Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis
Abstract
1. Introduction
2. Molecular Basis and Biological Function of IL-36R in Cells
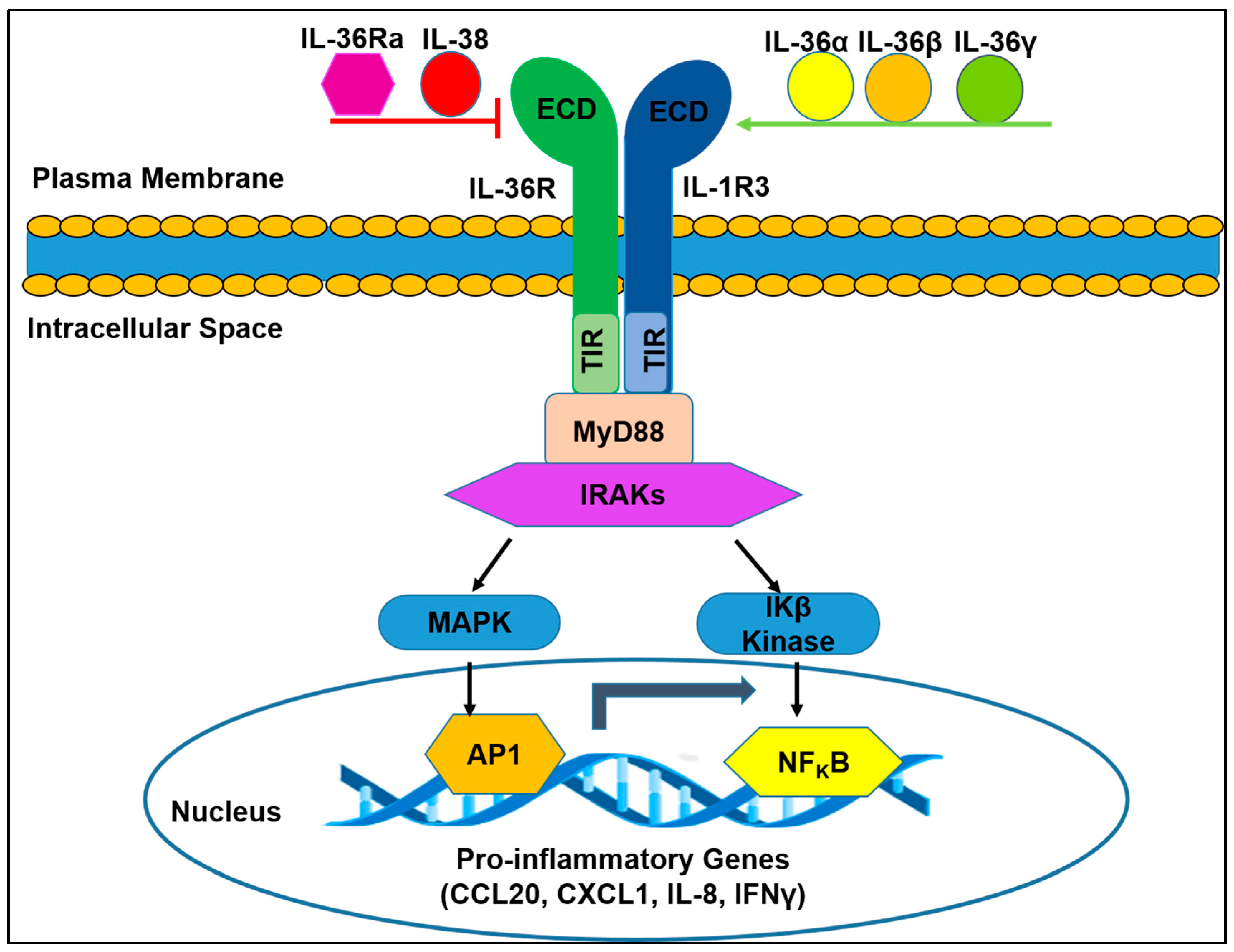
2.1. IL-36R Molecular Structure
2.2. IL-36R Function-Associated Molecules
2.3. Distribution and Biological Functions of IL-36R
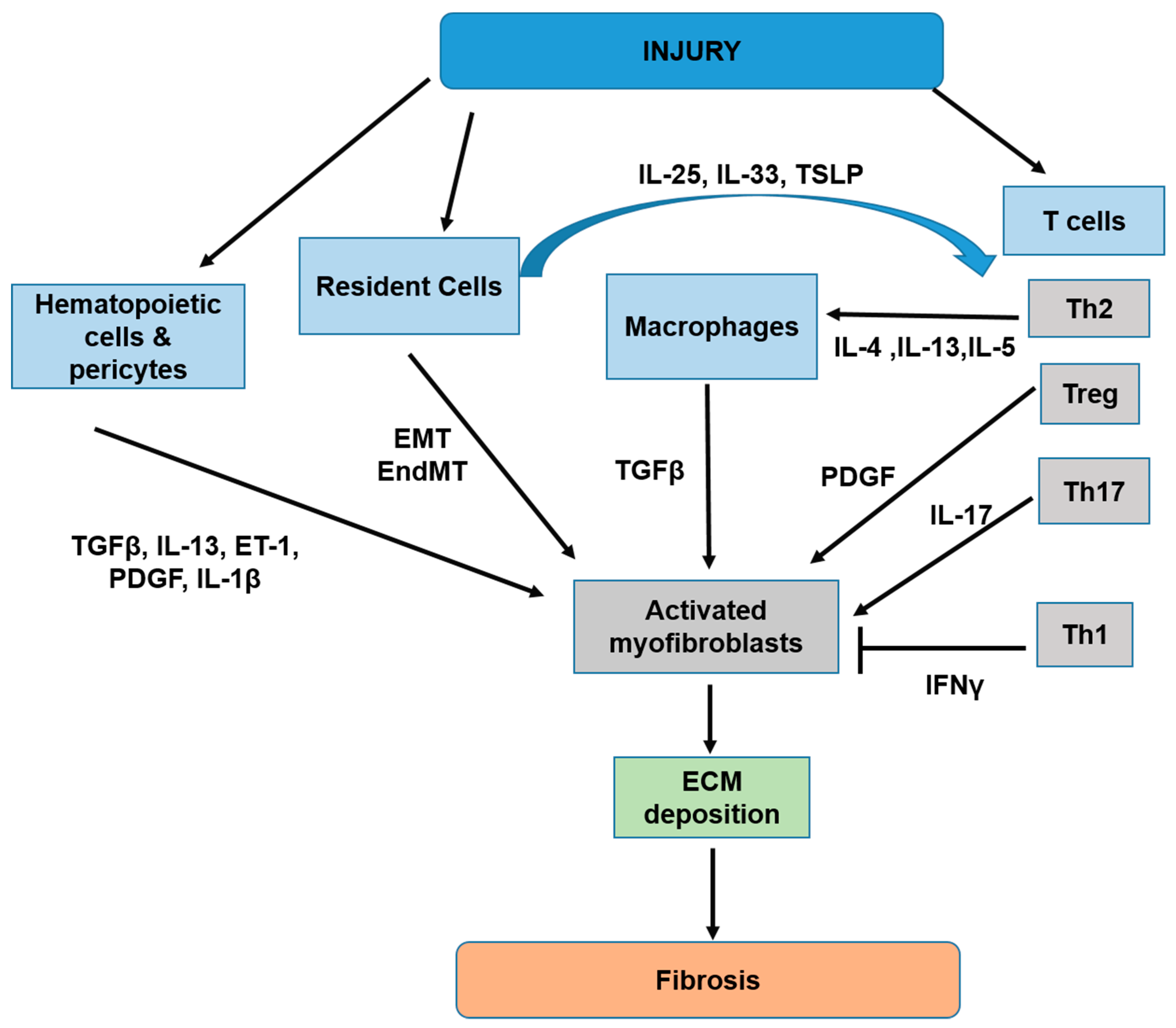
3. Cellular and Molecular Mechanisms of Tissue Fibrosis
3.1. Overview of Tissue Fibrosis Development
3.2. The Pathological Role of IL-36/IL-36R in the Development of Tissue Fibrosis
3.2.1. Stimulation of IL-36R Induces Lung Fibrosis
3.2.2. Enhanced IL-36R Ligand is Associated with Kidney Fibrosis
3.2.3. Repression of IL-36R Protects against Cardiac Fibrosis
3.2.4. Inhibition of IL-36R Activity Prevents Intestinal Fibrosis
3.2.5. IL-36/IL-36R Signaling is Involved in Pancreatic Fibrosis
3.3. Molecular Mechanisms of IL-36R Mediated Fibrosis
3.3.1. IL-36R Promotes Fibrosis via Regulation of Immune Cell Responses
3.3.2. IL-36R Promotes Fibrosis by Modulating Fibrogenic Factors in Fibroblasts
3.3.3. IL-36R Induces Tissue Fibrosis through Regulating Enzyme-Mediated Collagen Remodeling
4. Conclusions and Future Directions
Future Directions to Be Considered
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| AKI | Acute kidney injury |
| AP-1 | Activated Protein-1 |
| BUN | Blood urea nitrogen |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL20 | C-C motif chemokine ligand 20 |
| Col1a1 | Collagen type I alpha 1 |
| Col1a2 | Collagen type I alpha 2 |
| Col3a1 | Collagen type 3 alpha 1 |
| CKD | chronic kidney disease |
| CD | Crohns disease |
| CEBPβ | CCAAT/Enhancer-Binding Protein Beta |
| CXCL1 | C-X-C motif chemokine Ligand 1 |
| DITRA | Deficiency of the IL-36R antagonist |
| DT | Distal tubules |
| ECM | Extracellular matrix |
| ET-1 | Endothelin -1 |
| EMT | Epithelial to mesenchymal transformation |
| EndMT | endothelial to mesenchymal transformation |
| FOXP3 | Forkhead box protein P3 |
| G-CSF | Granulocyte colony-stimulating factor |
| HF | Heart failure |
| IBDs | Inflammatory bowel diseases |
| IFNγ | Interferon gamma |
| Ig | Immunoglobulin |
| IKβα | NFKB inhibitor |
| IKβ Kinase | IKβα kinase |
| IL-1 | Interleukin 1 |
| IL-1β | Interleukin 1 beta |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-17C | Interleukin 17C |
| IL-23 | Interleukin 23 |
| IL-33 | Interleukin 33 |
| IL-1R | Interleukin 1 receptor |
| IL-1RAcP | Interleukin 1 receptor accessory protein |
| IL-1RL2 | Interleukin 1 receptor like 2 |
| IL-1Rrp2 | Interleukin 1 receptor related protein 2 |
| IL-1R3 | Interleukin 1 receptor 3 |
| IL-36Ra | Interleukin 36 receptor antagonist |
| ILC2 | Lymphoid cells of group 2 |
| IRAK | Interleukin 1 receptor associated kinase |
| Kim-1 | Kidney injury molecule -1 |
| KD | Kilo dalton |
| MMPs | Metalloproteinases |
| MAPK | Mitogen-activated protein kinase |
| MyD88 | Myeloid differentiation primary response gene 88 |
| MI | Myocardial infarction |
| NLPR3 | Nod like Receptor family pyrin domain containing 3 |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PDGF | Platelet derived growth factor |
| Prss35 | Inactive serine protease 35 |
| RORγt | Retinoic acid receptor-related-orphan-receptor-gamma |
| sIL-33 | Soluble form of interleukin 33 |
| SMAD | Mothers against decapentaplegic |
| TAZ | Transcriptional co-activator with a PDZ binding domain |
| TECs | Renal tubular epithelial cells |
| TGF-β | Transforming growth factor beta |
| THP-1 cells | Human leukemic monocytes |
| Th 2 | T helper 2 cells |
| TILs | Tubulointerstitial lesions |
