Definition of a Novel Plasmid-Based Gene Transfection Protocol of Mammalian Skeletal Muscles by Means of In Vivo Electroporation
Abstract
1. Introduction
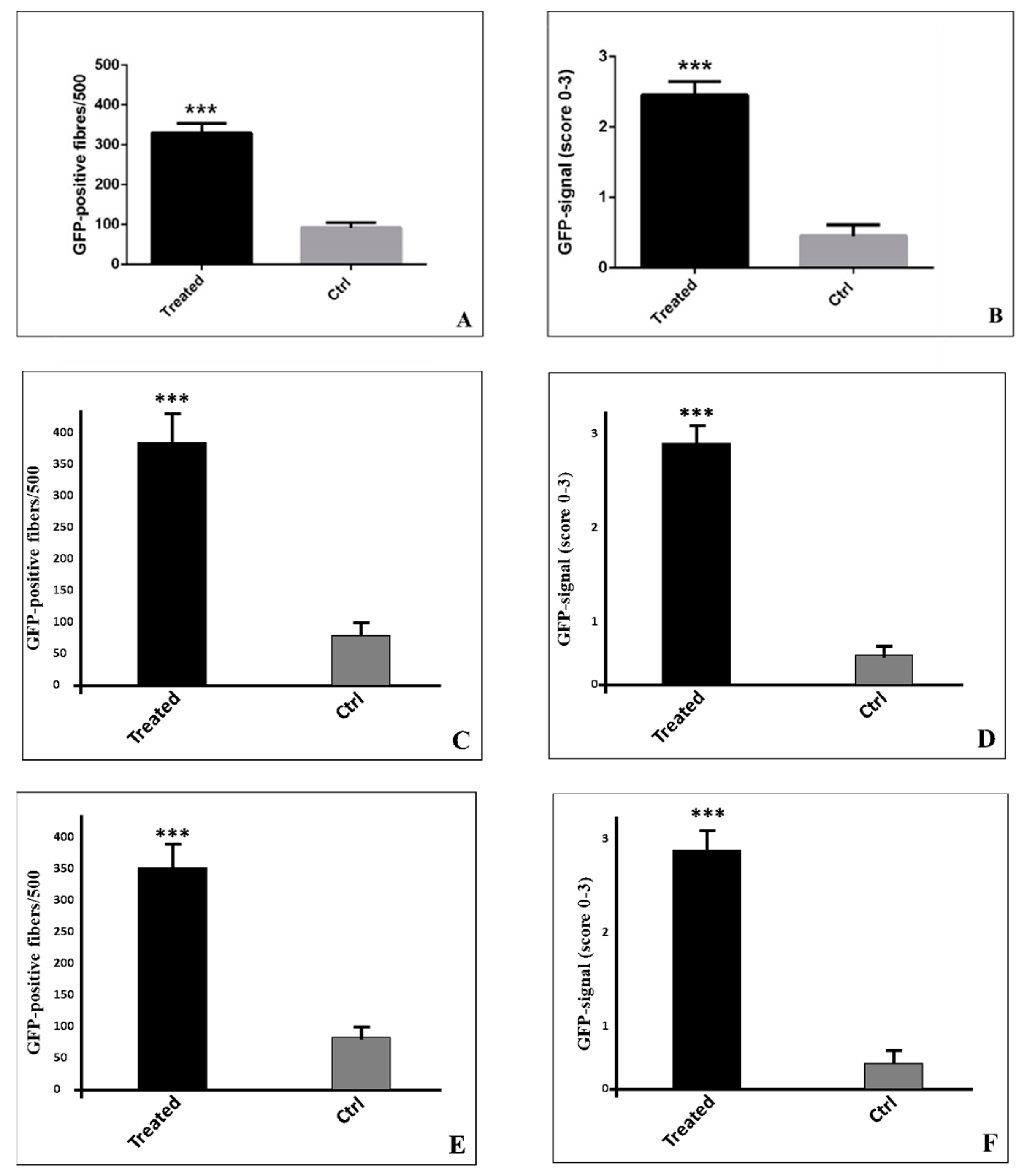
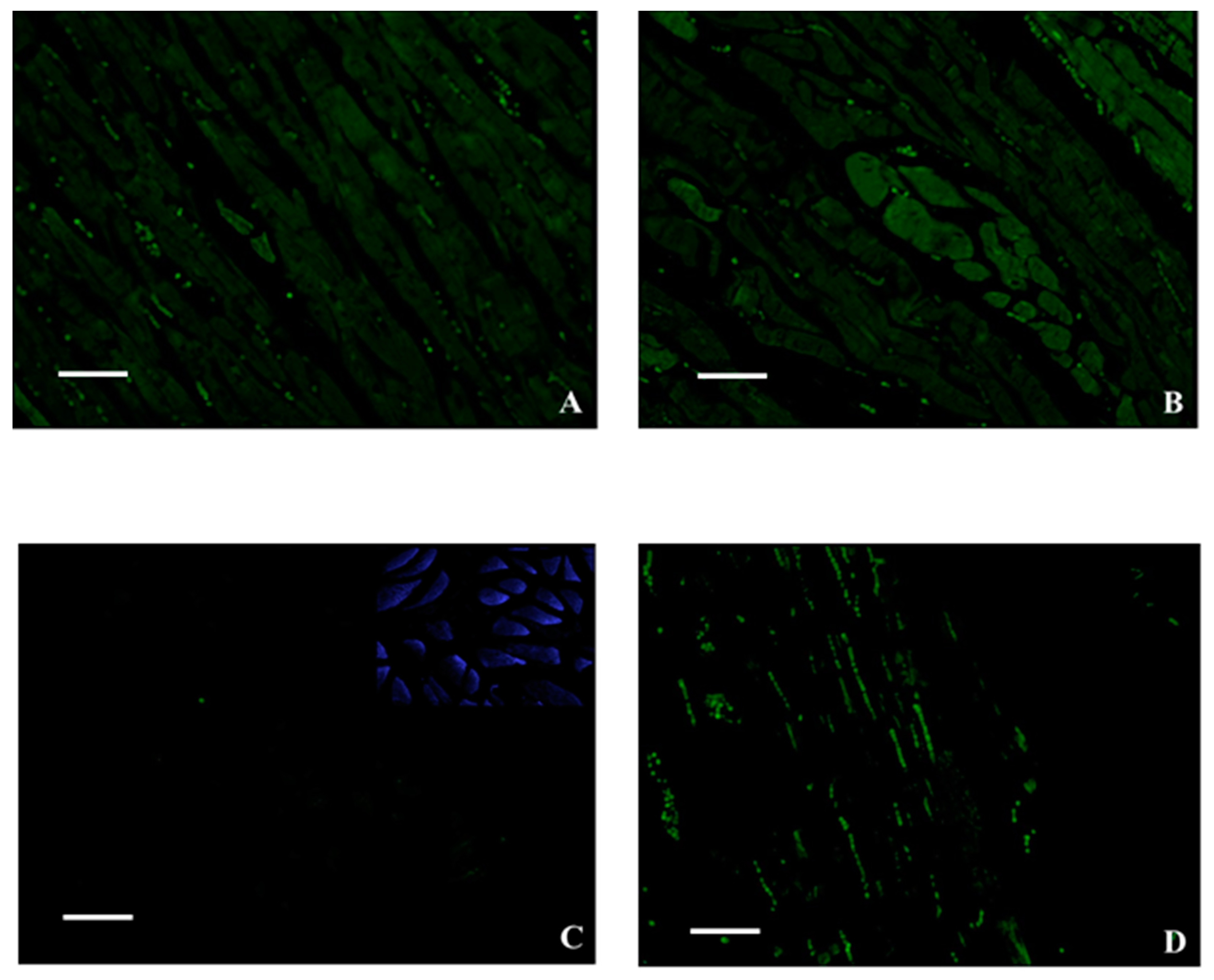
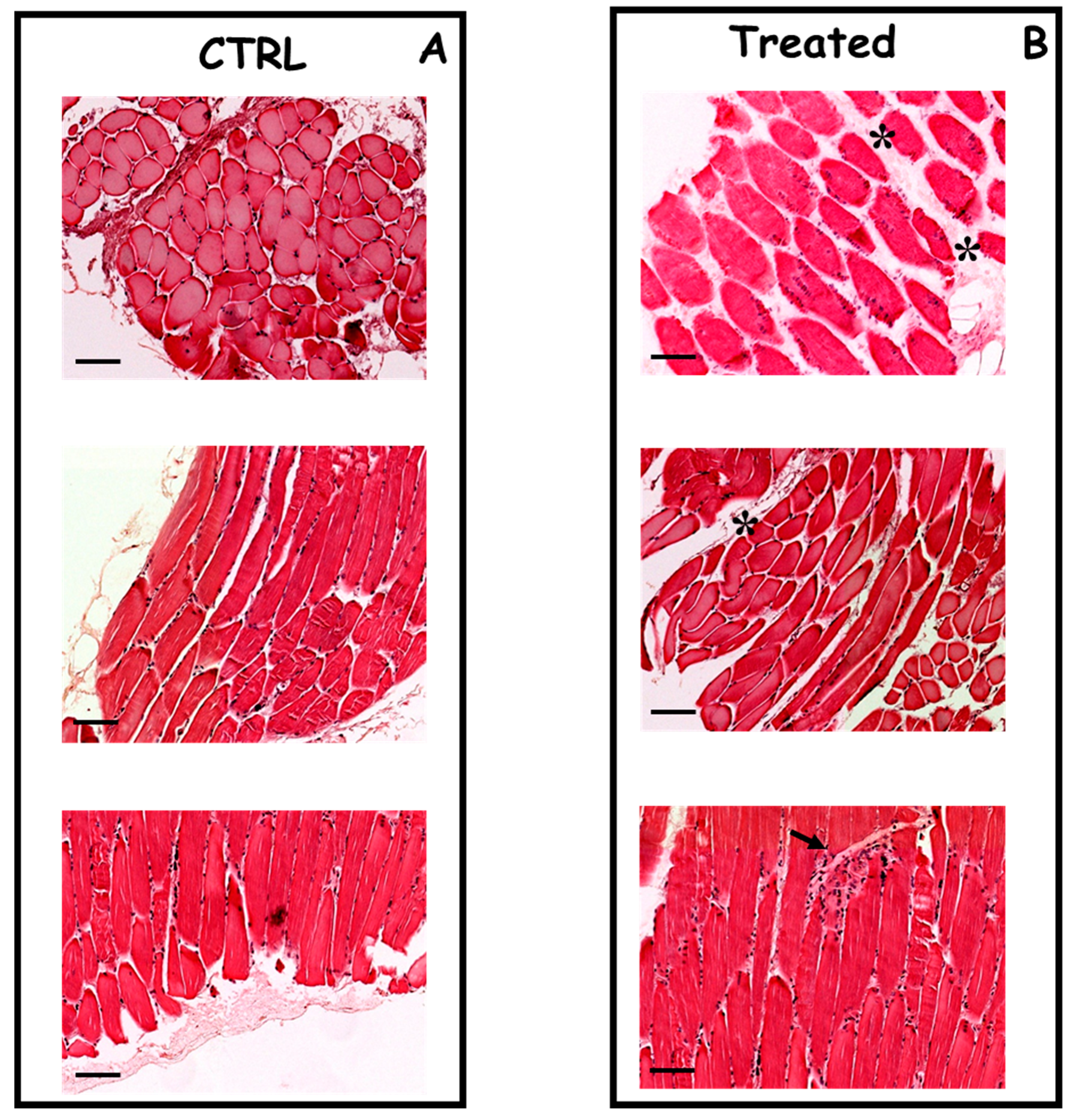
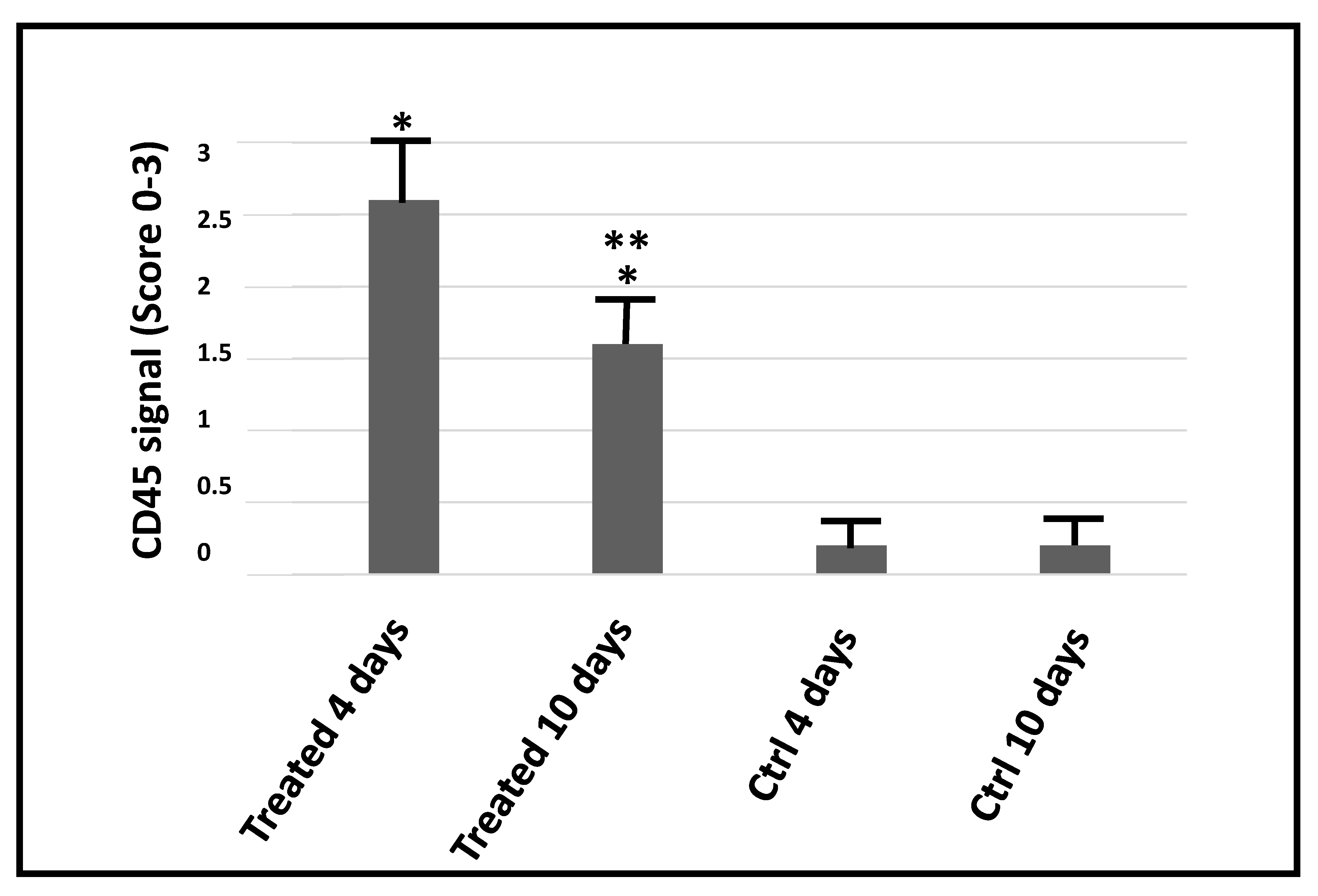
2. Results
3. Discussion
4. Materials and Methods
4.1. Plasmid DNA Preparation
4.2. Intra-Muscular Injection
4.3. Electrical and Instrumental Parameters for Electroporation
4.4. Evaluation of Electroporation-Associated Side Effects
4.5. Histology, Immunohistochemistry and GFP Analysis by Fluorescence
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Flingai, S.; Czerwonko, M.; Goodman, J.; Kudchodkar, S.B.; Muthumani, K.; Weiner, D.B. Synthetic DNA vaccines: Improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front. Immunol. 2013, 4, 354. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.J. Viral and non-viral methods for gene transfer into skeletal muscle. Curr. Opin. Drug Discov. Develop. 2006, 9, 163–168. [Google Scholar]
- Gregoveric, P.; Biankinship, M.J.; Chamberlain, J.S. Viral vectors for gene transfer to striated muscle. Curr. Opin. Mol. Ther. 2004, 6, 491–498. [Google Scholar]
- Schertzer, J.D.; Plant, D.R.; Lynch, G.S. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol. Ther. 2005, 13, 795–803. [Google Scholar] [CrossRef]
