Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis
Abstract
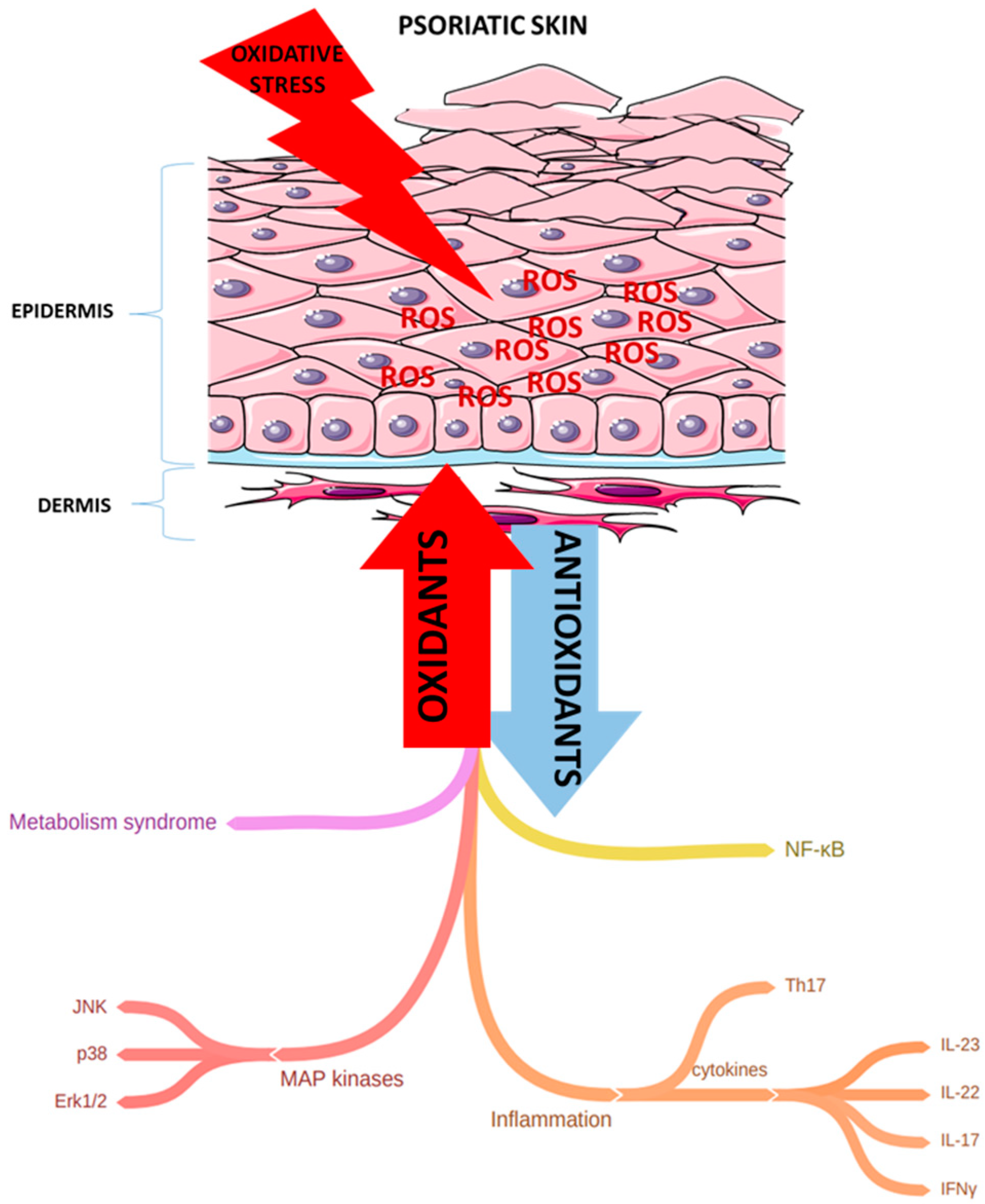
1. Introduction
2. Oxidative Stress and Reactive Oxidative Species in Psoriasis
2.1. Effects of Oxidative Stress and Dyslipidemia on Skin Cells
2.2. Antioxidant Enzymes (SOD, CAT, MDA, and GSH-Px)
3. Perspective Markers of Oxidative Stress in Ps
3.1. Paraoxonase-1
3.2. Thiol/Disulphide
3.3. MAP Kinases
3.4. Tec Kinases
3.5. Sirtuin
4. mTORC1 and Sestrins—Potential Stress Sensors in Ps
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| AP-1 | activator protein 1 |
| ARE | arylesterase |
| ASK1 | apoptosis signal-regulating kinase 1 |
| BTK | Burton’s tyrosine kinase |
| cAMP | cyclic adenosine monophosphate |
| CAT | catalase |
| cGMP | cyclic guanosine monophosphate |
| Cu | copper |
| DC | dendritic cells |
| EGF | epidermal growth factor |
| ERK | extracellular signal-regulated kinase |
| GATOR2 | positive regulator of mTORC1 signaling |
| GSH | glutathione |
| GSH-Px (also GPx) | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| HDL | high-density lipoprotein |
| IFNγ | interferon γ |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LDL | low-density lipoprotein |
| MAPK | mitogen-activated protein kinase |
| MAPKK | mitogen-activated protein kinase kinase |
| MDA | malondialdehyde |
| Mn | manganese |
| MPO | myeloperoxidase |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa-light-chain-enhancer |
| NLRP3 | nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein 3 |
| NO• | nitric oxide radical |
| NO | nitric oxide |
| NOS I | nitric oxide synthase I |
| NOS III | nitric oxide synthase III |
| OS | oxidative stress |
| OSI | oxidative stress index |
| OXPHOS | oxidative phosphorylation |
| PASI | psoriasis area severity index |
| PON | paraoxonase |
| PON1 | paraoxoase-1 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SESNs | sestrins |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| T2DM | type 2 diabetes mellitus |
| TAC | total oxidative capacity |
| Tec | tyrosine-protein kinase |
| TNFα | tumor necrosis factor alpha |
| TOS | total oxidative stress |
| Zn | zinc |
References
- Lin, X.; Huang, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic. Res. 2016, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.O.; Byamba, D.; Wu, W.H.; Kim, T.G.; Akinduro, M.G. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch. Dermatol. Res. 2012, 304, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Shilov, V.N.; Sergienko, V.I. Oxidative stress in keratinocytes as an etiopathogenetic factor of psoriasis. Bull. Exp. Biol. Med. 2000, 129, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Barygina, V.; Becatti, M.; Lotti, T.; Taddei, N.; Fiorillo, C. Low dose cytokines reduce oxidative stress in primary lesional fibroblasts obtained from psoriatic patients. J. Dermatol. Sci. 2016, 83, 242–244. [Google Scholar] [CrossRef]
- Kılıc, S.; Emre, S.; Metin, A.; Isıkoglu, S.; Erel, O. Effect of the systemic use of methotrexate on the oxidative stress and paraoxonase enzyme in psoriasis patients. Arch. Dermatol. Res. 2013, 305, 495–500. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Makavos, G.; Papadavid, E.; Varoudi, M.; Andreadou, I.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Parissis, J.; et al. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: The role of oxidative stress and inflammation. Can. J. Cardiol. 2015, 31, 287–295. [Google Scholar] [CrossRef]