| TIR | Toll-IL-1 receptor domain |
| TLRs | Toll Like receptors |
| TNFα | Tumor necrosis factor alpha |
| Tregs | Regulatory T cells |
| TSLP | Thymic stromal lymphopoietin |
| UC | Ulcerative colitis |
| UUO | Unilateral ureteral obstruction |
| VCAM 1 | Vascular cell adhesion molecule 1 |
| YAP | Yes-associated protein |
References
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 2013, 304, C216–C225. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Fibrogenesis of parenchymal organs. Proc. Am. Thorac. Soc. 2008, 5, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.J.; Shiwen, X.; Black, C.M.; Sa, S.; Xu, Y.; Leask, A. Tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J. Biol. Chem. 2000, 275, 15220–15225. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ratziu, V.; Choi, S.G.; Lalazar, A.; Theiss, G.; Dang, Q.; Kim, S.J.; Friedman, S.L. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J. Biol. Chem. 1998, 273, 33750–33758. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef]
- Helmig, S.; Belwe, A.; Schneider, J. Association of transforming growth factor beta1 gene polymorphisms and asbestos-induced fibrosis and tumors. J. Investig. Med. 2009, 57, 655–661. [Google Scholar] [CrossRef]
- Border, W.A.; Okuda, S.; Languino, L.R.; Sporn, M.B.; Ruoslahti, E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 1990, 346, 371–374. [Google Scholar] [CrossRef]
- Kuwahara, F.; Kai, H.; Tokuda, K.; Kai, M.; Takeshita, A.; Egashira, K.; Imaizumi, T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002, 106, 130–135. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M. Pirfenidone in idiopathic pulmonary fibrosis: The CAPACITY program. Expert Rev. Respir. Med. 2011, 5, 473–481. [Google Scholar] [CrossRef]
- Nakanishi, H.; Sugiura, T.; Streisand, J.B.; Lonning, S.M.; Roberts, J.D., Jr. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L151–L161. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Shamskhou, E.A.; Kratochvil, M.J.; Orcholski, M.E.; Nagy, N.; Kaber, G.; Steen, E.; Balaji, S.; Yuan, K.; Keswani, S.; Danielson, B.; et al. Hydrogel-based delivery of Il-10 improves treatment of bleomycin-induced lung fibrosis in mice. Biomaterials 2019, 203, 52–62. [Google Scholar] [CrossRef]
- Fields, J.K.; Gunther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Tagliabue, A. The interleukin-1 receptor family. Semin. Immunol. 2013, 25, 394–407. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [Google Scholar] [CrossRef]
- Wang, E.W.; Jia, X.S.; Ruan, C.W.; Ge, Z.R. miR-487b mitigates chronic heart failure through inhibition of the IL-33/ST2 signaling pathway. Oncotarget 2017, 8, 51688–51702. [Google Scholar] [CrossRef]
- Xu, D.; Mu, R.; Wei, X. The Roles of IL-1 Family Cytokines in the Pathogenesis of Systemic Sclerosis. Front. Immunol. 2019, 10, 2025. [Google Scholar] [CrossRef]
- Chi, H.H.; Hua, K.F.; Lin, Y.C.; Chu, C.L.; Hsieh, C.Y.; Hsu, Y.J.; Ka, S.M.; Tsai, Y.L.; Liu, F.C.; Chen, A. IL-36 Signaling Facilitates Activation of the NLRP3 Inflammasome and IL-23/IL-17 Axis in Renal Inflammation and Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.C.; Xu, W.D.; Liu, X.Y.; Liu, X.Y.; Huang, A.F.; Su, L.C. Biology of IL-36 Signaling and Its Role in Systemic Inflammatory Diseases. Front. Immunol. 2019, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Bassoy, E.Y.; Towne, J.E.; Gabay, C. Regulation and function of interleukin-36 cytokines. Immunol. Rev. 2018, 281, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, X.; Hong, X.; Lu, L.; Liu, D. IL-36 cytokines in autoimmunity and inflammatory disease. Oncotarget 2018, 9, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Crowe, P.D.; Liu, C.; Chalmers, D.T.; Liu, X.J.; Liaw, C.; Clevenger, W.; Oltersdorf, T.; De Souza, E.B.; Maki, R.A. Cloning of a cDNA encoding a novel interleukin-1 receptor related protein (IL 1R-rp2). J. NeuroImmunol. 