Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation
Abstract
1. Introduction
1.1. RF-Positive Polyarticular JIA
1.2. RF-Negative Polyarthritis
1.3. Systemic JIA
1.4. Enthesitis-Related Arthritis (ERA)
1.5. Psoriatic JIA
1.6. Oligoarticular JIA
1.7. Undifferentiated Arthritis
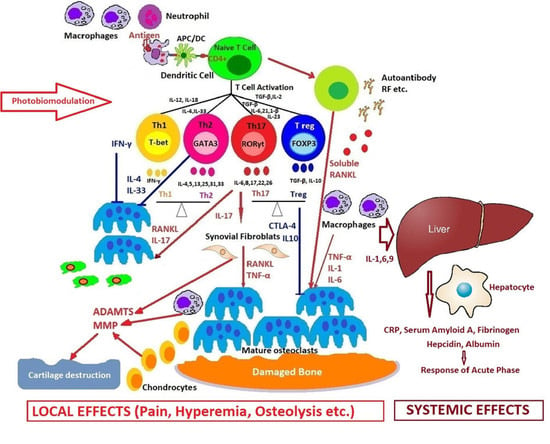
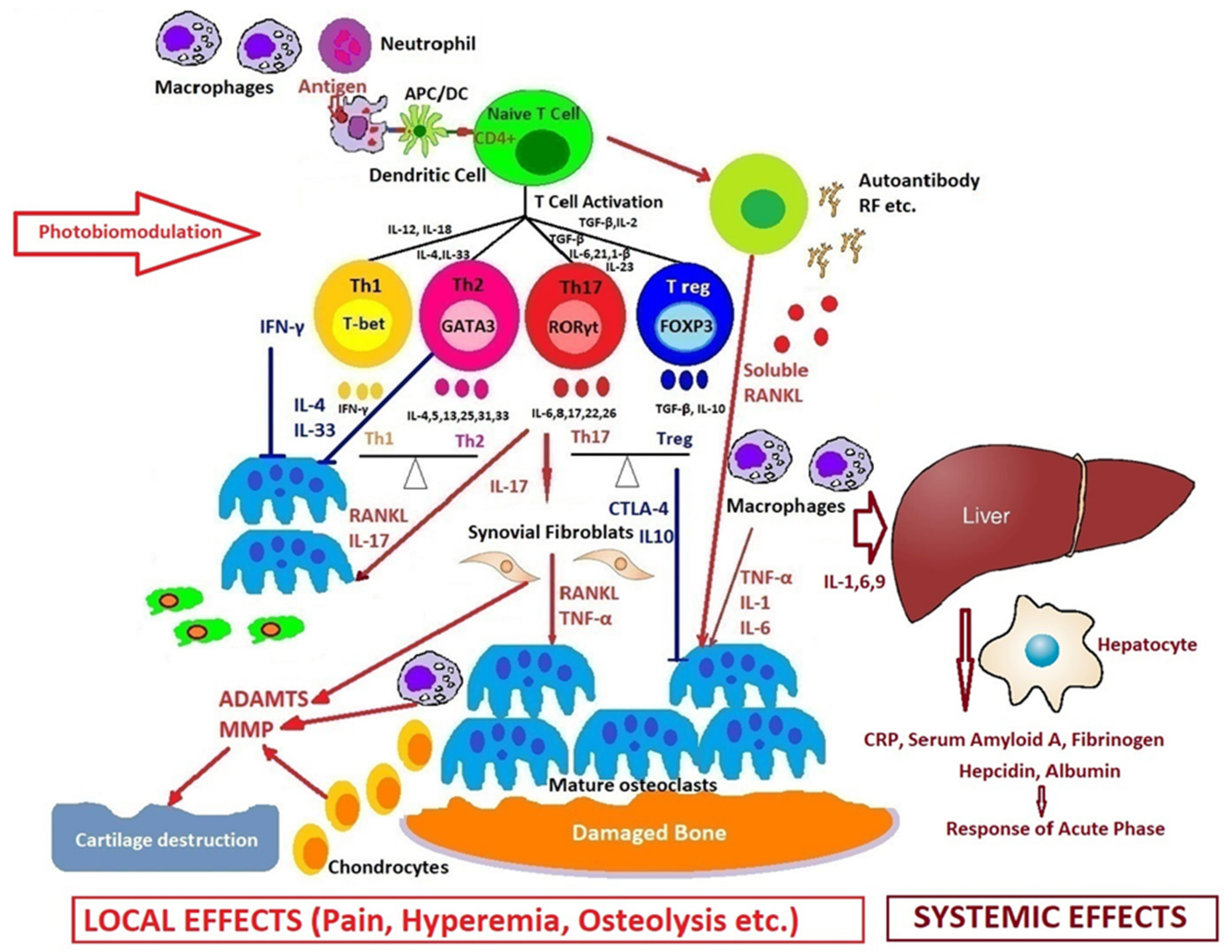
2. Molecular and Cellular Mechanisms of Systemic Arthritis
Macrophages Activation Syndrome
3. Comparative Pathogenesis of Rheumatoid Arthritis in Adults and Children
4. New Introspections and Perspectives on Photobiomodulation in Arthritis
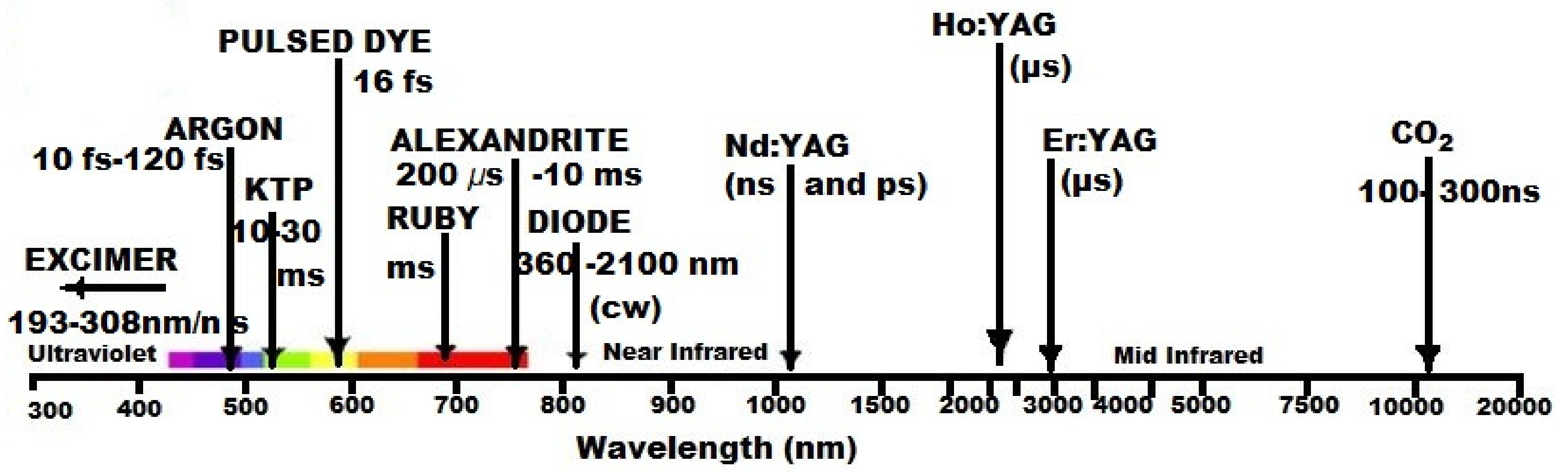
4.1. Photobiomodulation: Short History, Basic Concepts, and Current Applications
4.2. Novel Therapeutics Using Photobiomodulation in Arthritis. Where Are We?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sherry, D.D.; Bhaskar, R.S.A.; Poduval, M.; Rabinovich, C.L.; Myones, B.L. Juvenile Idiopathic Arthritis. Available online: https://emedicine.medscape.com/article/1007276-overview (accessed on 24 August 2020).
- Ailioaie, C.; Ailioaie, L.M. Juvenile idiopathic arthritis. In Management of Chronic Rheumatic Pain; PIM Publishing House: Iaşi, Romania, 2008; pp. 129–146. [Google Scholar]
- Haasnoot, A.J.W.; Sint Jago, N.F.M.; Tekstra, J.; de Boer, J.H. Impact of Uveitis on Quality of Life in Adult Patients with Juvenile Idiopathic Arthritis. Arthritis Care Res. 2017, 69, 1895–1902. [Google Scholar] [CrossRef]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology Classification of Juvenile Idiopathic Arthritis: Second Revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Cassidy, J.T.; Kivlin, J.; Lindsley, C.; Nocton, J. Ophthalmologic Examinations in Children with Juvenile Rheumatoid Arthritis. Pediatrics 2006, 117, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chronic rheumatic conditions. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 19 August 2020).
- Systemic juvenile idiopathic arthritis: Clinical manifestations and diagnosis. Available online: https://www.uptodate.com/contents/systemic-juvenile-idiopathic-arthritis-clinical-manifestations-and-diagnosis (accessed on 19 August 2020).
