Downregulation of TAP1 in Tumor-Free Tongue Contralateral to Squamous Cell Carcinoma of the Oral Tongue, an Indicator of Better Survival
Abstract
1. Introduction
2. Results
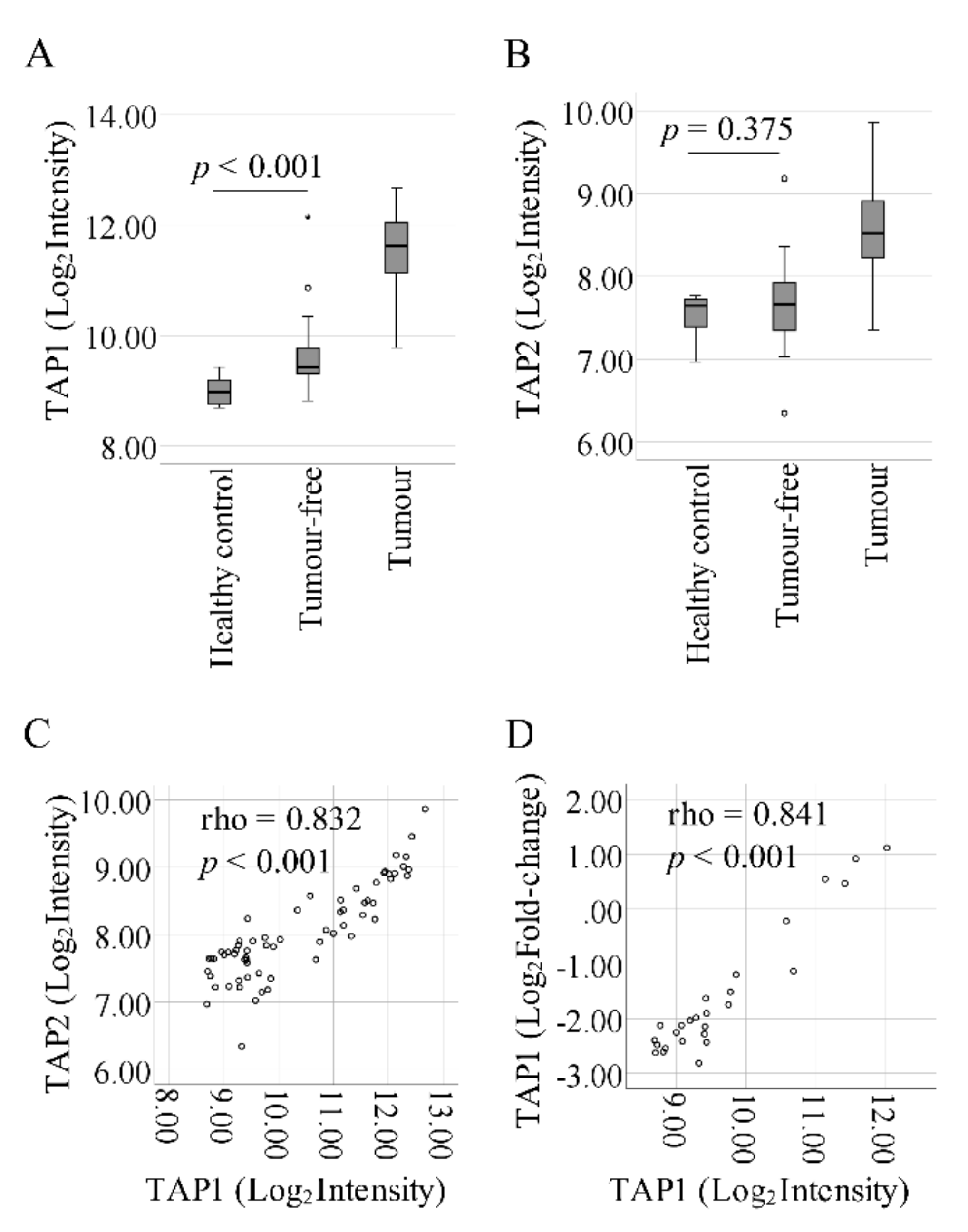
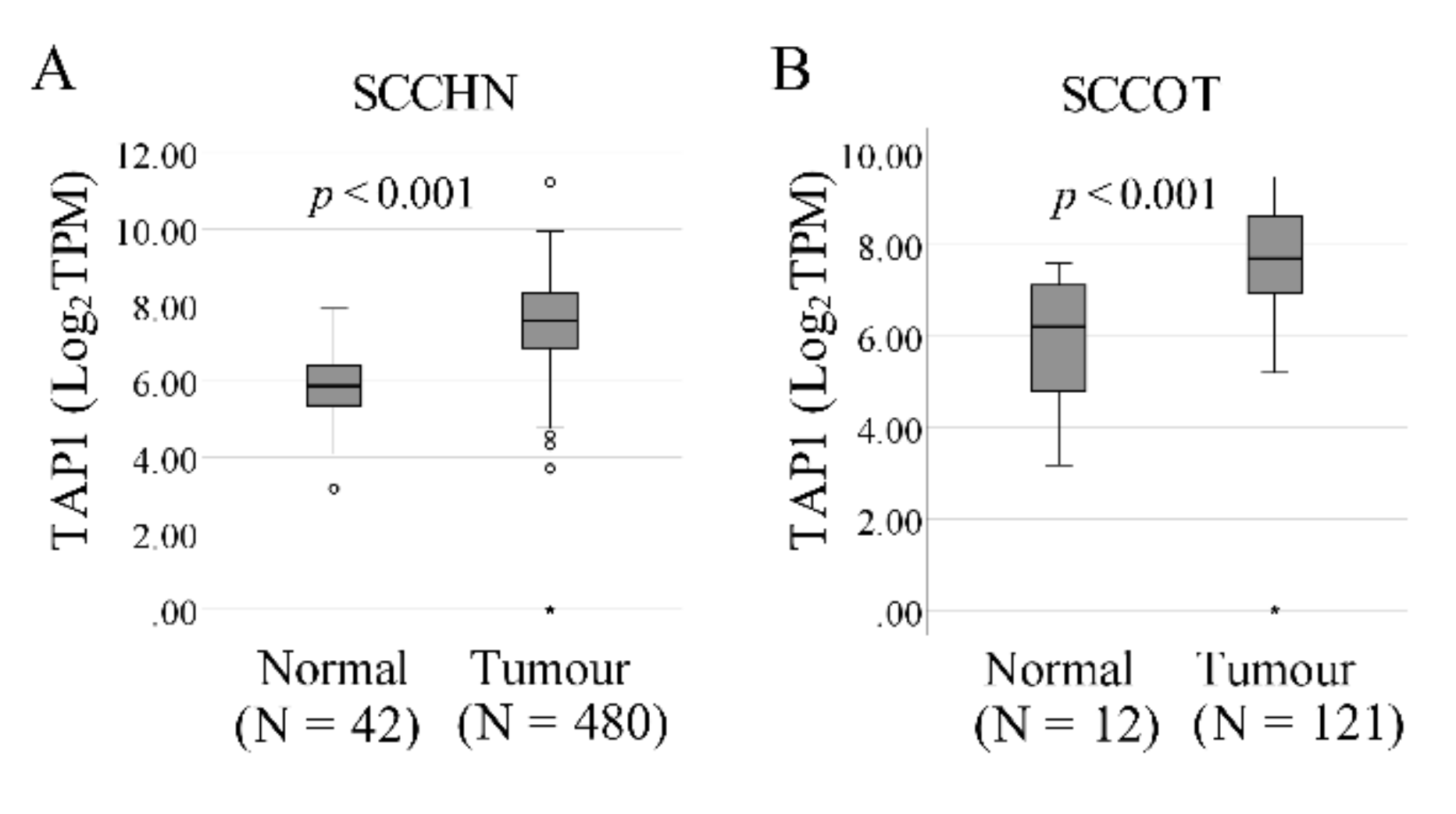
2.1. Progressive Upregulation of TAP1 from Control to Tumor-Free to Tumor
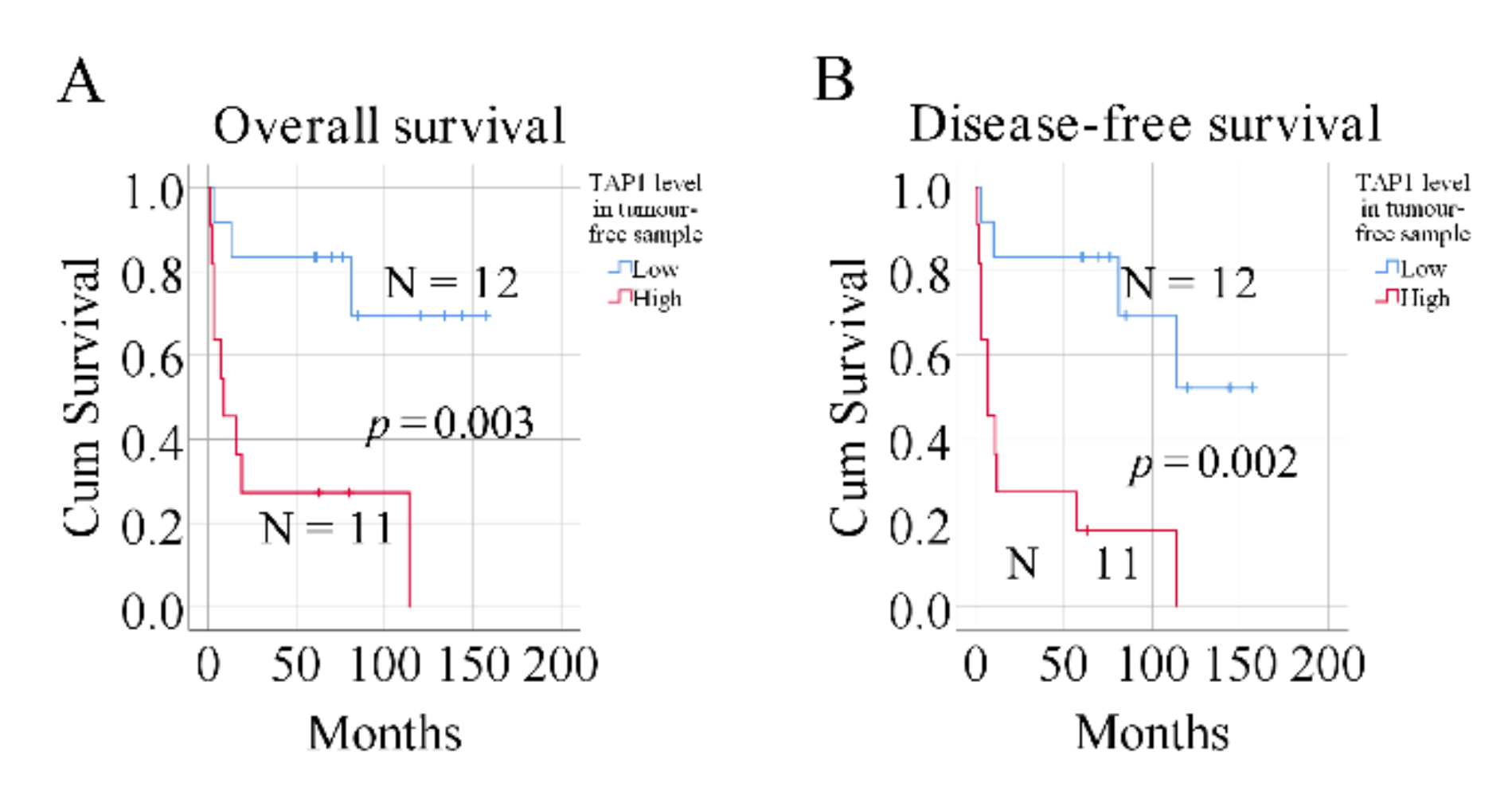
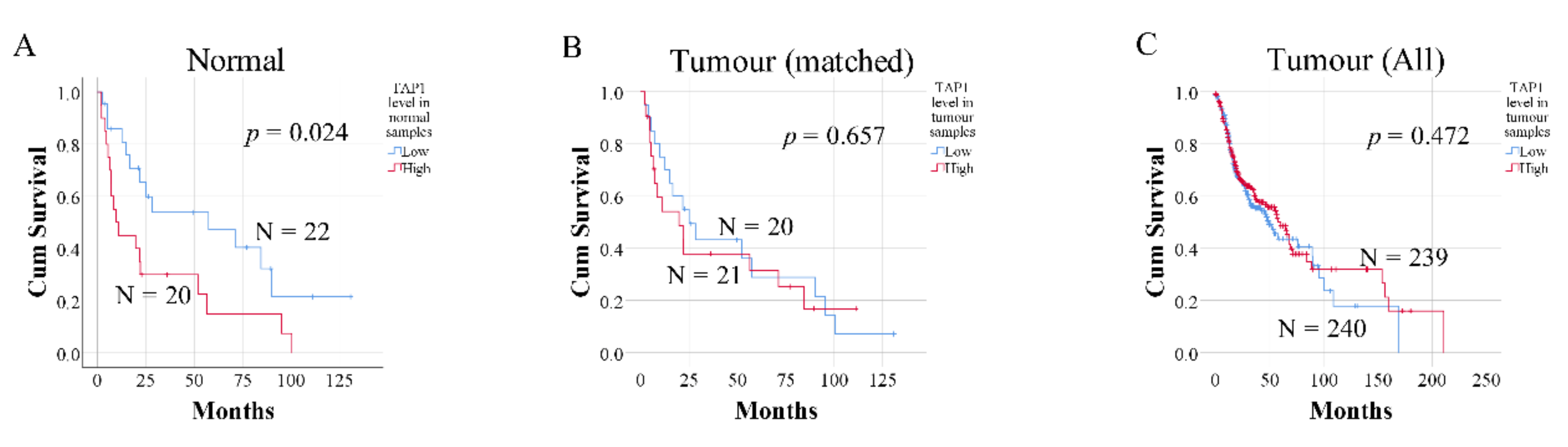
2.2. Expression of TAP1 in Tumor-Free Samples Confers Prognostic Information
3. Discussion
4. Materials and Methods
4.1. Patient Material and Ethical Approval
4.2. RNA Isolation and Gene Expression Profiling
4.3. Prognostic Factor Analysis
4.4. Confirmation of Microarray Data Using RT-qPCR
4.5. The Cancer Genome Atlas (TCGA) Data Collection and Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Sgaramella, N.; Gu, X.; Boldrup, L.; Coates, P.J.; Fåhraeus, R.; Califano, L.; Tartaro, G.; Colella, G.; Spaak, L.N.; Strom, A.; et al. Searching for New Targets and Treatments in the Battle Against Squamous Cell Carcinoma of the Head and Neck, with Specific Focus on Tumours of the Tongue. Curr. Top. Med. Chem. 2018, 18, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Myers, J.N. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007, 26, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar] [PubMed]
- Lochhead, P.; Chan, A.T.; Nishihara, R.; Fuchs, C.S.; Beck, A.H.; Giovannucci, E.; Ogino, S. Etiologic field effect: Reappraisal of the field effect concept in cancer predisposition and progression. Mod. Pathol. 