1996, 70, 113–122. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Daniels, M.J.D.; Colliver, I.; Robertson, D.L.; Brough, D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat. Commun. 2018, 9, 1156. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Born, T.L.; Smith, D.E.; Garka, K.E.; Renshaw, B.R.; Bertles, J.S.; Sims, J.E. Identification and characterization of two members of a novel class of the interleukin-1 receptor (IL-1R) family. Delineation Of a new class of IL-1R-related proteins based on signaling. J. Biol. Chem. 2000, 275, 41528. [Google Scholar] [CrossRef]
- Larson, E.T.; Brennan, D.L.; Hickey, E.R.; Ganesan, R.; Kroe-Barrett, R.; Farrow, N.A. X-ray crystal structure localizes the mechanism of inhibition of an IL-36R antagonist monoclonal antibody to interaction with Ig1 and Ig2 extra cellular domains. Protein Sci. 2020, 29, 1679–1686. [Google Scholar] [CrossRef]
- Towne, J.E.; Garka, K.E.; Renshaw, B.R.; Virca, G.D.; Sims, J.E. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J. Biol. Chem. 2004, 279, 13677–13688. [Google Scholar] [CrossRef] [PubMed]
- Muzio, M.; Ni, J.; Feng, P.; Dixit, V.M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 1997, 278, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Wesche, H.; Henzel, W.J.; Shillinglaw, W.; Li, S.; Cao, Z. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity 1997, 7, 837–847. [Google Scholar] [CrossRef]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-Derived Proteases Escalate Inflammation through Activation of IL-36 Family Cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Towne, J.E.; Renshaw, B.R.; Douangpanya, J.; Lipsky, B.P.; Shen, M.; Gabel, C.A.; Sims, J.E. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J. Biol. Chem. 2011, 286, 42594–42602. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Yi, G.; Ybe, J.A.; Saha, S.S.; Caviness, G.; Raymond, E.; Ganesan, R.; Mbow, M.L.; Kao, C.C. Structural and Functional Attributes of the Interleukin-36 Receptor. J. Biol. Chem. 2016, 291, 16597–16609. [Google Scholar] [CrossRef]
- Bridgewood, C.; Stacey, M.; Alase, A.; Lagos, D.; Graham, A.; Wittmann, M. IL-36gamma has proinflammatory effects on human endothelial cells. Exp. Dermatol 2017, 26, 402–408. [Google Scholar] [CrossRef]
- Chustz, R.T.; Nagarkar, D.R.; Poposki, J.A.; Favoreto, S., Jr.; Avila, P.C.; Schleimer, R.P.; Kato, A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 145–153. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G. New Insights in the Immunobiology of IL-1 Family Members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Vigne, S.; Palmer, G.; Lamacchia, C.; Martin, P.; Talabot-Ayer, D.; Rodriguez, E.; Ronchi, F.; Sallusto, F.; Dinh, H.; Sims, J.E.; et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood 2011, 118, 5813–5823. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, R.A.; Ewart, S.L.; Iwakura, Y.; Medoff, B.D.; LeVine, A.M. IL-36alpha exerts pro-inflammatory effects in the lungs of mice. PLoS ONE 2012, 7, e45784. [Google Scholar] [CrossRef] [PubMed]