- McMahon, J.M.; Wells, D.J. Electroporation for gene transfer to skeletal muscles: Current status. BioDrugs 2004, 18, 155–165. [Google Scholar] [CrossRef]
- Peri, D.; Deville, M.; Poignard, C.; Signori, E.; Natalini, R. Numerical optimization of plasmid DNA delivery combined with hyaluronidase injection for electroporation protocol. Comput. Methods Programs Biomed. 2020, 186, 105204. [Google Scholar] [CrossRef]
- Schertzer, J.D.; Lynch, G.S. Plasmid-based gene transfer in mouse skeletal muscle by electroporation. Methods Mol. Biol. 2008, 433, 115–125. [Google Scholar]
- Andre, F.M.; Cournil-Henrionnet, C.; Vernerey, D.; Opolon, P.; Mir, L.M. Variability of naked DNA expression after direct local injection: The influence of the injection speed. Gene Ther. 2006, 13, 1619–1627. [Google Scholar] [CrossRef]
- Hartikka, J.; Bozoukova, V.; Jones, D.; Mahajan, R.; Wloch, M.K.; Sawdey, M.; Buchner, C.; Sukhu, L.; Barnhart, K.M.; Abai, A.M.; et al. Sodium phosphate enhances plasmid DNA expression in vivo. Gene Ther. 2000, 7, 1171–1182. [Google Scholar] [CrossRef][Green Version]
- Lavigne, M.D.; Yates, L.; Coxhead, P.; Górecki, D.C. Nuclear-targeted chimeric vector enhancing nonviral gene transfer into skeletal muscle of Fabry mice in vivo. FASEB J. 2008, 22, 2097–2107. [Google Scholar] [CrossRef]
