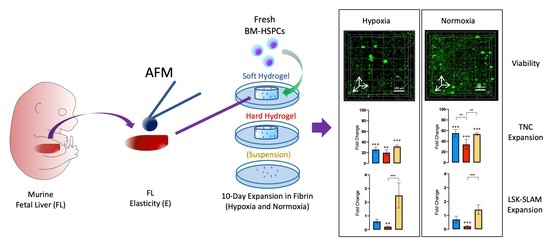
Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels
Abstract
1. Introduction
2. Results
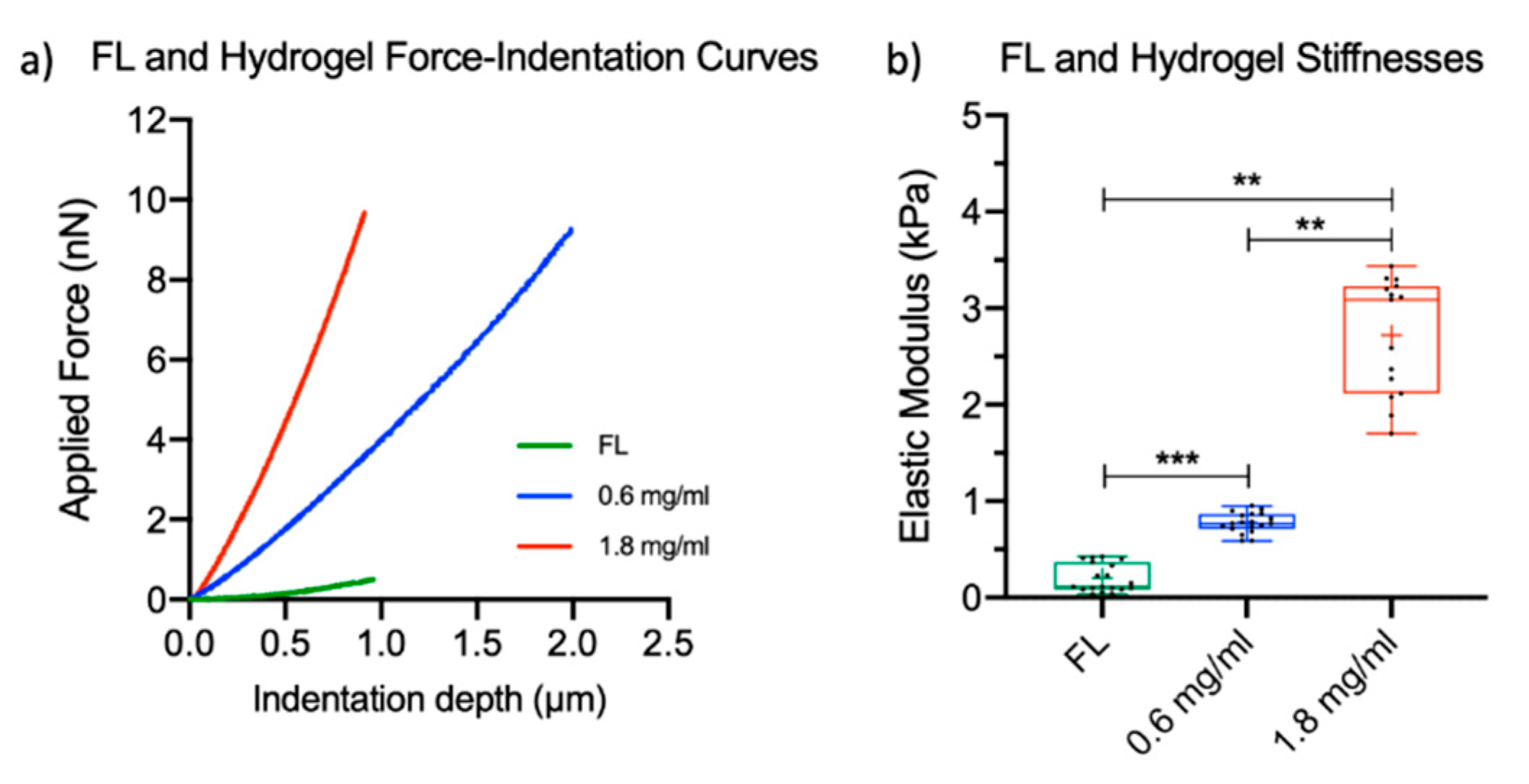
2.1. Characterization of the Elastic Properties of FL Tissue and Fibrin Hydrogels
2.2. Expansion of TNCs and of Lin−/cKit+, LSK, and LSK-SLAM Cell Populations
2.3. Cell Viability in Fibrin Hydrogels
2.4. LSK-SLAM Enriched Culture in Soft Hydrogels under Hypoxic Conditions
3. Discussion
4. Materials and Methods
4.1. FL Tissue Preparation and Elastic Modulus Quantification Via Atomic Force Microscopy (AFM)
4.2. Fibrin Hydrogel Preparation and Determination of Elastic Modulus Using AFM
4.3. Isolation of Murine Lin−/cKit+ and Lin−/Sca+/cKit+ (LSK) Cell Populations
4.4. Cell Encapsulation in Fibrin Hydrogels
4.5. Cell Harvesting from Hydrogels and Suspension Cultures
4.6. Live/Dead Assays and Quantification of TNCs and Viability
4.7. Flow Cytometry Staining and Data Acquisition
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Antibody |
| AGM | Aorta-Gonad-Mesonephros |
| AFM | Atomic Force Microscopy |
| AM | Acetoxymethyl |
| ANOVA | Analysis of Variance |
| APC | Allophycocyanin |
| APC-Cy7 | Allophycocyanin Cyanine-7 |
| BM | Bone Marrow |
| BM-HSC | Bone Marrow Derived Hematopoietic Stem Cell |
| BSA | Bovine Serum Albumin |
| DAPI | 4’,6-Diamidino-2-phenylindole |
| E | Young’s Modulus |
| ECD | Ethische Commissie Dierenwelzijn |
| EDTA | Ethylenediaminetetraacetic Acid |
| FACS | Fluorescence Activated Cell Sorting |
| FBS | Fetal Bovine Serum |
| FITC | Fluorescein Isothiocyanate |
| FL | Fetal Liver |
| FL-HSC | Fetal Liver Derived Hematopoietic Stem Cell |
| FLT3-L | FMS-related Tyrosine Kinase 3 Ligand |
| FMO | Fluorescence Minus One |
| FU/ml | Fibrin Degradation Units per Milliliter |
| FXIII | Factor XIII |
| H | Hypoxia |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic Acid |
| HPC | Hematopoietic Progenitor Cell |
| HSC | Hematopoietic Stem Cell |
| HSD | Honestly Significant Difference |
| HSPC | Hematopoietic Stem/Progenitor Cell |
| IGF-2 | Insulin like Growth Factor 2 |
| Lin | Lineage |
| LSK | Lin−/Sca+/cKit+ |
| MACS | Magnetic Activated Cell Sorting |
| N | Normoxia |
| PBS | Phosphate Buffered Saline |
| PE | Phycoerythrin |
| PECy7 | Phycoerythrin Cyanine-7 |
| PEG | Polyethylene Glycol |
| PI | Propidium Iodide |
| rcf | Relative Centrifugal Force |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| SCF | Stem Cell Factor |
| SFEM | Serum-Free Expansion Medium |
| SLAM | Signaling Lymphocyte Activated Molecules |
| Susp | Suspension |
| TNC | Total Nucleated Cell |
| TPO | Thrombopoietin |
References