- Mutamba, S.; Allison, A.; Mahida, Y.; Barrow, P.; Foster, N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur. J. Immunol. 2012, 42, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Harusato, A.; Abo, H.; Ngo, V.L.; Yi, S.W.; Mitsutake, K.; Osuka, S.; Kohlmeier, J.E.; Li, J.D.; Gewirtz, A.T.; Nusrat, A.; et al. IL-36gamma signaling controls the induced regulatory T cell-Th9 cell balance via NFkappaB activation and STAT transcription factors. Mucosal Immunol. 2017, 10, 1455–1467. [Google Scholar] [CrossRef]
- Marrakchi, S.; Guigue, P.; Renshaw, B.R.; Puel, A.; Pei, X.Y.; Fraitag, S.; Zribi, J.; Bal, E.; Cluzeau, C.; Chrabieh, M.; et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 2011, 365, 620–628. [Google Scholar] [CrossRef]
- Cowen, E.W.; Goldbach-Mansky, R. DIRA, DITRA, and new insights into pathways of skin inflammation: what’s in a name? Arch. Dermatol. 2012, 148, 381–384. [Google Scholar] [CrossRef]
- Bachelez, H.; Choon, S.E.; Marrakchi, S.; Burden, A.D.; Tsai, T.F.; Morita, A.; Turki, H.; Hall, D.B.; Shear, M.; Baum, P.; et al. Inhibition of the Interleukin-36 Pathway for the Treatment of Generalized Pustular Psoriasis. N. Engl. J. Med. 2019, 380, 981–983. [Google Scholar] [CrossRef]
- Blumberg, H.; Dinh, H.; Trueblood, E.S.; Pretorius, J.; Kugler, D.; Weng, N.; Kanaly, S.T.; Towne, J.E.; Willis, C.R.; Kuechle, M.K.; et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 2007, 204, 2603–2614. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or foe in organ fibrosis. Cytokine 2019, 120, 282–288. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68-69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Ueha, S.; Shand, F.H.; Matsushima, K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front. Immunol. 2012, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; O’Reilly, S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-beta signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef]
- Lin, S.L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef]
- Ronnov-Jessen, L.; Petersen, O.W.; Koteliansky, V.E.; Bissell, M.J. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J. Clin. Investig. 1995, 95, 859–873. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Munoz-Durango, N.; Riedel, C.A.; Echeverria, C.; Kalergis, A.M.; Cabello-Verrugio, C.; Simon, F. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Donnelly, S.C.; Peng, T.; Bucala, R.; Metz, C.N. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J. Immunol. 2001, 166, 7556–7562. [Google Scholar] [CrossRef] [PubMed]
- Galligan, C.L.; Keystone, E.C.; Fish, E.N. Fibrocyte and T cell interactions promote disease pathogenesis in rheumatoid arthritis. J. Autoimmun. 2016, 69, 38–50. [Google Scholar] [CrossRef]
- Hong, K.M.; Belperio, J.A.; Keane, M.P.; Burdick, M.D.; Strieter, R.M. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 22910–22920. [Google Scholar] [CrossRef]
- Wu, C.F.; Chiang, W.C.; Lai, C.F.; Chang, F.C.; Chen, Y.T.; Chou, Y.H.; Wu, T.H.; Linn, G.R.; Ling, H.; Wu, K.D.; et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 2013, 182, 118–131. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chang, F.C.; Wu, C.F.; Chou, Y.H.; Hsu, H.L.; Chiang, W.C.; Shen, J.; Chen, Y.M.; Wu, K.D.; Tsai, T.J.; et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011, 80, 1170–1181. [Google Scholar] [CrossRef]