2015, 28, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Boldrup, L.; Coates, P.J.; Fahraeus, R.; Wang, L.; Wilms, T.; Norberg-Spaak, L.; Sgaramella, N.; Nylander, K. High immune cytolytic activity in tumor-free tongue tissue confers better prognosis in patients with squamous cell carcinoma of the oral tongue. J. Pathol. Clin. Res. 2019, 5, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Huang, J. CD8+ T cells and NK cells: Parallel and complementary soldiers of immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Abele, R.; Tampe, R. The ABCs of Immunology: Structure and Function of TAP, the Transporter Associated with Antigen Processing. Physiology 2004, 19, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Seliger, B. The Transporter Associated With Antigen Processing (TAP): Structural Integrity, Expression, Function, and Its Clinical Relevance. Mol. Med. 2001, 7, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Eggensperger, S.; Tampe, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Lankat-Buttgereit, B.; Tampe, R. The Transporter Associated With Antigen Processing: Function and Implications in Human Diseases. Physiol. Rev. 2002, 82, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, N.; Ogino, T.; Katayama, A.; Takahara, M.; Katada, A.; Hayashi, T.; Harabuchi, Y. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol. Rep. 2010, 23, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Boldrup, L.; Gu, X.; Coates, P.J.; Norberg-Spaak, L.; Fåhraeus, R.; Laurell, G.; Wilms, T.; Nylander, K. Gene expression changes in tumor free tongue tissue adjacent to tongue squamous cell carcinoma. Oncotarget 2017, 8, 19389–19402. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Reichert, T.E.; Kunkel, M.; Gooding, W.; Whiteside, T.L.; Ferrone, S.; Seliger, B. Defects in the Human Leukocyte Antigen Class I Antigen Processing Machinery in Head and Neck Squamous Cell Carcinoma: Association with Clinical Outcome. Clin. Cancer Res. 2005, 11, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Pan, H.; Jiang, L.; Wei, K.; Zou, D.; Zhang, Z. A novel approach to rescue immune escape in oral squamous cell carcinoma: Combined use of interferon-γ and LY294002. Oncol. Rep. 2011, 25, 181–187. [Google Scholar] [PubMed]
- Wei, H.; Hongya, P.; Linlin, J.; Mujiang, A.; Kuijie, W.; Duohong, Z.; Qingang, H.; Zhiyuan, Z. IFN-γ enhances the anti-tumour immune response of dendritic cells against oral squamous cell carcinoma. Arch. Oral Biol. 2011, 56, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tsuchikawa, T.; Miyamoto, M.; Maki, T.; Ichinokawa, M.; Kubota, K.C.; Shichinohe, T.; Hirano, S.; Ferrone, S.; Dosaka-Akita, H.; et al. Down-regulation of Human Leukocyte Antigen class I heavy chain in tumors is associated with a poor prognosis in advanced esophageal cancer patients. Int. J. Oncol. 2012, 40, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Henle, A.M.; Nassar, A.; Puglisi-Knutson, D.; Youssef, B.; Knutson, K.L. Downregulation of TAP1 and TAP2 in early stage breast cancer. PLoS ONE 2017, 12, e0187323. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.; Löfgren-Burström, A.; Larsson, P.; Li, X.; Wikberg, M.L.; Öberg, Å.; Stenling, R.; Edin, S.; Palmqvist, R. TAP1 down-regulation elicits immune escape and poor prognosis in colorectal cancer. OncoImmunology 2017, 6, e1356143. [Google Scholar] [CrossRef] [PubMed]
| Sample | Gene | p-Value (Cox-Regression Overall Survival Analysis) | p-value (Cox-Regression Disease-Free Survival Analysis) |
|---|---|---|---|
| Tumor-free | TAP1 | 0.042 | 0.026 |
| TAP2 | 0.828 | 0.987 | |
| Tumor | TAP1 | 0.772 | 0.892 |
| TAP2 | 0.158 | 0.315 |
