Abstract
Our recent studies have implicated some passenger strands of miRNAs in the molecular pathogenesis of human cancers. Analysis of the microRNA (miRNA) expression signature in pancreatic ductal adenocarcinoma (PDAC) has shown that levels of miR-30a-3p, the passenger strand derived from pre-mir-30a, are significantly downregulated in PDAC tissues. This study aimed to identify the oncogenes closely involved in PDAC molecular pathogenesis under the regulation of miR-30a-3p. Ectopic expression assays showed that miR-30a-3p expression inhibited the aggressiveness of the PDAC cells, suggesting that miR-30a-3p acts as a tumor-suppressive miRNA in PDAC cells. We further identified 102 putative targets of miR-30a-3p regulation in PDAC cells by combining in silico analysis with gene expression data. Of these, ten genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM2, MGLL, SERPINE1, ITGA2, DTL, and UACA) were independent prognostic factors in multivariate analysis of survival of patients with PDAC (p < 0.01). We also investigated the oncogenic function of the integrin ITGA2 in PDAC cell lines. The integrin family comprises cell adhesion molecules expressed as heterodimeric, transmembrane proteins on the surface of various cells. Overexpression of ITGA2/ITGB1 (an ITGA2 binding partner) was detected in the PDAC clinical specimens. The knockdown of ITGA2 expression attenuated the malignant phenotypes of the PDAC cells. Together, results from these microRNA-based approaches can accelerate our understanding of PDAC molecular pathogenesis.
1. Introduction
Ductal carcinoma of the pancreas (PDAC) is derived from exocrine cells of the pancreatic duct and accounts for 90% of pancreatic cancer cases [1,2,3]. Due to the aggressive phenotype of PDAC cells, which includes high invasiveness and drug resistance, the 5-year relative survival rate for pancreatic cancer is only 3–10%, which is one of the lowest among human cancers [1,4]. Since many patients with PDAC are asymptomatic, most cases have reached an advanced stage at the time of diagnosis.
Current standard treatments for PDAC still consist of surgical resection and cytotoxic chemotherapies, although fewer than 20% of PDAC patients are candidates for complete surgical resection [1,5]. Gemcitabine is an essential drug for the treatment of PDAC. FOLFIRINOX (folinic acid, 5-FU, irinotecan, and oxaliplatin), as well as gemcitabine and nab-paclitaxel regimens, are given as first-line treatments for advanced PDAC with distant metastases. However, these treatment regimens typically offer insufficient therapeutic effects [6]. Thus, new diagnostic markers and the development of new therapeutic strategies based on the latest genomic analyses are needed for patients with PDAC.
In the post-human genome era, it has become clear that non-coding RNAs are functional and control pivotal biological functions in a wide range of biological processes [7,8]. MicroRNAs (miRNAs) are endogenous non-coding RNAs that are single-stranded molecules having lengths between 19 and 23 nt that function to fine-tune RNA expression control via degradation or translational inhibition of target RNA transcripts [7,8,9]. A single miRNA can control a large number of RNA transcripts for both protein-coding genes and non-coding genes. Expressions of around 60% of cellular RNAs are controlled by miRNAs [10,11]. Therefore, the aberrant expression of miRNAs can disrupt RNA networks and trigger the transformation of normal cells to diseased cells.
In the last decade, multiple studies have shown that aberrant expressions of miRNAs are involved in the molecular pathogenesis of human cancers and that their dysregulated expressions can enhance cancer cell aggressiveness via several pathways, such as loss of cell cycle regulation, suppression of apoptosis, promotion of cell invasion and metastasis, and acquisition of drug resistance [12,13,14]. In miRNA-based cancer research, the starting point of many studies is the identification of aberrantly expressed miRNAs in cancer cells and the investigation of cancer-related genes controlled by miRNAs in expression. Recently, we have applied RNA-sequencing methods to generate a miRNA expression signature of PDAC and identified downregulations of 122 miRNAs in PDAC tissues [15]. Based on this signature, we have sequentially identified the tumor-suppressive miRNAs, and the genes controlled by these miRNAs, that play important roles in the molecular pathogenesis of PDAC [15].
A mature miRNA duplex is derived from the hairpin structure of pri-miRNA. The orientation of the miRNA strand determines the name of the mature miRNA form (the 5p strand derived from the 5’end of the pre-miRNA and the 3p strand arises from the 3’end). During miRNA biogenesis, one strand is preferentially loaded into Argonaute (AGO) and is considered the guide strand to function. On the other hand, the unloaded strand is called the passenger strand and degraded. However, the proportion of 5p or 3p strands incorporated into AGO is greatly dependent on the cell types or cell environments [16]. Recently, both 5p and 3p miRNA strands have been recognized as functional but have not been fully investigated in cancer cells.
The salient point of our RNA-sequencing-based signature is that expressions of some passenger strands of miRNAs derived from pre-miRNAs were significantly dysregulated in PDAC tissues. Our ectopic expression assays demonstrated that miR-216a-3p, miR-216b-3p, miR-148a-5p and miR-130b-5p acted as tumor-suppressive miRNAs in PDAC cells by targeting several oncogenes [15,17,18]. In this study, we focused on miR-30a-3p, the passenger strand derived from pre-mir-30a, based on our analysis of the signature that revealed significantly reduced levels of miR-30a-3p in PDAC tissues. Moreover, compared to miR-30a-5p, the guide strand of pre-mir-30a, miR-30a-3p, has not been fully analyzed in pancreatic cancer cells.
Our data from the present study showed that the expression of miR-30a-3p inhibited the aggressive phenotype of cancer cells, suggesting that it may act as a tumor-suppressive miRNA in PDAC cells. Our miRNA target search strategy identified 102 putative targets for miR-30a-3p regulation in PDAC cells. Importantly, expression levels of 10 of these genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM, MGLL, SERPINE1, ITGA2, DTL, and UACA) were independent prognostic factors in the multivariate analysis of survival of patients with PDAC (p < 0.01). We also investigated the oncogenic function of ITGA2 in PDAC cells for this study.
2. Results
2.1. Downregulation of miR-30a-5p and miR-30a-3p in PDAC Cells and Their Tumor-Suppressive Roles in PDAC Cell Lines
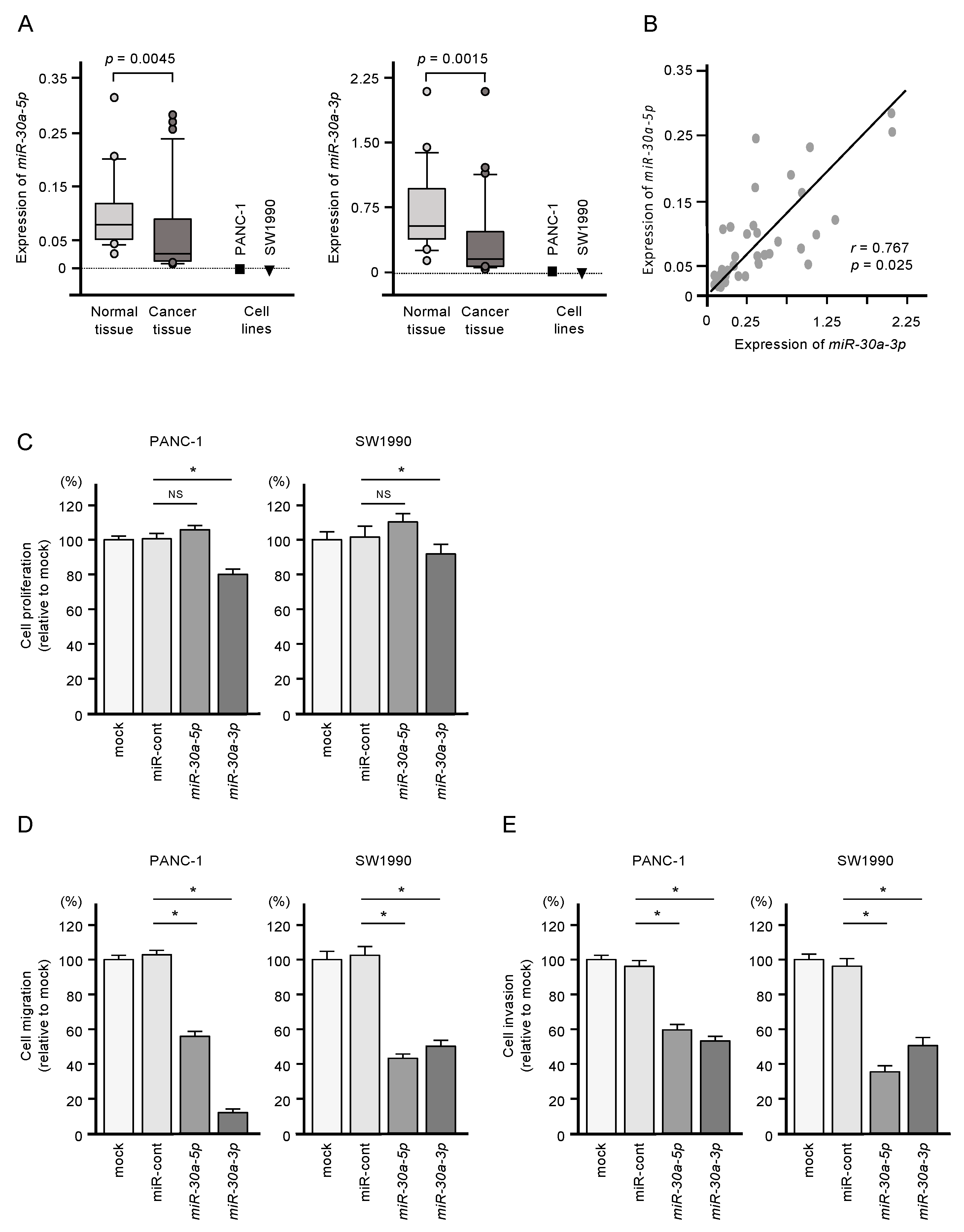
Expressions of miR-30a-5p and miR-30a-3p were significantly downregulated in PDAC clinical specimens (p = 0.0045 and p = 0.0015, respectively) and in two PDAC cell lines (PANC-1 and SW1990; Figure 1A). Pearson’s analysis showed a positive correlation between miR-30a-5p and miR-30a-3p expression levels in the clinical samples (r = 0.7672, p = 0.025; Figure 1B). The clinical features of patients with PDAC considered in this study are shown in Table S1.