- Angelis, A.; Kanavos, P.; López-Bastida, J.; Linertová, R.; Serrano-Aguilar, P.; BURQOL-RD Research Network. Socioeconomic costs and health-related quality of life in juvenile idiopathic arthritis: A cost-of-illness study in the United Kingdom. BMC Musculoskelet. Disord. 2016, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Ringold, S.; Khanna, D.; Neogi, T.; Johnson, S.R.; Miller, A.; Brunner, H.I.; Ogawa, R.; Felson, D.; Ogdie, A.; et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. 2015, 67, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Dewint, P.; Hoffman, I.E.A.; Rogge, S.; Joos, R.; Union, A.; Dehoorne, J.; Delanghe, J.; Veys, E.M.; De Keyser, F.; Elewaut, D. Effect of age on prevalence of anticitrullinated protein/peptide antibodies in polyarticular juvenile idiopathic arthritis. Rheumatology 2006, 45, 204–208. [Google Scholar] [CrossRef]
- Packham, J.C.; Hall, M.A.; Pimm, T.J. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: Predictive factors for mood and pain. Rheumatol. (Oxf.) 2002, 41, 1444–1449. [Google Scholar] [CrossRef]
- Gotia, S.; Ailioaie, C.; Ailioaie, L.M. Rheumatic Diseases and Physical Therapy in Children; Tehnopress: Iaşi, Romania, 2004; pp. 140–152. [Google Scholar]
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, ö. Juvenile Idiopathic Arthritis. Balkan Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef]
- Prieur, A.M.; Deslandre, C.J. Les arthrites juvéniles idiopathiques Maladies: Aspects nosologiques actuels. Presse med. 2000, 29, 499–501. [Google Scholar]
- Ailioaie, C. Contributions to the treatment of juvenile chronic arthritis. Ph.D. Thesis, University of Medicine and Pharmacy, Iaşi, Publishing House, Iaşi, Romania, 1996; pp. 102–127. [Google Scholar]
- Chkirate, B.; Aitouamar, H.; Bentahila, A.; Rouichi, A.; Belhadi Mouhid, A. Actualités dans les rheumatismes inflammatoires de l’enfant. Rev. Maghr Pediatr. 2001, 11, 3–8. [Google Scholar]
- Ailioaie, C.; Burdea, M.; Tansanu, I. Evolutionary considerations on juvenile chronic arthritis systemic form at onset. In Recent Advances in Pediatrics; Romanian Society of Pediatrics, Curtea Veche Publishing House: Bucharest, Romania, 1996; p. 603. [Google Scholar]
- Prieur, A.M. Progrès récents en rhumatologie pédiatrique. À propos des formes systèmiques d’arthrite chronique juvénile. Arch. Fr. Pediatr. 1991, 48, 287–289. [Google Scholar] [PubMed]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. For the Pediatric Rheumatology International Trials Organization (PRINTO). J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.G.; Bakker, P.A.; Van der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.G.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: Update by the ASAS MRI working group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Cassidy, J.T.; Petty, R.E. Chronic Arthritis in Childhood. In Textbook of Pediatric Rheumatology, 5th ed.; Cassidy, J.T., Petty, R.E., Laxer, R., Lindlsy, C., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 206–321. [Google Scholar]
- Kim, K.H.; Kim, D.S. Juvenile idiopathic arthritis: Diagnosis and differential diagnosis. Korean J. Pediatr. 2010, 53, 931–935. [Google Scholar] [CrossRef]
- Martini, A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann. Rheum. Dis. 2012, 71, 1437–1479. [Google Scholar] [CrossRef]
- Moretti, D.; Cianchi, I.; Vannucci, G.; Cimaz, R.; Simonini, G. Psoriatic Juvenile Idiopathic Arthritis Associated with Uveitis: A Case Report. Case Rep. Rheumatol. 2013, 595890. [Google Scholar] [CrossRef]
- Ravelli, A.; Varnier, G.C.; Oliveira, S.; Castell, E.; Arguedas, O.; Magnani, A.; Pistorio, A.; Ruperto, N.; Magni-Manzoni, S.; Galasso, R.; et al. Antinuclear antibody-positive patients should be grouped as a separate category in the classification of juvenile idiopathic arthritis. Arthritis Rheum. 2011, 63, 267–275. [Google Scholar] [CrossRef]
- Ailioaie, C.; Ailioaie, L.M. Progresses and perspectives in chronic arthritis in children. Rev. Med. Chir. Soc. Med. Nat. 2004, 108, 415–423. [Google Scholar]
- Merino, R.; de Inocencio, J.; García-Consuegra, J. Evaluation of revised International League of Associations for Rheumatology classification criteria for juvenile idiopathic arthritis in Spanish children (Edmonton 2001). J. Rheumatol. 2005, 32, 559–561. [Google Scholar]
- Ailioaie, C. Idiopathic Juvenile Arthritis Family Guide; CERMI Technical, Scientific and Didactic Publishing House: Iaşi, Romania, 2005; pp. 36–41. ISBN 973-667-109-7. [Google Scholar]
- Pascual, V.; Allantaz, F.; Arce, E.; Punaro, M.; Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005, 201, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Woo, P. Systemic juvenile idiopathic arthritis: Diagnosis, management, and outcome. Nat. Clin. Pract. Rheumatol. 2006, 2, 28–34. [Google Scholar] [CrossRef]
- Mellins, E.D.; Macaubas, C.; Grom, A.A. Pathogenesis of systemic juvenile idiopathic arthritis: Some answers, more questions. Nat. Rev. Rheumatol. 2011, 7, 416–426. [Google Scholar] [CrossRef]
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Schneider, R. Systemic Juvenile Idiopathic Arthritis and Adult Onset Still Disease. In Textbook of Autoinflammation; Hashkes, P., Laxer, R., Simon, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 587–616. [Google Scholar] [CrossRef]
- Singh-Grewal, D.; Schneider, R.; Bayer, N.; Feldman, B.M. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: Significance of early clinical and laboratory features. Arthritis Rheum. 2006, 54, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Ramanan, A.V. Recognition and management of macrophage activation syndrome in juvenile arthritis. Curr. Opin. Rheumatol. 2007, 19, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Behrens, E.M. Hyperinflammation, rather than hemophagocytosis, is the common link between macrophage activation syndrome and hemophagocytic lymphohistiocytosis. Curr. Opin. Rheumatol. 2014, 26, 562–569. [Google Scholar] [CrossRef]
- Put, K.; Avau, A.; Brisse, E.; Mitera, T.; Put, S.; Proost, P.; Bader-Meunier, B.; Westhovens, R.; Van den Eynde, B.J.; Orabona, C.; et al. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: Tipping the balance between interleukin-18 and interferon-γ. Rheumatol. (Oxf. Engl.) 2015, 54, 1507–1517. [Google Scholar] [CrossRef]
- Ramanan, A.V.; Grom, A.A. Does systemic-onset juvenile idiopathic arthritis belong under juvenile idiopathic arthritis? Rheumatol. (Oxf.) 2005, 44, 1350–1353. [Google Scholar] [CrossRef]
- Barnes, M.G.; Grom, A.A.; Thompson, S.D.; Griffin, T.A.; Pavlidis, P.; Itert, L.; Fall, N.; Sowders, D.P.; Hinze, C.H.; Aronow, B.J.; et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 2101–2112. [Google Scholar] [CrossRef]
- Fishman, D.; Faulds, G.; Jeffery, R.; Mohamed-Ali, V.; Yudkin, J.S.; Humphries, S.; Woo, P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 1998, 102, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, E.M.; Fife, M.S.; Thompson, S.D.; Twine, N.; Tsoras, M.; Moroldo, M.; Fisher, S.A.; Lewis, C.M.; Prieur, A.-M.; Glass, D.N.; et al. The -174G allele of the interleukin-6 gene confers susceptibility to systemic arthritis in children: A multicenter study using simplex and multiplex juvenile idiopathic arthritis families. Arthritis Rheum. 2003, 48, 3202–3206. [Google Scholar] [CrossRef] [PubMed]
- Fife, M.S.; Gutierrez, A.; Ogilvie, E.M.; Stock, C.J.; Samuel, J.M.; Thomson, W.; Mack, L.F.; Lewis, G.M.; Woo, P. Novel IL10 gene family associations with systemic juvenile idiopathic arthritis. Arthritis Res. Ther. 2006, 8, R148. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.C.; Paul, D.; Ganser, G.; Range, U.; Gahr, M.; Kelsch, R.; Rösen-Wolff, A.; Hedrich, C.M. IL10 promoter polymorphisms are associated with systemic onset juvenile idiopathic arthritis (SoJIA). Clin. Exp. Rheumatol. 2010, 28, 912–918. [Google Scholar] [PubMed]
- Fall, N.; Barnes, M.G.; Thornton, S.; Luyrink, L.; Olson, J.; Ilowite, N.T.; Gottlieb, B.S.; Griffin, T.; Sherry, D.D.; Thompson, S.; et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007, 56, 3793–3804. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, P.A. Review: Is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 2014, 66, 1405–1413. [Google Scholar] [CrossRef]
- Verbsky, J.; White, A. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J. Rheumatol. 2004, 31, 2071–2075. [Google Scholar]
- Irigoyen, P.I.; Olson, J.; Hom, C.; Ilowite, N.T. Treatment of systemic onset juvenile rheumatoid arthritis with anakinra. Arthritis Rheum. 2004, 50, S437. [Google Scholar]
- Henrickson, M. Efficacy of anakinra in refractory systemic arthritis. Arthritis Rheum. 2004, 50, S438. [Google Scholar]
- Sikora, K.A.; Grom, A.A. Update on the pathogenesis and treatment of systemic idiopathic arthritis. Curr. Opin. Pediatr. 2011, 23, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Toplak, N.; Blazina, Š.; Avčin, T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: Current status and future perspectives. Drug Des. Devel Ther. 2018, 12, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- De Benedetti, F.; Massa, M.; Robbioni, P.; Ravelli, A.; Burgio, G.R.; Martini, A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Tateiwa, D.; Yoshikawa, H.; Kaito, T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 2019, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Muller, K.; Herner, E.B.; Stagg, A.; Bendtzen, K.; Woo, P. Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Br. J. Rheumatol. 1998, 37, 562–569. [Google Scholar] [CrossRef][Green Version]
- Imbrechts, M.; Avau, A.; Vandenhaute, J.; Malengier-Devlies, B.; Put, K.; Mitera, T.; Berghmans, N.; Burton, O.; Junius, S.; Liston, A.; et al. Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis. J. Immunol. 2018, 201, 2654–2663. [Google Scholar] [CrossRef]
- Vandenhaute, J.; Avau, A.; Filtjens, J.; Malengier-Devlies, B.; Imbrechts, M.; Van den Berghe, N.; Ahmadzadeh, K.; Mitera, T.; Boon, L.; Leclercq, G.; et al. Regulatory Role for NK Cells in a Mouse Model of Systemic Juvenile Idiopathic Arthritis. J. Immunol. 2019, 203, 3339–3348. [Google Scholar] [CrossRef]
- Put, K. Interferon-Gamma en ‘Natural Killer’ Cellen in Systemische Juveniele Idiopathische Artritis en Macrofaagactivatiesyndroom. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2015. Available online: https://limo.libis.be/primo-explore/fulldisplay?docid=LIRIAS1907034&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1 (accessed on 24 August 2020).
- Put, K.; Vandenhaute, J.; Avau, A.; Van Nieuwenhuijze, A.; Brisse, E.; Dierckx, T.; Rutgeerts, O.; Garcia-Perez, J.; Toelen, J.; Waer, M.; et al. Inflammatory gene expression profile and defective IFN-gamma and granzyme K in natural killer cells of systemic juvenile idiopathic arthritis patients. Arthritis Rheumatol. 2017, 69, 213–224. [Google Scholar] [CrossRef]
- Lerkvaleekul, B.; Vilaiyuk, S. Macrophage activation syndrome: Early diagnosis is key. Open Access Rheumatol. 2018, 10, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Rago, C.; Breda, L.; Cipriani, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Di Battista, C.; Verrotti, A.; Giacomelli, R. Macrophage activation syndrome in Still’s disease: Analysis of clinical characteristics and survival in paediatric and adult patients. Clin. Rheumatol. 2017, 36, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.M.; Beukelman, T.; Paessler, M.; Cron, R.Q. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 2007, 34, 1133–1138. [Google Scholar]
- Ravelli, A.; Davi, S.; Minoia, F.; Martini, A.; Cron, R.Q. Macrophage activation syndrome. Hematol. Oncol. Clin. N. Am. 2015, 29, 927–941. [Google Scholar] [CrossRef]
- Grom, A.A.; Horne, A.; De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016, 12, 259–268. [Google Scholar] [CrossRef]
- Zhang, K.; Biroschak, J.; Glass, D.N.; Thompson, S.D.; Finkel, T.; Passo, M.H.; Binstadt, B.A.; Filipovich, A.; Grom, A.A. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13-4 polymorphisms. Arthritis Rheum. 2008, 58, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Vastert, S.J.; van Wijk, R.; D’Urbano, L.E.; de Vooght, K.M.; de Jager, W.; Ravelli, A.; Magni-Manzoni, S.; Insalaco, A.; Cortis, E.; van Solinge, W.W.; et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatol. (Oxf.) 2010, 49, 441–449. [Google Scholar] [CrossRef]
- Zhang, M.; Behrens, E.M.; Atkinson, T.P.; Shakoory, B.; Grom, A.A.; Cron, R.Q. Genetic defects in cytolysis in macrophage activation syndrome. Curr. Rheumatol. Rep. 2014, 16, 439. [Google Scholar] [CrossRef]
- Davì, S.; Consolaro, A.; Guseinova, D.; Pistorio, A.; Ruperto, N.; Martini, A.; Cron, R.Q.; Ravelli, A.; MAS Study Group. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 764–768. [Google Scholar] [CrossRef]
- Minoia, F.; Davì, S.; Horne, A.C.; Demirkaya, E.; Bovis, F.; Li, C.; Lehmberg, K.; Weitzman, S.; Insalaco, A.; Wouters, C.; et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014, 66, 3160–3169. [Google Scholar] [CrossRef]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.C.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann. Rheum. Dis. 2016, 68, 566–576. [Google Scholar] [CrossRef]
- Fournier, C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine 2005, 72, 527–532. [Google Scholar] [CrossRef]
- Pennock, D.N.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Kasama, T.; Isozaki, T.; Takahashi, R.; Miwa, Y. Clinical effects of tocilizumab on cytokines and immunological factors in patients with rheumatoid arthritis. Int. Immunopharmacol. 2016, 35, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Feist, E.; Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, F.; Goëb, V.; Parmar, R.; El-Sherbiny, Y.; Boissinot, M.; El Jawhari, J.; Burska, A.; Vital, E.M.; Harrison, S.; Conaghan, P.G.; et al. An immunological biomarker to predict MTX response in early RA. Ann. Rheum. Dis. 2014, 73, 2047–2053. [Google Scholar] [CrossRef]
- Nalbant, S.; Birlik, A.M. Cytokines in Rheumatoid Arthritis (RA). In New Developments in the Pathogenesis of Rheumatoid Arthritis; Sakkas, L.I., Ed.; IntechOpen: Rijeka, Croatia, 2017; Available online: https://www.intechopen.com/books/new-developments-in-the-pathogenesis-of-rheumatoid-arthritis/cytokines-in-rheumatoid-arthritis-ra- (accessed on 25 August 2020).