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| p-value | Hazard Ratio | 95% Confidence Interval | p-Value | Hazard Ratio | 95% Confidence Interval | ||||
| Lower | Upper | Lower | Upper | ||||||
| Overall survival | TAP1 | 0.042 | 1.836 | 1.023 | 3.294 | 0.031 | 12.650 | 1.267 | 126.283 |
| Cytolytic activity | 0.082 | 2.931 | 0.871 | 9.861 | 0.176 | 3.271 | 0.588 | 18.212 | |
| Age | 0.607 | 1.010 | 0.972 | 1.049 | 0.799 | 1.007 | 0.954 | 1.063 | |
| Sex | 0.124 | 2.531 | 0.775 | 8.271 | 0.217 | 2.877 | 0.538 | 15.394 | |
| Stage | 0.013 | 1.952 | 1.149 | 3.318 | 0.064 | 0.046 | 0.002 | 1.201 | |
| T | 0.012 | 1.912 | 1.152 | 3.172 | 0.034 | 33.982 | 1.308 | 882.785 | |
| N | 0.011 | 2.482 | 1.233 | 4.996 | 0.364 | 1.819 | 0.500 | 6.622 | |
| Disease-free survival | TAP1 | 0.029 | 1.844 | 1.066 | 3.189 | 0.005 | 19.886 | 2.480 | 159.446 |
| Cytolytic activity | 0.101 | 2.657 | 0.826 | 8.549 | 0.092 | 4.903 | 0.773 | 31.087 | |
| Age | 0.415 | 1.016 | 0.978 | 1.055 | 0.414 | 1.023 | 0.968 | 1.081 | |
| Sex | 0.099 | 2.509 | 0.841 | 7.486 | 0.236 | 2.399 | 0.565 | 10.188 | |
| Stage | 0.033 | 1.659 | 1.042 | 2.641 | 0.013 | 0.024 | 0.001 | 0.460 | |
| T | 0.028 | 1.674 | 1.057 | 2.650 | 0.010 | 52.561 | 2.581 | 1070.378 | |
| N | 0.016 | 2.285 | 1.163 | 4.490 | 0.176 | 2.373 | 0.679 | 8.285 | |
| TAP1 Level | p-Value | |||
|---|---|---|---|---|
| Low | High | |||
| Age (years) | ≤65 | 7 | 4 | 0.414 |
| >65 | 5 | 7 | ||
| Sex | Female | 4 | 7 | 0.220 |
| Male | 8 | 4 | ||
| Stage | I, II | 9 | 6 | 0.400 |
| III, IV | 3 | 5 | ||
| T | T1, T2 | 10 | 7 | 0.371 |
| T3, T4 | 2 | 4 | ||
| N | N = 0 | 10 | 8 | 0.640 |
| N > 0 | 2 | 3 | ||
| TAP1 Level | p-Value | |||
|---|---|---|---|---|
| Low | High | |||