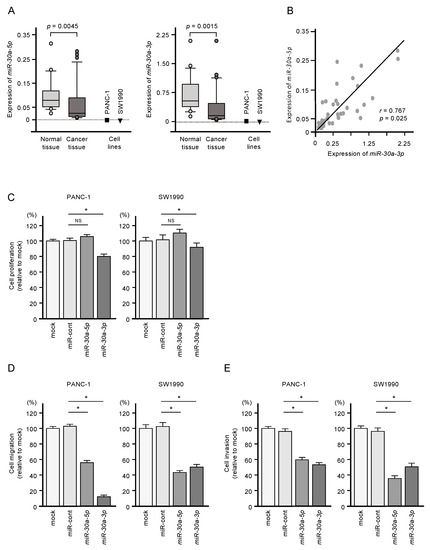
Figure 1.
Tumor-suppressive functions of miR-30a-5p and miR-30a-3p in pancreatic ductal adenocarcinoma (PDAC) cells. (A) Expression levels of miR-30a-5p and miR-30a-3p in PDAC clinical specimens and cell lines (PANC-1 and SW1990). Data were normalized relative to the expression of RNU48. (B) Pearson’s coefficient showed positive correlations between the expression levels of miR-30a-5p and miR-30a-3p in clinical specimens. (C) Cell proliferation assessed using XTT assays. Data were collected 72 h after miRNA transfection (* p < 0.0001). (D) Cell migration assessed with a membrane culture system. Data were collected 48 h after seeding the cells into the chambers (* p < 0.0001). (E) Cell invasion determined using Matrigel invasion assays conducted 48 h after the seeding of the miRNA-transfected cells into the chambers (* p < 0.0001).
Restoring expression of both miR-30a-5p and miR-30a-3p by transfection was associated with reduced malignant phenotypes of PANC-1 and SW1990 cells, as evidenced by suppressed cell proliferation, migration, and invasion compared to untransfected cells (Figure 1C,D). Meanwhile, increased expression of miR-30a-5p alone suppressed only cell migration and invasion (Figure 1C,D). Given the more substantial tumor-suppressive effect of miR-30a-3p, we focused on this miRNA in subsequent studies.
2.2. Identification of Genes Targeted by miR-30a-3p in PDAC Cells
To identify putative targets of miR-30a-3p regulation in PDAC cells, we assessed three datasets: (i) the TargetScan database, to identify putative targets of miR-30a-3p in silico; (ii) gene expression data for genes that were downregulated in miR-30a-3p-transfected PDAC cells; and (iii) gene expression data for genes that were upregulated in PDAC clinical specimens. A total of 102 genes were identified as putative targets of miR-30a-3p regulation in PDAC cells (Table 1).

Table 1.
Identification of putative targets regulated by miR-30a-3p in PDAC cells.
2.3. Clinical Significance of miR-30a-3p Target Genes in PDAC
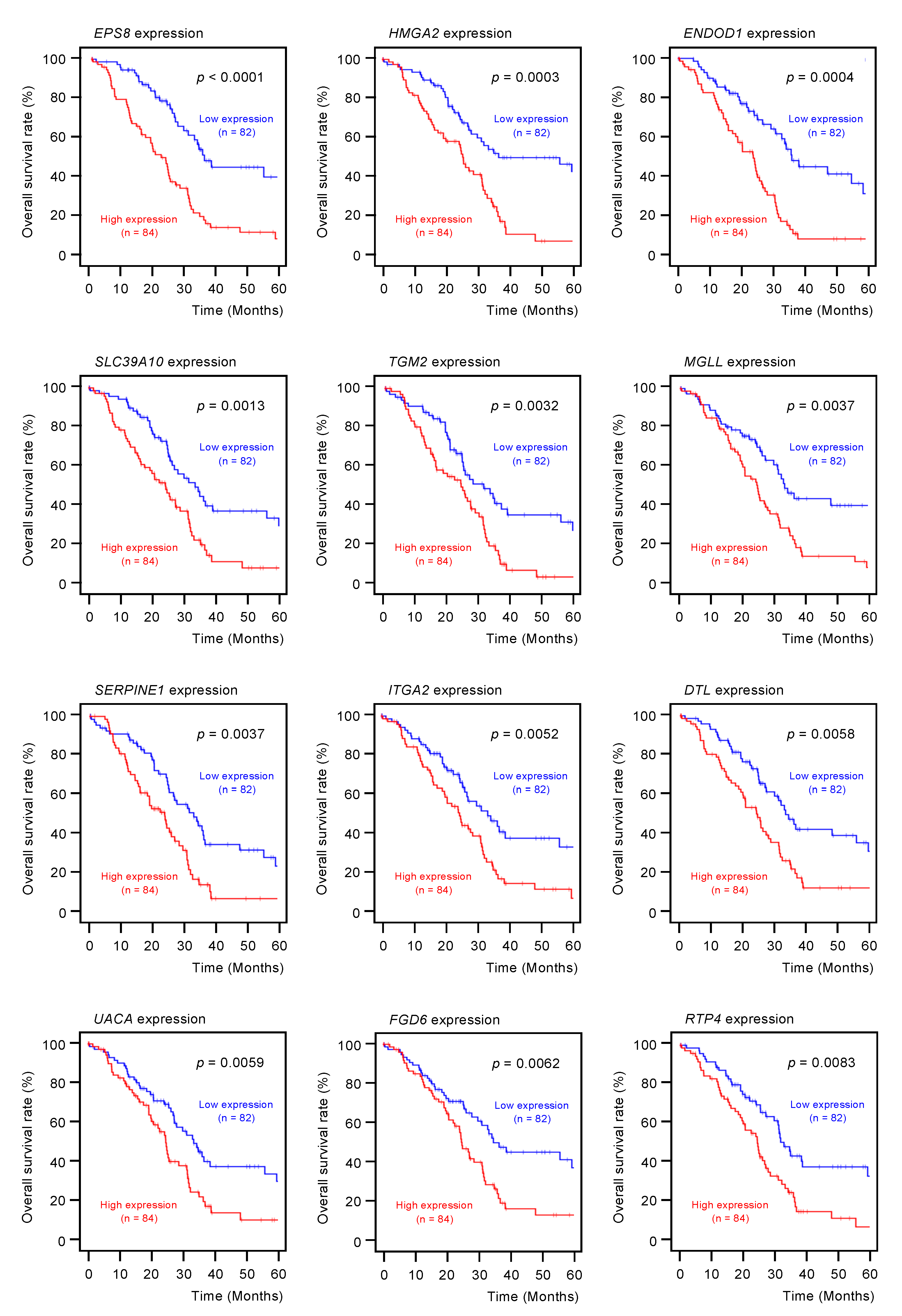
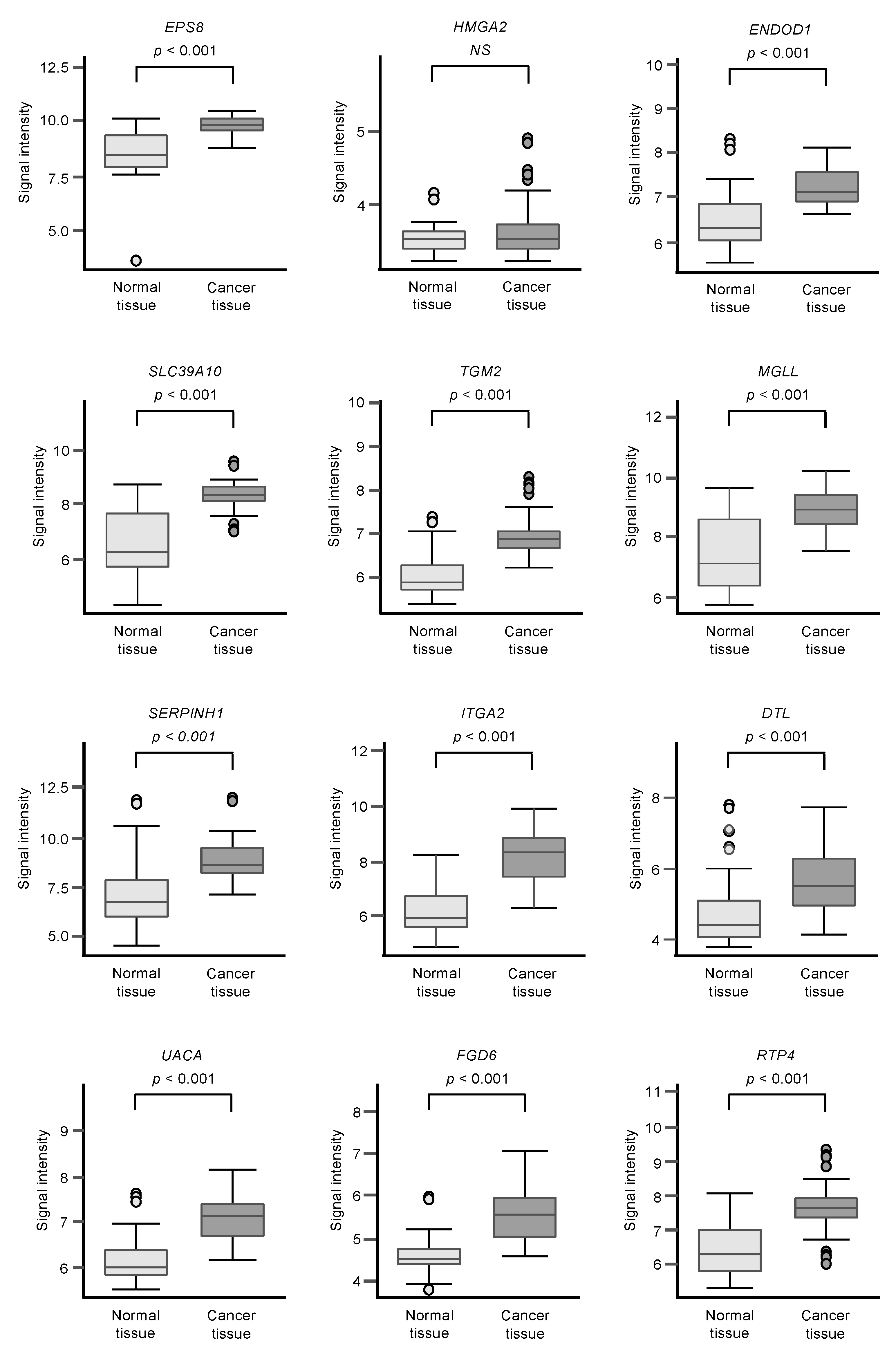