- Eaves, C.J. Hematopoietic stem cells: Concepts, definitions, and the new reality. Blood 2015, 125, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Göttgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 2018, 553, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Wagers, A.J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 643–655. [Google Scholar] [CrossRef]
- Mahony, C.B.; Bertrand, J.Y. How HSCs colonize and expand in the fetal niche of the vertebrate embryo: An evolutionary perspective. Front. Cell Dev. Biol. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Ivanovs, A.; Rybtsov, S.; Ng, E.S.; Stanley, E.G.; Elefanty, A.G.; Medvinsky, A. Human haematopoietic stem cell development: From the embryo to the dish. Development 2017, 144, 2323. [Google Scholar] [CrossRef] [PubMed]
- Ema, H.; Nakauchi, H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 2000, 95, 2284–2288. [Google Scholar] [CrossRef]
- Khan, J.A.; Mendelson, A.; Kunisaki, Y.; Birbrair, A.; Kou, Y.; Arnal-Estapé, A.; Pinho, S.; Ciero, P.; Nakahara, F.; Ma’ayan, A.; et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 2016, 351, 176. [Google Scholar] [CrossRef]
- Gao, S.; Liu, F. Fetal liver: An ideal niche for hematopoietic stem cell expansion. Sci. China Life Sci. 2018, 61, 885–892. [Google Scholar] [CrossRef]
- Schmelzer, E. Hepatic progenitors of the fetal liver: Interactions with hematopoietic stem cells. Differentiation 2019, 106, 9–14. [Google Scholar] [CrossRef]
- Chou, S.; Lodish, H.F. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 7799. [Google Scholar] [CrossRef]
- Wittig, O.; Paez-Cortez, J.; Cardier, J.E. Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells. Stem Cells Dev. 2009, 19, 341–350. [Google Scholar] [CrossRef]
- Ohneda, O.; Bautch, V.L. Murine endothelial cells support fetal liver erythropoiesis and myelopoiesis via distinct interactions. Br. J. Haematol. 1997, 98, 798–808. [Google Scholar] [CrossRef]
- Zhang, C.C.; Lodish, H.F. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 2004, 103, 2513–2521. [Google Scholar] [CrossRef]
- Broudy, V.C.; Lin, N.L.; Priestley, G.V.; Nocka, K.; Wolf, N.S. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood 1996, 88, 75–81. [Google Scholar] [CrossRef]
- Hannum, C.; Culpepper, J.; Campbell, D.; McClanahan, T.; Zurawski, S.; Kastelein, R.; Bazan, J.F.; Hudak, S.; Wagner, J.; Mattson, J.; et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 1994, 368, 643–648. [Google Scholar] [CrossRef]
- Timens, W.; Kamps, W.A. Hemopoiesis in human fetal and embryonic liver. Microsc. Res. Tech. 1997, 39, 387–397. [Google Scholar] [CrossRef]
- Porayette, P.; Paulson, R.F. BMP4/Smad5 dependent stress erythropoiesis is required for the expansion of erythroid progenitors during fetal development. Dev. Biol. 2008, 317, 24–35. [Google Scholar] [CrossRef]