- Hibi, M.; Murakami, M.; Saito, M.; Hirano, T.; Taga, T.; Kishimoto, T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 1990, 63, 1149–1157. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond.) 2012, 122, 143–159. [Google Scholar] [CrossRef]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- and IL-6 classic signaling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef]
- Yokota, S.; Tanaka, T.; Kishimoto, T. Efficacy, safety and tolerability of tocilizumab in patients with systemic juvenile idiopathic arthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Msihid, J.; Zilberstein, M.; Paccard, C.; Lin, Y.; Graham, N.M.H.; Boyapati, A. Identification of sarilumab pharmacodynamic and predictive markers in patients with inadequate response to TNF inhibition: A biomarker substudy of the phase 3 TARGET study. RMD Open 2018, 4, e000607. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Rauwel, B.; Degboé, Y.; Constantin, A.; Boyer, J.F.; Kruglov, A.; Cantagrel, A. Modulation of T-cell responses by anti-tumor necrosis factor treatments in rheumatoid arthritis: A review. Arthritis Res. Ther. 2018, 20, 229. [Google Scholar] [CrossRef] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, 3–11. [Google Scholar] [CrossRef]
- Jimenez-Boj, E.; Redlich, K.; Türk, B.; Hanslik-Schnabel, B.; Wanivenhaus, A.; Chott, A.; Ramiro, S.; Schett, G. Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J. Immunol. 2005, 175, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Alamgeer, H.U.; Uttra, A.M.; Qasim, S.; Ikram, J.; Saleem, M.; Niazi, Z.R. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 2020, 66, 153134. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Carubbi, F.; Giacomelli, R.; Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: New players and therapeutic targets. BMC Rheumatol. 2017, 1, 3. [Google Scholar] [CrossRef]
- Dinarello, C.; Arend, W.; Sims, J.; Smith, D.; Blumberg, H.; O’Neill, L.; Goldbach-Mansky, R.; Pizarro, T.; Hoffman, H.; Bufler, P.; et al. IL-1 family nomenclature. Nat. Immunol. 2010, 11, 973. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Okazaki, H.; Tamemoto, H.; Kimura, H.; Kamata, Y.; Nagatani, K.; Yoshio, T.; Nagashima, T.; Iwamoto, M.; Hayakawa, M.; et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J. Rheumatol. 2010, 37, 18–25. [Google Scholar] [CrossRef]
- Hong, Y.S.; Moon, S.J.; Joo, Y.B.; Jeon, C.H.; Cho, M.L.; Ju, J.H.; Oh, H.J.; Heo, Y.J.; Park, S.H.; Kim, H.Y.; et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J. Korean Med. Sci. 2011, 26, 1132–1139. [Google Scholar] [CrossRef]
- Xiangyang, Z.; Lutian, Y.; Lin, Z.; Liping, X.; Hui, S.; Jing, L. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine 2012, 58, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Talabot-Ayer, D.; McKee, T.; Gindre, P.; Bas, S.; Baeten, D.L.; Gabay, C.; Palmer, G. Distinct serum and synovial fluid interleukin (IL)-33 levels in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Joint Bone Spine 2012, 79, 32–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Miossec, P. IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front. Med. 2019, 5, 364. [Google Scholar] [CrossRef]
- Sweeney, S.E.; Firestein, G.S. Rheumatoid arthritis: Regulation of synovial inflammation. Int J. Biochem. Cell Biol. 2004, 36, 372–378. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Eljaafari, A.; Tartelin, M.L.; Aissaoui, H.; Chevrel, G.; Osta, B.; Lavocat, F.; Miossec, P. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: Contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 2012, 64, 2147–2157. [Google Scholar] [CrossRef]
- Honorati, M.C.; Neri, S.; Cattini, L.; Facchini, A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis Cartilage 2006, 14, 345–352. [Google Scholar] [CrossRef]
- Daoussis, D.; Andonopoulos, A.P.; Liossis, S.N. Wnt pathway and IL-17: Novel regulators of joint remodeling in rheumatic diseases. Looking beyond the RANK-RANKL-OPG axis. Semin. Arthritis Rheum. 2010, 39, 369–383. [Google Scholar] [CrossRef]
- Hot, A.; Miossec, P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 2011, 70, 727–732. [Google Scholar] [CrossRef]
- Hot, A.; Zrioual, S.; Toh, M.L.; Lenief, V.; Miossec, P. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann. Rheum. Dis. 2011, 70, 341–348. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; Papapoulos, S.E.; Löwik, C.W. Effect of interleukin-17 on nitric oxide production and osteoclastic bone resorption: Is there dependency on nuclear factor-kappaB and receptor activator of nuclear factor kappaB (RANK)/RANK ligand signaling? Bone 2001, 28, 378–386. [Google Scholar] [CrossRef]
- Lavocat, F.; Maggi, L.; Annunziato, F.; Miossec, P. T-cell clones from Th1, Th17 or Th1/17 lineages and their signature cytokines have different capacity to activate endothelial cells or synoviocytes. Cytokine 2016, 88, 241–250. [Google Scholar] [CrossRef]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheumatol. 2009, 5, 667–676. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Harada, Y.; Wang, J.T.; Gorn, A.H.; Thornhill, T.S.; Goldring, S.R. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am. J. Pathol. 1998, 152, 943–951. [Google Scholar]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Zwerina, J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Muller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt III, V.E.T.; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Scherle, P.A.; Collins, R.; Burn, T.C.; Li, Y.; Li, J.; Covington, M.B.; Thomas, B.; Collier, P.; Favata, M.F.; et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: Preclinical characterization of INCB028050. J. Immunol. 2010, 184, 5298–5307. [Google Scholar] [CrossRef]
- Kremer, J.M. Selective costimulation modulators: A novel approach for the treatment of rheumatoid arthritis. J. Clin. Rheumatol. 2005, 11, S55–S62. [Google Scholar] [CrossRef]
- Abatacept. DrugBank. Available online: https://www.drugbank.ca/drugs/DB01281 (accessed on 24 August 2020).
- Fukuyo, S.; Nakayamada, S.; Iwata, S.; Kubo, S.; Saito, K.; Tanaka, Y. Abatacept therapy reduces CD28+CXCR5+ follicular helper-like T cells in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35, 562–570. [Google Scholar]
- Ailioaie, L.M.; Ailioaie, C. Photobiomodulation—Targeting the quantum life. Newest implications for immunity, health, and youth. Invited Lecture. In Proceedings of the 11th International ISLA Congress for Medical Laser Applications, Lauenförde-Beverungen, Germany, 10–11 June 2016. [Google Scholar]
- Full-spectrum light. Available online: https://en.wikipedia.org/wiki/Full-spectrum_light (accessed on 23 August 2020).
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- NobelPrize.org; Nobel Media AB 2020. The Nobel Prize in Physiology or Medicine 1903. Available online: https://www.nobelprize.org/prizes/medicine/1903/summary/ (accessed on 3 August 2020).
- Keijzer, M.; Jacques, S.L.; Prahl, S.A.; Welch, A.J. Light distributions in artery tissue: Monte Carlo simulations for finite-diameter laser beams. Lasers Surg. Med. 1989, 9, 148–154. [Google Scholar] [CrossRef]
- Understanding the Differences between LED and Laser Therapy. Available online: https://www.lightforcemedical.com/understanding-the-differences-between-led-and-laser-therapy/ (accessed on 23 August 2020).
- Ailioaie, L.M. New clinical results in intravenous laser therapy. Invited lecture. In Proceedings of the 9th International ISLA Congress for Medical Laser Applications, Lauenförde-Beverungen, Germany, 27–29 June 2014. [Google Scholar]
- Ailioaie, C.; Ailioaie, L.M. Laser photobiostimulation and safety in pediatric diseases. In Lasers in Medicine, Science and Praxis; Simunovic, Z., Ed.; Printery Publishing House: Cakovec, Croatia, 2009; Chapter 32; pp. 467–504. [Google Scholar]
- All about High Intensity Laser. BTL High Intensity Laser. Available online: https://www.high-intensity-laser.com/subpage (accessed on 4 August 2020).
- Overman, D. Treating Pain with Low vs. High-Power Lasers: What is the Difference? Rehab Managagement, 4 April 2019. Available online: https://www.rehabpub.com/pain-management/products/treating-pain-low-vs-high-power-lasers-difference/ (accessed on 4 August 2020).
- Moskvin, S.V.; Kisselev, S.B. Laser Therapy for Joint and Muscle Pain; OOO Izdatelstvo “Triada”: Moscow/Tver, Russia, 2017; pp. 10, 169, 175–176. ISBN 978-5-94789-787-6. [Google Scholar]
- Moskvin, S.V.; Khadartsev, A.A. Basic Techniques of Low Level Laser Therapy; OOO Izdatelstvo “Triada”: Moscow/Tver, Russia, 2017; pp. 11–12. ISBN 978-5-94789-772-2. [Google Scholar]
- Chiran, D.A.; Litscher, G.; Weber, M.; Ailioaie, L.M.; Ailioaie, C.; Litscher, D. Intravenous laser blood irradiation increases efficacy of etanercept in selected subtypes of juvenile idiopathic arthritis: An innovative clinical research approach. Evid. Based Complement. Alternat. Med. 2013, 2013, 168134. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G.; Weber, M.; Ailioaie, C.; Litscher, D.; Chiran, D.A. Innovations and challenges by applying sublingual laser blood irradiation in juvenile idiopathic arthritis. Int. J. Photoenergy 2014, 2014, 130417. [Google Scholar] [CrossRef]
- Chiran, D.A.; Weber, M.; Ailioaie, L.M.; Moraru, E.; Ailioaie, C.; Litscher, D.; Litscher, G. Intravenous laser blood irradiation and tocilizumab in a patient with juvenile arthritis. Case Rep. Med. 2014, 2014, 923496. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Photobiomodulation. Available online: http://www.appliedbiophotonics.com/photobiomodulation/ (accessed on 4 August 2020).