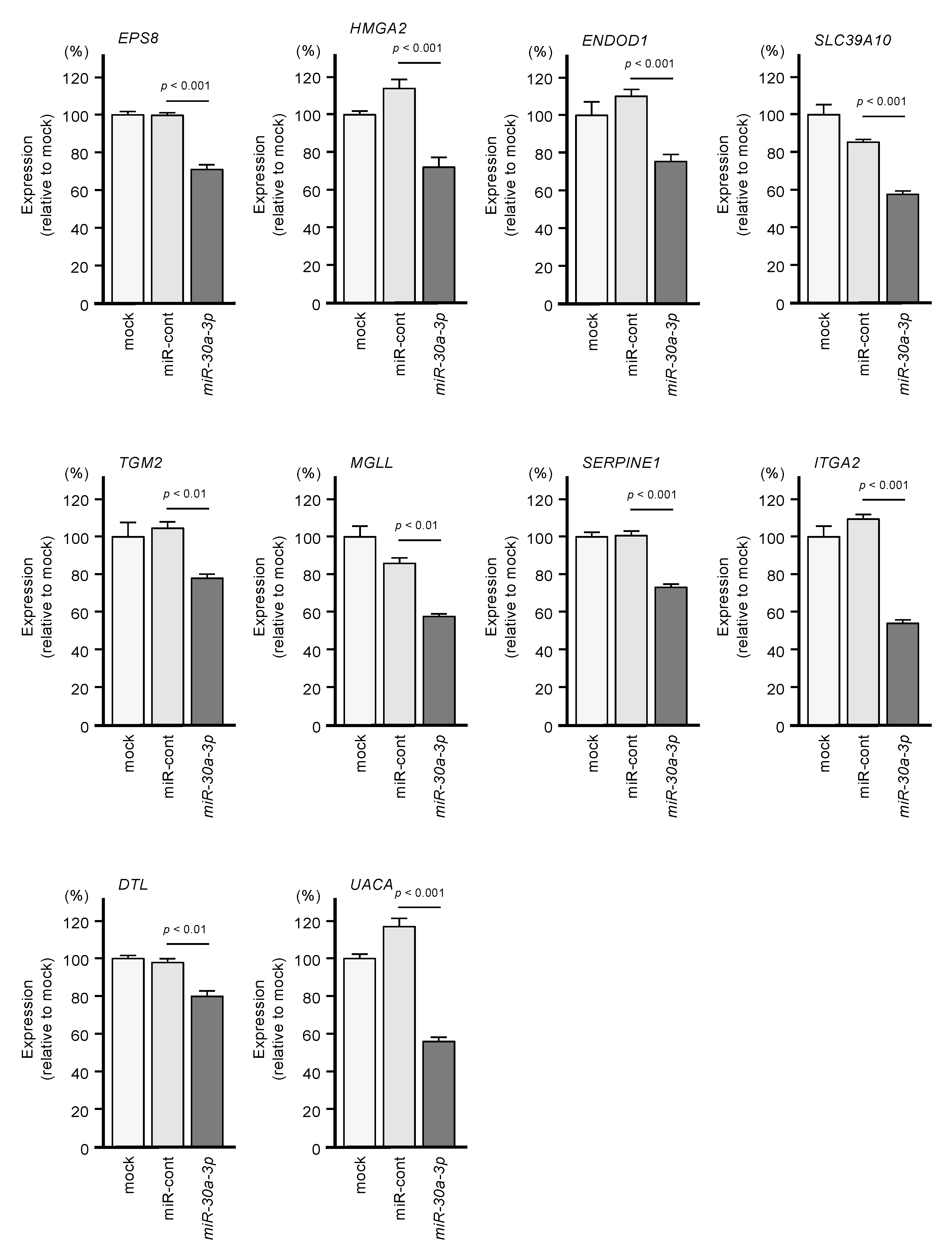
Among the 102 genes, expression levels of 12 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM2, MGLL, SERPINE1, ITGA2, DTL, UACA, FGD6, and RTP4) had significant impacts on the prognosis of PDAC patients (Figure 2; p < 0.01). We validated the expression levels of these 12 genes and found that, except for HMGA2, all had upregulated expressions in PDAC clinical specimens (Figure 3).
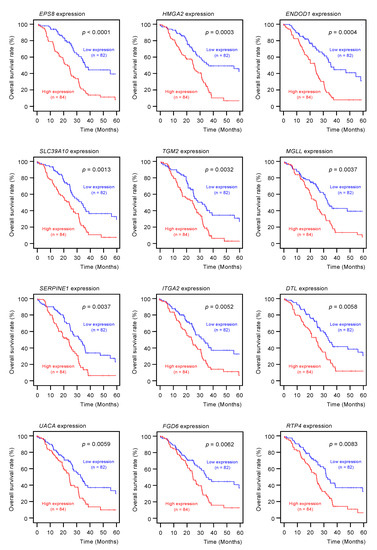
Figure 2.
Clinical significance of the miR-30a-3p target genes by TCGA database analysis.
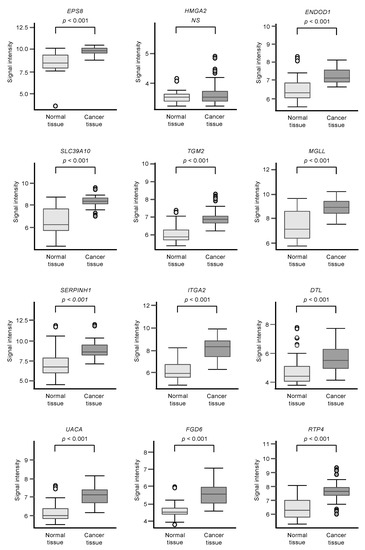
Figure 3.
GSE15471 dataset analyses of the expression levels of 12 genes (predicted 5-year survival) that are targets for miR-30a-3p regulation in PDAC clinical specimens.
Among the putative target genes for miR-30a-3p regulation in PDAC cells, high expression levels of 12 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM2, MGLL, SERPINE1, ITGA2, DTL, UAUC, FGD6 and RTP4) significantly predicted a worse prognosis in patients with PDAC (p < 0.01). Kaplan–Meier curves of the 5-year overall survival for each gene are presented.
Expression levels of 12 genes that are targets of miR-30a-3p regulation (Figure 2) were evaluated by analysis of the GSE15471 dataset. Expressions of all genes were upregulated in PDAC tissues (n = 39) compared to normal tissues (n = 39).
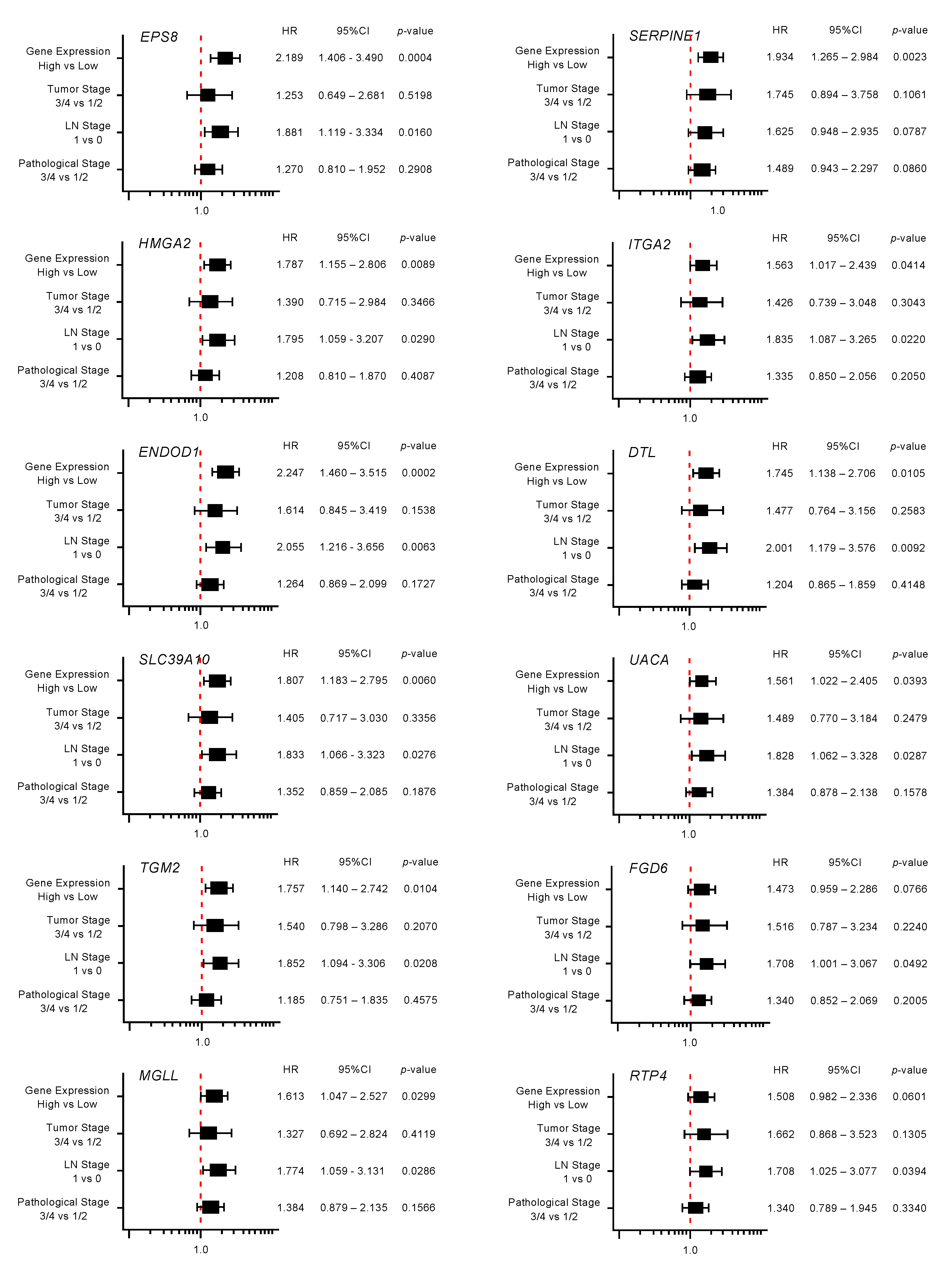
Moreover, expression levels of 10 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM, MGLL, SERPINE1, ITGA2, DTL, and UACA) were identified as independent prognostic factors in multivariate analyses for the survival of patients with PDAC (p < 0.01; Figure 4). In advance to the multivariate analysis, the following factors were selected, and a univariable analysis was performed: age, gender, tumor stage, LN stage, metastatic stage, pathological stage, tumor size, and alcohol history. Among these factors, the tumor stage, LN stage, and pathological stage affected the prognosis of the PDAC patients. From this result, we performed the multivariate analysis with the following four factors: tumor stage, LN stage, pathological stage, and gene expression.