- Molnar, M.J.; Gilbert, R.; Lu, Y.; Liu, A.B.; Guo, A.; Larochelle, N.; Orlopp, K.; Lochmuller, H.; Petrof, B.J.; Nalbantoglu, J.; et al. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 2004, 10, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Niedzinski, E.J.; Chen, Y.J.; Olson, D.C.; Parker, E.A.; Park, H.; Udove, J.A.; Scollay, R.; McMahon, B.M.; Bennett, M.J. Enhanced systemic transgene expression after nonviral salivary gland transfection using a novel endonuclease inhibitor/DNA formulation. Gene Ther. 2003, 10, 2133–2138. [Google Scholar] [CrossRef][Green Version]
- DiFranco, M.; Quinonez, M.; Capote, J.; Vergara, J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J. Vis. Exp. 2009, 32, 1520. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, P.; Freites-Martinez, A.; Gonzalez, S.; Spugnini, E.P.; Baldi, A. Successful treatment of plantar warts with intralesional bleomycin and electroporation: Pilot prospective study. Dermatol. Pract. Concept 2017, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, P.; Spugnini, E.P.; Baldi, A. Successful Treatment of a Keratoacanthoma with Electrochemotherapy: A Case Report. Dermatol. Ther. 2018, 8, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Fais, S.; Azzarito, T.; Baldi, A. Novel Instruments for the Implementation of Electrochemotherapy Protocols: From Bench Side to Veterinary Clinic. J. Cell Physiol. 2017, 232, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Baldi, A. Electrochemotherapy in Veterinary Oncology: State-of-the-Art and Perspectives. Vet. Clin. North Am. Small Animal Pract. 