| Age (years) | ≤65 | 15 | 10 | 0.346 |
| >65 | 7 | 10 | ||
| Sex | Female | 5 | 8 | 0.320 |
| Male | 17 | 12 | ||
| Stage | I, II | 17 | 17 | 0.700 |
| III, IV | 5 | 3 | ||
| T | T1, T2 | 9 | 9 | 1.000 |
| T3, T4 | 13 | 11 | ||
| N | N = 0 | 16 | 15 | 1.000 |
| N > 0 | 5 | 5 | ||
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| p-value | Hazard Ratio | 95% Confidence Interval | p-Value | Hazard Ratio | 95% Confidence Interval | |||
| Lower | Upper | Lower | Upper | |||||
| TAP1 (high vs. low) | 0.028 | 2.253 | 1.093 | 4.644 | 0.011 | 4.405 | 1.401 | 13.847 |
| Cytolytic activity | 0.447 | 1.318 | 0.647 | 2.683 | 0.217 | 0.476 | 0.146 | 1.547 |
| Age | 0.311 | 1.017 | 0.984 | 1.051 | 0.366 | 1.018 | 0.979 | 1.060 |
| Sex | 0.554 | 0.786 | 0.355 | 1.743 | 0.994 | 1.003 | 0.421 | 2.388 |
| Clinical stage | 0.125 | 1.340 | 0.922 | 1.950 | 0.632 | 1.275 | 0.472 | 3.445 |
| T stage | 0.194 | 1.310 | 0.871 | 1.971 | 0.801 | 1.105 | 0.509 | 2.397 |
| N stage | 0.074 | 1.711 | 0.949 | 3.086 | 0.408 | 1.441 | 0.606 | 3.426 |
| No | ID | Sample¤ | Age | Sex | TNM | Stage | Localization # | Status | Follow-up Months | Months to Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | p40 | 1 | 80 | Female | T4N2bM0 | IV | 3 | DWD | 1 | |
| 2 | p42 | 1 | 68 | Female | T2N0M0 | II | 1 | DWD | 9 | 7 |
| 3 | p14 | 2 | 77 | Female | T2N1M0 | III | 2 | DDF | 189 | |
| 4 | p24 | 2 | 64 | Male | T1N0M0 | I | 1 | ADF | 195 | |
| 5 | p29 | 2 | 64 | Female | T2N0M0 | II | 2 | DWD | 29 | 20 |
| 6 | p68 | 2 | 62 | Male | T2N0M0 | II | 1 | DOD | 9 | 6 |
| 7 | p70 | 2 | 71 | Male | T1N0M0 | I | 2 | ADF | 134 | |
| 8 | p82 | 2 | 19 | Female | T4N0M0 | IV | 2 | DOD | 18 | 12 |
| 9 | p83 | 2 | 64 | Female | T1N0M0 | I | 2 | ADF | 119 | |