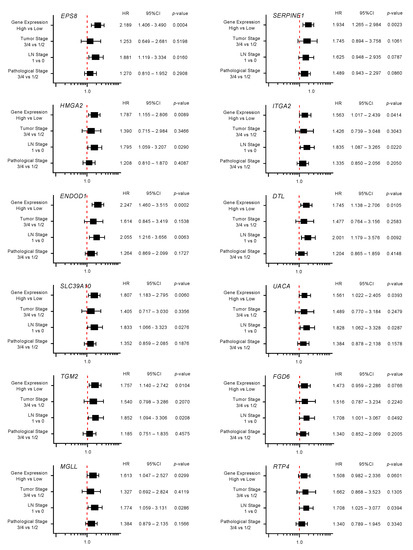
Figure 4.
Forest plot of the multivariate analysis of the 12 genes that are targets of miR-30a-3p regulation (predicted 5-year survival).
Multivariate analysis revealed that expression levels of 10 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM2, MGLL, SERPINE1, ITGA2, DTL and UAUC) were independent prognostic factors for 5-year overall survival after adjustment for tumor stage, lymph node metastasis, and pathological stage (p < 0.01).
We investigated whether expression levels of these 10 target genes would be suppressed by the transfection of PDAC cells with miR-30a-3p. After transfection of the PDAC cell line PANC-1 with miR-30a-3p, the expression levels of all 10 of these genes were reduced (Figure 5).
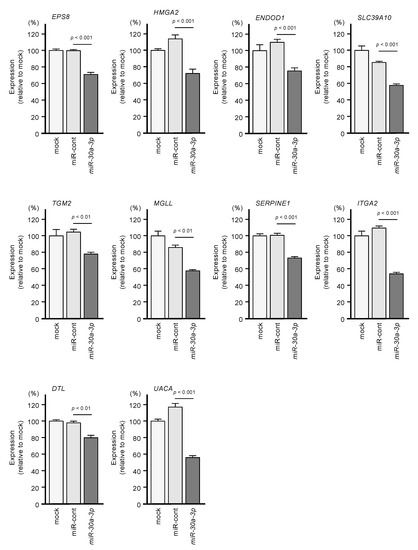
Figure 5.
Regulation of the expression of the 10 target genes following transfection of PANC-1 cells with miR-30a-3p.
Expression levels of the 10 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM2, MGLL, SERPINE1, ITGA2, DTL and UAUC) were reduced by miR-30a-3p transfection to PANC-1 cells (48 h after the transfection). GAPDH was used as an internal control.
2.4. Direct Regulation of ITGA2 Expression by miR-30a-3p in PDAC Cells
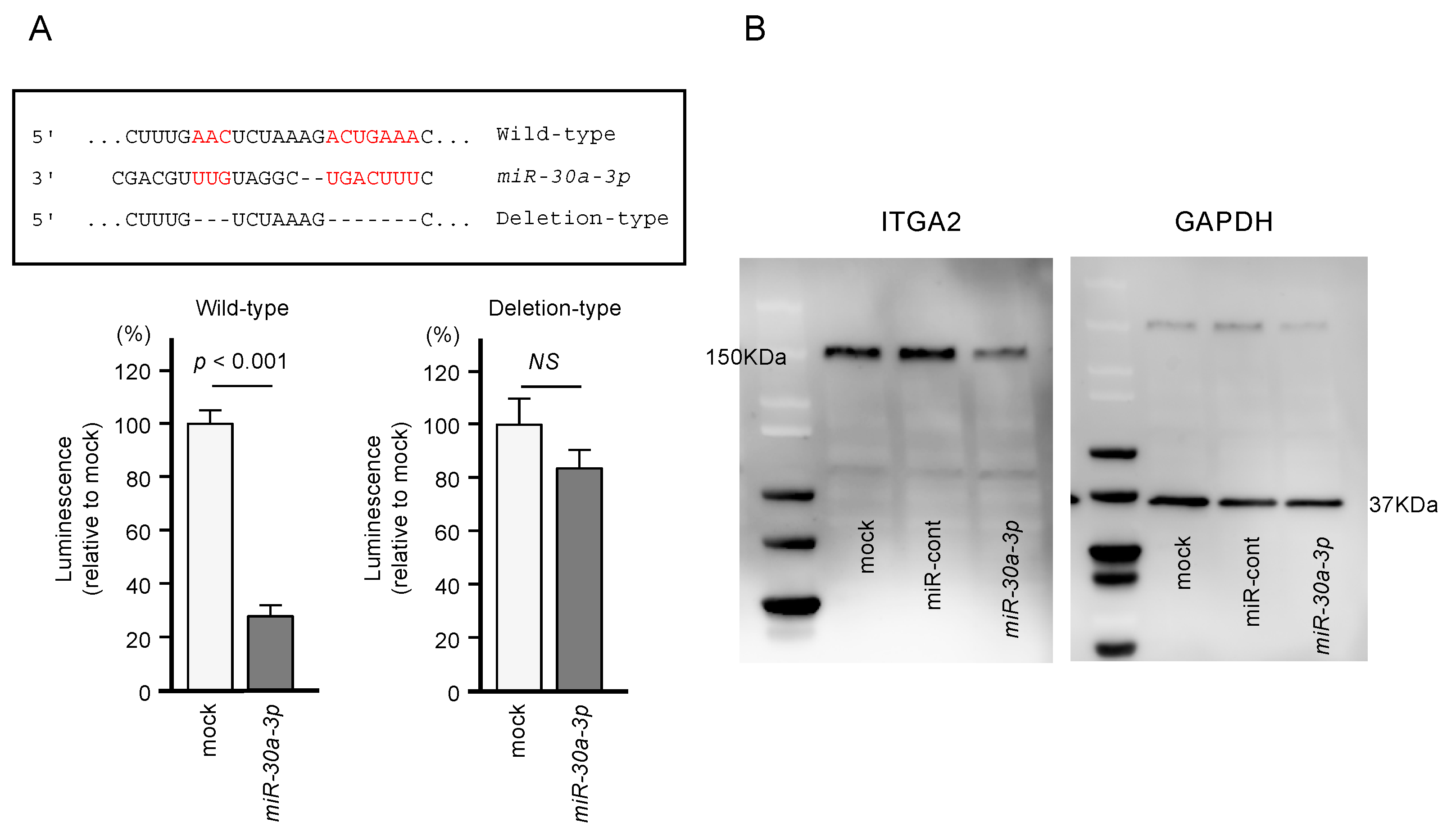
Using dual-luciferase reporter assays involving plasmid vectors carrying partial sequences of the ITGA2 3’-UTR, we determined whether miR-30a-3p can directly bind to ITGA2 in PDAC cells. We tested both the “Wild-type” ITGA2 3’-UTR sequence that contains the predicted miR-30a-3p target site and the “Deletion-type” sequence lacking the target site (Figure 6). A significant decrease in luciferase activity was seen in cells that were co-transfected with miR-30a-3p and the “Wild-type” vector, whereas no change in luciferase activity was observed for cells transfected with the “Deletion-type” vector and miR-30a-3p (Figure 6). These results indicate that miR-30a-3p directly binds to the 3’-UTR of ITGA2.
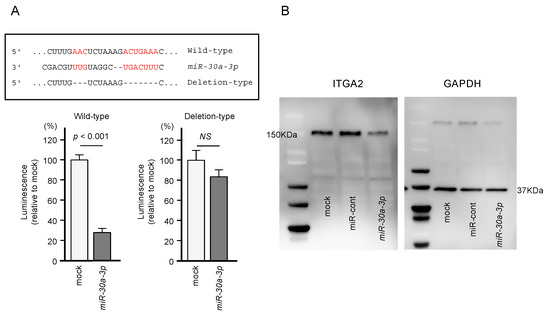
Figure 6.
Direct regulation of ITGA2 by miR-30a-3p in PANC-1 cells. (A) The TargetScan database showed that one putative binding site of miR-30a-3p was annotated in the 3′-UTR of ITGA2. Dual luciferase reporter assays showed that luminescence activities were reduced by co-transfection of PANC-1 cells with wild-type vector (containing miR-30a-3p binding sites) and miR-30a-3p. Normalized data were calculated as Renilla/firefly luciferase activity ratios. (B) Protein expression levels of ITGA2 were significantly reduced by miR-30a-3p transfection to PANC-1 cells (48 h after the transfection). Whole Western blotting images are shown. GAPDH was used as an internal control.