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The use of Low-Level Laser Therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015, 2, 188–194. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Santana-Blank, L.; Rodríguez-Santana, E.; Santana-Rodríguez, K.E.; Reyes, H. “Quantum Leap” in Photobiomodulation Therapy Ushers in a New Generation of Light-Based Treatments for Cancer and Other Complex Diseases: Perspective and Mini-Review. Photomed. Laser Surg. 2016, 34, 93–101. [Google Scholar] [CrossRef]
- Chiran, D.A.; Ailioaie, L.M.; Ailioaie, C. New challenges in treating pediatric rheumatic diseases with lasers in the age of biologic therapy. In Proceedings of the 9th World Association for Laser Therapy, Gold Coast, Australia, 28–30 September 2012; Laakso, E.L., Young, C., Eds.; World Association for Laser Therapy Congress (WALT): Gold Coast, Australia, 2013; pp. 25–27. [Google Scholar]
- Stoll, M.L.; Cron, R.Q. Treatment of juvenile idiopathic arthritis: A revolution in care. Pediatr. Rheumatol. Online J. 2014, 12, 13. [Google Scholar] [CrossRef]
- Wickenheisser, V.A.; Zywot, E.M.; Rabjohns, E.M.; Lee, H.H.; Lawrence, D.S.; Tarrant, T.K. Laser Light Therapy in Inflammatory, Musculoskeletal, and Autoimmune Disease. Curr. Allergy Asthma Rep. 2019, 19, 37. [Google Scholar] [CrossRef]
- Zak, M.; Pedersen, F.K. Juvenile chronic arthritis into adulthood: A long-term follow-up study. Rheumatology 2000, 39, 198–204. [Google Scholar] [CrossRef]
- Prieur, A.M.; Chèdeville, G. Prognostic factors in juvenile idiopathic arthritis. Curr. Rheumatol. Rep. 2001, 3, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, C.; Ailioaie, L.M. Beneficial effects of laser therapy in the early stages of rheumatoid arthritis onset. J. Laser Ther. 1999, 11, 79–87. [Google Scholar] [CrossRef]
- Tuner, J.; Hode, L. The Laser Therapy Handbook. Prima Books. Sweden. 2007, 292. [Google Scholar]
- Contraindications for Use of Therapeutic Laser. Available online: https://www.practicalpainmanagement.com/treatments/complementary/lasers/contraindications-use-therapeutic-laser (accessed on 4 August 2020).
- Meesters, A.A.; Pitassi, L.H.; Campos, V.; Wolkerstorfer, A.; Dierickx, C.C. Transcutaneous laser treatment of leg veins. Lasers Med. Sci. 2014, 29, 481–492. [Google Scholar] [CrossRef]
- Liu, T.C.Y.; Wu, D.F.; Gu, Z.Q.; Wu, M. Applications of intranasal low intensity laser therapy in sports medicine. J. Innov. Opt. Health Sci. 2010, 3, 1–16. [Google Scholar] [CrossRef]
- Wirz-Ridolfi, A. Comparison between Intravenous and Various Types of Transcutaneous Laser Blood Irradiation. Internet J. Laserneedle Med. 2013, 3. Available online: http://ispub.com/IJLNM/3/1/14462 (accessed on 4 August 2020).
- Mikhaylov, V.A. The use of Intravenous Laser Blood Irradiation (ILBI) at 630–640 nm to prevent vascular diseases and to increase life expectancy. Laser Ther. 2015, 24, 15–26. [Google Scholar] [CrossRef]
- Amjadi, A.; Mirmiranpor, H.; Khandani, S.; Sobhani, S.O.; Shafaee, Y. Intravenous laser wavelength irradiation effect on interleukins: IL-1α, IL-1β, IL6 in diabetic rats. Laser Ther. 2019, 28, 267–273. [Google Scholar]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Castano, A.P.; Dai, T.; Yaroslavsky, I.; Cohen, R.; Apruzzese, W.A.; Smotrich, M.H.; Hamblin, M.R. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg. Med. 2007, 39, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Alves, A.C.; Vieira, R.; Leal-Junior, E.; dos Santos, S.; Ligeiro, A.P.; Albertini, R.; Junior, J.; de Carvalho, P. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res. Ther. 2013, 15, R116. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Moretti, A.I.; Abrahão, T.B.; de Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med. Sci. 2013, 28, 947–955. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Cheng, Y.J.; Huang, F.C.; Yang, C.C. The fluence effects of low-level laser therapy on inflammation, fibroblast-like synoviocytes, and synovial apoptosis in rats with adjuvant-induced arthritis. Photomed. Laser Surg. 2014, 32, 669–677. [Google Scholar] [CrossRef]
- dos Santos, S.A.; Alves, A.C.; Leal-Junior, E.C.; Albertini, R.; de Paula Vieira, R.; Ligeiro, A.P.; Silva Junior, J.A.; de Carvalho, P. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med. Sci. 2014, 29, 1051–1058. [Google Scholar] [CrossRef]
- Torres-Silva, R.; Lopes-Martins, R.A.B.; Bjordal, J.M.; Frigo, L.; Rahouadj, R.; Arnold, G.; Leal-Junior, E.C.P.; Magdalou, J.; Pallotta, R.; Marcos, R.L. The low-level laser therapy (LLLT) operating in 660 nm reduce gene expression of inflammatory mediators in the experimental model of collagenase-induced rat tendinitis. Lasers Med. Sci. 2015, 30, 1985–1990. [Google Scholar] [CrossRef]
- Fernandes, K.P.; Souza, N.H.; Mesquita-Ferrari, R.A.; Silva, D.; Rocha, L.A.; Alves, A.N.; Sousa, K.; Bussadori, S.K.; Hamblin, M.R.; Nunes, F.D. Photobiomodulation with 660-nm and 780-nm laser on activated J774 macrophage-like cells: Effect on M1 inflammatory markers. J. Photochem. Photobiol. B Biol. 2015, 153, 344–351. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Al Musawi, M.S.; Jaafar, M.S.; Al-Gailani, B.; Ahmed, N.M.; Suhaimi, F.M.; Suardi, N. Effects of low-level laser irradiation on human blood lymphocytes in vitro. Lasers Med. Sci. 2017, 32, 405–411. [Google Scholar] [CrossRef]
- Baek, S.; Lee, K.P.; Cui, L.; Ryu, Y.; Hong, J.M.; Kim, J.; Jung, S.H.; Bae, Y.M.; Won, K.J.; Kim, B. Low-power laser irradiation inhibits PDGF-BB-induced migration and proliferation via apoptotic cell death in vascular smooth muscle cells. Lasers Med. Sci. 2017, 32, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, L.M.J.; da Fonseca, A.S.; Gameiro, J.; de Paoli, F. Apoptosis induced by low-level laser in polymorphonuclear cells of acute joint inflammation: Comparative analysis of two energy densities. Lasers Med. Sci. 2017, 32, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Tim, C.; Magri, A.; Fernandes, K.R.; Vassão, P.G.; Renno, A.C.M. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci 2018, 33, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Vescovi, P.; Belletti, S.; Uggeri, J.; Nammour, S.; Gatti, R. Effects of 915 nm laser irradiation on human osteoblasts: A preliminary in vitro study. Lasers Med. Sci 2018, 33, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Shakir, E.A.; Rasheed Naji, N.A. In vitro impact of laser irradiation on platelet aggregation. Lasers Med. Sci. 2018, 33, 1717–1721. [Google Scholar] [CrossRef]
- De Souza Costa, M.; Teles, R.H.G.; Dutra, Y.M.; Neto, J.C.R.M.; de Brito, T.V.; Queiroz, F.F.S.N.; do Vale, D.B.N.; de Souza, L.K.M.; Silva, I.S.; Barbosa, A.L.D.R.; et al. Photobiomodulation reduces neutrophil migration and oxidative stress in mice with carrageenan-induced peritonitis. Lasers Med. Sci. 2018, 33, 1983–1990. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef]
- Felizatti, A.L.; do Bomfim, F.R.C.; Bovo, J.L.; de Aro, A.A.; do Amaral, M.E.C.; Esquisatto, M.A.M. Effects of low-level laser therapy on the organization of articular cartilage in an experimental microcrystalline arthritis model. Lasers Med. Sci. 2019, 34, 1401–1412. [Google Scholar] [CrossRef]
- Han, B.; Fan, J.; Liu, L.; Tian, J.; Gan, C.; Yang, Z.; Jiao, H.; Zhang, T.; Liu, Z.; Zhang, H. Adipose-derived mesenchymal stem cells treatments for fibroblasts of fibrtic scar via downregulating TGF-β1 and Notch-1 expression enhanced by photobiomodulation therapy. Lasers Med. Sci. 2019, 34, 1–10. [Google Scholar] [CrossRef]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: In vitro study. Lasers Med. Sci. 2019, 34, 55–60. [Google Scholar] [CrossRef]
- Cardoso, L.M.; Pansani, T.N.; Hebling, J.; de Souza Costa, C.A.; Basso, F.G. Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers Med. Sci. 2020, 35, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Lee, G.; Tanushi, A.; Tsukada, K.; Choi, H.S.; Kashiwagi, S. High-throughput single-cell live imaging of photobiomodulation with multispectral near-infrared lasers in cultured T cells. J. Biomed. Opt. 2020, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.U.D.; Batista, G.A.; da Silva, P.L.P.; Araújo, D.N.; Sarmento, W.E.A.; Palomari, E.T. Photobiostimulation activity of different low-level laser dosage on masticatory muscles and temporomandibular joint in an induced arthritis rat model. Lasers Med. Sci. 2020, 35, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, Z.; Zhang, J.; Zuo, X.; Sun, J.; Zheng, Q.; Song, J.; Ding, T.; Hu, X.; Wang, Z. Attenuation of the inflammatory response and polarization of macrophages by photobiomodulation. Lasers Med. Sci. 2020, 35, 1509–1518. [Google Scholar] [CrossRef]
- Moreira, S.H.; Pazzini, J.M.; Álvarez, J.L.G.; Cassino, P.C.; Bustamante, C.C.; Bernardes, F.J.L.; Kajiura, C.Y.; De Nardi, A.B. Evaluation of angiogenesis, inflammation, and healing on irradiated skin graft with low-level laser therapy in rats (Rattus norvegicus albinus wistar). Lasers Med. Sci. 2020, 35, 1103–1109. [Google Scholar] [CrossRef]
- da Silva, J.G.F.; dos Santos, S.S.; de Almeida, P.; Marcos, R.L.; Lino-Dos-Santos-Franco, A. Effect of systemic photobiomodulation in the course of acute lung injury in rats. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Lopes-Martins, R.A.; Iversen, V.V. A randomised, placebo-controlled trial of low-level laser therapy for activated achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br. J. Sports Med. 2006, 40, 76–80. [Google Scholar] [CrossRef]
- Nakamura, T.; Ebihara, S.; Ohkuni, I.; Izukura, H.; Harada, T.; Ushigome, N.; Ohshiro, T.; Musha, Y.; Nakamura, T.; Takahashi, H.; et al. Low Level Laser Therapy for chronic knee joint pain patients. Laser Ther. 2014, 23, 273–277. [Google Scholar] [CrossRef]
- Soleimanpour, H.; Gahramani, K.; Taheri, R.; Golzari, S.E.; Safari, S.; Esfanjani, R.M.; Iranpour, A. The effect of low-level laser therapy on knee osteoarthritis: Prospective, descriptive study. Lasers Med. Sci. 2014, 29, 1695–1700. [Google Scholar] [CrossRef]