- Tang, Y.; Peitzsch, C.; Charoudeh, H.N.; Cheng, M.; Chaves, P.; Jacobsen, S.E.W.; Sitnicka, E. Emergence of NK-cell progenitors and functionally competent NK-cell lineage subsets in the early mouse embryo. Blood 2012, 120, 63–75. [Google Scholar] [CrossRef]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Ema, H.; Nakauchi, H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J. Exp. Med. 2010, 207, 1173–1182. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, Ö.H.; Iwashita, T.; Yilmaz, O.H.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Bowie, M.B.; Kent, D.G.; Dykstra, B.; McKnight, K.D.; McCaffrey, L.; Hoodless, P.A.; Eaves, C.J. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl. Acad. Sci. USA 2007, 104, 5878. [Google Scholar] [CrossRef]
- Gao, X.; Xu, C.; Asada, N.; Frenette, P.S. The hematopoietic stem cell niche: From embryo to adult. Development 2018, 145, dev139691. [Google Scholar] [CrossRef]
- Foudi, A.; Hochedlinger, K.; Van Buren, D.; Schindler, J.W.; Jaenisch, R.; Carey, V.; Hock, H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009, 27, 84–90. [Google Scholar] [CrossRef]
- Takano, H.; Ema, H.; Sudo, K.; Nakauchi, H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: Inference from differentiation in daughter cell and granddaughter cell pairs. J. Exp. Med. 2004, 199, 295–302. [Google Scholar] [CrossRef]
- Raic, A.; Rödling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef]
- Cuchiara, M.L.; Coşkun, S.; Banda, O.A.; Horter, K.L.; Hirschi, K.K.; West, J.L. Bioactive poly(ethylene glycol) hydrogels to recapitulate the HSC niche and facilitate HSC expansion in culture. Biotechnol. Bioeng. 2016, 113, 870–881. [Google Scholar] [CrossRef]
- Leisten, I.; Kramann, R.; Ventura Ferreira, M.S.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef]
- Braham, M.V.J.; Li Yim, A.S.P.; Garcia Mateos, J.; Minnema, M.C.; Dhert, W.J.A.; Öner, F.C.; Robin, C.; Alblas, J. A human hematopoietic niche model supporting hematopoietic stem and progenitor cells in vitro. Adv. Healthc. Mater. 2019, 8, 1801444. [Google Scholar] [CrossRef]
- Yuan, Y.; Tse, K.T.; Sin, F.W.Y.; Xue, B.; Fan, H.H.; Xie, Y.; Xie, Y. Ex vivo amplification of human hematopoietic stem and progenitor cells in an alginate three-dimensional culture system. Int. J. Lab. Hematol. 2011, 33, 516–525. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Pedron Haba, S.; Skertich, L.J.; Harley, B.A.C. The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel. Biomaterials 2015, 67, 297–307. [Google Scholar] [CrossRef]
- Ventura Ferreira, M.S.; Jahnen-Dechent, W.; Labude, N.; Bovi, M.; Hieronymus, T.; Zenke, M.; Schneider, R.K.; Neurs, S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 2012, 33, 6987–6997. [Google Scholar] [CrossRef]