2.5. Functional Significance of ITGA2 in PDAC Cells
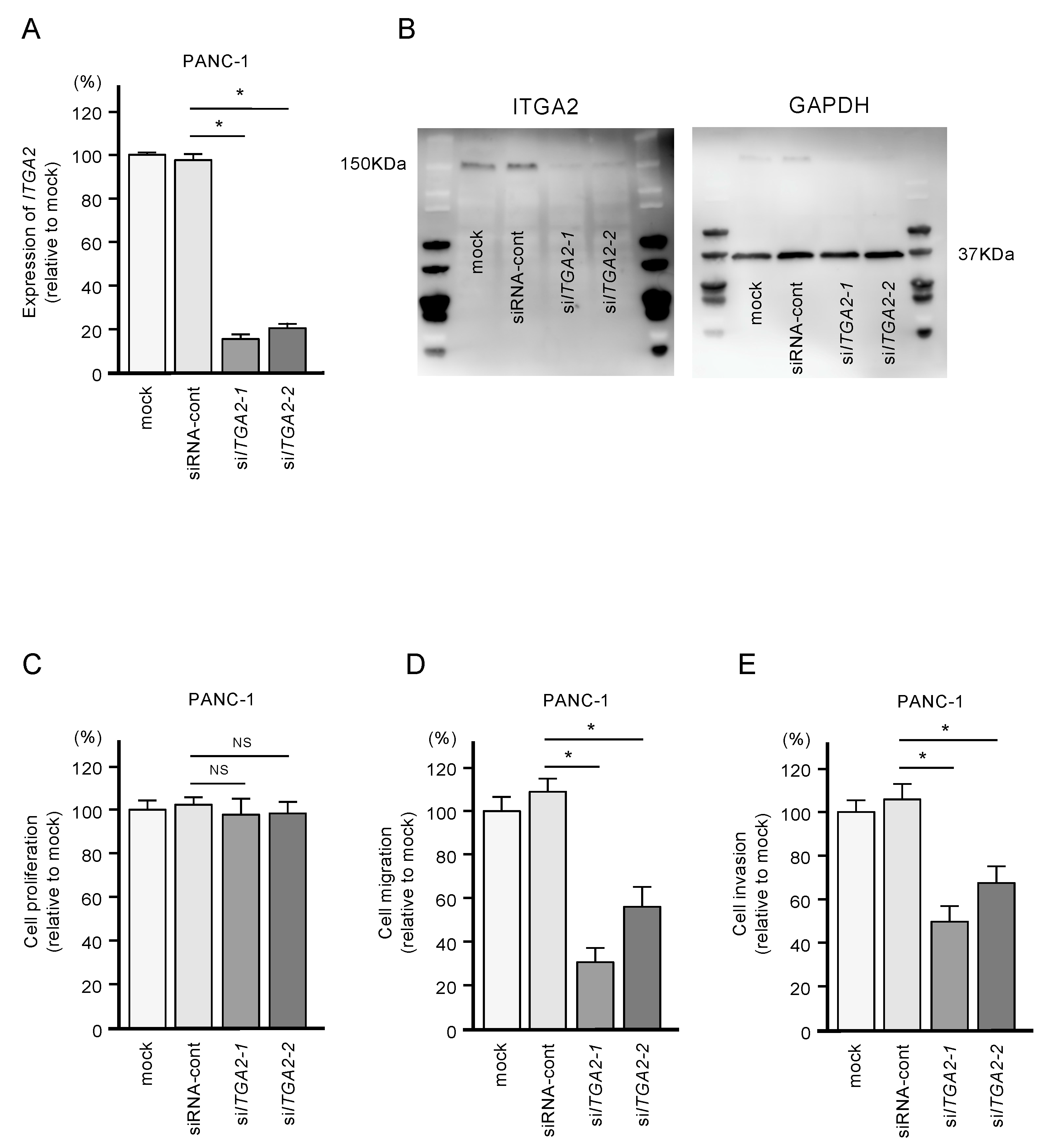
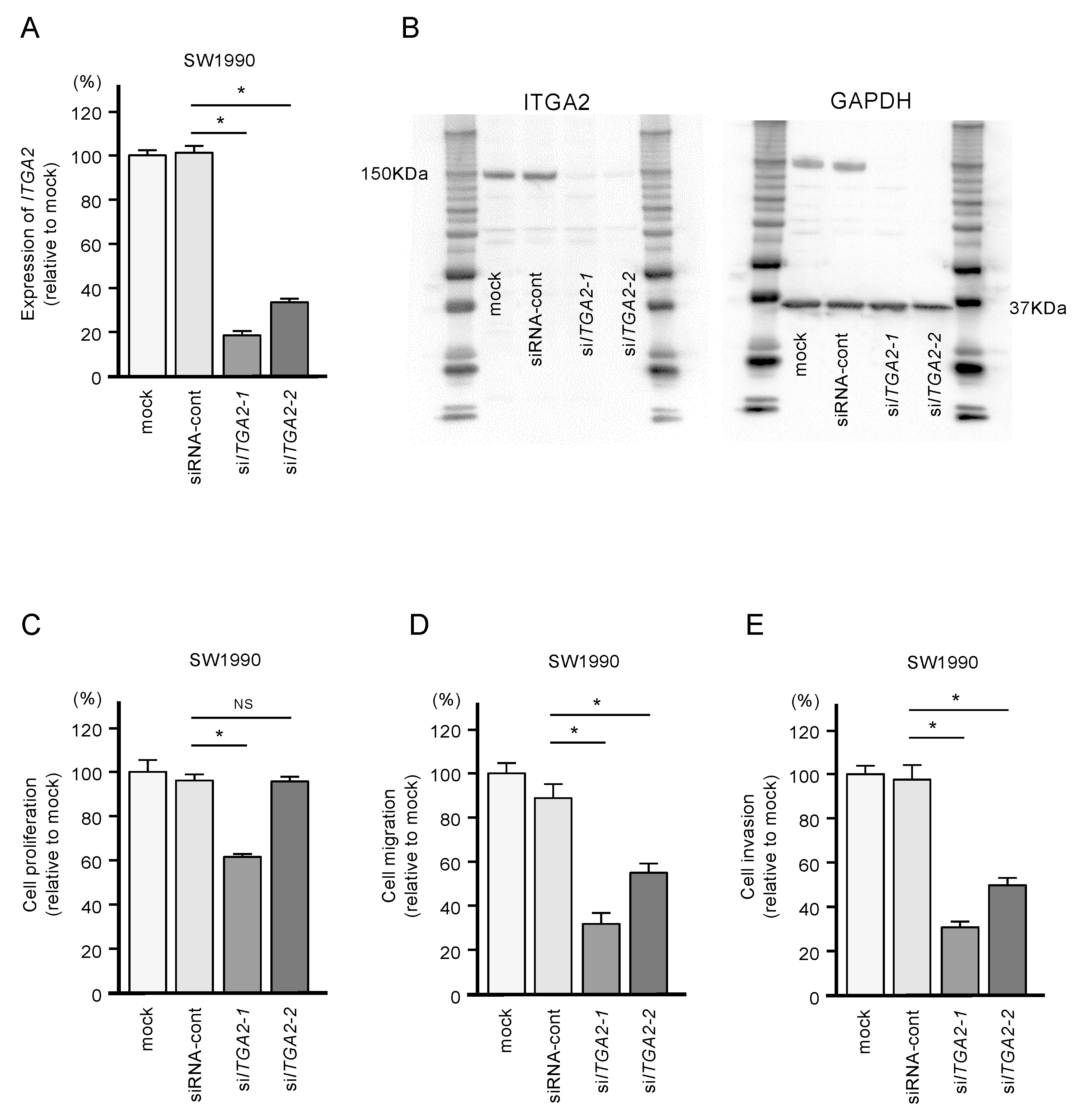
To confirm the oncogenic functions of ITGA2 in PDAC cells, we performed knockdown assays using two types of siRNAs: si-ITGA2-1 and si-ITGA2-2. After confirming knockdown of ITGA2/ITGA2 by siRNA transfection of PANC-1 cells (Figure 7A,B), we showed that cells with siRNA-mediated knockdown of ITGA2 gene expression suppressed cell migration and invasion activity, but not cell proliferation (Figure 7C,D). Similarly, ITGA2 knockdown assays showed that cancer cell migration and invasive abilities were significantly suppressed in the SW1990 cells (Figure 8A–D).
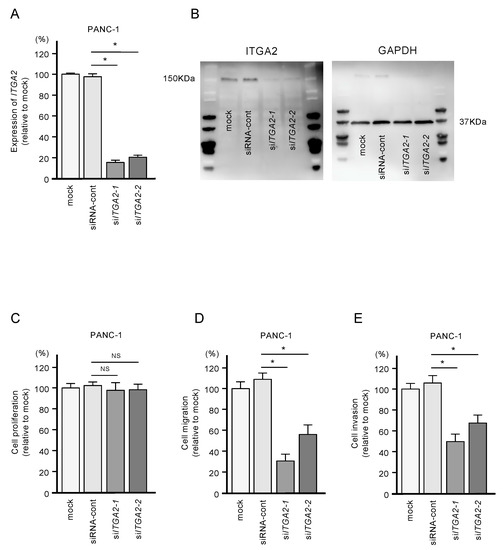
Figure 7.
Effects of ITGA2 knockdown in PANC-1 cells. (A) mRNA and (B) protein expression levels of ITGA2 after transfection of two types of siITGA2 (siITGA2-1 and siITGA2-2; 72 h after transfection). GAPDH/GAPDH was used as an internal control. (C) Cell proliferation using XTT assays. Data were collected at 72 h after miRNA transfection. (D) Cell migration with a membrane culture system. Data were collected at 48 h after seeding the cells into the chambers (* p < 0.0001). (E) Cell invasion using Matrigel invasion assays at 48 h after seeding miRNA-transfected cells into the chambers. (* p < 0.0001)
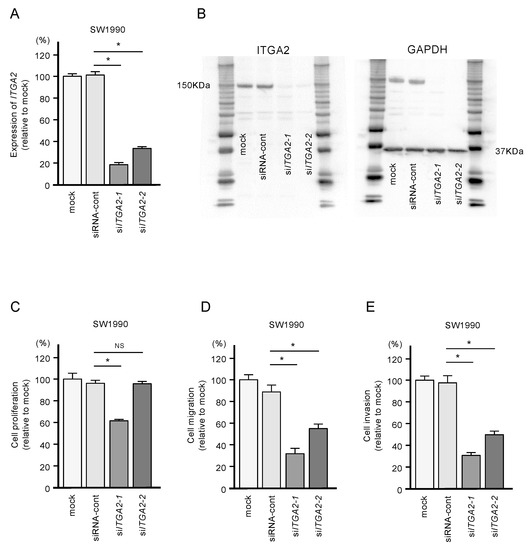
Figure 8.
