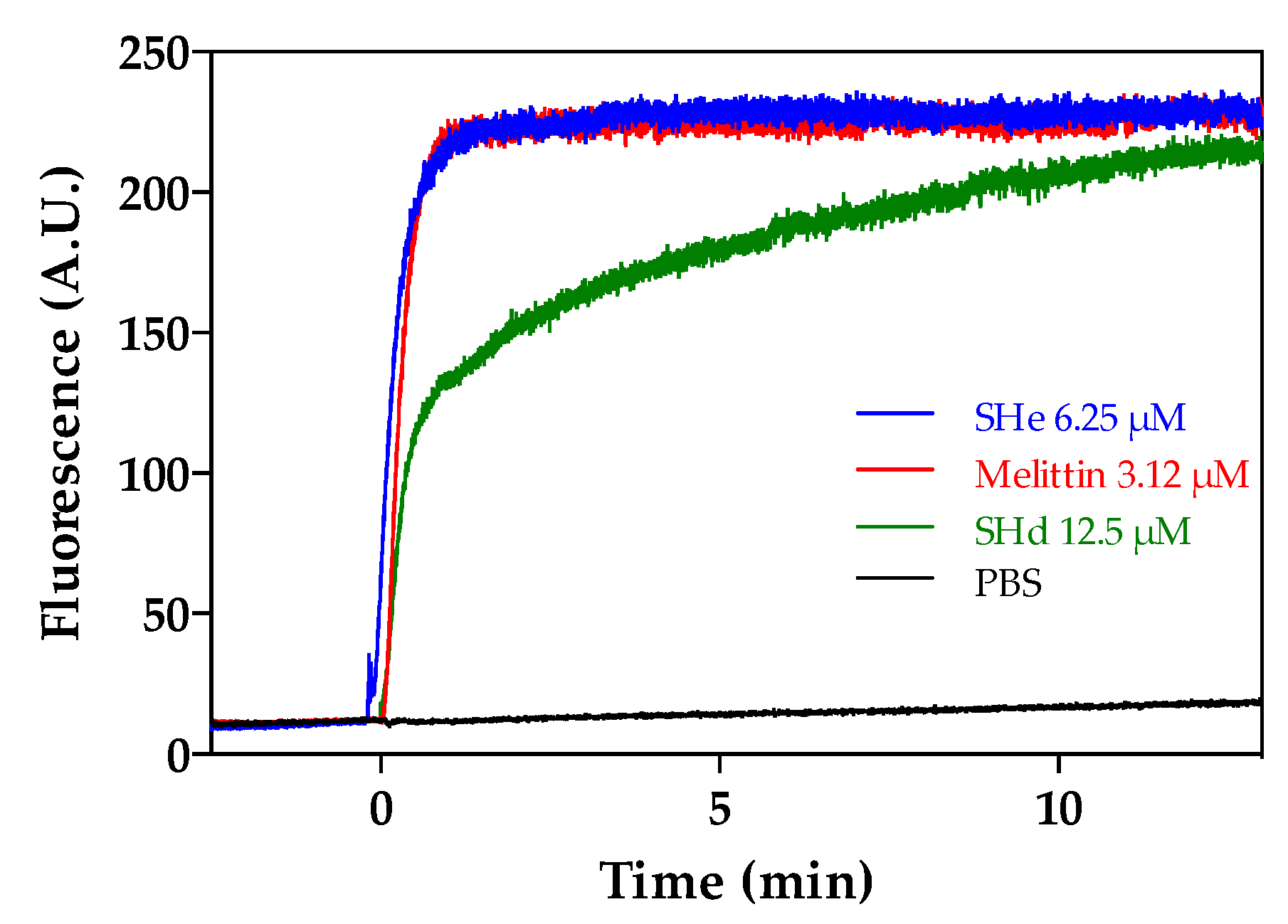
2.2. Interaction of Temporin-SHe with a Bacterial Membrane Model
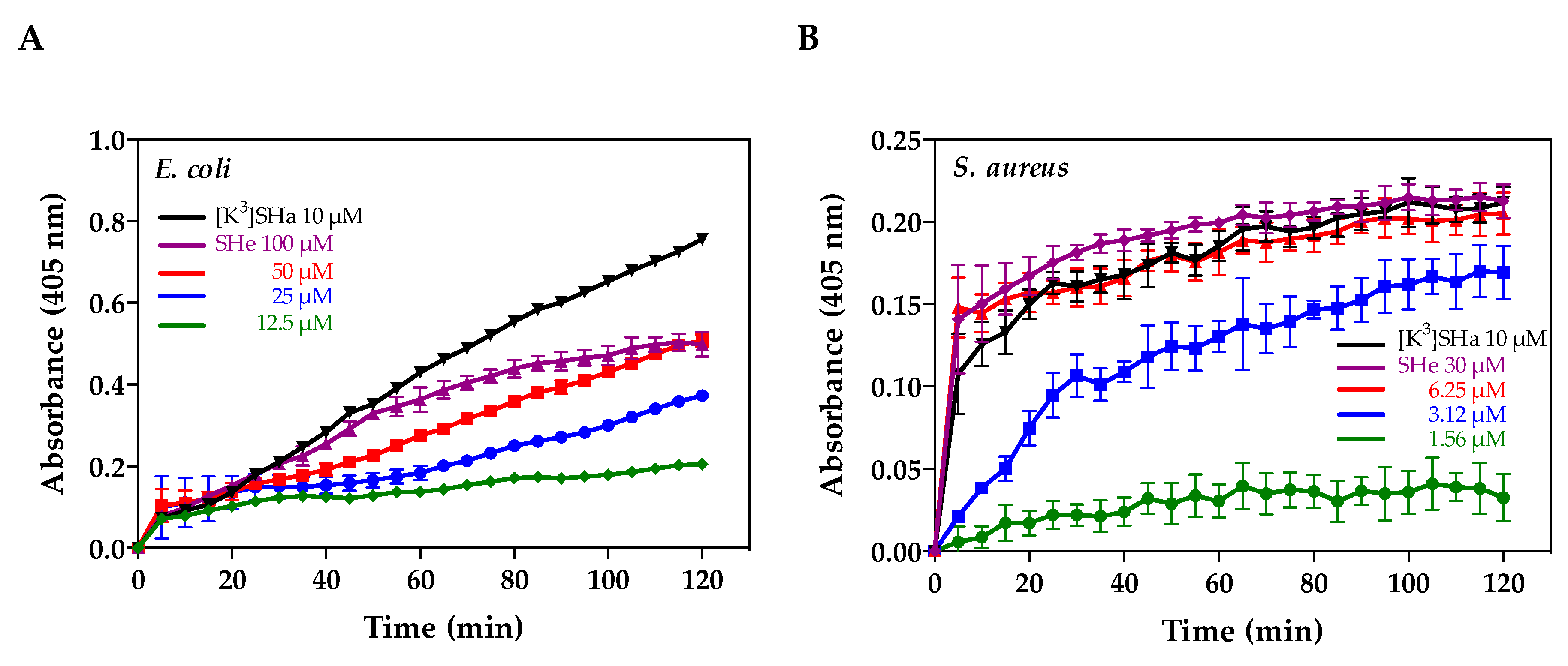
2.3. Antimicrobial Activities
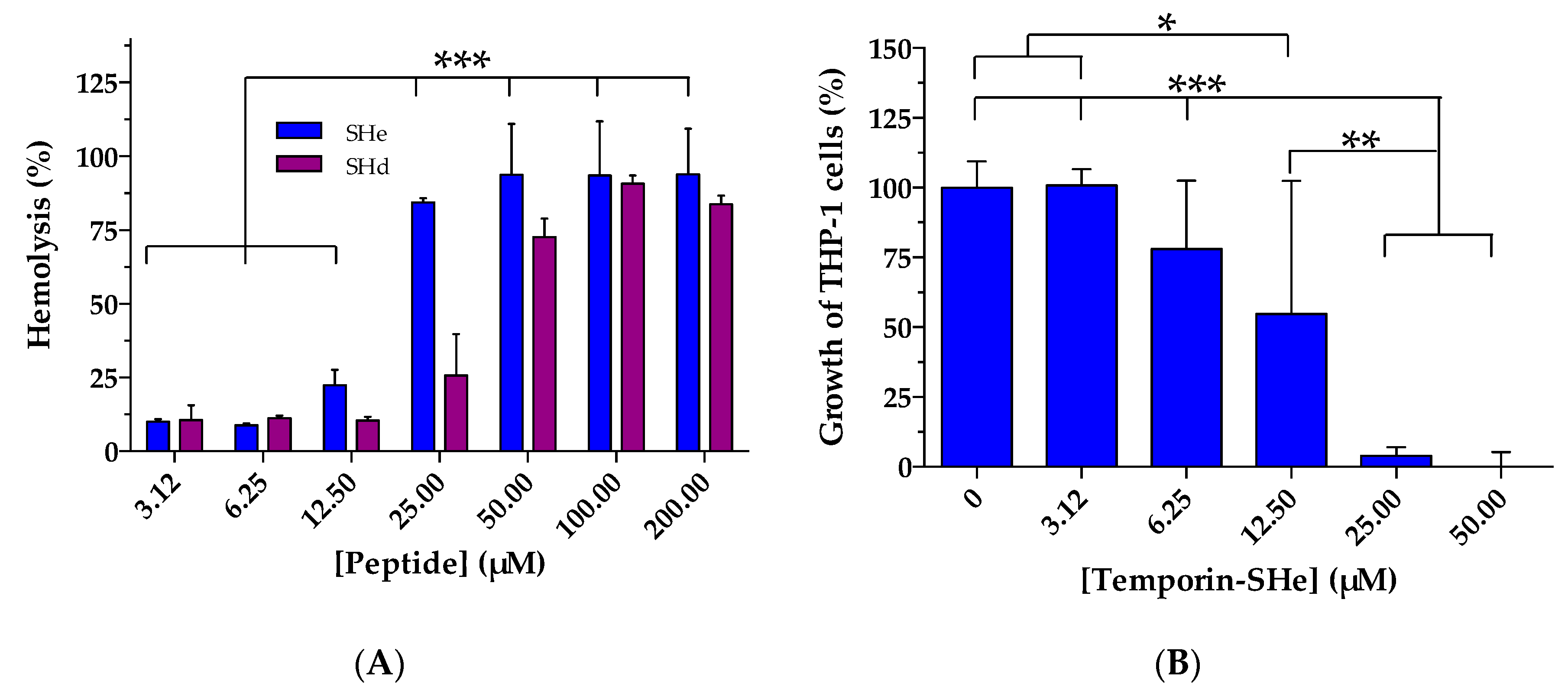
2.4. Cytotoxic Activities
2.5. Alteration of Bacterial Membranes
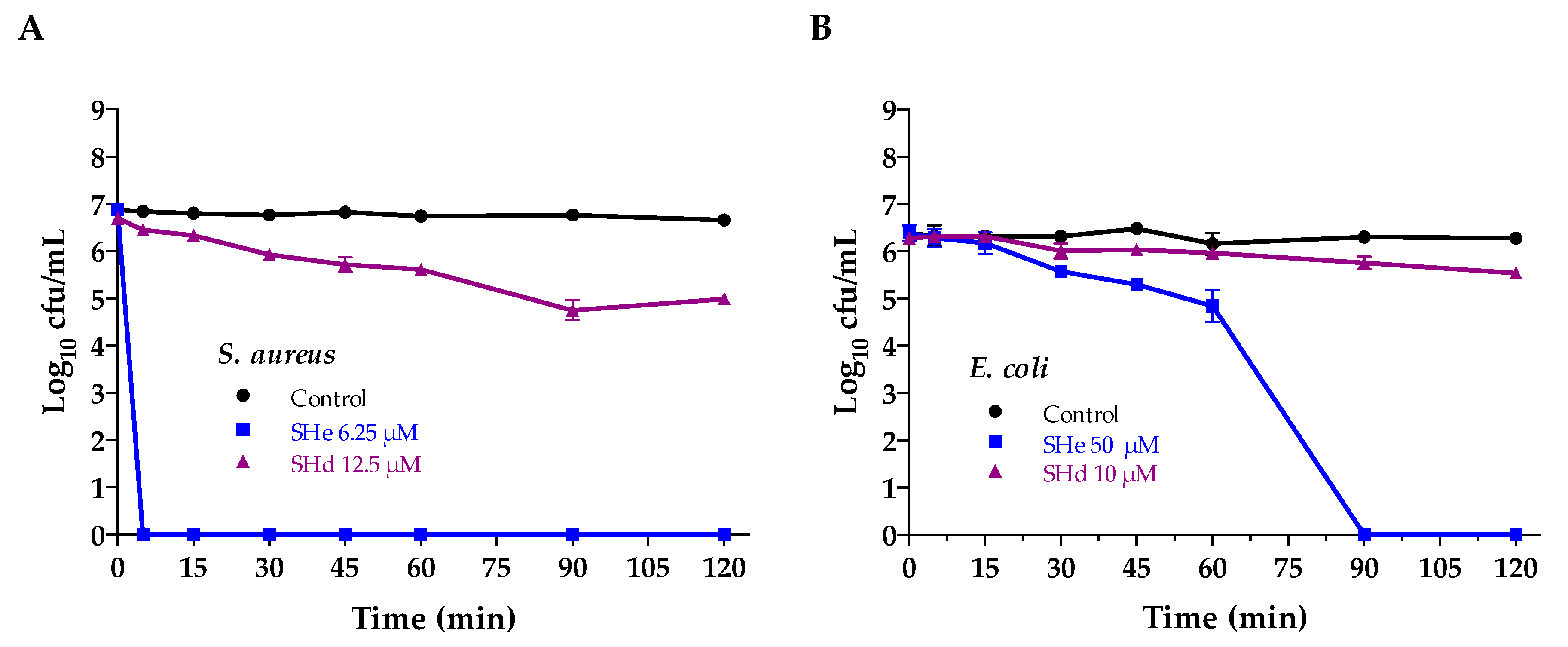
2.6. Bacterial Killing
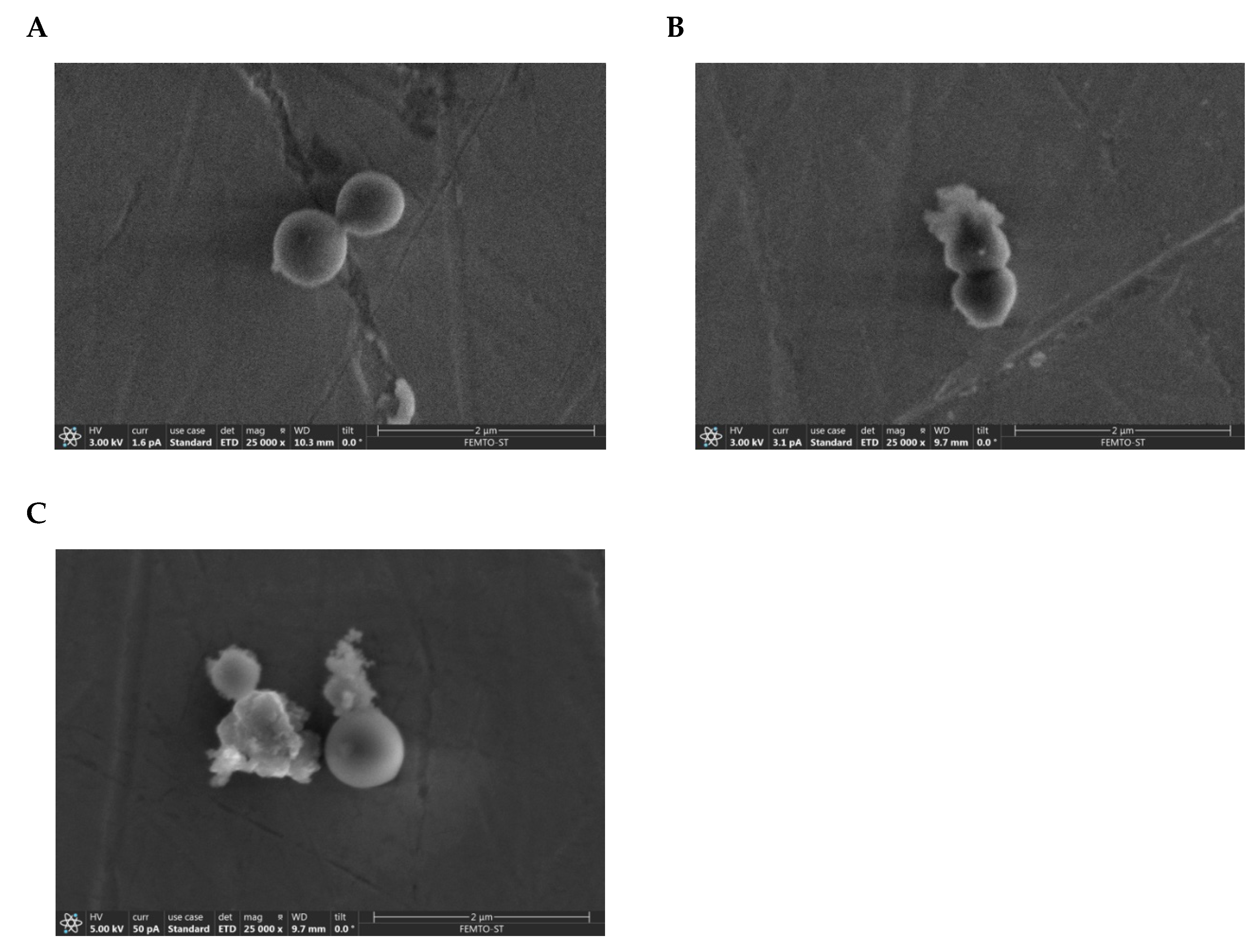
2.7. Visualization of the Membranolytic Effect of Temporin-SHe on S. aureus Bacteria
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Conformational Study
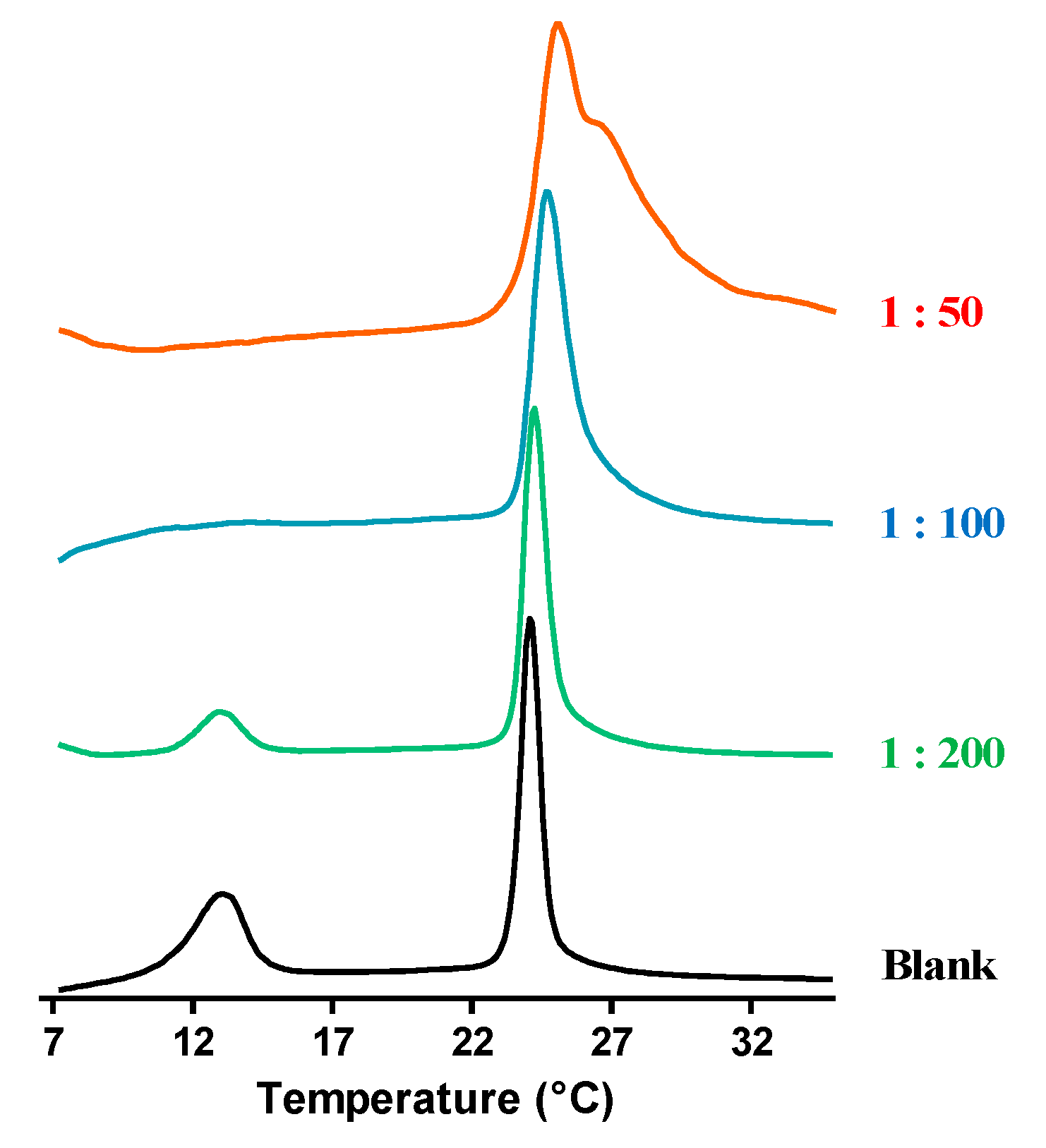
4.3. Differential Scanning Calorimetry
4.4. Microorganisms and Cells
4.5. Antibacterial and Antifungal Activities
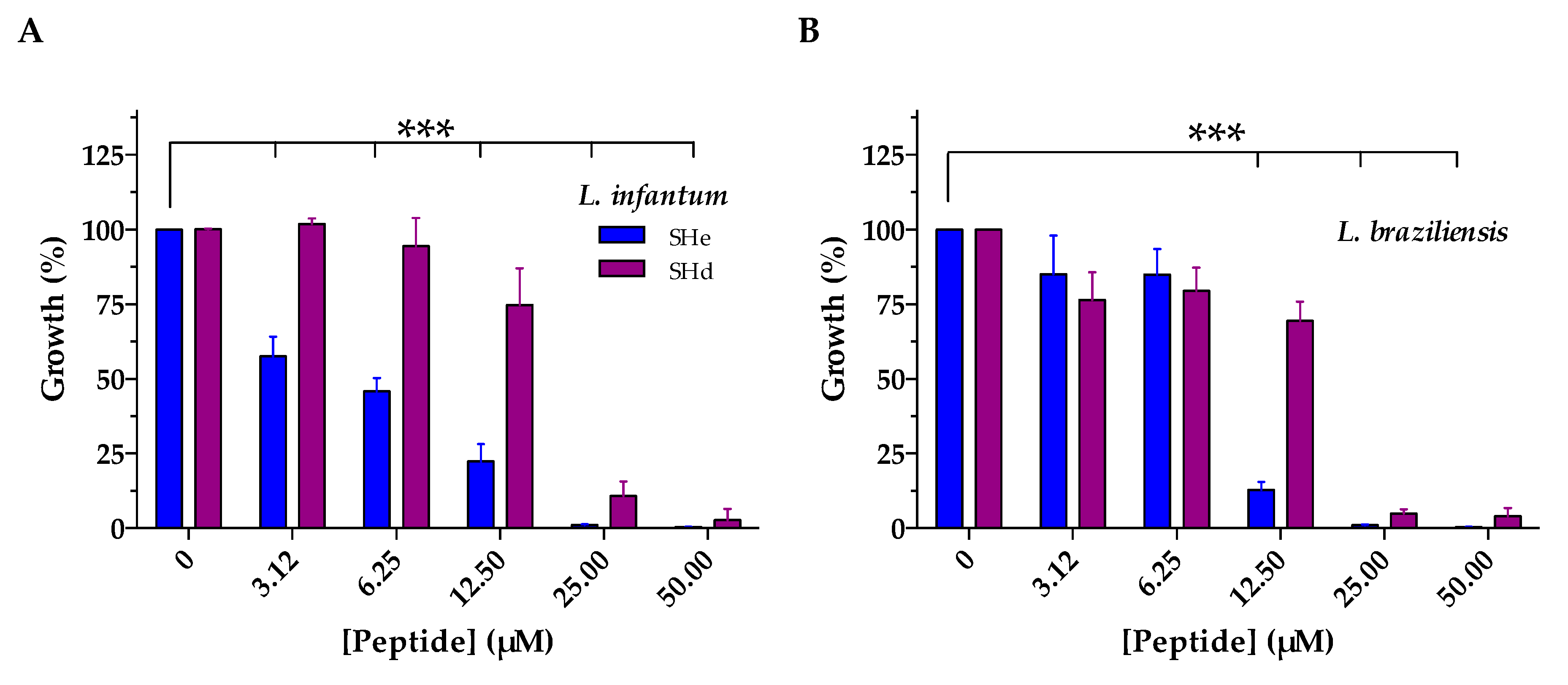
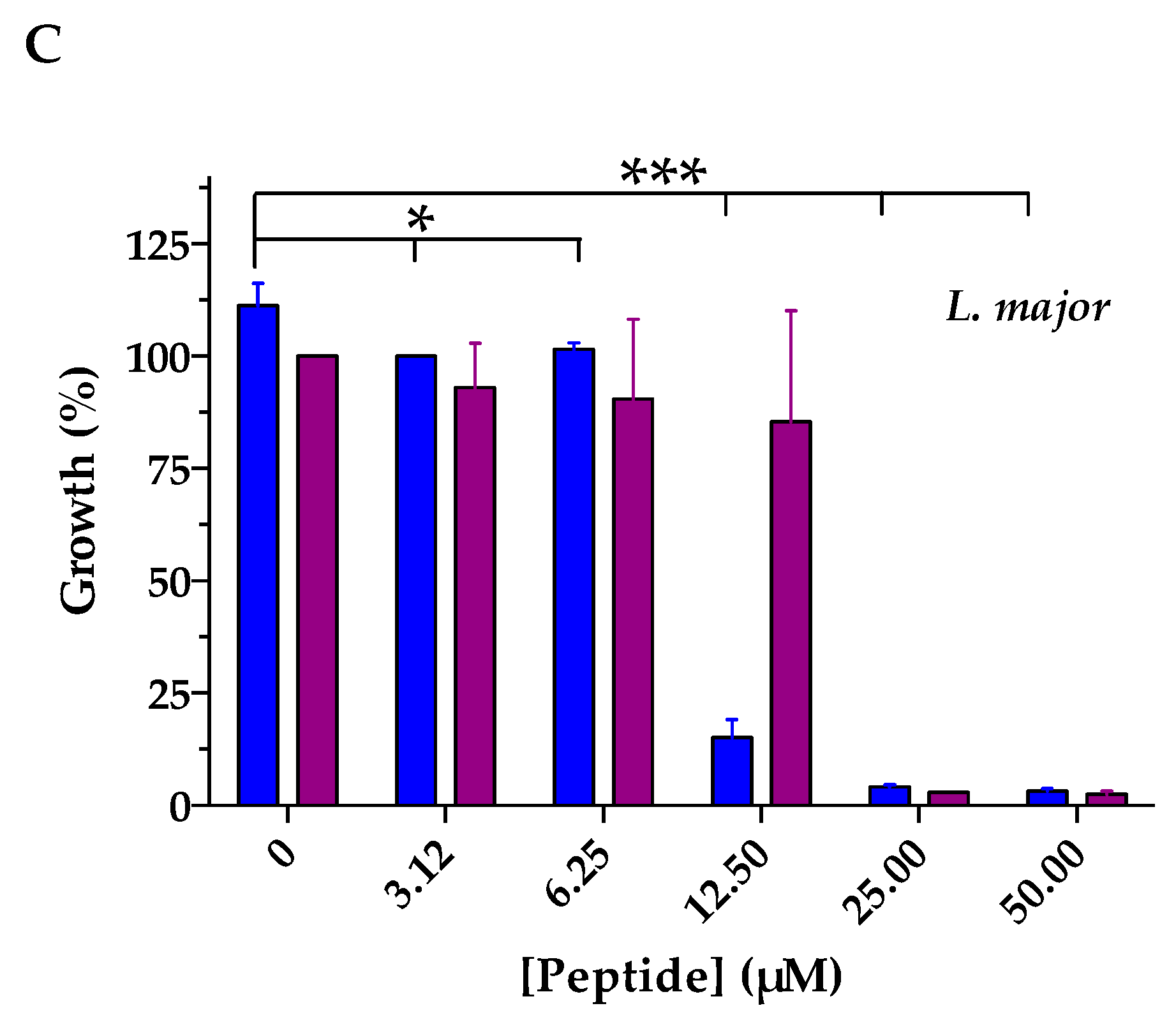
4.6. Antiparasitic Activity
4.7. Cytotoxic Activity
4.8. Membrane Permeabilization Assay
4.9. Membrane Depolarization Assay
4.10. Time-Killing Assay
4.11. Scanning Electron Microscopy (SEM) Imaging
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial peptide action on parasites. Curr. Drug Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Grazia, A.D.; Cappiello, F.; Casciaro, B.; Luca, V. Naturally occurring peptides from Rana temporaria: Antimicrobial properties and more. Curr. Top. Med. Chem. 2016, 16, 54–64. [Google Scholar] [CrossRef]
- Guimarães, A.B.; Costa, F.J.; Pires, O.R.; Fontes, W.; Castro, M.S. The amazing world of peptide engineering: The example of antimicrobial peptides from frogs and their analogues. Protein Pept. Lett. 2016, 23, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from ranid frogs: Taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta 2004, 1696, 1–14. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Abbassi, F.; Lequin, O.; Piesse, C.; Goasdoué, N.; Foulon, T.; Nicolas, P.; Ladram, A. Temporin-SHf, a new type of phe-rich and hydrophobic ultrashort antimicrobial peptide. J. Biol. Chem. 2010, 285, 16880–16892. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’amato, G.; Silvestri, C.; Del Prete, M.S.; Licci, A.; Lukasiak, J.; Scalise, G. In vitro activity and killing effect of temporin A on nosocomial isolates of Enterococcus faecalis and interactions with clinically used antibiotics. J. Antimicrob. Chemother. 2005, 55, 272–274. [Google Scholar] [CrossRef]
- Wade, D.; Silberring, J.; Soliymani, R.; Heikkinen, S.; Kilpeläinen, I.; Lankinen, H.; Kuusela, P. Antibacterial activities of temporin A analogs. FEBS Lett. 2000, 479, 6–9. [Google Scholar] [CrossRef]
- Ghiselli, R.; Giacometti, A.; Cirioni, O.; Mocchegiani, F.; Orlando, F.; Kamysz, W.; Del Prete, M.S.; Lukasiak, J.; Scalise, G.; Saba, V. Temporin A as a prophylactic agent against methicillin sodium-susceptible and methicillin sodium-resistant Staphylococcus epidermidis vascular graft infection. J. Vasc. Surg. 2002, 36, 1027–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbassi, F.; Oury, B.; Blasco, T.; Sereno, D.; Bolbach, G.; Nicolas, P.; Hani, K.; Amiche, M.; Ladram, A. Isolation, characterization and molecular cloning of new temporins from the skin of the North African ranid Pelophylax saharica. Peptides 2008, 29, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Raja, Z.; André, S.; Abbassi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef] [Green Version]
- Abbassi, F.; Raja, Z.; Oury, B.; Gazanion, E.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Ladram, A. Antibacterial and leishmanicidal activities of temporin-SHd, a 17-residue long membrane-damaging peptide. Biochimie 2013, 95, 388–399. [Google Scholar] [CrossRef]