- Priya, R.; Kumar, U.; Saran, A.; Kumari, R.; Kishore, C. Oxidative stress in psoriasis. Biomed. Res. 2014, 25, 132–134. [Google Scholar]
- Khmaladze, I.; Kelkka, T.; Guerard, S.; Wing, K.; Pizzolla, A.; Saxena, A.; Lundqvist, K.; Holmdahl, M.; Nandakumar, K.S.; Holmdahl, R. Mannan induces ROS-regulated, IL-17A–dependent psoriasis arthritis-like disease in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 3669–3678. [Google Scholar] [CrossRef]
- Abdel-Mawla, M.Y.; Nofal, E.; Khalifa, N.; Abdel-Shakoor, R.; Nasr, M. Role of oxidative stress in psoriasis: An evaluation study. J. Am. Sci. 2013, 9, 151–155. [Google Scholar]
- Kanda, N.; Watanabe, S. 17β-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J. Investig. Dermatol. 2003, 121, 1500–1509. [Google Scholar] [CrossRef]
- Lisse, T.S.; King, B.L.; Rieger, S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: Implications toward conservation, migration and wound healing. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barygina, V.; Becatti, M.; Prignano, F.; Lotti, T.; Taddei, N.; Fiorillo, C. Fibroblasts to keratinocytes redox signaling: The possible role of ROS in psoriatic plaque formation. Antioxidants 2019, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.B.; Krueger, G.G.; Griffiths, C.E.M. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.; Mkrtchyan, G.; Grabarska, A.; Oppermann, H.; Daloso, D.; Araujo, W.L.; Juszczak, M.; Rzeski, W.; Bettendorff, L.; Fernie, R.A.; et al. Inhibition of mitochondrial 2-oxoglutarate dehydrogenase impairs viability of cancer cells in a cell-specific metabolism-dependent manner. Oncotarget 2016, 7, 26400–26421. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Oszukowska, M.; Kozłowska, M.; Kaszuba, A. Paraoxonase-1 and other factors related to oxidative stress in psoriasis. Adv. Dermatol. Allergol. 2020, 37, 92–96. [Google Scholar] [CrossRef]
- Candel, S.; de Oliveira, S.; López-Muñoz, A.; García-Moreno, D.; Espín-Palazón, R.; Tyrkalska, S.D.; Cayuela, M.L.; Renshaw, S.A.; Corbalán-Vélez, R.; Vidal-Abarca, I.; et al. Tnfa signaling through tnfr2 protects skin against oxidative stress–induced inflammation. PLoS Biol. 2014, 12, e1001855. [Google Scholar] [CrossRef]
- Shah, A.A.; Sinha, A.A. Oxidative stress and autoimmune skin disease. Eur. J. Dermatol. 2013, 23, 5–13. [Google Scholar] [CrossRef]
- Sander, C.S.; Thiele, J.J. Oxidative stress. In Irritant Dermatitis; Chew, A.L., Maibach, H.I., Eds.; Springer: Heidelberg/Berlin, Germany, 2006; pp. 375–382. [Google Scholar]
- Gupta, M.; Chari, S.; Borkar, M.; Chandankhede, M. Dyslipidemia and oxidative stress in patients of psoriasis. Biomed. Res. 2011, 22, 221–224. [Google Scholar]
- Wagener, F.A.; Carels, C.E.; Lundvig, D. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; Bakheet, S.A.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Cayuela, M.L.; Renshaw, S.A.; Corbalán-Vélez, R.; Vidal-Abarca, I.; et al. Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur. J. Pharmacol. 2020, 877, 173088. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Dellambra, E.; Ciarapica, R.; Capogrossi, M.C. Oxidative stress, microRNAs and cytosolic calcium homeostasis. Cell Calcium 2016, 60, 207–217. [Google Scholar] [CrossRef]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-α-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Investig. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef]