- Holst, J.; Watson, S.; Lord, M.S.; Eamegdool, S.S.; Bax, D.V.; Nivison-Smith, L.B.; Kondyurin, A.; Ma, L.; Oberhauser, A.F.; Weiss, A.S.; et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 2010, 28, 1123–1128. [Google Scholar] [CrossRef]
- Kumar, S.S.; Hsiao, J.-H.; Ling, Q.-D.; Dulinska-Molak, I.; Chen, G.; Chang, Y.; Chang, Y.; Chen, Y.H.; Chen, D.-C.; Hsu, S.-T.; et al. The combined influence of substrate elasticity and surface-grafted molecules on the ex vivo expansion of hematopoietic stem and progenitor cells. Biomaterials 2013, 34, 7632–7644. [Google Scholar] [CrossRef]
- Choi, J.S.; Harley, B.A.C. The combined influence of substrate elasticity and ligand density on the viability and biophysical properties of hematopoietic stem and progenitor cells. Biomaterials 2012, 33, 4460–4468. [Google Scholar] [CrossRef]
- Gvaramia, D.; Müller, E.; Müller, K.; Atallah, P.; Tsurkan, M.; Freudenberg, U.; Bornhäuser, M.; Werner, C. Combined influence of biophysical and biochemical cues on maintenance and proliferation of hematopoietic stem cells. Biomaterials 2017, 138, 108–117. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Rauch, N.; Fiammengo, R.; Klein, G.; Spatz, J.P. Impact of substrate elasticity on human hematopoietic stem and progenitor cell adhesion and motility. J. Cell Sci. 2012, 125, 3765. [Google Scholar] [CrossRef]
- Choi, J.S.; Harley, B.A.C. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 2017, 3, e1600455. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef]
- Jansen, L.E.; Birch, N.P.; Schiffman, J.D.; Crosby, A.J.; Peyton, S.R. Mechanics of intact bone marrow. J. Mech. Behav. Biomed. Mater. 2015, 50, 299–307. [Google Scholar] [CrossRef]
- Koller, M.R.; Bender, J.G.; Miller, W.M.; Papoutsakis, E.T. Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Exp. Hematol. 1992, 20, 264–270. [Google Scholar]
- Ivanović, Z.; Sbarba, P.D.; Trimoreau, F.; Faucher, J.-L.; Praloran, V. Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion 2000, 40, 1482–1488. [Google Scholar] [CrossRef]
- Jang, Y.-Y.; Sharkis, S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef]
- Galluzzi, M.; Biswas, C.S.; Wu, Y.; Wang, Q.; Du, B.; Stadler, F.J. Space-resolved quantitative mechanical measurements of soft and supersoft materials by atomic force microscopy. NPG Asia Mater. 2016, 8, e327. [Google Scholar] [CrossRef]
- Tang, G.; Galluzzi, M.; Biswas, C.S.; Stadler, F.J. Investigation of micromechanical properties of hard sphere filled composite hydrogels by atomic force microscopy and finite element simulations. J. Mech. Behav. Biomed. Mater. 2018, 78, 496–504. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Motwani, M.S.; Faull, P.A.; Seymour, A.J.; Yu, T.T.L.; Enayati, M.; Taheem, D.K.; Salzlechner, C.; Haghighi, T.; Kania, E.M.; et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nat. Commun. 2018, 9, 4049. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Chevalier, N.R.; Gazguez, E.; Dufour, S.; Fleury, V. Measuring the micromechanical properties of embryonic tissues. Methods 2016, 94, 120–128. [Google Scholar] [CrossRef]