- Rinaldi, A.C.; Mangoni, M.L.; Rufo, A.; Luzi, C.; Barra, D.; Zhao, H.; Kinnunen, P.K.; Bozzi, A.; Di Giulio, A.; Simmaco, M. Temporin L: Antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 2002, 368, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Urbán, E.; Nagy, E.; Pál, T.; Sonnevend, A.; Conlon, J.M. Activities of four frog skin-derived antimicrobial peptides (temporin-1DRa, temporin-1Va and the melittin-related peptides AR-23 and RV-23) against anaerobic bacteria. Int. J. Antimicrob. Agents 2007, 29, 317–321. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Saugar, J.M.; Dellisanti, M.; Barra, D.; Simmaco, M.; Rivas, L. Temporins, small antimicrobial peptides with leishmanicidal activity. J. Biol. Chem. 2005, 280, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Eggimann, G.A.; Sweeney, K.; Bolt, H.L.; Rozatian, N.; Cobb, S.L.; Denny, P.W. The role of phosphoglycans in the susceptibility of Leishmania mexicana to the temporin family of anti-microbial peptides. Molecules 2015, 20, 2775–2785. [Google Scholar] [CrossRef] [Green Version]
- Chinchar, V.G.; Bryan, L.; Silphadaung, U.; Noga, E.; Wade, D.; Rollins-Smith, L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 2004, 323, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The amphibian antimicrobial peptide temporin B inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62, e02367-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Lebeau, L.; Chessa, C.; Damour, A.; Ladram, A.; Oury, B.; Boutolleau, D.; Bodet, C.; Lévêque, N. Comparison of anti-viral activity of frog skin anti-microbial peptides temporin-Sha and [K³]SHa to LL-37 and temporin-Tb against herpes simplex virus type 