- Aksoy, M.; Kirmit, A. Thiol/disulphide balance in patients with psoriasis. Adv. Dermatol. Allergol. 2020, 37, 52–55. [Google Scholar] [CrossRef]
- Dimon-Gadal, S.; Gerbaud, P.; Guibourdenche, J.; Evain-Brion, D.; Raynaud, F.; Thérond, P.; Anderson, W.B. Increased oxidative damage to fibroblasts in skin with and without lesions in psoriasis. J. Investig. Dermatol. 2000, 114, 984–989. [Google Scholar] [CrossRef]
- Becatti, M.; Barygina, V.; Mannucci, A.; Emmi, G.; Prisco, D.; Lotti, T.; Fiorillo, C.; Taddei, N. Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: A new insight into the pathogenetic mechanisms of psoriasis. Int. J. Mol. Sci. 2018, 19, 1572. [Google Scholar] [CrossRef]
- Simonetti, O.; Ferretti, G.; Salvi, A.; Offidani, A.M.; Bossi, G. Plasma lipid changes in psoriatic children. Dermatology 1992, 185, 96–100. [Google Scholar] [CrossRef]
- Kokcam, I.; Naziroglu, M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin. Chim. Acta 1999, 289, 23–31. [Google Scholar] [CrossRef]
- Drewa, G.; Krzyzynska-Malinowska, E.; Wozniak, A.; Protas-Drozd, F.; Mila-Kierzenkowska, C.; Rozwodowska, M.; Kowaliszyn, B.; Czajkowski, R. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med. Sci. Monit. 2002, 8, 338–343. [Google Scholar]
- Bacchetti, T.; Simonetti, O.; Ricotti, F.; Offidani, A.; Ferretti, G. Plasma oxidation status and antioxidant capacity in psoriatic children. Arch. Dermatol. Res. 2020, 312, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kiluk, P.; Myśliwiec, H.; Flisiak, I. The role of lipids in psoriasis. Dermatol. Rev. 2017, 104, 619–635. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of oxidative stress in various stages of psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, P.; Petzold, L.; Therond, P.; Anderson, W.B.; Evain-Brion, D.; Raynaud, F. Differential regulation of Cu, Zn- and Mn-superoxide dismutases by retinoic acid in normal and psoriatic human fibroblasts. J. Autoimmun. 2005, 24, 69–78. [Google Scholar] [CrossRef]
- Kharaeva, Z.; Gostova, E.; De Luca, C.; Raskovic, D.; Korkina, L. Clinical and biochemical effects of coenzyme Q (10),vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009, 25, 295–302. [Google Scholar] [CrossRef]
- Gabr, S.A.; Al-Ghadir, A.H. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch. Dermatol. Res. 2012, 304, 451–457. [Google Scholar] [CrossRef]
- Therond, P.; Gerbaud, P.; Dimon, S.; Anderson, W.B.; Evain-Broin, D.; Raynaud, F. Antioxidant enzymes in psoriatic fibroblasts and erythrocytes. J. Investig. Dermatol. 1996, 106, 1325–1328. [Google Scholar] [CrossRef]
- Yildirim, M.; Inaloz, H.S.; Baysal, V.; Delibas, N. The role of oxidants and antioxidants in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 34–36. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug. Targets. Inflamm. Allergy. 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Kukreja, S.; Kaur, J.; Bassi, R. Study of oxidative stress in patients of psoriasis. Int. J. Res. Dermatol. 2016, 2, 95–98. [Google Scholar] [CrossRef]