Effects of ITGA2 knockdown in SW1990 cells. (A) mRNA and (B) protein expression levels of ITGA2 by transfection with two types of siITGA2 in SW1990 cells. (C,D,E) Cell proliferation, migration and invasion assays in SW1990 cells. (* p < 0.0001)
We analyzed the ITGA2-related molecules with Ingenuity Pathway Analysis (IPA) software (Figure S1). Many of the molecules were associated with the migration and invasion of cells, respectively (Figures S2 and S3). These data indicate that ITGA2, in combination with the connected molecules, induces cancer cell migration and invasion firmly.
2.6. Overexpression of ITGA2 in Immunohistochemical Staining of PDAC Clinical Specimens
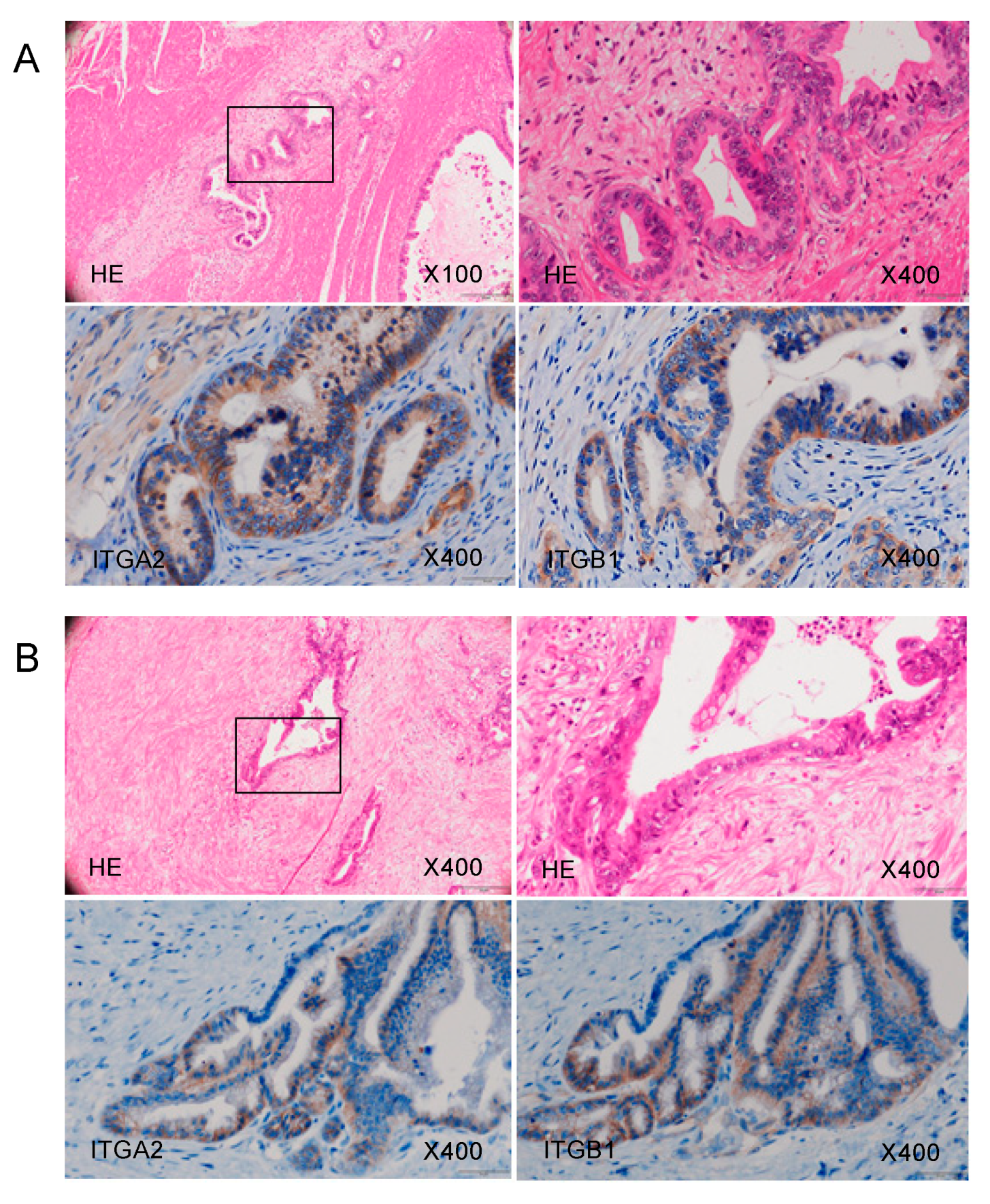
Using immunohistochemistry, we confirmed the expression of the ITGA2 and ITGB1 proteins, binding partner of ITGA2, in clinical specimens from patients with PDAC. Overexpression of both ITGA2 and ITGB1 was detected in these cancer lesions (Figure 9).
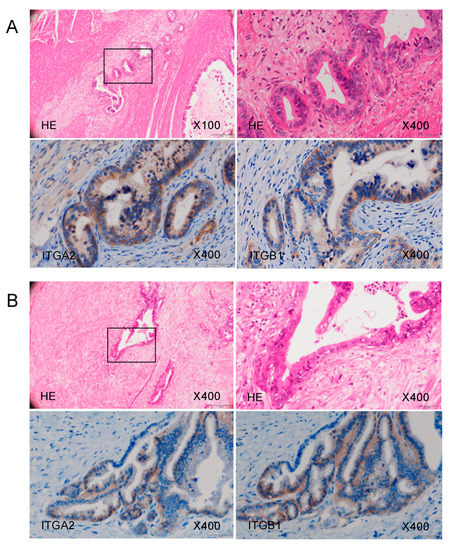
Figure 9.
ITGA2 overexpression in clinical PDAC specimens. Representative immunohistochemical images with staining of ITGA2 and ITGB1 in clinical samples. Overexpression of ITGA2 and ITGB1 was detected in the cancer lesions. Images of samples from Patient (A) number 20 and (B) number 17 (Table S1) are shown.
3. Discussion
Aberrantly expressed miRNAs in cancer cells are known to contribute to malignant transformation, metastasis, and treatment resistance [19]. In the last decade, genomics analysis methods have been actively used to identify miRNAs aberrantly expressed in various cancer cells [20,21]. Our miRNA expression profile generated by RNA sequencing provided us with new information about dysregulated miRNAs in PDAC cells [22,23,24]. Our miRNA signatures have revealed that some passenger strands of miRNAs were significantly downregulated in cancerous tissues, including miR-30a-3p and miR-30c-2-3p [25,26,27]. Recently, other groups reported that passenger strands of other miRNAs are functional in cancer cells [28,29,30,31]. Together, these findings strongly suggest that analyses that consider both strands of miRNAs are essential for cancer research.
In the human genome, mir-30a and mir-30c-2 form a cluster of 4 miRNAs on chromosome 6q13: miR-30a-5p, miR-30a-3p, miR-30c-2-5p, and miR-30c-2-3p [32,33,34]. The seed sequences of miR-30a-5p and miR-30c-2-5p are identical (GUAAACA), suggesting that the genes they control are similar. On the other hand, the seed sequences of miR-30a-3p (UUUCAGU) and miR-30c-2-3p (UGGGAGA) differ, suggesting that they control different genes. In this study, we demonstrated that the expression of miR-30a-3p significantly inhibited the malignant phenotypes of cancer cells, such as cell proliferation, migration, and invasion.
To our knowledge, no studies have reported a tumor-suppressive function of miR-30a-3p in PDAC cells. Previous studies showed the tumor-suppressive function of miR-30a-3p in several types of cancers, including gastric cancer, lung adenocarcinoma, and renal cell carcinoma [35,36,37]. Recent studies showed that some non-coding RNAs, such as Linc00483, Linc00460, and Linc01436, adsorbed miR-30a-3p and weakened the tumor-suppressive effects in gastric cancer, nasopharyngeal carcinoma, and non-small cell lung cancer, respectively [38,39,40]. In esophageal squamous cell carcinoma, expressions of both strands of miR-30a-5p and miR-30a-3p were downregulated and the low expression levels enhanced cancer cell proliferation by activating the WNT signaling pathway [41]. A recent study has shown that the WNT pathway is closely involved in lymph node metastasis-positive PDAC patients, and possibility of novel immune-based therapeutic strategies targeting WNT [42]. Our present study demonstrated that the ectopic expression of miR-30a-5p inhibited cancer cell proliferation. In contrast, the expression of miR-30a-5p did not significantly affect cell migration and invasion in PDAC cells. A previous study demonstrated that miR-30a-5p expression was downregulated in PDAC clinical specimens, and ectopic expression of miR-30a-5p increases the sensitivity of PDAC cells to gemcitabine through targeting of FOXD1 [43]. Previous reports, as well as our present data, indicate that downregulation of both strands of miRNAs derived from pre-mir-30a are involved in malignant transformation of PDAC cells.
We also elucidated which genes associated with the promotion of PDAC are regulated by these tumor-suppressive miRNAs. In this study, we identified 135 genes (33 genes as miR-30a-5p targets and 102 genes as miR-30a-3p targets) as putative targets for pre-mir-30a regulation in PDAC cells. Among the miR-30a-3p targets, expression levels of 10 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM, MGLL, SERPINE1, ITGA2, DTL and UACA) significantly predicted the 5-year overall survival rates of patients with PDAC (p < 0.01). However, prospective studies are essential to confirm the effectiveness of these genes as prognostic markers. It is also an important issue to unify and analyze the clinical setting of the patients (i.e., nodal status, TNM, grade and histological subtype, R0 or R1 if surgery was performed, neo- or adjuvant therapy received, etc.).
