Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling
Abstract
1. Introduction
2. Results
2.1. Chronic Restraint Stress Induced Cognitive Impairment in Adult Male Rats
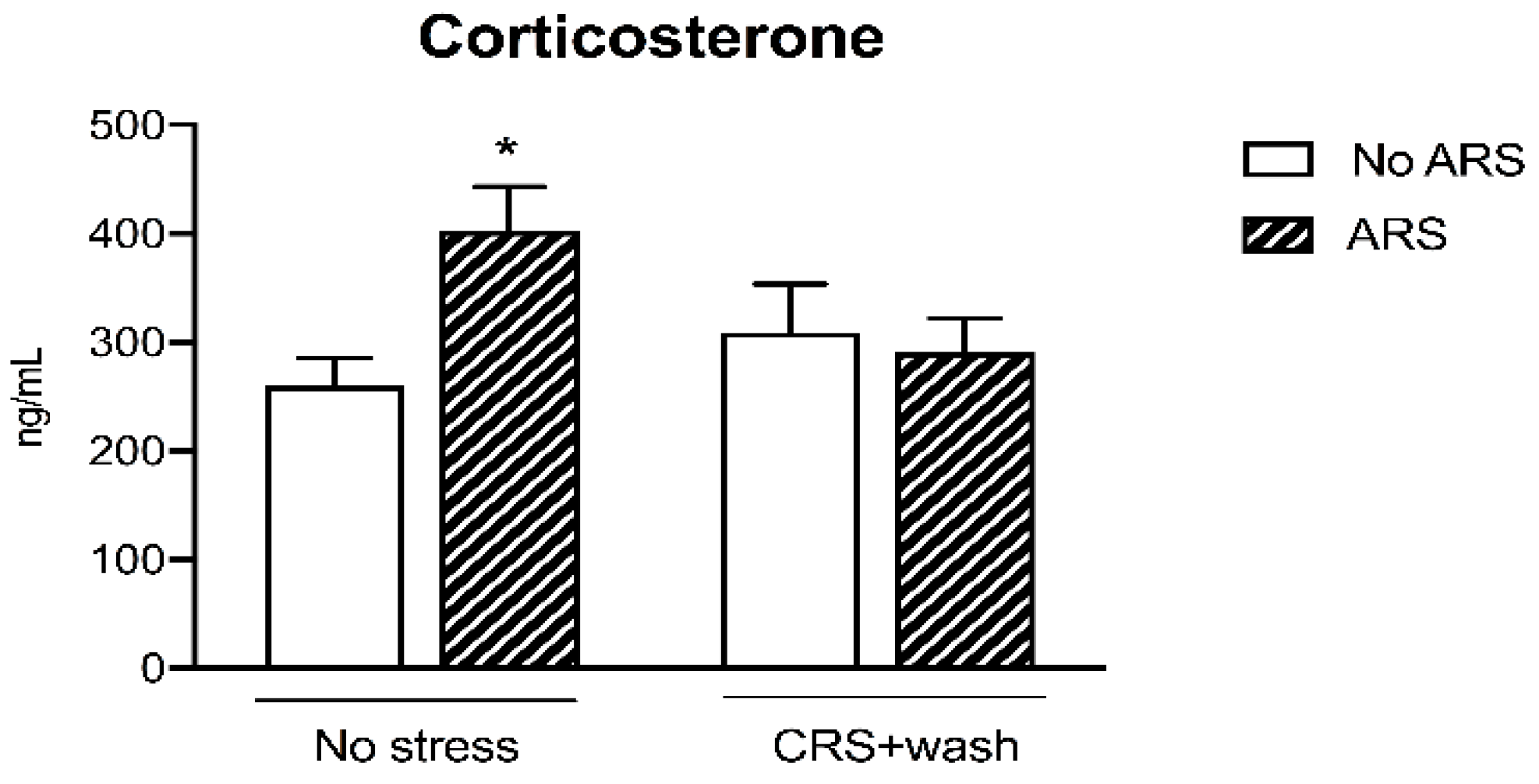