1. Viruses 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbassi, F.; Galanth, C.; Amiche, M.; Saito, K.; Piesse, C.; Zargarian, L.; Hani, K.; Nicolas, P.; Lequin, O.; Ladram, A. Solution structure and model membrane interactions of temporins-SH, antimicrobial peptides from amphibian skin. A NMR spectroscopy and differential scanning calorimetry study. Biochemistry 2008, 47, 10513–10525. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Piesse, C.; Foulon, T.; Nicolas, P.; Ladram, A. Effects of residue 5-point mutation and N-terminus hydrophobic residues on temporin-SHc physicochemical and biological properties. Mol. Cell. Biochem. 2014, 394, 91–99. [Google Scholar] [CrossRef]
- Carotenuto, A.; Malfi, S.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.; Mangoni, M.L.; Gaddi, L.M.; Novellino, E.; Grieco, P. A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins A and L. J. Med. Chem. 2008, 51, 2354–2362. [Google Scholar] [CrossRef]
- Malgieri, G.; Avitabile, C.; Palmieri, M.; D’Andrea, L.D.; Isernia, C.; Romanelli, A.; Fattorusso, R. Structural basis of a temporin 1b analogue antimicrobial activity against Gram negative bacteria determined by CD and NMR techniques in cellular environment. ACS Chem. Biol. 2015, 10, 965–969. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [Green Version]
- Mahalka, A.K.; Kinnunen, P.K. Binding of amphipathic alpha-helical antimicrobial peptides to lipid membranes: Lessons from temporins B and L. Biochim. Biophys. Acta 2009, 1788, 1600–1609. [Google Scholar] [CrossRef] [Green Version]
- André, S.; Washington, S.K.; Darby, E.; Vega, M.M.; Filip, A.D.; Ash, N.S.; Muzikar, K.A.; Piesse, C.; Foulon, T.; O’Leary, D.J.; et al. Structure-activity relationship-based optimization of small temporin-SHf analogs with potent antibacterial activity. ACS Chem. Biol. 2015, 10, 2257–2266. [Google Scholar]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef] [Green Version]