Notably, the expression of EPS8 (epidermal growth factor receptor kinase substrate 8) is a highly effective prognostic marker for patients with PDAC. Our recent study of PDAC showed that the expression of EPS8 was directly regulated to tumor-suppressive miR-130b-5p [18]. Moreover, EPS8 was aberrantly expressed in PDAC clinical specimens, and its overexpression enhanced the aggressive phenotype of PDAC cells [18]. Another target, HMGA2 (high-mobility group A2), is a member of the non-histone chromosomal high-mobility group protein family and acts as a transcription factor [44,45]. Multiple studies have shown that overexpression of HMGA2 affected malignant features in multiple types of cancer [46]. Detailed analyses of these target genes will lead to a better understanding of the malignant features of PDAC.
In this study, we focused on ITGA2 (integrin subunit alpha 2) and showed that miR-30a-3p directly regulates this gene in PDAC cells. Integrins are transmembrane receptors composed of one alpha subunit and one beta subunit, and have affinities for different extracellular membrane components [47]. Integrins activate several signal transduction pathways, and aberrant expressions of integrins have been detected in several types of cancers; overexpression of integrins can enhance cancer cell development and progression [48,49,50,51]. ITGA2 forms a homodimer with ITGB1, and dysregulated levels of ITGA2/ITGB1 can mediate the signal pathways that enhance malignant transformation in multiple types of cancer cells [52,53,54,55]. In hepatocellular carcinoma cells, ITGA2 inhibits MST1 kinase phosphorylation and activates YAP pro-oncogenic activities [52]. In PDAC cells, ITGA2 promotes PDAC cell progression through activation of a focal adhesion pathway [53]. In ovarian cancer cells, expressions of ITGA2 enhance AKT phosphorylation and further accelerate the phosphorylation of the oncogenic protein FOXO1. Moreover, a knockdown assay of ITGA2 has shown that sensitivity of paclitaxel is improved in paclitaxel-resistant ovarian cancer cells [54]. Furthermore, ITGA2 controls the MAPK pathways and EMT in gastric cancer cells, and these events closely contribute to the chemo-resistance of gastric cancer [55]. A recent study has shown that ITGA2 increases the expression of PD-L1 by activating the STAT3 pathway in pancreatic cancer [56]. These studies indicate that overexpression of ITGA2 activates various molecular pathways and important effects on malignant transformation of cancer cells. Our recent study of PDAC revealed that the tumor-suppressive miR-124-3p directly regulates ITGA3 and ITGB1 expression, and aberrant expressions of these integrins were closely involved in PDAC molecular pathogenesis [57]. Taken together, tumor-suppressive miRNAs controls the expression of ITGA2/ITGB1 and ITGA3/ITGB1 in PDAC cells, and the aberrant expressions of these integrins can play pivotal roles in the malignant features of PDAC. Thus, signaling pathways that are activated by ITGA2/ITGB1 and ITGA3/ITGB1 could be therapeutic targets for PDAC.
4. Materials and Methods
4.1. Collection of Clinical Human PDAC Specimens, Pancreas Tissue Specimens, and PDAC Cell Lines
The present study was approved by the Bioethics Committee of Kagoshima University (Kagoshima, Japan; approval no. 160,038 28-65). Written prior informed consent and approval were obtained from all of the patients.
In this study, 31 PDAC clinical samples were collected from patients with PDAC who underwent resection at Kagoshima University Hospital from 1997 to 2016. Fifteen normal pancreatic tissue specimens were collected from noncancerous regions. The clinical samples were staged according to the American Joint Committee on Cancer/Union Internationale Contre le Cancer (UICC) TNM classification. Clinical features in PDAC specimens are shown in Table S1.
PDAC cell lines SW1990 and PANC-1 were purchased from the American Type Culture Collection (Manassas, VA, USA) and RIKEN Cell Bank (Tsukuba, Ibaraki, Japan), respectively.
4.2. RNA Extraction and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
The methods for RNA extraction from clinical specimens and cell lines, and qRT-PCR have been described previously [25,26,55]. TaqMan probes and primers used in this study are listed in Table S2.
4.3. Transfection of PDAC Cells with miRNAs, siRNAs, and Plasmid Vectors
The procedures for transfection of PDAC cells with miRNAs, siRNAs, and plasmid vectors were described previously [25,26,55]. The reagents used in this study are listed in Table S2.
4.4. Functional Assays in PDAC Cells (Cell Proliferation, Migration, and Invasion Assays)
The procedures for functional assays in cancer cells (proliferation, migration, and invasion) are described in our previous studies [25,26,55]. Cells were transfected with 10 nM miRNAs or siRNAs.
Cell proliferation was evaluated with XTT assays. Migration assays were performed with uncoated trans-well polycarbonate membrane filters, and invasion assays were carried out using modified Boyden chambers.
4.5. Identification of the miR-30a-5p and miR-30a-3p Targets in PDAC Cells
We selected putative target genes having binding sites for miR-30a-5p and miR-30a-3p using TargetScanHuman ver.7.2 (http://www.targetscan.org/vert_72/; data was downloaded on 13 July 2018). Our microarray data (miR-30a-5p or miR-30a-3p transfected cells) were deposited in the GEO repository under accession number GSE113066. To examine upregulated genes in PDAC clinical specimens, expression data was obtained from the GEO database (GSE15471).
4.6. Clinical Database Analysis of miRNA Target Genes in PDAC Clinical Specimens
For analysis of differential gene expression between normal tissues and cancer tissues, we utilized GSE15471 datasets obtained from the Gene Expression Omnibus (GEO). Briefly, GSE15471 contains mRNA array data from 36 PDAC tumors and matching normal pancreatic tissue samples. The data was obtained using Affymetrix U133 Plus 2.0 whole-genome chips. Expression levels are shown in signal intensities, and for genes that had multiple probes, the mean value was used.
For the Kaplan–Meier survival analysis, we downloaded TCGA clinical data (TCGA, Firehose Legacy) from cBioportal (https://www.cbioportal.org). Gene expression grouping data for each gene was collected from OncoLnc (http://www.oncolnc.org). R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
4.7. Plasmid Construction and Dual-Luciferase Reporter Assays
Plasmid vectors, including vectors carrying ITGA2 with the wild-type sequences of the miR-30a-3p binding sites in the 3′-UTR or with these sequences deleted, were prepared. The procedures for transfection and dual-luciferase reporter assays were described in our previous studies [25,26,55]. The reagents used in this study are listed in Table S2.
4.8. Western Blotting and Immunohistochemistry
The procedures for western blotting and immunohistochemistry were described in our previous studies [25,26,55]. The antibodies used in this study are listed in Table S2.
4.9. Downregulation of miR-30a-5p and miR-30a-3p in PDAC Cells and Their Tumor-Suppressive Roles in PDAC Cell Lines
Mann–Whitney U tests were applied for comparisons between two groups. To compare multiple groups, one-way analysis of variance and Dunnett’s test were applied. These analyses were carried out using JMP Pro 14 (SAS Institute Inc., Cary, NC, USA).
5. Conclusions
Analysis of our PDAC miRNA expression signature revealed that expression of both strands of pre-mir-30a (miR-30a-5p and miR-30a-3p) was downregulated in PDAC tissues. Ectopic expression assays showed that both miRNAs act as tumor-suppressive miRNAs in PDAC cells. A total of 102 genes were identified as targets for control by miR-30a-3p in PDAC cells. Among these targets, the expression levels of 10 genes (EPS8, HMGA2, ENDOD1, SLC39A10, TGM, MGLL, SERPINE1, ITGA2, DTL, and UACA) significantly predicted the 5-year overall survival rates of patients with PDAC (p < 0.01). Aberrant expression of ITGA2 contributed to the malignant transformation of PDAC cells. Our miRNA-based analysis strategy provides important insights into the role of miRNA in the molecular pathogenesis of PDAC.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/18/6459/s1, Table S1: Clinicopathological characteristics of the clinical specimens, Table S2: Reagents used in this study, Figure S1: Molecules predicted to be closely related with ITGA2 by Ingenuity Pathway Analysis (IPA), Figure S2: IPA of the canonical pathways for ITGA2 related molecules. Colored molecules are predicted to be associated with the migration of cells, Figure S3: IPA of the canonical pathways for ITGA2 related molecules. Colored molecules are predicted to be associated with the invasion of cells.