- Leaf, I.A.; Nakagawa, S.; Johnson, B.G.; Cha, J.J.; Mittelsteadt, K.; Guckian, K.M.; Gomez, I.G.; Altemeier, W.A.; Duffield, J.S. Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. J. Clin. Investig. 2017, 127, 321–334. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Parker, M.W.; Rossi, D.; Peterson, M.; Smith, K.; Sikstrom, K.; White, E.S.; Connett, J.E.; Henke, C.A.; Larsson, O.; Bitterman, P.B. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 2014, 124, 1622–1635. [Google Scholar] [CrossRef]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The immunology of fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lo Re, S.; Lecocq, M.; Uwambayinema, F.; Yakoub, Y.; Delos, M.; Demoulin, J.B.; Lucas, S.; Sparwasser, T.; Renauld, J.C.; Lison, D.; et al. Platelet-derived growth factor-producing CD4+ Foxp3+ regulatory T lymphocytes promote lung fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L. Recent advances in the IL-17 cytokine family. Curr. Opin. Immunol. 2011, 23, 613–619. [Google Scholar] [CrossRef]
- Cortez, D.M.; Feldman, M.D.; Mummidi, S.; Valente, A.J.; Steffensen, B.; Vincenti, M.; Barnes, J.L.; Chandrasekar, B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3356–H3365. [Google Scholar] [CrossRef]
- Leung, G.; Wang, A.; Fernando, M.; Phan, V.C.; McKay, D.M. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: Participation of IL-10. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G781–G792. [Google Scholar] [CrossRef]
- Baroni, G.S.; D’Ambrosio, L.; Curto, P.; Casini, A.; Mancini, R.; Jezequel, A.M.; Benedetti, A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology 1996, 23, 1189–1199. [Google Scholar] [CrossRef]
- Oldroyd, S.D.; Thomas, G.L.; Gabbiani, G.; El Nahas, A.M. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int. 1999, 56, 2116–2127. [Google Scholar] [CrossRef]
- Iimuro, Y.; Nishio, T.; Morimoto, T.; Nitta, T.; Stefanovic, B.; Choi, S.K.; Brenner, D.A.; Yamaoka, Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology 2003, 124, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Foronjy, R.F.; Sun, J.; Lemaitre, V.; D’Armiento, J.M. Transgenic expression of matrix metalloproteinase-1 inhibits myocardial fibrosis and prevents the transition to heart failure in a pressure overload mouse model. Hypertens. Res. 2008, 31, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Zhou, Y.; Gaggar, A.; Duncan, S.R. Fibrosis: Ultimate and proximate causes. J. Clin. Investig. 2014, 124, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinkovic, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Tschumperlin, D.J.; Ligresti, G.; Hilscher, M.B.; Shah, V.H. Mechanosensing and fibrosis. J. Clin. Investig. 2018, 128, 74–84. [Google Scholar] [CrossRef]
- Giannoudaki, E.; Hernandez-Santana, Y.E.; Mulfaul, K.; Doyle, S.L.; Hams, E.; Fallon, P.G.; Mat, A.; O’Shea, D.; Kopf, M.; Hogan, A.E.; et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat. Commun. 2019, 10, 4003. [Google Scholar] [CrossRef]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carle, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; Zheng, J.; et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, X.; Gao, Q.; Li, Y.; Li, M. Interleukin-38 overexpression prevents bleomycin-induced mouse pulmonary fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, Y.; Zhong, Y.; Yu, K.; Xu, W.; Zhu, R.; Sun, H.; Ding, Y.; Wang, Y.; Zeng, Q. Interleukin-38 alleviates cardiac remodelling after myocardial infarction. J. Cell Mol. Med. 2020, 24, 371–384. [Google Scholar] [CrossRef]