- Crépin, A.; Jégou, J.F.; André, S.; Ecale, F.; Croitoru, A.; Cantereau, A.; Berjeaud, J.M.; Ladram, A.; Verdon, J. In vitro and intracellular activities of frog skin temporins against Legionella pneumophila and its eukaryotic hosts. Sci. Rep. 2020, 10, 3978. [Google Scholar]
- Lombana, A.; Raja, Z.; Casale, S.; Pradier, C.M.; Foulon, T.; Ladram, A.; Humblot, V. Temporin-SHa peptides grafted on gold surfaces display antibacterial activity. J. Pept. Sci. 2014, 20, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.P.; Lewis, R.N.; McElhaney, R.N. Calorimetric and spectroscopic studies of the thermotropic phase behavior of the n-saturated 1, 2-diacylphosphatidylglycerols. Biophys. J. 1997, 72, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Rex, S. Pore formation induced by the peptide melittin in different lipid vesicle membranes. Biophys. Chem. 1996, 58, 75–85. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic alpha helical antimicrobial peptides. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef]
- Seto, G.W.; Marwaha, S.; Kobewka, D.M.; Lewis, R.N.; Separovic, F.; McElhaney, R.N. Interactions of the Australian tree frog antimicrobial peptides aurein 1.2, citropin 1.1 and maculatin 1.1 with lipid model membranes: Differential scanning calorimetric and Fourier transform infrared spectroscopic studies. Biochim. Biophys. Acta 2007, 1768, 2787–2800. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.F.; Schmitt, M.A.; Gellman, S.H.; Epand, R.M. Role of membrane lipids in the mechanism of bacterial species selective toxicity by two alpha/beta-antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.W. Action of antimicrobial peptides: Two-state model. Biochemistry 2000, 39, 8347–8352. [Google Scholar] [CrossRef]