- Astolfi, M.; Péant, B.; Lateef, M.A.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.; Mes-Masson, A.M.; et al. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab Chip 2016, 16, 312–325. [Google Scholar] [CrossRef]
- Markert, C.D.; Guo, X.; Skardal, A.; Wang, Z.; Bharadwaj, S.; Zhang, Y.; Bonin, K.; Guthold, M. Characterizing the micro-scale elastic modulus of hydrogels for use in regenerative medicine. J. Mech. Behav. Biomed. Mater. 2013, 27, 115–127. [Google Scholar] [CrossRef]
- Murphy, K.C.; Leach, J.K. A reproducible, high throughput method for fabricating fibrin gels. BMC Res. Notes 2012, 5, 423. [Google Scholar] [CrossRef] [PubMed]
- Leonidakis, K.A.; Bhattacharya, P.; Patterson, J.; Vos, B.E.; Koenderink, G.H.; Vermant, J.; Lambrechts, D.; Roeffaers, M.; Van Oosterwyck, H. Fibrin structural and diffusional analysis suggests that fibers are permeable to solute transport. Acta Biomater. 2017, 47, 25–39. [Google Scholar] [CrossRef]
- Kurniawan, N.A.; Grimbergen, J.; Koopman, J.; Koenderink, G.H. Factor XIII stiffens fibrin clots by causing fiber compaction. J. Thromb. Haemost. 2014, 12, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Megone, W.; Roohpour, N.; Gautrot, J.E. Impact of surface adhesion and sample heterogeneity on the multiscale mechanical characterisation of soft biomaterials. Sci. Rep. 2018, 8, 6780. [Google Scholar] [CrossRef]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; O’Kelly, M.B.; Nourigat, C.; Jain, P.; Delrow, J.J.; et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef]
- Oguro, H.; Ding, L.; Morrison, S.J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 2013, 13, 102–116. [Google Scholar] [CrossRef]
- Kobayashi, H.; Morikawa, T.; Okinaga, A.; Hamano, F.; Hashidate-Yoshida, T.; Watanuki, S.; Hishikawa, D.; Shindou, H.; Arai, F.; Kabe, Y.; et al. Environmental optimization enables maintenance of quiescent hematopoietic stem cells ex vivo. Cell Rep. 2019, 28, 145–158.e149. [Google Scholar] [CrossRef]
- Eliasson, P.; Rehn, M.; Hammar, P.; Larsson, P.; Sirenko, O.; Flippin, L.A.; Cammenga, J.; Jönsson, J.-I. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term–reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 2010, 38, 301–310.e302. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015. [Google Scholar] [CrossRef]
- van Oss, C.J. Surface properties of fibrinogen and fibrin. J. Protein Chem. 1990, 9, 487–491. [Google Scholar] [CrossRef]