- Sommerfeld, S.D.; Cherry, C.; Schwab, R.M.; Chung, L.; Maestas, D.R., Jr.; Laffont, P.; Stein, J.E.; Tam, A.; Ganguly, S.; Housseau, F.; et al. Interleukin-36gamma-producing macrophages drive IL-17-mediated fibrosis. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Maron-Gutierrez, T.; Castiglione, R.C.; Xisto, D.G.; Oliveira, M.G.; Cruz, F.F.; Pecanha, R.; Carreira-Junior, H.; Ornellas, D.S.; Moraes, M.O.; Takiya, C.M.; et al. Bone marrow-derived mononuclear cell therapy attenuates silica-induced lung fibrosis. Eur. Respir. J. 2011, 37, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Rosenthal, M. IL-17 in lung disease: Friend or foe? Thorax 2013, 68, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin ImmunoPathol. 2016, 38, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Towne, J.E. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J. Leukoc. Biol. 2015, 97, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Parsanejad, R.; Fields, W.R.; Steichen, T.J.; Bombick, B.R.; Doolittle, D.J. Distinct regulatory profiles of interleukins and chemokines in response to cigarette smoke condensate in normal human bronchial epithelial (NHBE) cells. J. Interferon Cytokine Res. 2008, 28, 703–712. [Google Scholar] [CrossRef]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Wu, L.; Ong, S.; Talor, M.V.; Barin, J.G.; Baldeviano, G.C.; Kass, D.A.; Bedja, D.; Zhang, H.; Sheikh, A.; Margolick, J.B.; et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 2014, 211, 1449–1464. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef]
- Ichii, O.; Otsuka, S.; Sasaki, N.; Yabuki, A.; Ohta, H.; Takiguchi, M.; Hashimoto, Y.; Endoh, D.; Kon, Y. Local overexpression of interleukin-1 family, member 6 relates to the development of tubulointerstitial lesions. Lab. Investig. 2010, 90, 459–475. [Google Scholar] [CrossRef]
- Nishikawa, H.; Taniguchi, Y.; Matsumoto, T.; Arima, N.; Masaki, M.; Shimamura, Y.; Inoue, K.; Horino, T.; Fujimoto, S.; Ohko, K.; et al. Knockout of the interleukin-36 receptor protects against renal ischemia-reperfusion injury by reduction of proinflammatory cytokines. Kidney Int. 2018, 93, 599–614. [Google Scholar] [CrossRef]
- Ichii, O.; Kimura, J.; Okamura, T.; Horino, T.; Nakamura, T.; Sasaki, H.; Elewa, Y.H.A.; Kon, Y. IL-36alpha Regulates Tubulointerstitial Inflammation in the Mouse Kidney. Front. Immunol. 2017, 8, 1346. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, S.; Ueda, R.; Urayama, K.; Sagawa, F.; Endo, S.; Shiizaki, K.; Kurosu, H.; Maria de Almeida, G.; Hasan, S.M.; Nakazato, K.; et al. The Body-wide Transcriptome Landscape of Disease Models. iScience 2018, 2, 238–268. [Google Scholar] [CrossRef] [PubMed]
- Cowling, R.T.; Kupsky, D.; Kahn, A.M.; Daniels, L.B.; Greenberg, B.H. Mechanisms of cardiac collagen deposition in experimental models and human disease. Transl. Res. 2019, 209, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Daniels, L.B.; Bayes-Genis, A. Using ST2 in cardiovascular patients: A review. Future Cardiol. 2014, 10, 525–539. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef]
- Martinez, E.C.; Lilyanna, S.; Wang, P.; Vardy, L.A.; Jiang, X.; Armugam, A.; Jeyaseelan, K.; Richards, A.M. MicroRNA-31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J. Mol. Cell Cardiol. 2017, 112, 27–39. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, K.; Wang, X.; Wang, X.; Ji, Q.; Zeng, Q. Elevated Plasma IL-38 Concentrations in Patients with Acute ST-Segment Elevation Myocardial Infarction and Their Dynamics after Reperfusion Treatment. Mediators Inflamm. 2015, 2015, 490120. [Google Scholar] [CrossRef]
- Ippolito, C.; Colucci, R.; Segnani, C.; Errede, M.; Girolamo, F.; Virgintino, D.; Dolfi, A.; Tirotta, E.; Buccianti, P.; Di Candio, G.; et al. Fibrotic and Vascular Remodelling of Colonic Wall in Patients with Active Ulcerative Colitis. J. Crohns Colitis 2016, 10, 1194–1204. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Goldblum, J.R.; Fiocchi, C.; Rieder, F. Fibrosis in ulcerative colitis: Mechanisms, features, and consequences of a neglected problem. Inflamm. Bowel Dis. 2014, 20, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Keir, M.E.; Scherl, A.; Zhao, R.; de Hertogh, G.; Faubion, W.A.; Lu, T.T. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut 2017, 66, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Wu, F.; Chakravarti, S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J. Immunol. 2007, 179, 6988–7000. [Google Scholar] [CrossRef]