- Chou, H.T.; Kuo, T.Y.; Chiang, J.C.; Pei, M.J.; Yang, W.T.; Yu, H.C.; Lin, S.B.; Chen, W.J. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int. J. Antimicrob. Agents 2008, 32, 130–138. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Hollmann, A.; Martínez, M.; Noguera, M.E.; Augusto, M.T.; Disalvo, A.; Santos, N.C.; Semorile, L.; Maffía, P.C. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids Surf. B Biointerfaces 2016, 141, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Luca, V.; Segev-Zarko, L.A.; Shai, Y.; Mangoni, M.L. Temporins A and B stimulate migration of HaCaT keratinocytes and kill intracellular Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 2520–2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golda, A.; Kosikowska-Adamus, P.; Kret, A.; Babyak, O.; Wójcik, K.; Dobosz, E.; Potempa, J.; Lesner, A.; Koziel, J. The bactericidal activity of temporin analogues against methicillin resistant Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 4761. [Google Scholar] [CrossRef] [Green Version]
- Simmaco, M.; De Biase, D.; Severini, C.; Aita, M.; Erspamer, G.F.; Barra, D.; Bossa, F. Purification and characterization of bioactive peptides from skin extracts of Rana esculenta. Biochim. Biophys. Acta 1990, 1033, 318–323. [Google Scholar] [CrossRef]
- Ali, M.F.; Knoop, F.C.; Vaudry, H.; Conlon, J.M. Characterization of novel antimicrobial peptides from the skins of frogs of the Rana esculenta complex. Peptides 2003, 24, 955–961. [Google Scholar] [CrossRef]