- Liu, W.F.; Ma, M.; Bratlie, K.M.; Dang, T.T.; Langer, R.; Anderson, D.G. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials 2011, 32, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Mullin, N.; Hobbs, J.K. A non-contact, thermal noise based method for the calibration of lateral deflection sensitivity in atomic force microscopy. Rev. Sci. Instrum. 2014, 85, 113703. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L. One hundred years of Hertz contact. Proc. Inst. Mech. Eng. 1982, 196, 363–378. [Google Scholar] [CrossRef]
- Baldwin, F.P.; Ivory, J.E.; Anthony, R.L. Experimental examination of the statistical theory of rubber elasticity. Low extension studies. Rubber Chem. Technol. 1956, 29, 227–239. [Google Scholar] [CrossRef]
- Dimitriadis, E.K.; Horkay, F.; Maresca, J.; Kachar, B.; Chadwick, R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002, 82, 2798–2810. [Google Scholar] [CrossRef]
- Carrion, B.; Janson, I.A.; Kong, Y.P.; Putnam, A.J. A safe and efficient method to retrieve mesenchymal stem cells from three-dimensional fibrin gels. Tissue Eng. Part C Methods 2013, 20, 252–263. [Google Scholar] [CrossRef] [PubMed]



| Component | 0.6 mg/mL Fibrin Hydrogel | 1.8 mg/mL Fibrin Hydrogel |
|---|---|---|
| Fibrinogen (mg/mL) | 0.6 | 1.8 |
| Thrombin (U/mL) | 0.12 | 0.12 |
| FXIII (U/mL) | 0.12 | 0.12 |
| CaCl2 (mM) | 20 | 20 |
| HEPES (mM) | 20 | 20 |
| NaCl (mM) | 150 | 150 |
| BSA (wt.%) | 0.05 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Abrego, C.; Zaunz, S.; Toprakhisar, B.; Subramani, R.; Deschaume, O.; Jooken, S.; Bajaj, M.; Ramon, H.; Verfaillie, C.; Bartic, C.; et al. Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels. Int. J. Mol. Sci. 2020, 21, 6367. https://doi.org/10.3390/ijms21176367
Garcia-Abrego C, Zaunz S, Toprakhisar B, Subramani R, Deschaume O, Jooken S, Bajaj M, Ramon H, Verfaillie C, Bartic C, et al. Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels. International Journal of Molecular Sciences. 2020; 21(17):6367. https://doi.org/10.3390/ijms21176367
Chicago/Turabian StyleGarcia-Abrego, Christian, Samantha Zaunz, Burak Toprakhisar, Ramesh Subramani, Olivier Deschaume, Stijn Jooken, Manmohan Bajaj, Herman Ramon, Catherine Verfaillie, Carmen Bartic, and et al. 2020. "Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels" International Journal of Molecular Sciences 21, no. 17: 6367. https://doi.org/10.3390/ijms21176367
APA StyleGarcia-Abrego, C., Zaunz, S., Toprakhisar, B., Subramani, R., Deschaume, O., Jooken, S., Bajaj, M., Ramon, H., Verfaillie, C., Bartic, C., & Patterson, J. (2020). Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels. International Journal of Molecular Sciences, 21(17), 6367. https://doi.org/10.3390/ijms21176367