- Rieder, F.; Zimmermann, E.M.; Remzi, F.H.; Sandborn, W.J. Crohn’s disease complicated by strictures: A systematic review. Gut 2013, 62, 1072–1084. [Google Scholar] [CrossRef]
- Russell, S.E.; Horan, R.M.; Stefanska, A.M.; Carey, A.; Leon, G.; Aguilera, M.; Statovci, D.; Moran, T.; Fallon, P.G.; Shanahan, F.; et al. IL-36alpha expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016, 9, 1193–1204. [Google Scholar] [CrossRef]
- Nishida, A.; Hidaka, K.; Kanda, T.; Imaeda, H.; Shioya, M.; Inatomi, O.; Bamba, S.; Kitoh, K.; Sugimoto, M.; Andoh, A. Increased Expression of Interleukin-36, a Member of the Interleukin-1 Cytokine Family, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 303–314. [Google Scholar] [CrossRef]
- Neufert, C.; Neurath, M.F.; Atreya, R. Rationale for IL-36 receptor antibodies in ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 339–342. [Google Scholar] [CrossRef]
- Nishida, A.; Inatomi, O.; Fujimoto, T.; Imaeda, H.; Tani, M.; Andoh, A. Interleukin-36alpha Induces Inflammatory Mediators From Human Pancreatic Myofibroblasts Via a MyD88 Dependent Pathway. Pancreas 2017, 46, 539–548. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rios-Navarro, C.; de Pablo, C.; Collado-Diaz, V.; Orden, S.; Blas-Garcia, A.; Martinez-Cuesta, M.A.; Esplugues, J.V.; Alvarez, A. Differential effects of anti-TNF-alpha and anti-IL-12/23 agents on human leukocyte-endothelial cell interactions. Eur. J. Pharmacol. 2015, 765, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Zaba, L.C.; Cardinale, I.; Gilleaudeau, P.; Sullivan-Whalen, M.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Novitskaya, I.; Khatcherian, A.; Bluth, M.J.; Lowes, M.A.; et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007, 204, 3183–3194. [Google Scholar] [CrossRef] [PubMed]
- Indramohan, M.; Sieve, A.N.; Break, T.J.; Berg, R.E. Inflammatory monocyte recruitment is regulated by interleukin-23 during systemic bacterial infection. Infect. Immun. 2012, 80, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Yuan, J.; Zhu, Z.F.; Zhang, W.C.; Xiao, H.; Xia, N.; Yan, X.X.; Nie, S.F.; Liu, J.; Zhou, S.F.; et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 2012, 107, 232. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Hirano, Y.; Kurosu, H.; Shiizaki, K.; Iwazu, Y.; Tsuruoka, S.; Kuro, O.M. Interleukin-36alpha as a potential biomarker for renal tubular damage induced by dietary phosphate load. FEBS Open Bio 2020, 10, 894–903. [Google Scholar] [CrossRef]
- Ge, S.; Hertel, B.; Susnik, N.; Rong, S.; Dittrich, A.M.; Schmitt, R.; Haller, H.; von Vietinghoff, S. Interleukin 17 receptor A modulates monocyte subsets and macrophage generation in vivo. PLoS ONE 2014, 9, e85461. [Google Scholar] [CrossRef]