- Youssef, E.F.; Muaidi, Q.I.; Shanb, A.A. Effect of Laser Therapy on Chronic Osteoarthritis of the Knee in Older Subjects. J. Lasers Med. Sci. 2016, 7, 112–119. [Google Scholar] [CrossRef]
- Nambi, S.G.; Kamal, W.; George, J.; Manssor, E. Radiological and biochemical effects (CTX-II, MMP-3, 8, and 13) of low-level laser therapy (LLLT) in chronic osteoarthritis in Al-Kharj, Saudi Arabia. Lasers Med. Sci. 2017, 32, 297–303. [Google Scholar] [CrossRef]
- Alayat, M.S.; Ali, M.M. Efficacy of class IV diode laser on pain and dysfunction in patients with knee osteoarthritis: A randomized placebo-control trial. Bull. Fac. Phys. Ther. 2017, 22, 40–45. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Costa, L.; Joensen, J.; Stausholm, M.B.; Naterstad, I.F.; Leal-Junior, E.; Bjordal, J.M. Effects of photobiomodulation therapy on inflammatory mediators in patients with chronic non-specific low back pain: Protocol for a randomized placebo-controlled trial. Medicine 2019, 98, e15177. [Google Scholar] [CrossRef] [PubMed]
- Tsuk, S.; Lev, Y.H.; Fox, O.; Carasso, R.; Dunsky, A. Does Photobiomodulation Therapy Enhance Maximal Muscle Strength and Muscle Recovery? J. Hum. Kinet. 2020, 73, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Tomazoni, S.S.; Costa, L.O.P.; Joensen, J.; Stausholm, M.B.; Naterstad, I.F.; Ernberg, M.; Leal-Junior, E.C.P.; Bjordal, J.M. Photobiomodulation Therapy is Able to Modulate PGE2 Levels in Patients With Chronic Non-Specific Low Back Pain: A Randomized Placebo-Controlled Trial. Lasers Surg. Med. 2020. [Google Scholar] [CrossRef]
- Aimbire, F.; Albertini, R.; Pacheco, M.T.; Castro-Faria-Neto, H.C.; Leonardo, P.S.L.M.; Iversen, V.V.; Lopes-Martins, R.A.B.; Bjordal, J.M. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed. Laser Surg. 2006, 24, 33–37. [Google Scholar] [CrossRef]
- Albertini, R.; Aimbire, F.; Villaverde, A.B.; Silva, J.A., Jr.; Costa, M.S. COX-2 mRNA expression decreases in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low level laser therapy. Inflamm. Res. 2007, 56, 228–229. [Google Scholar] [CrossRef]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.B.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Leal-Junior, E.; Lopes-Martins, R.; Bjordal, J.M. Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: Current evidence and future directions. Braz. J. Physic. Ther. 2019, 23, 71–75. [Google Scholar] [CrossRef]
- Stausholm, M.B.; Naterstad, I.F.; Lopes-Martins, R.A.B.; Sæbø, H.; Lund, H.; Fersum, K.V.; Bjordal, J.M. Efficacy of low-level laser therapy on pain and disability in knee osteoarthritis: Systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 2019, 9, e031142. [Google Scholar] [CrossRef]
- Weber, M.H.; Fussgänger-May, T.; Wolf, T. The intravenous laser blood irradiation - introduction of a new therapy. Dt. Ztschr. f Akup. 2007, 50, 12–23. [Google Scholar] [CrossRef]
- Dube, A.; Bansal, H.; Gupta, P.K. Modulation of macrophage structure and function by low level He-Ne laser irradiation. Photochem. Photobiol. Sci. 2003, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Agaiby, A.D.; Ghali, L.R.; Wilson, R.; Dyson, M. Laser modulation of angiogenic factor production by T-lymphocytes. Lasers Surg. Med. 2000, 26, 357–363. [Google Scholar] [CrossRef]
- Momenzadeh, S.; Abbasi, M.; Ebadifar, A.; Aryani, M.; Bayrami, J.; Nematollahi, F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J. Lasers Med. Sci. 2015, 6, 6. [Google Scholar] [PubMed]
- Yamaura, M.; Yao, M.; Yaroslavsky, I.; Cohen, R.; Smotrich, M.; Kochevar, I.E. Low-level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg. Med. 2009, 41, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.O.; Kruse, A.; Kirchner, H. Cytokine production after helium neon laser irradiation in cultures of human peripheral blood mononuclear cells. J. Photochem. Photobiol. B. 1992, 16, 347–355. [Google Scholar] [CrossRef]
- Hashmi, J.T.; Huang, Y.Y.; Osmani, B.Z.; Sharma, S.K.; Naeser, M.A.; Hamblin, M.R. Role of low-level laser therapy in neurorehabilitation. PM & R: J. Injury Func. Rehabil. 2010, 2, S292–S305. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Chiran, D.A. Laser therapy in neurophatic pain. In Proceedings of the Annual Symposium on Mathematics Applied in Biology & Biophisics, Iaşi, Romania, 28–29 May 2004; Volume XL VII, pp. 303–308. [Google Scholar]
- Sobol, E.; Baum, O.; Shekhter, A.; Wachsmann-Hogiu, S.; Shnirelman, A.; Alexandrovskaya, Y.; Sadovskyy, I.; Vinokur, V. Laser-induced micropore formation and modification of cartilage structure in osteoarthritis healing. J. Biomed. Opt. 2017, 22, 091515. [Google Scholar] [CrossRef]
- Ip, D. Does addition of low-level laser therapy (LLLT) in conservative care of knee arthritis successfully postpone the need for joint replacement? Lasers Med. Sci. 2015, 30, 2335–2339. [Google Scholar] [CrossRef]
- Brosseau, L.; Robinson, V.; Wells, G.; Debie, R.; Gam, A.; Harman, K.; Morin, M.; Shea, B.; Tugwell, P. Withdrawn: Low-level laser therapy (Classes III) for treating osteoarthritis. Cochrane Database Syst. Rev. 2007, 1, CD002046. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L.; Robinson, V.; Wells, G.; Debie, R.; Gam, A.; Harman, K.; Morin, M.; Shea, B.; Tugwell, P. Low-level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2005, 19, CD002049. [Google Scholar] [CrossRef] [PubMed]

| No | References | Type of Study | PBM Properties | Immune Cells/Signaling Pathways | Brief Results |
|---|---|---|---|---|---|
| 1. | Castano, A.P.; Dai, T.; Yaroslavsky, I. et al. Low-level laser therapy for zymosan-induced arthritis in rats: Importance of illumination time. Lasers Surg Med. 2007, 39, 543–550. doi:10.1002/lsm.20516 [155] | Animal Model | 810 nm; 5 and 50 mW/cm2; 3 and 30 J/cm2 | Pathway of prostanoids/PGE2 | Light regimen (30 J/cm2 at 50 mW/cm2) effective in reducing swelling of the knees and a greater reduction in the serum PGE2. |
| 2. | Chen, A.C.; Arany, P.R.; Huang, Y.Y. et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, 22453. doi:10.1371/journal.pone.0022453 [156] | Animal Model | 810 nm; different fluences (0.003, 0.03, 0.3, 3, and 30 J/cm2); 1 mW/cm2 to 30 mW/cm2 | Murine embryonic fibroblasts/NF-kB | Significant activation of NF-kB at fluences higher than 0.3 J/cm2. NF-kB was activated earlier (1 h) by LLLT compared to conventional lipopolysaccharide treatment. Increase in ATP. |
| 3. | Alves, A.C.; Vieira, R.; Leal-Junior, E. et al. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013, 15, R116. doi:10.1186/ar4296 [157] | Animal Model | GaAlAs (808 nm); 50 mW; 0.028 cm2 1.78 W/cm2; 4 J; 142.4 J/cm2; 80 s/point. 100 mW; GaAlAs (808 nm); 0.028 cm2 3.57 W/cm2; 4 J; 142.4 J/cm2; 40 s/point | Inflammatory cells (macrophages and neutrophils); gene expression of IL-1β, IL-6, TNFα. | LLLT with 50 mW was more efficient in modulating inflammatory mediators (IL-1β, IL-6) and inflammatory cells (macrophages and neutrophils). |
| 4. | Assis L.; Moretti, A.I.; Abrahão T.B.; de Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2013, 28, 947–955. doi:10.1007/s10103-012-1183-3 [158] | Experimental groups and freezing muscle injury (cryoinjury) Adult male Wistar rats were randomly divided. | 808 nm; CW; 30 mW power output, 47 s irradiation time, 0.00785 cm2 spot area, dose 180 J/cm2, irradiance 3.8 W/cm2 and 1.4 J total energy per point. | Myogenic regulatory factors (myoD and myogenin), vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β) 1 and type I collagen mRNA | LLLT improved skeletal muscle regeneration by reducing the injured area, increasing myoD, myogenin, and VEGF gene expression and, simultaneously, reducing TGF-β mRNA and type I collagen deposition in the injured tissue. Therefore, LLLT can increase muscle regeneration markers and reduce scar tissue formation, which should favor tissue repair in muscle injuries. |
| 5. | Hsieh, Y.L.; Cheng, Y.J.; Huang, F.C.; Yang, C.C. The fluence effects of low-level laser therapy on inflammation, fibroblast-like synoviocytes, and synovial apoptosis in rats with adjuvant-induced arthritis. Photomed Laser Surg. 2014, 32, 669–677. doi:10.1089/pho.2014.3821 [159] | Animal Model | 780-nm GaAlAs, 30 mW; spot size 0.2 cm2, power density 0.15 W/cm2. 30 s and 3 min laser irradiation, total fluences at the lower and higher energy densities (power density×irradiation time) of 4.5 and 27 J/cm2 were applied daily for five successive days. The accumulated energies delivered from all sessions were 0.9 and 5.4 J, respectively | β-endorphin (β-ep) and TNF-α; substance P and COX-2 | This study determined that the fluence provided by LLLT is one of the factors affecting biochemicals related to pain in the treatment of myofascial pain. LLLT irradiation with fluences of 4.5 and 27 J/cm2 at myofascial trigger spots can significantly reduce substance P level in dorsal root ganglion. LLLT with lower fluence of 4.5 J/cm2 exerted lower levels of TNF-α and COX-2 expression in laser-treated muscle, but LLLT with a higher fluence of 27 J/cm2 elevated the levels of β-ep in serum, DRG, and muscle. |
| 6. | dos Santos S.A.; Alves, A.C.; Leal-Junior, E.C. et al. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med Sci. 2014, 29, 1051–1058. doi:10.1007/s10103-013-1467-2 [160] | Animal Model | AsGaAl-type diode laser with a wavelength (λ) of 808 nm LLLT at doses of 2 and 4 J on joint papain-induced inflammation in rats; Mean power output (mW = 50); 50; Spot size (cm2) 0.028; Power density (W/cm2) 1.78; 1.78; Energy (J); 2 and 4 Energy density (J/cm2) 71.4; 142.8 Time per point (s) 40; 80. | Inflammatory cells (macrophages and neutrophils); gene expression of IL-1β, IL-6, and IL-10; and TNF-α | Dose of 2 J is more efficient in modulating inflammatory mediators (IL-1β, IL-6, TNF-α, and IL-10) and inflammatory cells (macrophages and neutrophils) and its effects can be observed by histological signs of attenuation of inflammatory processes. |