2.2. Chronic Restraint Stress Inhibited the Release of Corticosterone Levels Following the Acute Restraint Stress
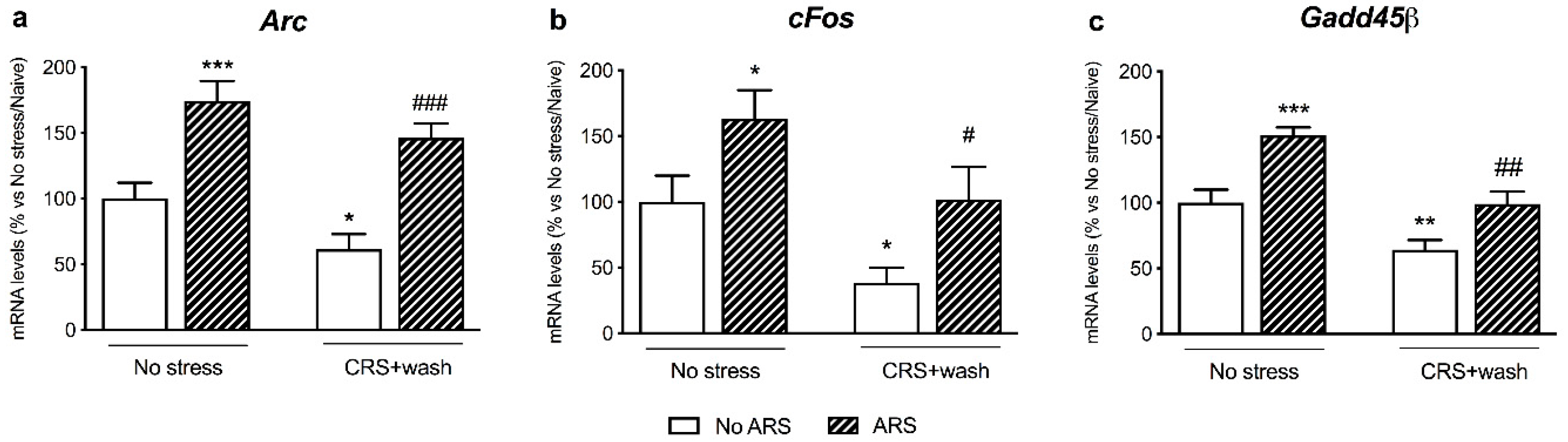
2.3. Chronic Stress Pre-Exposure Did Not Interfere with the Immediate-Early Genes Expression Following an Acute Restraint Stress