Author Contributions
Conceptualization, N.S.; methodology, N.S.; validation, R.O., T.T., and Y.H.; formal analysis, H.S. and R.O.; investigation, H.S., R.O., T.T., Y.H., and S.M.; resources, T.I., Y.K., H.K., and T.O.; data curation, N.S. and R.O.; writing—original draft preparation, N.S. and R.O.; writing—review and editing, R.O., S.M., and Y.H.; visualization, H.S., R.O., and N.S.; supervision, N.S.; project administration, N.S.; funding acquisition, M.W., T.I., Y.K., H.K., N.S., and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers; 17H04285, 18K08626, 18K08687, 18K09338, 18K16322, 19K09077.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic ductal adenocarcinoma: Current and evolving therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Gustafsson, A.; Andersson, R. Update on the management of pancreatic cancer: Surgery is not enough. World J. Gastroenterol. 2015, 21, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer Zum Buschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef]
- Du, J.; Gu, J.; Li, J. Mechanisms of drug resistance of pancreatic ductal adenocarcinoma at different levels. Biosci. Rep. 2020. [Google Scholar] [CrossRef]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs–An update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Tarang, S.; Weston, M.D. Macros in microRNA target identification: A comparative analysis of in silico, in vitro, and in vivo approaches to microRNA target identification. RNA Biol. 2014, 11, 324–333. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Chen, C.Z. MicroRNAs as oncogenes and tumor suppressors. N. Engl. J. Med. 2005, 353, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Seki, N.; Idichi, T.; Kurahara, H.; Osako, Y.; Koshizuka, K.; Arai, T.; Okato, A.; Kita, Y.; Arigami, T.; et al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: Anti-tumour functions of the microRNA-216 cluster. Oncotarget 2017, 8, 70097–70115. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef]
- Idichi, T.; Seki, N.; Kurahara, H.; Fukuhisa, H.; Toda, H.; Shimonosono, M.; Okato, A.; Arai, T.; Kita, Y.; Mataki, Y.; et al. Molecular pathogenesis of pancreatic ductal adenocarcinoma: Impact of passenger strand of pre-miR-148a on gene regulation. Cancer Sci. 2018, 109, 2013–2026. [Google Scholar] [CrossRef]
- Fukuhisa, H.; Seki, N.; Idichi, T.; Kurahara, H.; Yamada, Y.; Toda, H.; Kita, Y.; Kawasaki, Y.; Tanoue, K.; Mataki, Y.; et al. Gene regulation by antitumor miR-130b-5p in pancreatic ductal adenocarcinoma: The clinical significance of oncogenic EPS8. J. Hum. Genet. 2019, 64, 521–534. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673–677. [Google Scholar] [CrossRef]
- Chevalier, D.; Borchert, G.M. Genome-wide analysis of MicroRNA-regulated transcripts. Methods Mol. Biol. 2017, 1617, 93–107. [Google Scholar]
- Tafrihi, M.; Hasheminasab, E. MiRNAs: Biology, biogenesis, their web-based tools, and databases. Microrna 2019, 8, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Kurahara, H.; Maemura, K.; Natsugoe, S. MicroRNA in pancreatic cancer. J. Hum. Genet. 2017, 62, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Kadian, K.; Gupta, Y.; Kumar, A.; Chain, P.S.G.; Kovbasnjuk, O.; Kumar, S.; Parasher, G. MicroRNA in Pancreatic Cancer: From Biology to Therapeutic Potential. Genes (Basel) 2019, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.Z.; Mulholland, E.J.; Cole, G.; McCarthy, H.O. MicroRNAs in Pancreatic Cancer: Biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019, 19, 1130. [Google Scholar] [CrossRef]
- Toda, H.; Seki, N.; Kurozumi, S.; Shinden, Y.; Yamada, Y.; Nohata, N.; Moriya, S.; Idichi, T.; Maemura, K.; Fujii, T.; et al. RNA-sequence-based microRNA expression signature in breast cancer: Tumor-suppressive miR-101-5p regulates molecular pathogenesis. Mol. Oncol. 2020, 14, 426–446. [Google Scholar] [CrossRef]
- Toda, H.; Kurozumi, S.; Kijima, Y.; Idichi, T.; Shinden, Y.; Yamada, Y.; Arai, T.; Maemura, K.; Fujii, T.; Horiguchi, J.; et al. Molecular pathogenesis of triple-negative breast cancer based on microRNA expression signatures: Antitumor miR-204-5p targets AP1S3. J. Hum. Genet. 2018, 63, 1197–1210. [Google Scholar] [CrossRef]
- Wada, M.; Goto, Y.; Tanaka, T.; Okada, R.; Moriya, S.; Idichi, T.; Noda, M.; Sasaki, K.; Kita, Y.; Kurahara, H.; et al. RNA sequencing-based microRNA expression signature in esophageal squamous cell carcinoma: Oncogenic targets by antitumor miR-143-5p and miR-143-3p regulation. J. Hum. Genet. 2020. [Google Scholar] [CrossRef]
- Yonemori, M.; Seki, N.; Yoshino, H.; Matsushita, R.; Miyamoto, K.; Nakagawa, M.; Enokida, H. Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016, 107, 1233–1242. [Google Scholar] [CrossRef]
- Matsushita, R.; Seki, N.; Chiyomaru, T.; Inoguchi, S.; Ishihara, T.; Goto, Y.; Nishikawa, R.; Mataki, H.; Tatarano, S.; Itesako, T.; et al. Tumour-suppressive microRNA-144-5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br. J. Cancer 2015, 113, 282–289. [Google Scholar] [CrossRef]
- Goto, Y.; Kurozumi, A.; Arai, T.; Nohata, N.; Kojima, S.; Okato, A.; Kato, M.; Yamazaki, K.; Ishida, Y.; Naya, Y.; et al. Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br. J. Cancer 2017, 117, 409–420. [Google Scholar] [CrossRef]
- Mitra, R.; Adams, C.M.; Jiang, W.; Greenawalt, E.; Eischen, C.M. Pan-cancer analysis reveals cooperativity of both strands of microRNA that regulate tumorigenesis and patient survival. Nat. Commun. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Cui, H.; Liang, J.; Su, X. Role of MicroRNA-30c in cancer progression. J. Cancer 2020, 11, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, S.; Hu, L.; Jia, L.; Wang, H.; Guo, M.; Chen, C.; Liu, Y.; Xu, L. miR-30 Family: A promising regulator in development and disease. Biomed Res. Int. 2018, 2018, 9623412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Zhang, H.D.; Tang, J.H. MiR-30a: A novel biomarker and potential therapeutic target for cancer. J. Oncol. 2018, 2018, 5167829. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; He, J.; Du, J.; Zhang, H.; Shi, H.; Chen, Y.; Wei, Y.; Xue, W.; Yan, J.; et al. miR-30a-3p targets MAD2L1 and regulates proliferation of gastric cancer cells. Onco Targets Ther. 2019, 12, 11313–11324. [Google Scholar] [CrossRef]
- Wang, H.; Kanmangne, D.; Li, R.; Qian, Z.; Xia, X.; Wang, X.; Wang, T. miR-30a-3p suppresses the proliferation and migration of lung adenocarcinoma cells by downregulating CNPY2. Oncol. Rep. 2020, 43, 646–654. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wu, X.; Huang, J.; Chen, W.; Liu, D.; Zhang, J.; Huang, Y.; Xue, W. miR-30a-3p inhibits renal cancer cell invasion and metastasis through targeting ATG12. Transl. Urol. 2020, 9, 646–653. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Liao, A.; Zeng, B.; Liu, D.; Yao, Y.; Hu, G.; Chen, X.; Feng, Z.; Du, Y.; et al. Linc00483 as ceRNA regulates proliferation and apoptosis through activating MAPKs in gastric cancer. J. Cell Mol. Med. 2018, 22, 3875–3886. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Jiang, X.; Wang, B.; Li, L.; Wang, J.; Ma, J. Long non-coding RNA LINC00460 aggravates invasion and metastasis by targeting miR-30a-3p/Rap1A in nasopharyngeal carcinoma. Hum. Cell 2019, 32, 465–476. [Google Scholar] [CrossRef]
- Yuan, S.; Xiang, Y.; Wang, G.; Zhou, M.; Meng, G.; Liu, Q.; Hu, Z.; Li, C.; Xie, W.; Wu, N.; et al. Hypoxia-sensitive LINC01436 is regulated by E2F6 and acts as an oncogene by targeting miR-30a-3p in non-small cell lung cancer. Mol. Oncol. 2019, 13, 840–856. [Google Scholar] [CrossRef]
- Qi, B.; Wang, Y.; Chen, Z.-J.; Li, X.-N.; Qi, Y.; Yang, Y.; Cui, G.-H.; Guo, H.-Z.; Li, W.-H.; Zhao, S. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J. Gastroenterol. 2017, 23, 7965–7977. [Google Scholar] [CrossRef] [PubMed]
- Argentiero, A.; De Summa, S.; Di Fonte, R.; Iacobazzi, R.M.; Porcelli, L.; Da Vià, M.; Brunetti, O.; Azzariti, A.; Silvestris, N.; Solimando, A.G. Gene expression comparison between the lymph node-positive and -negative reveals a peculiar immune microenvironment signature and a theranostic role for WNT targeting in pancreatic ductal adenocarcinoma: A pilot study. Cancers (Basel) 2019, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jia, S.; Ding, G.; Zhang, M.; Yu, W.; Wu, Z.; Cao, L. Down-regulation of miR-30a-5p is associated with poor prognosis and promotes chemoresistance of gemcitabine in pancreatic ductal adenocarcinoma. J. Cancer 2019, 10, 5031–5040. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Narita, M. Oncogenic HMGA2: Short or small? Genes. Dev. 2007, 21, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuche, K.; Summer, H.; Li, O.; Hescheler, J.; Dröge, P. The high mobility group protein HMGA2: A co-regulator of chromatin structure and pluripotency in stem cells? Stem. Cell Rev. Rep. 2009, 5, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mo, Q.; Wang, X. Oncological role of HMGA2 (Review). Int. J. Oncol. 2019, 55, 775–788. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Cruz da Silva, E.; Dontenwill, M.; Choulier, L.; Lehmann, M. Role of integrins in resistance to therapies targeting growth factor receptors in cancer. Cancers (Basel) 2019, 11, 692. [Google Scholar] [CrossRef]
- Wong, K.F.; Liu, A.M.; Hong, W.; Xu, Z.; Luk, J.M. Integrin α2β1 inhibits MST1 kinase phosphorylation and activates Yes-associated protein oncogenic signaling in hepatocellular carcinoma. Oncotarget 2016, 7, 77683–77695. [Google Scholar] [CrossRef] [PubMed]
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin α2 in cell adhesion and disease. Genes Dis. 2019, 6, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Y.; Li, D.; Li, H.; Jin, X.; Ren, D. Overexpressed ITGA2 contributes to paclitaxel resistance by ovarian cancer cells through the activation of the AKT/FoxO1 pathway. Aging (Albany NY) 2020, 12, 5336–5351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, T.; Guo, K.; Zhou, Y.; Liu, H.; Pan, Y.; Hou, Q.; Nie, Y.; Fan, D.; Lu, Y.; et al. Regulation of integrin subunit alpha 2 by miR-135b-5p modulates chemoresistance in gastric cancer. Front. Oncol. 2020, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhao, J.; Sun, Y.; Li, D.; Meng, Z.; Wang, B.; Fan, P.; Liu, Z.; Jin, X.; Wu, H. Overexpressed ITGA2 promotes malignant tumor aggression by up-regulating PD-L1 expression through the activation of the STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 485. [Google Scholar] [CrossRef]
- Idichi, T.; Seki, N.; Kurahara, H.; Fukuhisa, H.; Toda, H.; Shimonosono, M.; Yamada, Y.; Arai, T.; Kita, Y.; Kijima, Y.; et al. Involvement of anti-tumor miR-124-3p and its targets in the pathogenesis of pancreatic ductal adenocarcinoma: Direct regulation of ITGA3 and ITGB1 by miR-124-3p. Oncotarget 2018, 9, 28849–28865. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).