| 10 | p92 | 2 | 63 | Female | T2N0M0 | II | 2 | DOD | 20 | 6 |
| 11 | p11 | 3 | 78 | Male | T2N0M0 | II | 2 | DWD | 3 | |
| 12 | p35 | 3 | 24 | Female | T2N0M0 | II | 1 | DOD | 13 | 10 |
| 13 | p49 | 3 | 52 | Female | T4N2cM0 | IV | 3 | DWD | 3 | |
| 14 | p51 | 3 | 74 | Male | T2N0M0 | II | 1 | ADF | 157 | |
| 15 | p56 | 3 | 40 | Female | T2N2bM0 | IV | 3 | DOD | 16 | 12 |
| 16 | p58 | 3 | 61 | Male | T1N0M0 | I | 1 | ADF | 144 | |
| 17 | p59 | 3 | 68 | Female | T2N0M0 | II | 1 | DOD | 7 | |
| 18 | p61 | 3 | 69 | Male | T4aN0M0 | IV | 3 | DDF | 81 | |
| 19 | p65 | 3 | 81 | Female | T2N0M0 | II | 3 | ADF | 134 | 114 |
| 20 | p73 | 3 | 80 | Male | T4aN0M0 | IV | 3 | DOD | 19 | 11 |
| 21 | p76 | 3 | 58 | Male | T4aN0M0 | IV | 3 | DDF | 114 | |
| 22 | p79 | 3 | 60 | Male | T1N0M0 | I | 2 | ADF | 120 | |
| 23 | p85 | 3 | 87 | Female | T2N0M0 | II | 1 | DOD | 2 | 2 |
| 24 | p98 | 3 | 31 | Male | T2N0M0 | II | 3 | ADF | 85 | |
| 25 | p105 | 3 | 63 | Male | T1N0M0 | I | 2 | ADF | 80 | 57 |
| 26 | p111 | 3 | 31 | Female | T1N0M0 | I | 2 | ADF | 76 | |
| 27 | p119 | 3 | 66 | Male | T2N0M0 | II | 2 | ADF | 70 | |
| 28 | p124 | 3 | 54 | Male | T4aN2bM0 | IV | 3 | DOD | 3 | |
| 29 | p131 | 3 | 74 | Female | T2N0M0 | II | 2 | ADF | 63 | |
| 30 | p137 | 3 | 71 | Female | T2N0M0 | II | 2 | ADF | 61 | |
| 31 | p138 | 3 | 50 | Male | T2N1M0 | III | 2 | ADF | 60 | |
| 32 | NT1 | 4 | 32 | Female | ||||||
| 33 | NT2 | 4 | 49 | Female | ||||||
| 34 | NT3 | 4 | 25 | Female | ||||||
| 35 | NT4 | 4 | 30 | Male | ||||||
| 36 | NT5 | 4 | 27 | Male | ||||||
| 37 | NT6 | 4 | 42 | Female | ||||||
| 38 | NT7 | 4 | 32 | Female | ||||||
| 39 | NT8 | 4 | 41 | Female | ||||||
| 40 | NT9 | 4 | 35 | Female | ||||||