- Holmannova, D.; Borska, L.; Andrys, C.; Borsky, P.; Kremlacek, J.; Hamakova, K.; Rehacek, V.; Malkova, A.; Svadlakova, T.; Palicka, V.; et al. The impact of psoriasis and metabolic syndrome on the systemic inflammation and oxidative damage to nucleic acids. J. Immunol. Res. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary antioxidants and oxidative stress in psoriatic patients: Can salivary total oxidant status and oxidative status index be a plaque psoriasis biomarker? Oxid. Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pujari, V.M.; Suryakar, A.N.; Ireddy, S. Oxidants and antioxidant status in psoriasis patients. Biomed. Res. 2010, 21, 221–224. [Google Scholar]
- Gornicki, A.; Gutsze, A. Erythrocyte membrane fluidity changes in psoriasis: An EPR study. J. Dermatol. Sci. 2001, 27, 27–30. [Google Scholar] [CrossRef]
- Yildirim, M.; Baysal, V.; Inaloz, H.S.; Kesici, D.; Delibas, N. The role of oxidants and antioxidants in generalized vitiligo. J. Dermatol. 2003, 30, 104–108. [Google Scholar] [CrossRef]
- Vanizor, K.B.; Orem, A.; Cimşit, G.; Yandi, Y.E.; Calapoglu, M. Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationships with oxidant–antioxidant system in patients with psoriasis. Clin. Chim. Acta. 2003, 328, 71–82. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef]
- Aktürk, A.Ş.; Özdoğan, H.K.; Bayramgürler, D.; Çekmen, M.B.; Bilen, N.; Kıran, R. Nitric oxide and malondialdehyde levels in plasma and tissue of psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 833–837. [Google Scholar] [CrossRef]
- Demir, H.D.; Aydın, E.; Sezer, E.; Yardım, H. Evaluation of plasma vitamin A and E levels and tear film changes in patients with Psoriasis Vulgaris. Korean J. Ophthalmol. 2013, 27, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Singal, A.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 708–713. [Google Scholar] [PubMed]
- Taha, M.M.; Al-Asady, Z.T.S. Evaluation of the effectiveness of antioxidants and TNF-α in Iraqi patients with psoriasis treated with Etanercept. Res. J. Pharm. Technol. 2019, 12, 665–668. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H. Requirement of aquaporin-3-mediated hydrogen peroxide for NF-κB cell signaling and psoriasis pathogenesis. J. Dermatol. Sci. 2016, 84, e5. [Google Scholar] [CrossRef]
- Usta, M.; Turan, E.; Aral, H.; Inal, B.B.; Gurel, M.S.; Guvenen, G. Serum paraoxonase-1 activities and oxidative status in patients with plaque-type psoriasis with/without metabolic syndrome. J. Clin. Lab. Anal. 2011, 25, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Campanati, A.; Simonetti, O.; Liberati, G.; Offidani, A. Correlation between lipoprotein (a) and lipid peroxidation in psoriasis: Role of the enzyme paraoxonase-1. Br. J. Dermatol. 2012, 166, 204–207. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campanati, A.; Ferretti, G.; Simonetti, O.; Liberati, G.; Offidani, A.M. Oxidative stress and psoriasis: The effect of antitumour necrosis factor-α inhibitor treatment. Br. J. Dermatol. 2013, 168, 984–989. [Google Scholar] [CrossRef]
- Pektas, S.D.; Akoglu, G.; Metin, A.; Neselioglu, S.; Erel, O. Evaluation of systemic oxidant/antioxidant status and paraoxonase 1 enzyme activities in psoriatic patients treated by narrow band ultraviolet B phototherapy. Redox Rep. 2013, 18, 200–204. [Google Scholar] [CrossRef]