| 7. | Torres-Silva, R.; Lopes-Martins, R.A.; Bjordal, J.M. et al. The low-level laser therapy (LLLT) operating in 660 nm reduce gene expression of inflammatory mediators in the experimental model of collagenase-induced rat tendinitis. Lasers Med Sci. 2015, 30, 1985–1990. doi:10.1007/s10103-014-1676-3 [161] | Animal Model | 100 mW, 660 nm, 1 J or 3 J, comparatively. | Gene expression for COX-2; TNF-α; IL-6; and IL-10. | Reduction of important pro-inflammatory IL-6 and TNF-α, at 3 J. |
| 8. | Fernandes, K.P.; Souza, N.H.; Mesquita-Ferrari, R.A.; Silva, D.; Rocha, L.A.; Alves, A.N.; Sousa, K.; Bussadori, S.K.; Hamblin, M.R.; Nunes, F.D. Photobiomodulation with 660-nm and 780-nm laser on activated J774 macrophage-like cells: Effect on M1 inflammatory markers. Journal of photochemistry and photobiology. B, Biology, 2015, 153, 344–351. doi:10.1016/j.jphotobiol.2015.10.015 [162] | Cells Culture J774 were derived from a BALB/c mouse. | 660 nm (InGaAlP diode); 780 nm (GaAlAs diode) laser; CW. Average radiant power: 15 and 70 mW. Beam spot size at target: 0.04 cm2. Total radiant energy 0.22 J and 0.16 J | Inflammatory cells (macrophages and neutrophils)/mRNA expression of TNF-α and iNOS; production of TNF-α and COX-2 proteins in M1 J774 cells. | 660 nm and 780 nm lasers strongly reduced the mRNA expression of TNF-α and iNOS and down-regulated the production of TNF-α and COX-2 proteins in M1 J774 cells. |
| 9. | Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthritis and Cartilage, 2016, 24, 169–177. doi:10.1016/j.joca.2015.07.020 [163] | Animal Model | Diode laser (GaAlAs) 808 nm, cw, 50 mW output power, 28 s irradiation time, 0.028 cm2 spot area, 50 J/cm2, 1.7 W/cm2, 1.4 J total energy per point/section. 3 days/week, at two points on left knee joint, contact technique, for 24 sessions (8 weeks). | IL-1, Caspase-3, and MMP-13 expression in nucleus of chondrocytes | 808 nm laser prevented articular degenerative morphological modifications and modulated inflammatory process in OA rats. |
| 10. | Al Musawi, M.S.; Jaafar, M.S.; Al-Gailani, B.; et al. Effects of low-level laser irradiation on human blood lymphocytes in vitro. Lasers Med Sci 2017, 32, 405–411. doi:10.1007/s10103-016-2134-1 [164] | Irradiation on human blood lymphocytes in vitro | Diode pump solid state (DPSS) laser, with wavelengths of 405, 589, and 780 nm, with an output power of 10 mW and irradiance rate fixed at 30 mW/cm2. | Effect of laser at peripheral blood lymphocyte subsets | The effect of laser irradiation fluences of 36, 54, 72, and 90 J/cm2 for each wavelength of 405, 589, or 780 nm, with an output power of 10 mW on human blood lymphocyte count in vitro are: no significant differences in lymphocyte count were observed before and after irradiation with the above fluences at wavelengths of 405 and 780 nm; however, a laser wavelength of 589 nm was associated with a significant increase in the lymphocyte count at a radiation fluence of 72 (by 1.6%) and increase in the NK cell lymphocyte subset. |
| 11. | Baek, S.; Lee, K.P.; Cui, L.; et al. Low-power laser irradiation inhibits PDGF-BB-induced migration and proliferation via apoptotic cell death in vascular smooth muscle cells. Lasers Med Sci 2017, 32, 2121–2127. doi:10.1007/s10103-017-2338-z [165] | Animal experiment In vivo vascular smooth muscle cells (VSMCs) | Low-power laser (LPL) green diode laser 532-nm pulsed wave of 300 mW at a spot diameter of 1 mm. | Apoptosis, migration, and proliferation in vascular smooth muscle cells (VSMCs)/ Caspase-3, Bax, and p38 mitogen-activated protein kinase in PDGF-BB-treated VSMCs. | The study demonstrated that 532 nm LPL irradiation inhibited VSMC proliferation and migration in response to platelet-derived growth factor (PDGF)-BB. LPL irradiation induced apoptosis and enhanced activation of caspase-3, Bax, and p38 mitogen-activated protein kinase in PDGF-BB-treated VSMCs. Based on these results, 532 nm LPL irradiation may inhibit PDGF-BB stimulated proliferation and migration, likely resulting from apoptosis associated with the interaction between 532 nm LPL irradiation and PDGF-BB in VSMCs. Therefore, this study provides a foundation for therapeutic strategies for vascular restenosis via 532 nm LPL irradiation as an alternative treatment against restenosis. |
| 12. | Dos Anjos, L.M.J.; da Fonseca, A.S.; Gameiro, J.; de Paoli, F. Apoptosis induced by low-level laser in polymorphonuclear cells of acute joint inflammation: comparative analysis of two energy densities. Lasers Med Sci. 2017, 32, 975–983. doi:10.1007/s10103-017-2196-8 [166] | Animal Model/randomly distributed | 830 nm, output power 10 mW, 0.05 cm2 laser beam area, power density 0.2 W/cm2, energy densities:3 and 30 J/cm2 (total energy of 150 and 1500 mJ were delivered after 15 and 150 s, respectively), in continuous wave emission mode. | Apoptotic cells in mouse ankle joint samples/DNA fragmentation rate of inflammatory cells Gene expression of proteins involved in apoptosis pathways Bcl2 protein and mRNA expression in PMN cells | The higher energy density (30 Jcm−2) can reduce the inflammatory process by PMN apoptosis induction, while the lower energy density (3 Jcm−2) could also induce apoptosis in PMN; however, this process seems to be slower. The results suggest that apoptosis in PMN cells comprises part of LLLT anti-inflammatory mechanisms and could be a consequence of the balance alteration between expression of proapoptotic (Bax and p53) and anti-apoptotic (Bcl-2) proteins in these cells. |
| 13. | Assis, L.; Tim, C.; Magri, A. et al. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med Sci 2018, 33, 1875–1882. doi:10.1007/s10103-018-2541-6 [167] | Study of the experimental animals. The degenerative process related to osteoarthritis (OA) in the articular cartilage in rats. | Diode laser GaAIAs 808 nm; CW; power output 50 mW; irradiance 1.7 W/cm2; spot area 0.28 cm2; dose 50 J/cm2; total energy 1.4 J per point/section. Irradiation time: 28 s; local: 2 points (medial and lateral side of the left knee joint) Technique: punctual contact | Chondrocytes/IL-10 expression; transforming growth factor beta (TGF-β) expression; collagen type I (Col I) and II (Col II). | PBM associated with aerobic and aquatic exercise were effective in promoting chondroprotective effects and maintaining the integrity of the articular tissue in the knees of OA rats. PBM and aerobic exercises produced an increase in the expression of TGF-β, which is a member of a superfamily of cytokines; increased Col II and IL-10 expression, which may interfere in cell abnormal metabolism, preventing the matrix degradation and OA progression. |
| 14. | Mergoni, G.; Vescovi, P.; Belletti, S. et al. Effects of 915 nm laser irradiation on human osteoblasts: a preliminary in vitro study. Lasers Med Sci 2018, 33, 1189–1195. doi:10.1007/s10103-018-2453-5 [168] | A primary culture of human osteoblasts was isolated from mandibular cortical bone of a young health donor. | 915-nm GaAs diode laser in the different samples, was administered at 5, 15 and 45 J/cm2 with a power output of 1.5 W in continuous wave. Using two different power densities: 0.12 and 1.25 W/cm2. Irradiation time was 41.7, and 375 s using a power density of 0.12 W/cm2 and 4, 12 and 36 s using a power density of 1.25 W/cm2. | Osteoblast proliferation Osteoblast differentiation (bone nodule production) | Osteoblasts treated with a single irradiation per day for 3 days at doses of 5, 15, and 45 J/cm2 (power density: 0.12 W/cm2 showed no significant differences in terms of cell count compared to controls. PBM at parameters tested in the present study positively modulated the mineralization process in human osteoblasts, inducing the formation of a greater amount of bone nodules but did not increase cell proliferation. |
| 15. | Shakir, E.A.; Rasheed Naji, N.A.; In vitro impact of laser irradiation on platelet aggregation. Lasers Med Sci. 2018, 33, 1717–1721. doi:10.1007/s10103-018-2527-4 [169] | In vitro blood platelets from 30 healthy volunteers | 532 nm; power 100 mW; CW; 4-mm-diameter irradiation beam spot. Irradiation times: 1.8, 3.7, and 6.2 s giving doses of irradiation 1.5, 3, and 5 J/cm2, respectively. The divergence was <1.5 m rad, the crystal type of this source was Nd:VYO4:KTP, the laser spot diameter was 0.4 cm, and the power density was 796.17 W/cm2. | Platelet aggregation response to laser irradiation /ADP/ATP | Low laser irradiation induced significant changes in platelet aggregation in the presence of weak agonists such as adenosine diphosphate (ADP) and epinephrine. PBM has no influence on platelet count; however, it promotes platelet aggregation in response to weak agonists, specifically ADP and epinephrine. |
| 16. | De Souza Costa, M.; Teles, R.H.G.; Dutra, Y.M. et al. Photobiomodulation reduces neutrophil migration and oxidative stress in mice with carrageenan-induced peritonitis. Lasers Med Sci. 2018, 33, 1983–1990. doi:10.1007/s10103-018-2569-7 [170] | Animal Model (28 animals were randomly divided) | 904 nm ± 5% Operating mode Pulsed. Frequency 1000 Hz; Pulse duration 100 ns; Peak radiant power 50 W; Average radiant power 50 mW. | Cell migration and oxidative stress, in a model of carrageenan-induced inflammation | Treatment with laser decreased the number of leukocytes, especially the neutrophils, in the PBM group and reduced the concentrations of MDA (malondialdehyde), GSH (glutathione), and NO3/NO2 (nitrate/nitrite) in the peritoneal fluid. |