- Conlon, J.M. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 2008, 29, 1815–1819. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Guo, C.; Zhang, W.; Zhang, X.; Zhang, B.; Li, S.; Ren, J.; Hu, Y.; Wang, H. Identification and functional analyses of novel antioxidant peptides and antimicrobial peptides from skin secretions of four East Asian frog species. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Hu, Y.; Li, J.; Liu, Y.; Li, S.; Yan, K.; Wang, X.; Liu, J.; Wang, H. Identification of multiple peptides with antioxidant and antimicrobial activities from skin and its secretions of Hylarana taipehensis, Amolops lifanensis, and Amolops granulosus. Biochimie 2014, 105, 192–201. [Google Scholar] [CrossRef]
- Raja, Z.; André, S.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Oury, B.; Ladram, A. Structure, antimicrobial activities and mode of interaction with membranes of novel phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii. PLoS ONE 2013, 8, e70782. [Google Scholar]

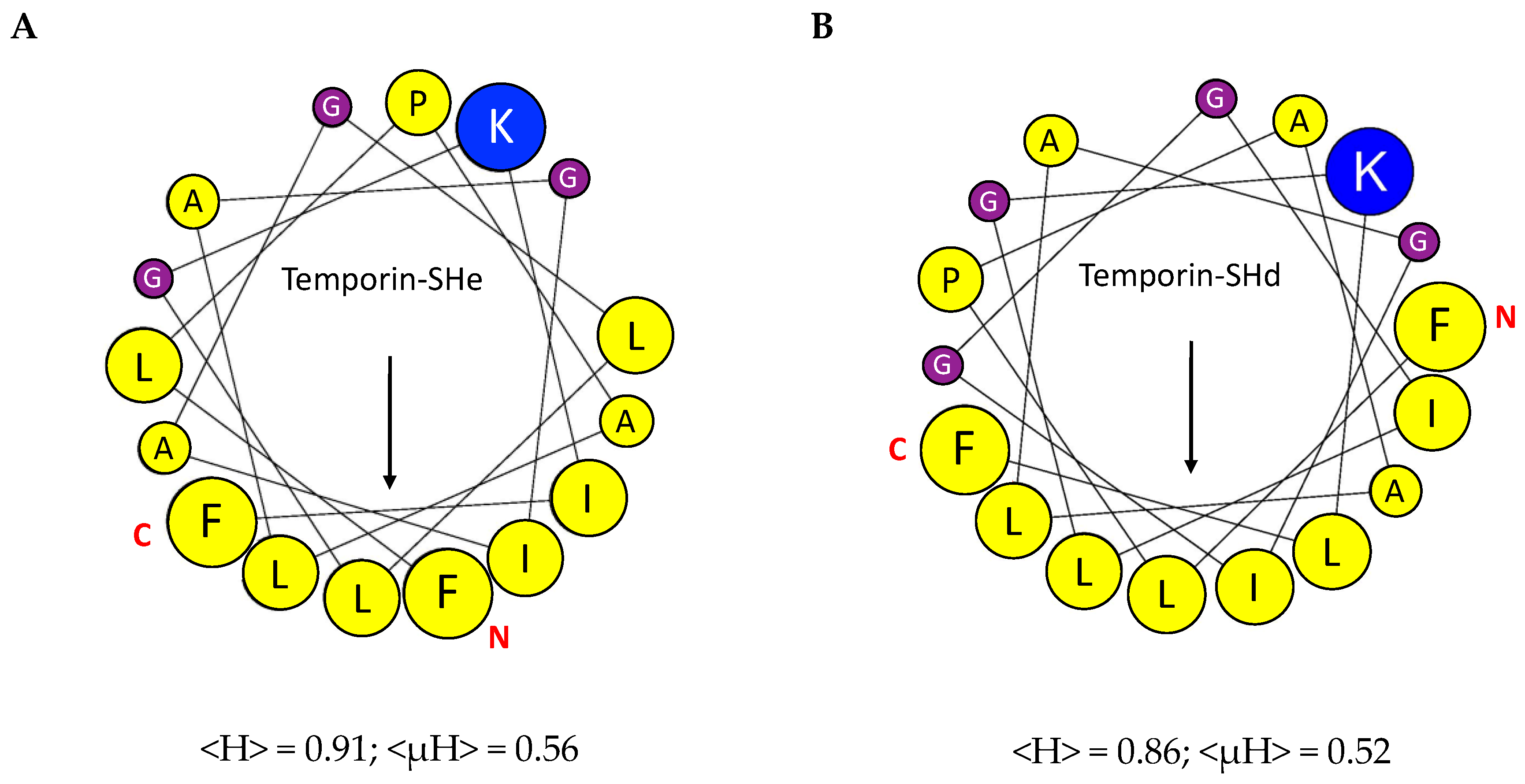
| Temporin | Sequence Alignment 1 | Reference | Residue | Net Charge 2 | Mw 3 | GRAVY 4 |
|---|---|---|---|---|---|---|
| SHa | FLSGIVGMLGKLFamide | [13,14,23] | 13 | +2 | 1381.74 | 1.67 |
| SHb | FLPIVTNLLSGLLamide | [13,23] | 13 | +1 | 1399.74 | 1.81 |
| SHc | FLSHIAGFLSNLFamide | [13,23,24] | 13 | +1 | 1465.71 | 1.34 |
| SHd | FLPAALAGIGGILGKLFamide | [15] | 17 | +2 | 1658.06 | 1.65 |
| SHe | FLP-ALAGIAGLLGKIFamide | [8] | 16 | +2 | 1601.01 | 1.78 |
| SHf | FFFLSRIFamide | [8] | 8 | +2 | 1076.31 | 1.77 |
| MIC (µM) 1 | ||
|---|---|---|
| Temporin-SHe | Temporin-SHd | |
| Gram-negative bacteria | ||
| E. coli ATCC 25922 | 25 | 5 * |
| E. coli ATCC 35218 | 50 | 50 * |
| E. coli ML-35p | 50 | 25 * |
| P. aeruginosa ATCC 27853 | 60 | >200 * |
| S. enterica2 | 100 | >200 * |