2019, 49, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Lakes, E.H.; Allen, K.D. Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthr. Cartil. 2016, 24, 1837–1849. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Grunwald, T.; Ulbert, S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: Vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.A.; Ford-Speelman, D.L.; Ru, L.W.; Densmore, A.L.; Roche, R.; Reed, P.W.; Bloch, R.J. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am. J. Physiol. Cell Physiol. 2011, 301, C1239–C1250. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Blachnio-Zabielska, A.U. A critical review of electroporation as a plasmid delivery system in mouse skeletal muscle. Int. J. Mol. Sci. 2019, 20, 2776. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Lester, G.M.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef]
- Esposito, V.; Manente, L.; Lucariello, A.; Perna, A.; Viglietti, R.; Gargiulo, M.; Parrella, R.; Parrella, G.; Baldi, A.; De Luca, A.; et al. Role of FAP48 in HIV-associated lipodystrophy. J. Cell Biochem. 2012, 113, 3446–3454. [Google Scholar] [CrossRef]
- Khokhlova, O.N.; Tukhovskaya, E.A.; Kravchenko, I.N.; Sadovnikova, E.S.; Pakhomova, I.A.; Kalabina, E.A.; Lobanov, A.V.; Shaykhutdinova, E.R.; Ismailova, A.M.; Murashev, A.N. Using Tiletamine-Zolazepam-Xylazine Anesthesia Compared to CO2-inhalation for Terminal Clinical Chemistry, Hematology, and Coagulation Analysis in Mice. J. Pharmacol. Toxicol. Methods 2017, 84, 11–19. [Google Scholar] [CrossRef]