| 17. | Amaroli, A.; Ravera, S.; Baldini, F. et al. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med Sci 2019, 34, 495–504. doi:10.1007/s10103-018-2623-5 [171] | In vitro Human Endothelial Cells (HECV) | 808-nm diode laser light emitted by the flat-top handpiece using 1 W of power energy, 1 W/cm2 of power density, single dose of 60 J, irradiation of 60 s, fluence of 60 J/cm2, mode CW (corresponding to the measured laser therapy of 0.95 W, 0.95 W/cm2, 57 J, 60 s, 57 J/cm2). To assess the effect of 808-nm laser light irradiation on cell viability, also longer irradiations were performed (100 s and 150 s) corresponding to a final fluences of 100 J/cm2 and 150 J/cm2, respectively. | HECV/Oxidative phosphorylation aerobic metabolism of HECV, NF-κB/ROS and NO production in endothelial cells. | The present report demonstrated that the short irradiation of 60 s, by the laser setup of 1 W, 1 W/cm2, 60 J, 60 J/cm2, CW (real measured energy = 0.95 W, 0.95 W/cm2, 57 J, 57 J/cm2, CW), of HECV in vitro with 808-nm diode laser light was able to stimulate endothelial cell proliferation and oxidative metabolism, which resulted in a more efficient wound repair ability; increase in NO; activate NF-κB; NIR treatment is able to increase the aerobic metabolism, enhancing the O2 consumption and the aerobic ATP synthesis. |
| 18. | Felizatti, A.L.; do Bomfim, F.R.C.; Bovo, J.L. et al. Effects of low-level laser therapy on the organization of articular cartilage in an experimental microcrystalline arthritis model. Lasers Med Sci. 2019, 34, 1401–1412. doi:10.1007/s10103-019-02740-5 [172] | Animal Model | The gallium arsenide laser device AsGa (λ = 830 nm), CW, fluence = 18 J/cm2, power = 40 mW, total energy = 0.36 J, beam area = 0.02 cm2, by 9 s. The therapies were applied punctually in the right knee patellar region. After 7, 14, and 21 days of treatment, the animals from the three groups were euthanized (xylazine = 20 mg/kg/ketamine = 40 mg/kg associated with cardiac exsanguination) and the knees were removed and processed for structural and biochemical analysis (n = 4/experimental time/analysis) of the AC of the femur and tibia. | Morphometric parameters evaluated in the articular cartilage in male rats. Biochemical parameters evaluated in the articular cartilage (Glycosaminoglycans, Hydroxyproline, Non-collagen proteins) Non-collagen proteins | The present study shows that the phototherapy protocol, using AsGa (λ = 830 nm) in the experimental period employed, was able to revert tissue injuries produced by the microcrystalline arthritis (MA) model in young adult rats. |
| 19. | Han, B.; Fan, J.; Liu, L. et al. Adipose-derived mesenchymal stem cells treatments for fibroblasts of fibrotic scar via downregulating TGF-β1 and Notch-1 expression enhanced by photobiomodulation therapy. Lasers Med Sci. 2019, 34, 1–10. doi:10.1007/s10103-018-2567-9 [173] | Culture of cells | The total surface of the culture dishes was irradiated for 152 s each time; the energy density of the laser was 4 J/cm2. The dual model device emitted florida 6 laser beams (beam diameter <5 mm) at a wavelength of 655 nm (± 5%) and 6 laser beams at a wavelength of 635 nm. | Fibroblasts/TGF-β1 and Notch-1 expression Cell proliferation (CCK-8), cell apoptosis (MUSE), and cytotoxicity (LDH) assays | Results obtained from experiments showed that cell culture supernatant of post-PBM, adipose-derived mesenchymal stem cells (ADSCs) has much more potential as a fibrotic treatment of keloid fibroblasts (KFs) and hypertrophic scar fibroblasts (HSFs), and acting by inhibition of the proliferation, migration, and profibrotic genes synthesis via downregulating TGF-β1 and Notch-1 expression. |
| 20. | Tsuka, Y.; Kunimatsu, R.; Gunji, H. et al. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Lasers Med Sci 2019, 34, 55–60. doi:10.1007/s10103-018-2586-6 [174] | In vivo and In vitro was studied a variety of cell types | Nd:YAG laser (wavelength of 1064 nm) for 60 s at 0.3 W (10 pps, 30 mJ). The total energy density was about 10.34 J/cm2. | Migration of cultured human osteoblasts; ATP synthesis | This study showed that Nd:YAG laser irradiation (wavelength of 1064 nm, 0.3W, 10 pps, 30 mJ, 10.34 J/cm2, irradiation time 60 s) may contribute to the regeneration of bone tissues owing to enhanced osteoblast cell migration. ATP synthesis was significantly increased in the laser irradiation group compared to the control group. |
| 21. | Cardoso, L.M.; Pansani, T.N.; Hebling, J.; de Souza Costa C.A.; Basso, F.G. Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers Med Sci. 2020, 35, 1205–1212. doi:10.1007/s10103-020-02974-8 [175] | Primary cell culture Gingival fibroblast isolation | 12 units of laser diode DL-7140-201S, InGaAsP laser. Center wavelength (nm) 780 nm; spectral band width 780 nm ± 5 nm; Operating mode Continuous wave Frequency 1012 Hz to 1015 Hz; Pulse on duration 40 s; Pulse of duration or duty cycle 40 s; Energy per pulse 0.5 J Peak radiant power 0.07 W; Average radiant power 0.025 W. Number and frequency of treatment sessions 1 irradiation per day, over 3 days. Total radiant energy 1.5 J | Cytokine exposure Cell viability Gene expression of collagen type I and vascular endothelial growth factor (VEGF); Synthesis of VEGF, TNF-α, IL-1β | PBM on the selected parameters (0.5 J/cm2, 0.025 W, 780 nm) was capable of adequately penetrating the collagen matrix and positively stimulating human gingival fibroblasts (HGF) wound healing-related functions and decreasing TNF-α synthesis, even in the presence of inflammatory challenge. IL-6 and IL-8 decreased cell viability, the synthesis of VEGF, and gene expression of collagen type I. PBM enhanced cell density in the matrices and stimulated VEGF expression, even after IL-6 challenge. |
| 22. | Katagiri, W.; Lee, G.; Tanushi, A.; Tsukada, K.; Choi, H.S.; Kashiwagi, S. High-throughput single-cell live imaging of photobiomodulation with multispectral near-infrared lasers in cultured T cells. Journal of biomedical optics, 2020, 25, 1–18. doi:10.1117/1.JBO.25.3.036003 [176] | In vitro T cells culture | Two lasers were adjusted from 200 to 400 mW/cm2 for 1064 nm and 50 to 100 mW/cm2 for 1270 nm at the focal plane. Dual laser irradiation at an irradiance of 400 mW/cm2 for 1064 nm and 100 mW/cm2 for 1270 nm was monitored using an IR camera (FLIR Systems). | T cells/PBM on T cells and imaging of intracellular calcium levels and ROS generation nitric oxide binding to cytochrome c oxidase/mitochondrial retrograde signaling | A specific combination of wavelengths at low irradiances (250 to 400 mW/cm2 for 1064 nm and 55 to 65 mW/cm2 for 1270 nm) modulates mitochondrial retrograde signaling, including intracellular calcium and reactive oxygen species in T cells. The time-dependent density functional theory computation of binding of nitric oxide (NO) to cytochrome c oxidase indicates that the illumination with NIR light could result in the NO release, which might be involved in these changes. |
| 23. | Lemos, G.A., Batista, A.U.D., da Silva, P.L.P. et al. Photobiostimulation activity of different low-level laser dosage on masticatory muscles and temporomandibular joint in an induced arthritis rat model. Lasers Med Sci. 2020, 35, 1129–1139. doi:10.1007/s10103-019-02933-y [177] | Animal Model | (GaAlAs), 830 nm, 30 mW, 0.116 cm2, irradiance 0.259 W/cm2, CW, divided as follows: LG5: 5 J/cm2, 0.6 J, 20 s/session LG10: 10 J/cm2, 1.2 J, 40 s /session LG20: 20 J/cm2, 2.4 J, 80 s /session Ten sessions, with 48-h intervals. | Pro-inflammatory cells/IL-1β and TNF-α/ Matrix metalloproteinases (MMPs) family MMP 9 and MMP 2 activity | Results suggest that in this experimental model of joint inflammation, PBM can modulate pro-inflammatory mediators, reducing IL-1β and TNF-α concentrations in affected tissues. LLLT doses promoted better organization of articular disc collagen fibers, a greater number of proteoglycans in articular cartilage, increased area and diameter of left lateral pterygoid fibers, reduced latent and active MMP 9 and 2 activity, and lower IL-1β concentration. |
| 24. | Li, K., Liang, Z., Zhang, J. et al. Attenuation of the inflammatory response and polarization of macrophages by photobiomodulation. Lasers Med Sci. 2020, 35, 1509–1518. [178] | Culture bone marrow-derived macrophages (BMDMs) | GaAlAs; 810 nm, 2 mW/cm2, 4 J and 10 J. | Inflammatory cells (macrophages and neutrophils)/NF-κB p65 | PBM suppressed the expression of a marker of classically activated macrophages, inducible nitric oxide synthase; decreased the mRNA expression and secretion of pro-inflammatory cytokines, TNF-α, iNOS, and IL-1β; increased the secretion of monocyte chemotactic protein 1; significantly decreased NF-κB p65 expression in the 4J and 10 J PBM groups. |
| 25. | Moreira, S.H.; Pazzini, J.M.; Álvarez, J.L.G. et al. Evaluation of angiogenesis, inflammation, and healing on irradiated skin graft with low-level laser therapy in rats (Rattus norvegicus albinus wistar). Lasers Med Sci. 2020. 35, 1103–1109 doi:10.1007/s10103-019-02917-y [179] | Animal Model | AlGaInP 660 nm, 30 mW; its local action area of 2 cm2. The laser tip was positioned at a 90° angle in contact with the skin at each predetermined point of the graft, and it was kept for 12 s/point in 6 J/cm2 dose and 20 s/point in 10 J/cm2 dose. The animals were seen for 15 days, being the sessions performed every 3 or 5 days with 6 J/cm2 or 10 J/cm2 dose. The groups were G1—control; G2—6 J/cm2 every 3 days; G3—10 J/cm2 every 3 days; G4—6 J/cm2 every 5 days; and G5—10 J/cm2 every 5 days. | Fibroblasts/skin grafts/the expression of collagen type III the inflammatory response/COX-2 expression/CD31 expression | It is concluded the exhibition of the skin grafts to 6 J/cm2 or 10 J/cm2 dose every 5 days improved the healing and the modulation of the local inflammation. The results showed the LLLT may modulate the COX-2 expression in G3, when it has lower average; a greater trend of CD31 expression in G1 was observed as well as less expression in G2. The greater collagen type III–green expression was observed in grafts from G4 in association with greater fibroblasts count. However, in grafts from G5, the collagen type I–red expression was better seen. It was possible to deduce the 10 J/cm2 dose every 5 days in G5 resulted in the collagen ripeness. |