- Ramadan, R.; Tawdy, A.; Hay, R.A.; Rashed, L.; Tawfik, D. The antioxidant role of paraoxonase 1 and vitamin E in three autoimmune diseases. Skin Pharmacol. Physiol. 2013, 26, 2–7. [Google Scholar] [CrossRef]
- Schiattarella, M.; Caiazzo, G.; Di Caprio, R.; Lembo, S.; Raimondo, A.; Ayala, F.; Balato, N.; Monfrecola, G.; Fortunato, G.; Balato, A. Paraoxonases and psoriasis: Negative imbalance of anti-oxidant endogenous mechanisms. G. Ital. Dermatol. Venereol. 2017, 154, 192–196. [Google Scholar]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef]
- Demir, Y.; Köksal, Z. The inhibition effects of some sulfonamides on human serum paraoxonase-1 (hPON1). Pharmacol. Rep. 2019, 71, 545–549. [Google Scholar] [CrossRef]
- Feingold, K.R.; Memon, R.A.; Moser, A.H.; Grunfeld, C. Paraoxo-nase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 1998, 139, 307–315. [Google Scholar] [CrossRef]
- Husni, M.E.; Tang, W.H.W.; Lucke, M.; Chandrasekharan, U.M.; Brennan, D.M.; Hazen, S.L. Correlation of high-density lipoprotein–associated Paraoxonase 1 activity with systemic inflammation, disease activity, and cardiovascular risk factors in psoriatic disease. Arthritis Rheum. 2018, 70, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Kadı, M.; Yıldırım, A.K.; Aksoy, H.; Akçay, F. Serum lipid profile paraoxonase and arylesterase activities in psoriasis. Cell Biochem. Funct. 2009, 27, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Houshang, N.; Reza, K.; Masoud, S.; Ali, E.; Mansour, R.; Vaisi-Raygani, A. Antioxidant status in patients with psoriasis. Cell Biochem. Funct. 2014, 32, 268–273. [Google Scholar] [CrossRef]
- Asefi, M.; Vaisi-Raygani, A.; Bahrehmand, F.; Kiani, A.; Rahimi, Z.; Nomani, H.; Ebrahimi, A.; Tavilani, H.; Pourmotabbed, T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br. J. Dermatol. 2012, 167, 1279–1286. [Google Scholar] [CrossRef]
- Keihan, G.S.; Gharib, M.H.; Momeni, A.; Hemati, Z.; Sedighin, R. A comparison between the effect of cuminum cyminum and vitamin E on the level of leptin, paraoxonase 1, Hba1c and oxidized LDL in diabetic patients. Int. J. Mol. Cell. Med. 2016, 5, 229–235. [Google Scholar]
- Dilek, N.; Dilek, A.R.; Taskin, Y.; Erkinuresin, T.; Yalcin, O.; Saral, Y. Contribution of myeloperoxidase and inducible nitric oxide synthase to pathogenesis of psoriasis. Adv. Dermatol. Allergol. 2006, 33, 435–439. [Google Scholar] [CrossRef]
- Pektas, S.D.; Pektas, G.; Tosun, K.; Dogan, G.; Neselioglu, S.; Erel, O. Evaluation of erythroid disturbance and thiol-disulphide homeostasis in patients with psoriasis. Biomed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Bal, C.; Buyuksekerci, M.; Koca, C.; Ağış, E.R.; Erdoğan, S.; Baran, P.; Gündüzöz, M.; Yilmaz, Ö. The compromise of dynamic disulphide/thiol homeostasis as a biomarker of oxidative stres in trichloroethylene exposure. Hum. Exp. Toxicol. 2016, 35, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Ates, I.; Ozkayar, N.; Inan, B.; Yilmaz, F.M.; Topcuoglu, C.; Neselioglu, S.; Erel, O.; Dede, F.; Yilmaz, N. Dinamic thiol/disulphide homeostasis in patients with newly diagnosed primary hypertension. J. Am. Soc. Hypertens. 2016, 10, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ozyazıcı, S.; Karateke, F.; Turan, U.; Kuvvetli, A.; Kilavuz, H.; Karakaya, B.; Ozaltun, P.; Alışık, M.; Erel, O. A novel oxidative stress mediator in acute appendicitis: Thiol/disulphide homeostasis. Mediat. Inflamm. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.Z.; Zawilska, J.B. Receptory i Mechanizmy Przekazywania Sygnału; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2004; pp. 382–414. [Google Scholar]