- Esposito, T.; Lucariello, A.; Hay, E.; Contieri, M.; Tammaro, P.; Varriale, B.; Guerra, G.; De Luca, A.; Perna, A. Effects of curcumin and its adjuvant on TPC1 thyroid cell line. Chem. Biol. Interact. 2019, 305, 112–118. [Google Scholar] [CrossRef]
- Lucariello, A.; Trabucco, E.; Boccia, O.; Perna, A.; Sellitto, C.; Castaldi, M.A.; De Falco, M.; De Luca, A.; Cobellis, L. Small leucine rich proteoglycans are differently distributed in normal and pathological endometrium. In Vivo 2015, 29, 217–222. [Google Scholar]
- De Luca, A.; De Falco, M.; Fedele, V.; Cobellis, L.; Mastrogiacomo, A.; Laforgia, V.; Tuduce, I.L.; Campioni, M.; Giraldi, D.; Paggi, M.G.; et al. The serine protease HtrA1 is upregulated in the human placenta during pregnancy. J. Histochem. Cytochem. 2004, 52, 885–892. [Google Scholar] [CrossRef]
- Baldi, A.; Lombardi, D.; Russo, P.; Palescandolo, E.; De Luca, A.; Santini, D.; Baldi, F.; Rossiello, L.; Dell’Anna, M.L.; Mastrofrancesco, A.; et al. Ferritin contributes to melanoma progression by modulating cell growth and sensitivity to oxidative stress. Clin. Cancer Res. 2005, 11, 3175–3183. [Google Scholar] [CrossRef][Green Version]

| Gait Score | Description |
|---|---|
| 4 | Normal |
| 3 | Minimal impairment |
| 2 | Moderate impairment |
| 1 | Significant impairment |