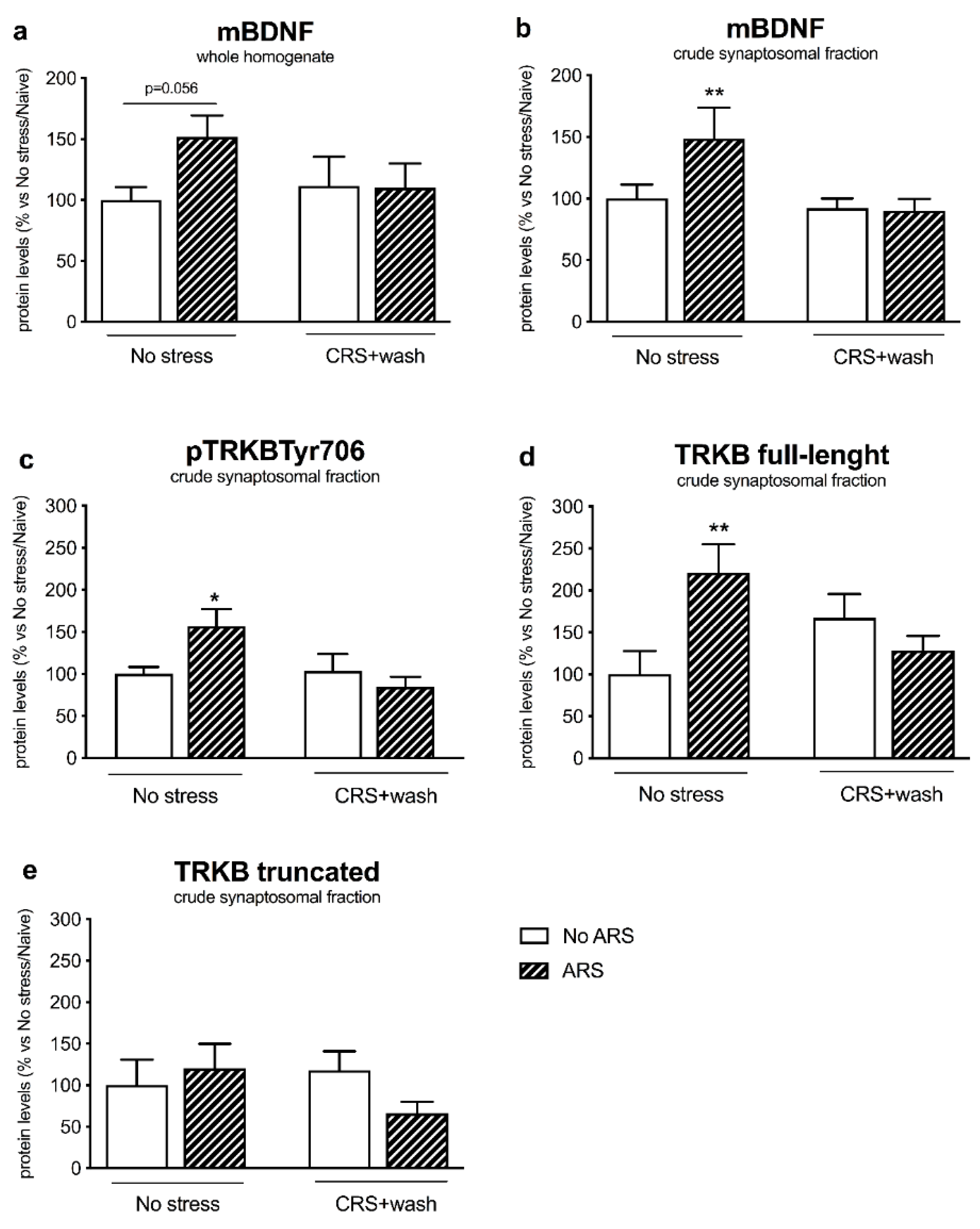
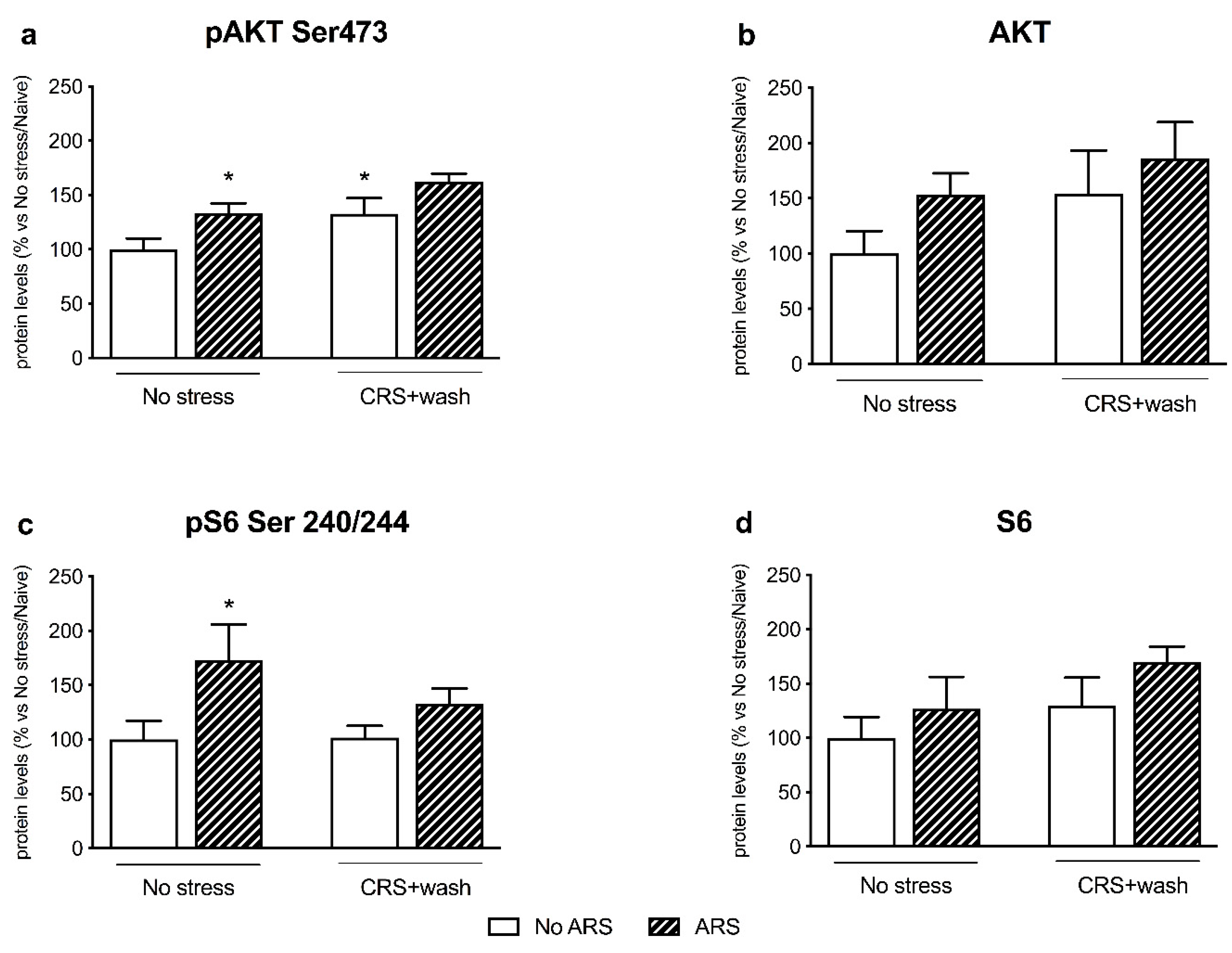
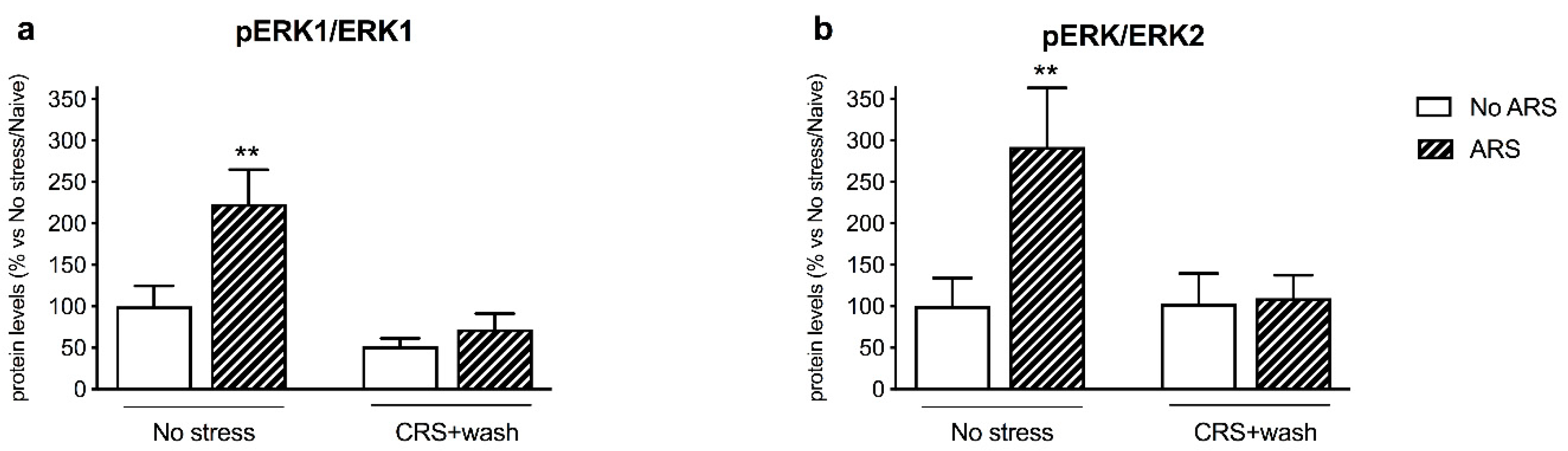
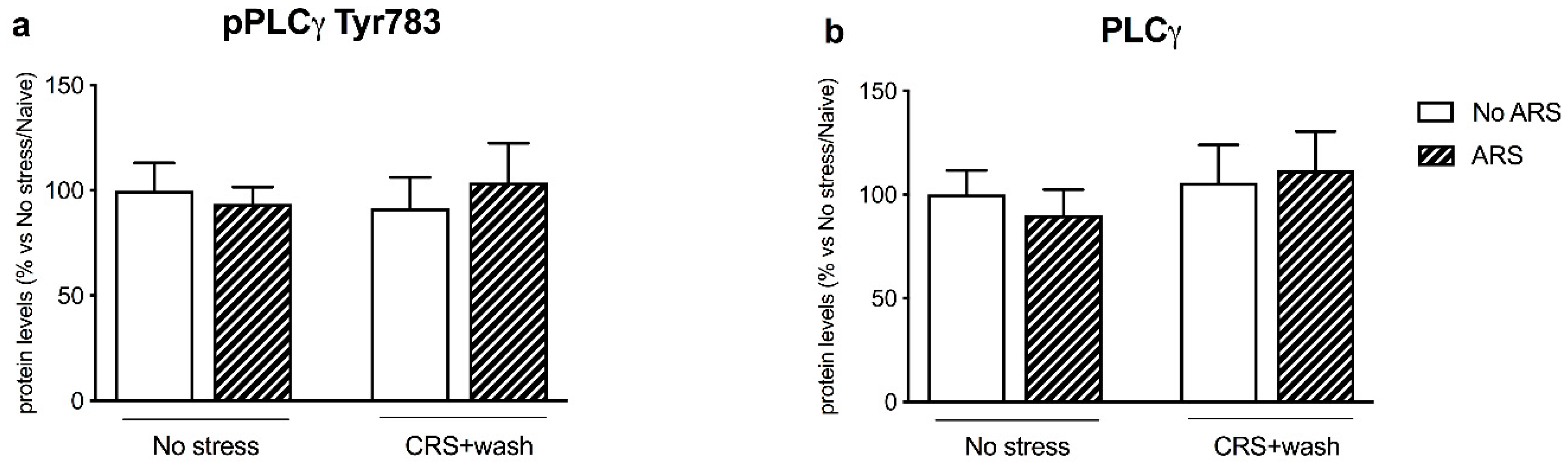
2.4. Chronic Stress Interfered with BDNF Signaling Following a Second Hit
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stress Procedure and Behavioral Test
4.3. Measurement of Plasma Corticosterone Levels
4.4. RNA Preparation and Gene Expression Analysis by Quantitative Real-Time PCR
4.5. Protein Extraction and Western Blot Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization, Depression and Other Common Mental Disorders. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2eng.pdf;jsessionid=73E8D4C73FE47D255CCB4E8D54794B63?sequence=1 (accessed on 29 August 2020).