- Klein, A. Molekularne Podstawy Regulacji Hormonalnej: Sygnalizacja Międzykomórkowa i Wewnatrzkomórkowa; Wydział Biotechnologii UJ: Cracow, Poland, 2002; pp. 103–109. [Google Scholar]
- Takahashi, H.; Ibe, M.; Nakamura, S.; Ishida-Yamamoto, A.; Hashimoto, Y.; Iizuka, H. Extracellular regulated kinase and c-Jun N-terminal kinase are activated in psoriatic involved epidermis. J. Dermatol. Sci. 2002, 30, 94–99. [Google Scholar] [CrossRef]
- Zhou, Q.; Mrowietz, U.; Rostami-Yazdi, M. Oxidative stress in the pathogenesis of psoriasis. Free Radic. Biol. Med. 2009, 47, 891–905. [Google Scholar] [CrossRef]
- Kozina, L.S.; Borzova, I.V.; Arutiunov, V.A.; Ryzhak, G.A. Role of oxidative stress in skin aging. Adv. Gerontol. 2013, 3, 18–22. [Google Scholar] [CrossRef]
- Gerald, D.; Berra, E.; Frapart, Y.M.; Chan, D.A.; Giaccia, A.J.; Mansuy, D.; Pouysségur, J.; Yaniv, M.; Mechta-Grigoriou, F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 2004, 118, 781–794. [Google Scholar] [CrossRef]
- Zenz, R.; Wagner, E.F. Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors. Int. J. Biochem. Cell Biol. 2006, 38, 1043–1049. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Shin, I.; Kim, S.; Song, H.; Kim, H.R.; Moon, A. H-Ras-specific activation of Rac- MKK3/6-p38 pathway: Its critical role in invasion and migration of breast epithelial cells. J. Biol. Chem. 2005, 280, 14675–14683. [Google Scholar] [CrossRef]
- Matsukawa, J.; Matsuzawa, A.; Takeda, K.; Ichijo, H. The ASK1–MAP kinase cascades in mammalian stress response. J. Biochem. 2004, 136, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Schonthaler, H.B.; Guinea-Viniegra, J.; Tschachler, E. Psoriasis: What we have learned from mouse models. Nat. Rev. Rheumatol. 2010, 6, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kusuba, N.; Kitoh, A.; Miyachi, Y.; Kabashima, K. Role of neutrophils in the pathogenesis of imiquimod-induced psoriasis-like skin lesions. J. Dermatol. Sci. 2016, 84, e73. [Google Scholar] [CrossRef]
- Papagrigoraki, A.; Maurelli, M.; Del Giglio, M.; Gisondi, P.; Girolomoni, G. Advanced glycation end products in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2017, 18, 2471. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Becatti, M.; Barygina, V.; Emmi, G.; Silvestri, E.; Taddei, N.; Lotti, T.; Fiorillo, C. SIRT1 activity is decreased in lesional psoriatic skin. Intern. Emerg. Med. 2016, 11, 891–893. [Google Scholar] [CrossRef]
- Rasheed, H.; El-Komy, M.H.M.; Hegazy, R.A.; Gawdat, H.I.; AlOrbani, A.M.; Shaker, O.G. Expression of sirtuins 1, 6, tumor necrosis factor, and interferon-γ in psoriatic patients. Int. J. Immunopathol. Pharmacol. 2016, 29, 764–768. [Google Scholar] [CrossRef]
- Balatol, A.; Caprio, R.D.I.; Lembol, S.; Mattii, M.; Megna, M.; Schiattarella, M.; Tarantino, G.; Balato, N.; Ayala, F.; Monfrecola, G. Mammalian target of rapamycin in inflammatory skin conditions. Eur. J. Inflamm. 2014, 12, 341–350. [Google Scholar] [CrossRef]