| 0 | Unable to walk on the examined limb |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spugnini, E.P.; Scimeca, M.; Amadio, B.; Cortese, G.; Fanciulli, M.; Vincenzi, B.; De Luca, A.; Baldi, A. Definition of a Novel Plasmid-Based Gene Transfection Protocol of Mammalian Skeletal Muscles by Means of In Vivo Electroporation. Int. J. Mol. Sci. 2020, 21, 6494. https://doi.org/10.3390/ijms21186494
Spugnini EP, Scimeca M, Amadio B, Cortese G, Fanciulli M, Vincenzi B, De Luca A, Baldi A. Definition of a Novel Plasmid-Based Gene Transfection Protocol of Mammalian Skeletal Muscles by Means of In Vivo Electroporation. International Journal of Molecular Sciences. 2020; 21(18):6494. https://doi.org/10.3390/ijms21186494
Chicago/Turabian StyleSpugnini, Enrico P., Manuel Scimeca, Bruno Amadio, Giancarlo Cortese, Maurizio Fanciulli, Bruno Vincenzi, Antonio De Luca, and Alfonso Baldi. 2020. "Definition of a Novel Plasmid-Based Gene Transfection Protocol of Mammalian Skeletal Muscles by Means of In Vivo Electroporation" International Journal of Molecular Sciences 21, no. 18: 6494. https://doi.org/10.3390/ijms21186494
APA StyleSpugnini, E. P., Scimeca, M., Amadio, B., Cortese, G., Fanciulli, M., Vincenzi, B., De Luca, A., & Baldi, A. (2020). Definition of a Novel Plasmid-Based Gene Transfection Protocol of Mammalian Skeletal Muscles by Means of In Vivo Electroporation. International Journal of Molecular Sciences, 21(18), 6494. https://doi.org/10.3390/ijms21186494