- Keller, M.B. Past, Present, and future directions for defining optimal treatment outcome in depression. JAMA 2003, 289, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.I.; Leon, A.C.; Keller, M.B.; Solomon, D.A.; Endicott, J.; Coryell, W.; Warshaw, M.; Maser, J.D. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am. J. Psychiatry 1999, 156, 1000–1006. [Google Scholar]
- Baldessarini, R.J.; Lau, W.K.; Sim, J.; Sum, M.Y.; Sim, K. Duration of initial antidepressant treatment and subsequent relapse of major depression. J. Clin. Psychopharmacol. 2015, 35, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. Animal models of depression: Molecular perspectives. Curr. Top. Behav. Neurosci. 2011, 121–147. [Google Scholar] [CrossRef]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of stress in the brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Nemeroff, C.B. Early life stress, mood, and anxiety disorders. Chronic Stress 2017, 1. [Google Scholar] [CrossRef]
- McDowell, A.L.; Fransen, K.M.H.; Elliott, K.S.; Elghouche, A.; Kostylev, P.V.; O’Dea, P.K.; Garraghty, P.E. Sex differences and the impact of chronic stress and recovery on instrumental learning. Neurosci. J. 2015, 1–12. [Google Scholar] [CrossRef]
- Alves, N.D.; Correia, J.S.; Patrício, P.; Mateus-Pinheiro, A.; Machado-Santos, A.R.; Loureiro-Campos, E.; Morais, M.; Bessa, J.; Sousa, N.; Pinto, L. Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl. Psychiatry 2017, 7, e1058. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Altar, C. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–62. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.-I.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Boil. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Dunham, J.; Deakin, J.F.W.; Miyajima, F.; Payton, A.; Toro, C. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J. Psychiatr. Res. 2009, 43, 1175–1184. [Google Scholar] [CrossRef]
- Qi, X.-R.; Zhao, J.; Liu, J.; Fang, H.; Swaab, D.F.; Zhou, J.-N. Abnormal retinoid and trkb signaling in the prefrontal cortex in mood disorders. Cereb. Cortex 2013, 25, 75–83. [Google Scholar] [CrossRef]
- Tripp, A.; Oh, H.; Guilloux, J.-P.; Martinowich, K.; Lewis, D.A.; Sibille, E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am. J. Psychiatry 2012, 169, 1194–1202. [Google Scholar] [CrossRef]
- Deyama, S.; Duman, R.S. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 2020, 188, 172837. [Google Scholar] [CrossRef]
- Larsen, M.H.; Mikkelsen, J.D.; Hay-Schmidt, A.; Sandi, C. Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J. Psychiatr. Res. 2010, 44, 808–816. [Google Scholar] [CrossRef]
- Luoni, A.; Macchi, F.; Papp, M.; Molteni, R.; Riva, M.A. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef]
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Riva, M.A.; Calabrese, F. Acute stress induces cognitive improvement in the novel object recognition task by transiently modulating bdnf in the prefrontal cortex of male rats. Cell. Mol. Neurobiol. 2020, 40, 1037–1047. [Google Scholar] [CrossRef]
- Calabrese, F.; Brivio, P.; Gruca, P.; Lason-Tyburkiewicz, M.; Papp, M.; Riva, M.A. Chronic mild stress-induced alterations of local protein synthesis: A role for cognitive impairment. ACS Chem. Neurosci. 2017, 8, 817–825. [Google Scholar] [CrossRef]