- Buerger, C.; Malisiewicz, B.; Eiser, A.; Hardt, K.; Boehncke, W.H. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 2013, 169, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C.; Shirsath, N.; Lang, V.; Berard, A.; Diehl, S.; Kaufmann, R.; Boehncke, W.-H.; Wolf, P. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS ONE 2017, 12, e0180853. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C. Epidermal mTORC1 signaling contributes to the pathogenesis of psoriasis and could serve as a therapeutic target. Front. Immunol. 2018, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F.; Pavlis, M. Dysregulation of the mTOR pathway secondary to mutations or a hostile microenvironment contributes to cancer and poor wound healing. J. Investig. Dermatol. 2009, 129, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Yin, N.; Li, M.O. Sestrins function as guanine nucleotide dissociation inhibitors for rag GTPases to control mTORC1 signaling. Cell 2014, 159, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The sestrins interact with gator2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Budanov, A.V.; Karin, M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013, 18, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, K.M.; Kim, M.G.; Seo, K.H.; Han, J.Y.; Ka, S.O.; Park, B.; Shin, S.M.; Ku, S.K.; Cho, I.J.; et al. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic. Biol. Med. 2015, 78, 156–167. [Google Scholar] [CrossRef]
- Zhao, B.; Shah, P.; Budanov, A.V.; Qiang, L.; Ming, M.; Aplin, A.; Sims, D.M.; He, Y.-Y. Sestrin2 protein positively regulates AKT enzyme signaling and survival in human squamous cell carcinoma and melanoma cells. J. Biol. Chem. 2014, 289, 35806–35814. [Google Scholar] [CrossRef]
- Mlitz, V.; Gendronneau, G.; Berlin, I.; Buchberger, M.; Eckhart, L.; Tschachler, E. The expression of the endogenous mtorc1 inhibitor sestrin 2 is induced by UVB and balanced with the expression level of sestrin 1. PLoS ONE 2016, 11, e0166832. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C.; Lejeune, F.; Sok, J.; Thomas, M.; Bernerd, F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS ONE 2010, 5, e12059. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Maggs, D.G.; Spollett, G.R.; Page, S.L.; Rife, F.S.; Walton, V.; Shulman, G.I. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N. Engl. J. Med. 1998, 338, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Tzatsos, A.; Kandror, K.V. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol. 2006, 26, 63–76. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, T.; Gao, J.; Liu, X.; Hou, H.; Cao, R.; Li, B.; Quan, M.; Guo, L. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J. Neuroimmunol. 2016, 292, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tomczynska, M.; Bijak, M.; Saluk, J. Metformin-the drug for the treatment of autoimmune diseases; a new use of a known anti-diabetic drug. Curr. Top. Med. Chem. 2016, 16, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Yen, V.H.; Takemura, M.; Yumine, A.; Furue, K.; Nakahara, T. Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: Implications for preventing the development of psoriasis. Cell Death Discov. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ba, W.; Xu, Y.; Yin, G.; Yang, J.; Wang, R.; Chi, S.; Li, C. Metformin inhibits pro-inflammatory responses via targeting nuclear factor-κB in HaCaT cells. Cell Biochem. Funct. 2019, 37, 4–10. [Google Scholar] [CrossRef]