| 41 | NT10 | 4 | 57 | Male | ||||||
| 42 | NT11 | 4 | 45 | Male | ||||||
| 43 | NT12 | 4 | 37 | Male | ||||||
| 44 | NT13 | 4 | 48 | Female | ||||||
| 45 | NT14 | 4 | 59 | Female |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attaran, N.; Gu, X.; Coates, P.J.; Fåhraeus, R.; Boldrup, L.; Wilms, T.; Wang, L.; Sgaramella, N.; Zborayova, K.; Nylander, K. Downregulation of TAP1 in Tumor-Free Tongue Contralateral to Squamous Cell Carcinoma of the Oral Tongue, an Indicator of Better Survival. Int. J. Mol. Sci. 2020, 21, 6220. https://doi.org/10.3390/ijms21176220
Attaran N, Gu X, Coates PJ, Fåhraeus R, Boldrup L, Wilms T, Wang L, Sgaramella N, Zborayova K, Nylander K. Downregulation of TAP1 in Tumor-Free Tongue Contralateral to Squamous Cell Carcinoma of the Oral Tongue, an Indicator of Better Survival. International Journal of Molecular Sciences. 2020; 21(17):6220. https://doi.org/10.3390/ijms21176220
Chicago/Turabian StyleAttaran, Nima, Xiaolian Gu, Philip J. Coates, Robin Fåhraeus, Linda Boldrup, Torben Wilms, Lixiao Wang, Nicola Sgaramella, Katarina Zborayova, and Karin Nylander. 2020. "Downregulation of TAP1 in Tumor-Free Tongue Contralateral to Squamous Cell Carcinoma of the Oral Tongue, an Indicator of Better Survival" International Journal of Molecular Sciences 21, no. 17: 6220. https://doi.org/10.3390/ijms21176220
APA StyleAttaran, N., Gu, X., Coates, P. J., Fåhraeus, R., Boldrup, L., Wilms, T., Wang, L., Sgaramella, N., Zborayova, K., & Nylander, K. (2020). Downregulation of TAP1 in Tumor-Free Tongue Contralateral to Squamous Cell Carcinoma of the Oral Tongue, an Indicator of Better Survival. International Journal of Molecular Sciences, 21(17), 6220. https://doi.org/10.3390/ijms21176220