- Peng, X.; Xiao, Z.; Zhang, J.; Li, Y.; Dong, Y.; Du, J. IL-17A produced by both gammadelta T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J. Pathol. 2015, 235, 79–89. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Stoeckman, A.K.; Wu, G.; Boeckermann, A.N.; Azam, T.; Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Hao, R.; Kalabokis, V.; et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 2012, 109, 3001–3005. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Weng, D.; Song, L.; Tang, W.; Dai, W.; Yu, Y.; Liu, F.; Zhao, M.; Lu, C.; et al. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2014, 275, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Okamoto, M.; Kawayama, T.; Matsuoka, M.; Kaieda, S.; Sakazaki, Y.; Kinoshita, T.; Mori, D.; Inoue, A.; Hoshino, T. Overexpression of IL-38 protein in anticancer drug-induced lung injury and acute exacerbation of idiopathic pulmonary fibrosis. Respir. Investig. 2017, 55, 293–299. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Teng, Y.; O’Connell, J.T.; Charytan, D.; Muller, G.A.; Muller, C.A.; Sugimoto, H.; Kalluri, R. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat. Med. 2013, 19, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D.; Pluznick, J.L. How does your kidney smell? Emerging roles for olfactory receptors in renal function. Pediatr. Nephrol. 2016, 31, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Wei, Y.; Szekeres, C.; Kugler, M.C.; Wolters, P.J.; Hill, M.L.; Frank, J.A.; Brumwell, A.N.; Wheeler, S.E.; Kreidberg, J.A.; et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Investig. 2009, 119, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Broekema, M.; Harmsen, M.C.; van Luyn, M.J.; Koerts, J.A.; Petersen, A.H.; van Kooten, T.G.; van Goor, H.; Navis, G.; Popa, E.R. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J. Am. Soc. Nephrol. 2007, 18, 165–175. [Google Scholar] [CrossRef]
- Zhou, B.; von Gise, A.; Ma, Q.; Hu, Y.W.; Pu, W.T. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev. Biol. 2010, 338, 251–261. [Google Scholar] [CrossRef]

| Organ | Disease Model | IL-36R Ligand Involved | IL-36 Producing Cells | Possible Downstream Targets | Outcome of IL-36R Inhibition | Refs |
|---|---|---|---|---|---|---|
| Lung | Bleomycin induced IPF | IL-36γ | IL-17A expressing Macrophages (CD9hi, IL3γ+) | Th17 T cells | IL-38 overexpression reduced lung inflammation, IL-17A production, and fibrosis | [88,90] |
| Kidney | UUO | IL-36α | Renal TECs | BMDCs, Th17 T cells | IL-36R KO prevented T cell activation/infiltration, and suppressed the occurrence of renal TILs | [21] |
| Heart | LAD coronary artery ligation model of MI | IL-38 | Cardiomyocytes | DCs | Recombinant IL-38 treatment protected against cardiac fibrosis and cardiomyocyte apoptosis. IL-38 also altered DC differentiation and T cell responses | [89] |
| Intestine | DSS and TNBS induced intestinal colitis and fibrosis | IL-36α | CD14+CD64+ CD163+ Macrophages | α-SMA+ Fibroblasts | IL-36R KO protected mice from colitis and fibrosis. IL-36R deficiency also reduced collagen production and blunted the presence of activated fibroblasts (CD90+, α-SMA+) | [87] |
| Pancreas | Chronic Pancreatitis | IL-36α | -------- | Myofibroblasts | --------- | [120] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melton, E.; Qiu, H. Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis. Int. J. Mol. Sci. 2020, 21, 6458. https://doi.org/10.3390/ijms21186458
Melton E, Qiu H. Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis. International Journal of Molecular Sciences. 2020; 21(18):6458. https://doi.org/10.3390/ijms21186458
Chicago/Turabian StyleMelton, Elaina, and Hongyu Qiu. 2020. "Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis" International Journal of Molecular Sciences 21, no. 18: 6458. https://doi.org/10.3390/ijms21186458
APA StyleMelton, E., & Qiu, H. (2020). Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis. International Journal of Molecular Sciences, 21(18), 6458. https://doi.org/10.3390/ijms21186458