- Calabrese, F.; Brivio, P.; Sbrini, G.; Gruca, P.; Lason, M.; Litwa, E.; Niemczyk, M.; Papp, M.; Riva, M.A. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: The potential role for glucocorticoid receptor signalling. J. Psychopharmacol. 2020, 34, 420–428. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar] [CrossRef]
- Lupien, S.J.; Juster, R.-P.; Raymond, C.; Marin, M.-F. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrinol. 2018, 49, 91–105. [Google Scholar] [CrossRef]
- Ons, S.; Rotllant, D.; Armario, A.; Marín-Blasco, I.J. Immediate-early gene response to repeated immobilization: Fos protein and arc mRNA levels appear to be less sensitive than c-fos mRNA to adaptation. Eur. J. Neurosci. 2010, 31, 2043–2052. [Google Scholar] [CrossRef]
- Grassi, D.; Franz, H.; Vezzali, R.; Bovio, P.; Heidrich, S.; Dehghanian, F.; Lagunas, N.; Belzung, C.; Krieglstein, K.; Vogel, T. Neuronal Activity, TGFβ-Signaling and unpredictable chronic stress modulate transcription of Gadd45 family members and DNA methylation in the hippocampus. Cereb. Cortex 2017, 27, 4166–4181. [Google Scholar] [CrossRef]
- Moench, K.M.; Breach, M.R.; Wellman, C.L. Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression. Neurobiol. Stress 2019, 10, 100147. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic model for stress-related mood disorders. Boil. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Calabrese, F.; Molteni, R.; Racagni, G.; Riva, M.A. Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinology 2009, 34, S208–S216. [Google Scholar] [CrossRef]
- Brivio, P.; Corsini, G.; Riva, M.A.; Calabrese, F. Chronic vortioxetine treatment improves the responsiveness to an acute stress acting through the ventral hippocampus in a glucocorticoid-dependent way. Pharmacol. Res. 2019, 142, 14–21. [Google Scholar] [CrossRef]
- Molteni, R.; Calabrese, F.; Cattaneo, A.; Mancini, M.; Gennarelli, M.; Racagni, G.; Riva, M.A. Acute stress responsiveness of the neurotrophin bdnf in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology 2008, 34, 1523–1532. [Google Scholar] [CrossRef]
- Fumagalli, F.; Calabrese, F.; Luoni, A.; Bolis, F.; Racagni, G.; Riva, M.A. Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int. J. Neuropsychopharmacol. 2011, 15, 235–246. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Peeva, P.; Todiras, M.; Bader, M.; Alenina, N.; Calabrese, F. TPH2 deficiency influences neuroplastic mechanisms and alters the response to an acute stress in a sex specific manner. Front. Mol. Neurosci. 2018, 11, 389. [Google Scholar] [CrossRef]
- Shen, C.-P.; Tsimberg, Y.; Salvadore, C.; Meller, E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004, 5, 36. [Google Scholar] [CrossRef]
- Xu, H.; Luo, C.; Richardson, J.S.; Li, X.M. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J. 2004, 4, 322–331. [Google Scholar] [CrossRef]
- Lakshminarasimhan, H.; Chattarji, S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLOS ONE 2012, 7, e30481. [Google Scholar] [CrossRef]