| 26. | da Silva, J.G.F.; dos Santos, S.S.; de Almeida, P. et al. Effect of systemic photobiomodulation in the course of acute lung injury in rats. Lasers Med Sci. 2020. doi:10.1007/s10103-020-03119-7 [180] | Animal Model | Red light-emitting diode (LED) (660 nm) 100 mW; 5 J/cm; Energy density 5.35 J/cm2; Power density = 33.3 mW/cm2; Area = 2.8 cm2; total energy= 15 J; time= 150 sec. | Inflammatory cells (macrophages and neutrophils)/ myeloperoxidase activity/ (IL) 1β, IL-6, and IL-17. | PBM on the systemic lipopolysaccharide induced acute lung injury, as it reduced the number of neutrophils recruited into the bronchoalveolar lavage, myeloperoxidase activity, and reduced interleukins (IL) 1β, IL-6, and IL-17 in the lung. |
| Authors/year | Type of Clinical Pathology | Type of Laser/ Wavelength (nm) | Mean Output Power (mW) | Energy Density (J/cm2) | Power Density mW/cm2/Beam Spot Size (cm2) | Area/Pulse (ns) | Time (s or min) | Total E (J) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Bjordal, J.M.; Lopes-Martins, R.A.; Iversen, V.V. A randomized, placebo controlled trial of low-level laser therapy for activated achilles tendinitis with microdialysis measurement of peritendinous prostaglandin E2 concentrations. Br J Sports Med. 2006, 40, 76–80. [181] | Bilateral Achilles tendinitis | GaAs Infrared 904 nm | Peak power 10 W/Freq. 5000 Hz | 20 mW/cm2 | Fluence 20 mW/cm2/0.5 cm2 | 1.8 J for each of 3 points along the tendon | 200 ns | In total 5.4 J per tendon | LLLT at a dose of 5.4 J per point can reduce inflammation and pain in activated Achilles tendinitis. LLLT may have potential in the management of diseases with an inflammatory component. |
| Nakamura, T.; Ebihara, S.; Ohkuni, I. et al. Low Level Laser Therapy for chronic knee joint pain patients. Laser therapy 2014, 23, 273–277. doi:10.5978/islsm.14-OR-21 [182] | 35 subjects with of chronic knee joint pain caused by OA- induced degenerative meniscal tear. | Ga-Al-AS: 830 nm ± 15 nm; CW | 1000 mW ± 20% | 20.1 J/cm2/point | - | 20.1 J/cm2/point | 30 s/point | - | This study confirmed that 830 nm diode laser LLLT was an effective treatment for pain related to knee osteoarthritis. |
| Soleimanpour, H.; Gahramani, K.; Taheri, R. et al. The effect of low-level laser therapy on knee osteoarthritis: prospective, descriptive study. Lasers Med Sci. 2014; 29, 1695-1700. doi:10.1007/s10103-014-1576-6 [183] | 18 patients with knee osteoarthritis | Gal-Al-As: 810 nm; 50 mW pulse; radiation mode F = 3000 Hz, Δt = 200 ns. | 80 W | 6 J/cm2 | 0.05 W/cm2 | 1 cm2 | 120 s | 36 J | Significant reduction of the nocturnal pain, pain on walking and ascending the steps, knee circumference, distance between the hip and heel, and knee to horizontal hip to heel distance at the end of the treatment course. |
| 890 nm; 30 mW; F = 3000 Hz Δt = 200 ns | peak power = 50 W | 10 J/cm2 | 17 mW /cm2 | 1.765 cm2 | 588 s | 53.6 J | |||
| Youssef, E.F.; Muaidi, Q.I.; Shanb, A.A. Effect of Laser Therapy on Chronic Osteoarthritis of the Knee in Older Subjects. J Lasers Med Sci. 2016, 7, 112–119. doi:10.15171/jlms.2016.19 [184] | Patients with OA, Group 1 (n = 18) | 880 nm, 2 times/ week for 8 weeks = 16 sessions; | 50 mW, CW | - | - | - | 6 J/point for 60 s, for 8 points | 48 J in each session | LLLT to exercise training program is more effective than exercise training alone in the treatment of patients with chronic knee OA and the rate of improvements may be dose dependent, as with 6 J/cm2 or 3 J/cm2. |
| Group 2 (n = 18) | 904 nm, frequency 700 Hz; 2 times/ week for 8 weeks = 16 sessions- | 60 mW | 3 J/cm2 | Peak power 20 WSpot: 0.5 cm2 | Pulse duration 4.3 ms | 50 s per point | 27 J per session | ||
| Group 3 (placebo laser) (n = 15) | - | - | - | - | - | - | - | ||
| Nambi, S.G.; Kamal, W.; George, J.; Manssor, E. Radiological and biochemical effects (CTX-II, MMP-3, 8, and 13) of low-level laser therapy (LLLT) in chronic osteoarthritis in Al-Kharj, Saudi Arabia. Lasers Med Sci. 2017, 32, 297–303. doi:10.1007/s10103-016-2114-5 [185] | Thirty-four subjects with knee OA were randomized into two groups: active group (n = 17) and | GaAs super pulsed laser: 905 nm | 25 mW | 1.5 J per point for 8 points in total. | - | 1 cm2 | 60 s | Total dose 12 J | This study provides the evidence of PBM in reduction of pain and the therapy’s ability to inhibit the proliferation of collagen type II C-telopeptide and other proteins MMP-3 (stromelysin), MMP-8 (collagenase-2), and MMP-13 (collagenase-3), making it an ideal treatment for subjects in the later stages of OA. |
| Placebo (n = 17). | - | - | - | - | - | - | - | ||
| Alayat, M.S.; Ali, M.M. Efficacy of class IV diode laser on pain and dysfunction in patients with knee osteoarthritis: a randomized placebo-control trial. Bull Fac Phys Ther 2017, 22, 40–5. doi:10.4103/1110-6611.209880 [186] | Randomized blinded placebo-controlled trial 50 patients with knee OA | Ga–Al–Ar 808 nm CW 3 sessions /week/for 4 consecutive weeks | 1000 mW | Mean power 500 mW | Spot diam = 2 cm; Aria= 3.14 cm2 | 2.14 J/cm2 | 6 min and 17 s per session | 150 J | Class IV diode laser combined with exercise was more effective than exercise alone in the treatment of knee OA. PBM combined with exercise effectively decreased pain and WOMAC index (Western Ontario and McMaster Universities Osteoarthritis Index), as compared with exercise alone. |
| 905 nm pulsed emission, frequency: 1500 Hz. 3 sessions /week/for 4 consecutive weeks | Peak power 25 W | Mean power 54 mW | Spot diam = 2 cm; Aria= 3.14 cm2 | Scan on 100 cm2; Trigg. Points 6.175 J on each point in an average time of 16 s | 9 min. | 214 J | |||
| Tomazoni, S.S.; Costa, L.; Joensen, J.; Stausholm, M.B.; Naterstad, I.F.; Leal-Junior, E.; Bjordal, J.M. Effects of photobiomodulation therapy on inflammatory mediators in patients with chronic non-specific low back pain: Protocol for a randomized placebo-controlled trial. Medicine 2019, 98, e15177. doi:10.1097/MD.0000000000015177 [187] | Randomized placebo-controlled trial PBMT with 5 lasers, skin contact | 905 ± 1 nm, Super pulsed infrared 3000 Hz | 25 W | 1,35 J | 17.05 mW/cm2 0.44 cm2 (spot) | 3.07 J/cm2 | - | This is the first study that will investigate a possible biological mechanism behind the positive clinical effects of PBMT on non-specific chronic low back pain. We strongly believe that this investigation can be helpful in the management of this condition. | |
| 905 ± 1 nm, Super pulsed infrared1000 Hz | Laser shower 12.5 W | 0.225 J | 2.84 mW/cm2 0.44 cm2 (spot) | 0.511 J/cm2 | - | ||||
| 640 ± 10 nm,2 Hz | 15 mW | 2.7 J | 0.9 cm2 | - | - | 4 LEDs Red 24.75 J | |||
| 640 ± 10 nm, 2 Hz2 Hz | 15 mW | 2.7 J | 0.9 cm2 | - | - | 4 LEDs Red 24.30 J | |||
| 875 ± 10 nm,16 Hz | 17.5 mW | 3.15 J | 0.9 cm2 | - | 180 s | 4 LEDs Infrared idem | |||
| 875 ± 10 nm,16 Hz | 17.5 mW | 3.15 J | 0.9 cm2 | - | 180 s | ||||
| Tsuk, S.; Lev, Y.H.; Fox, O.; Carasso, R.; Dunsky, A. Does Photobiomodulation Therapy Enhance Maximal Muscle Strength and Muscle Recovery? J Hum Kinet. 2020, 73, 135–144. Published 2020 Jul 21. doi:10.2478/hukin-2019-0138 [188] | Randomized double-blinded placebo-controlled trial | GaAlAs: 808 nm, Pulse frequency: 13 kHz | 250 mW | - | Average power: 84.5 mW, Beam size: 1 × 4,5 cm2 on 3 quadriceps points located in parallel | Pulse width26 μs | Dose rate: 5.07 J/min (1.13 J/cm2/ min) | ≈150 J overall treatment | Photobiomodulation protocol of irradiation that was used (20 min, 808 nm, energy of 150 J) did not show beneficial effects on quadriceps muscle performance or recovery after induction of fatigue, when applied immediately after exercise. |
| Tomazoni, S.S.; Costa, L.O.P.; Joensen, J.; Stausholm, M.B.; Naterstad, I.F.; Ernberg, M.; Leal-Junior, E.C.P. and Bjordal, J.M. Photobiomodulation Therapy is Able to Modulate PGE2 Levels in Patients With Chronic Non-Specific Low Back Pain: A Randomized Placebo-Controlled Trial. Lasers Surg Med. 2020, doi:10.1002/lsm.23255 [189] | Randomized placebo-controlled trial PBMT with multiple lasers, skin contact | SE 25TM Super pulsed infrared: 905 ± 1 nm, 3000 Hz | Peak power 25 W | 7.5 mW | 0.44 cm2 (spot) | 17.05 mW/cm2 | 3.07 J/cm2 | 1.35 J | Our results suggest that PBMT was able to modulate PGE2 levels. Results are compatible with the hypothesis that modulating inflammation by decreasing PGE2 levels may be one of the mechanisms involved in the effects of PBMT in patients with LBP. |
| Laser Shower 4 Super pulsed infrared: 905 ± 1 nm, 1000 Hz | 12.5 W | 1.25 mW | 0.44 cm2 (spot) | 2.84 mW/cm2 | 0.51 J/cm2 | 0.225 J | |||
| SE 25TM 4 RED LEDs (nm): 640 ± 10 nm, 2 Hz | - | 15 mW | 0.9 cm2 (spot) | 16.67 mW/cm2 | 3 J/cm2 | 2.7 J | |||
| Laser Shower 640 ± 10 nm, 2 Hz | - | 15 mW | 0.9 cm2 | 16.67 mW/cm2 | 3 J/cm2 | 2.7 J | |||
| SE 25TM 4 Infrared LEDs (nm): 875 ± 10 nm, 16 Hz; Magnetic field: 35 mT | - | 17.5 mW | 0.9 cm2/spot | 19.44 mW/cm2 | 3.5 J/cm2/ 180 s | Total dose/site 24.75 J | |||
| Laser Shower 4 Infrared LEDs (nm): 875 ± 10 nm, 16 Hz | - | 17.5 mW | 0.9 cm2/spot | 19.44 mW/cm2 | 3.5 J/cm2/180 s | Total dose/site 24.3 J |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailioaie, L.M.; Litscher, G. Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation. Int. J. Mol. Sci. 2020, 21, 6565. https://doi.org/10.3390/ijms21186565
Ailioaie LM, Litscher G. Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation. International Journal of Molecular Sciences. 2020; 21(18):6565. https://doi.org/10.3390/ijms21186565
Chicago/Turabian StyleAilioaie, Laura Marinela, and Gerhard Litscher. 2020. "Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation" International Journal of Molecular Sciences 21, no. 18: 6565. https://doi.org/10.3390/ijms21186565
APA StyleAilioaie, L. M., & Litscher, G. (2020). Molecular and Cellular Mechanisms of Arthritis in Children and Adults: New Perspectives on Applied Photobiomodulation. International Journal of Molecular Sciences, 21(18), 6565. https://doi.org/10.3390/ijms21186565