- Ogawa, E.; Sato, Y.; Minagawa, A.; Okuyama, R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: A literature review. Int. J. Mol. Sci. 2020, 21, 1690. [Google Scholar] [CrossRef]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol. 2019, 80, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Christensen, R.; Zachariae, C.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: A prospective observational follow-up study. Am. J. Clin. Nutr. 2016, 104, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed]
| Lp. | Markers of Oxidative Stress | Fibroblasts | Keratinocytes | Serum | Plasma | Erythrocytes | Saliva | Author | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ENZYMATIC | SOD | ↑ | ↓ | ↑/↓ | ↓ | ↓ | ↑ | Therond et al., 1996 [40]; Dimon-Gadal et al., 2000 [28]; Gornicki and Gutsze, 2001 [47]; Yildirim et al., 2003 [48]; Vanizor et al., 2003 [49]; Gerbaud et al., 2005 [37]; Kaharaeva et al., 2009 [38]; Pujari et al., 2010 [46]; Gabr and Al-Ghadir, 2012 [39]; Wagener et al., 2013 [22] |
| 2 | CAT | ↑ | ↑ | ↑/↓ | ↓ | ↑/↓ | ↑ | Thérond et al., 1996 [40]; Gornicki and Gutsze, 2001 [47]; Yildirim et al., 2003 [48]; Vanizor et al., 2003 [49]; Pujari et al., 2010 [46]; Skutnik-Radziszewska et al., 2020 [45]; Jarocka-Karpowicz et al., 2020 [50] | |
| 3 | GSH-Px | ↑ | ↓ | n. d. | ↑ | ↑/↓ | ↑ | Thérond et al., 1996 [40]; Pujari et al., 2010 [46]; Kaur et al., 2016 [43]; Holmannova et al., 2020 [44]; Skutnik-Radziszewska et al., 2020 [45]; Jarocka-Karpowicz et al., 2020 [50] | |
| 4 | MDA | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | Gornicki and Gutsze, 2001 [47]; Yildirim et al., 2003 [48]; Vanizor et al., 2003 [49]; Pujari et al., 2010 [46]; Gabr and Al-Ghadir, 2012 [39]; Şikar Aktürk et al., 2012 [51]; Skutnik-Radziszewska et al., 2020 [45] | |
| 5 | NON-ENZYMATIC | Vitamin E | n.d. | n.d. | ↓ | ↓ | n.d. | n.d. | Pujari et al., 2010 [46]; Demir et al., 2013 [52]; Skutnik-Radziszewska et al., 2020 [45]; Oszukowska et al., 2020 [17] |
| 6 | GSH | ↓ | ↓ | ↓ | ↓ | n.d. | ↓ | Thérond et al., 1996 [40]; Asha et al., 2017 [53]; Taha and Al-Asady, 2019 [54]; Skutnik-Radziszewska et al., 2020 [45]; Jarocka-Karpowicz et al., 2020 [50] | |
| 7 | ROS/RNS | H2O2 | ↑ | ↑ | n.d. | n.d. | n.d. | n.d. | Dimon-Gadal et al., 2000 [28]; Hara-Chikuma and Satooka, 2016 [55]; Barygina et al., 2019 [12] |
| 8 | O2•− | ↑ | ↑ | n.d. | n.d. | n.d. | n.d. | Dimon-Gadal et al., 2000 [28]; Gabr and Al-Ghadir, 2012 [39] | |
| 9 | NO• | ↑ | ↑ | ↑ | n.d. | n.d. | n.d. | Vanizor et al., 2003 [49]; Kadam et al., 2010 [36]; Barygina et al., 2019 [12] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. https://doi.org/10.3390/ijms21176206
Pleńkowska J, Gabig-Cimińska M, Mozolewski P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. International Journal of Molecular Sciences. 2020; 21(17):6206. https://doi.org/10.3390/ijms21176206
Chicago/Turabian StylePleńkowska, Joanna, Magdalena Gabig-Cimińska, and Paweł Mozolewski. 2020. "Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis" International Journal of Molecular Sciences 21, no. 17: 6206. https://doi.org/10.3390/ijms21176206
APA StylePleńkowska, J., Gabig-Cimińska, M., & Mozolewski, P. (2020). Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. International Journal of Molecular Sciences, 21(17), 6206. https://doi.org/10.3390/ijms21176206