- Ortiz, J.B.; Conrad, C.D. The impact from the aftermath of chronic stress on hippocampal structure and function: Is there a recovery? Front. Neuroendocrinol. 2018, 49, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, S.; Kubota, K. Working memory and prefrontal cortex. Neurosci. Res. 1994, 21, 1–11. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates-The New Coronal Set; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Brivio, P.; Homberg, J.R.; Riva, M.A.; Calabrese, F. Alterations of glutamatergic markers in the prefrontal cortex of serotonin transporter knockout rats: A developmental timeline. Cell. Mol. Neurobiol. 2019, 39, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sbrini, G.; Brivio, P.; Fumagalli, M.; Giavarini, F.; Caruso, D.; Racagni, G.; Dell’Agli, M.; SanGiovanni, E.; Calabrese, F. Centella asiatica L. Phytosome improves cognitive performance by promoting Bdnf expression in rat prefrontal cortex. Nutrients 2020, 12, 355. [Google Scholar] [CrossRef]
| No Stress/No ARS | No Stress/ARS | CRS/No ARS | CRS/ARS | |
|---|---|---|---|---|
| TRKB fl | 100 ± 9 | 78 ± 17 | 117 ± 10 | 91 ± 18 |
| TRKB tr | 100 ± 14 | 79 ± 19 | 103 ± 7 | 77 ± 9 |
| (A) | Forward Primer | Reverse Primer | Probe |
| Arc | GGTGGGTGGCTCTGAAGAAT | ACTCCACCCAGTTCTTCACC | GATCCAGAACCACATGAATGGG |
| cFos | TCCTTACGGACTCCCCAC | CTCCGTTTCTCTTCCTCTTCAG | TGCTCTACTTTGCCCCTTCTGCC |
| 36b4 | TCAGTGCCTCACTCCATCAT | AGGAAGGCCTTGACCTTTTC | TGGATACAAAAGGGTCCTGG |
| (B) | Accession Number | Assay ID | |
| Gadd45β | BC085337.1 | Rn01452530_gI |
| Primary Antibody | Secondary Antibody | |
|---|---|---|
| mBDNF (14kDa) | 1:1000 M 3% (Icosagen), 4° O/N | Anti-mouse1:3000 M3%, 1h RT |
| TRKB (145–95 kDa) | 1:750 M5% (Cell signaling), 4° O/N | Anti-rabbit 1:2000 M5%, 1h RT |
| TRKB Tyr 706 (145 kDa) | 1:500 M5% (Cell signaling), 4° O/N | Anti-rabbit 1:1000 M5%, 1h RT |
| AKT Ser473 (60 kDa) | 1:1000 M5% (Cell signaling), 2h RT | Anti-rabbit 1:1000 M5%, 1h RT |
| AKT (60 kDa) | 1:1000 M10% (Cell signaling), 4° O/N | Anti-rabbit 1:1000 M5%, 1h RT |
| pS6 Ser240/244 (32 kDa) | 1:1000 M5% (Cell signaling), 4° O/N | Anti-rabbit 1:2000 M5%, 1h RT |
| S6 (32 kDa) | 1:1000 M5% (Cell signaling), 4° O/N | Anti-rabbit 1:2000 M5%, 1h RT |
| pERK1/2 Thr202/ Tyr204 (44 kDa) | 1:1000 M3% (Cell signaling), 4° O/N | Anti-rabbit 1:2000 M3%, 1h RT |
| ERK1/2 (44 kDa) | 1:1000 M3% (Santa Cruz Biotechnology), 4° O/N | Anti-rabbit 1:5000 M3%, 1h RT |
| pPLCγ Tyr783 (155 kDa) | 1:1000 BSA5% (Cell signaling), 4° O/N | Anti-rabbit 1:2000 M5%, 1h RT |
| PLCγ (155 kDa) | 1:1000 M5% (Cell signaling), 4° O/N | Anti-rabbit 1:1000 M5%, 1h RT |
| β-ACTIN (43 kDa) | 1:10,000 M3% (Sigma-aldrich), 4° O/N | Anti-mouse 1:10,000 M3%, 1h RT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brivio, P.; Sbrini, G.; Corsini, G.; Paladini, M.S.; Racagni, G.; Molteni, R.; Calabrese, F. Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling. Int. J. Mol. Sci. 2020, 21, 6261. https://doi.org/10.3390/ijms21176261
Brivio P, Sbrini G, Corsini G, Paladini MS, Racagni G, Molteni R, Calabrese F. Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling. International Journal of Molecular Sciences. 2020; 21(17):6261. https://doi.org/10.3390/ijms21176261
Chicago/Turabian StyleBrivio, Paola, Giulia Sbrini, Giulia Corsini, Maria Serena Paladini, Giorgio Racagni, Raffaella Molteni, and Francesca Calabrese. 2020. "Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling" International Journal of Molecular Sciences 21, no. 17: 6261. https://doi.org/10.3390/ijms21176261
APA StyleBrivio, P., Sbrini, G., Corsini, G., Paladini, M. S., Racagni, G., Molteni, R., & Calabrese, F. (2020). Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling. International Journal of Molecular Sciences, 21(17), 6261. https://doi.org/10.3390/ijms21176261