| A. baumannii ATCC 19606 | 25 | 25 * |
| K. pneumoniae ATCC 13883 | 100 | 100 * |
| Gram-positive bacteria | ||
| S. aureus ATCC 25923 | 3.12 | 6.25 * |
| S. aureus ATCC 43300 3 | 3.12 | 6.25 * |
| S. aureus ATCC BAA-44 4 | 3.12 | 6.25 * |
| S. aureus ST1065 | 3.12 | 6.25 * |
| L. ivanovii | 5 | 10 |
| E. faecalis ATCC 29212 | 12.5 | 25 * |
| B. megaterium | 1.56 | 1.56 * |
| Yeasts/fungi | ||
| C. albicans ATCC 90028 | >100 | 100 * |
| C. parapsilosis ATCC 22019 | 50 | >200 * |
| S. cerevisiae | 12.5 | 25 * |
| IC50 (µM) 1 | ||
|---|---|---|
| Temporin-SHe | Temporin-SHd | |
| L. infantum | 4.6 | 16.5 * |
| L. braziliensis | 10.5 | 17.9 * |
| L. major | 11.6 | 14.6 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, S.; Raja, Z.; Humblot, V.; Piesse, C.; Foulon, T.; Sereno, D.; Oury, B.; Ladram, A. Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus). Int. J. Mol. Sci. 2020, 21, 6713. https://doi.org/10.3390/ijms21186713
André S, Raja Z, Humblot V, Piesse C, Foulon T, Sereno D, Oury B, Ladram A. Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus). International Journal of Molecular Sciences. 2020; 21(18):6713. https://doi.org/10.3390/ijms21186713
Chicago/Turabian StyleAndré, Sonia, Zahid Raja, Vincent Humblot, Christophe Piesse, Thierry Foulon, Denis Sereno, Bruno Oury, and Ali Ladram. 2020. "Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus)" International Journal of Molecular Sciences 21, no. 18: 6713. https://doi.org/10.3390/ijms21186713
APA StyleAndré, S., Raja, Z., Humblot, V., Piesse, C., Foulon, T., Sereno, D., Oury, B., & Ladram, A. (2020). Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus). International Journal of Molecular Sciences, 21(18), 6713. https://doi.org/10.3390/ijms21186713





