Close Temporal Relationship between Oscillating Cytosolic K+ and Growth in Root Hairs of Arabidopsis
Abstract
1. Introduction
2. Results
2.1. Application of NK3 in Monitoring K+ Levels in Arabidopsis Root Hairs
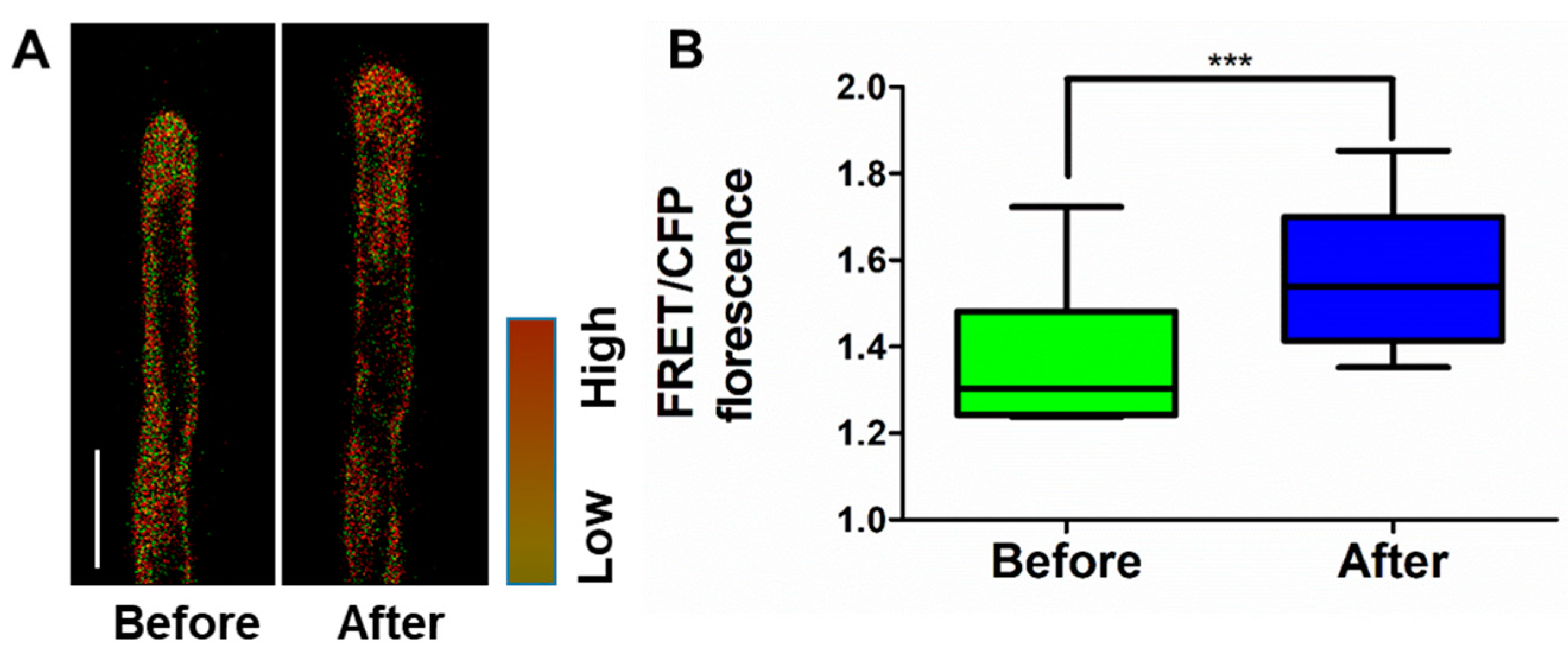
2.2. Relationship between Cytoplasmic K+ Level and Root Hair Growth
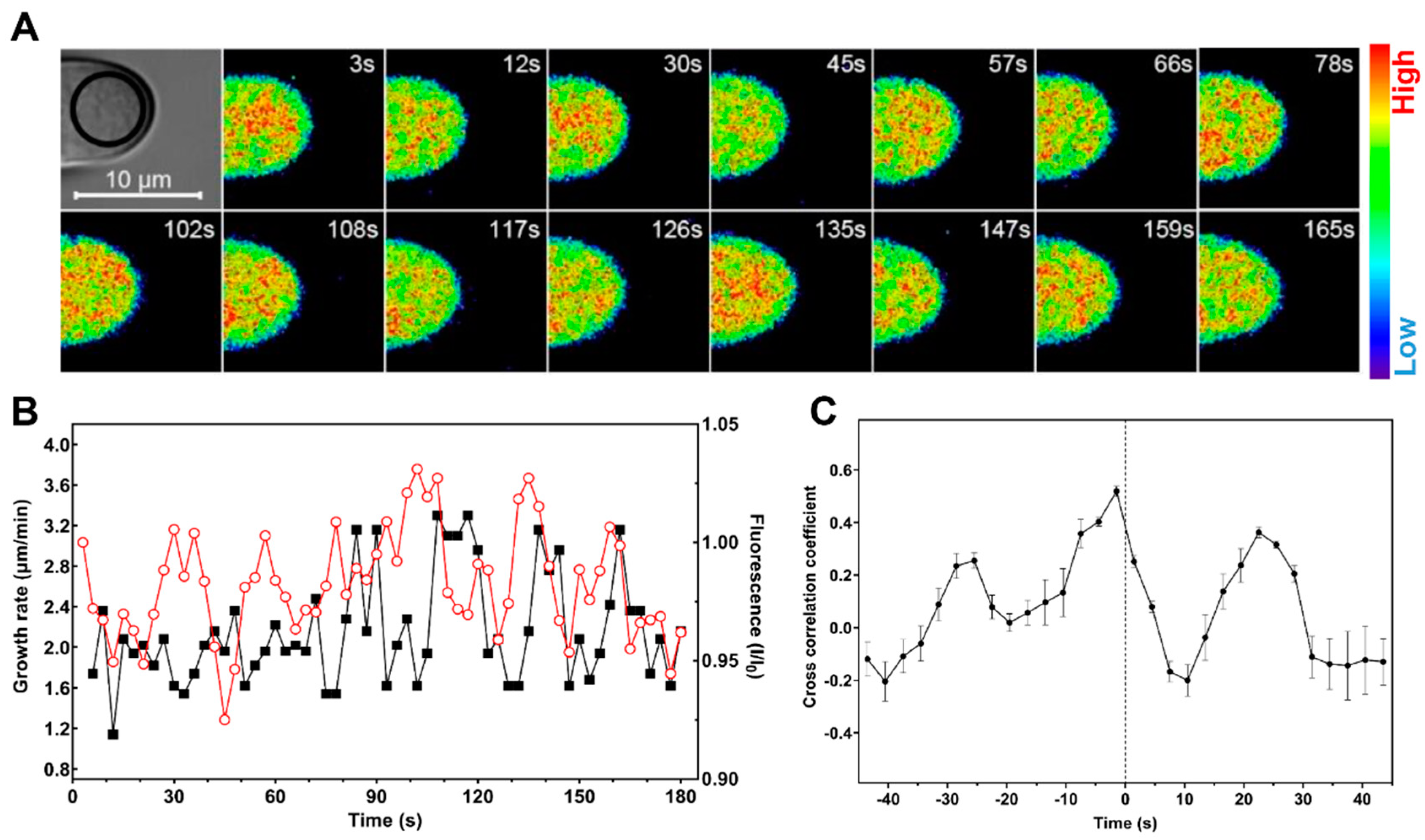
2.3. Cytosolic Level of K+ Oscillates during Root Hair Rapid Elongation
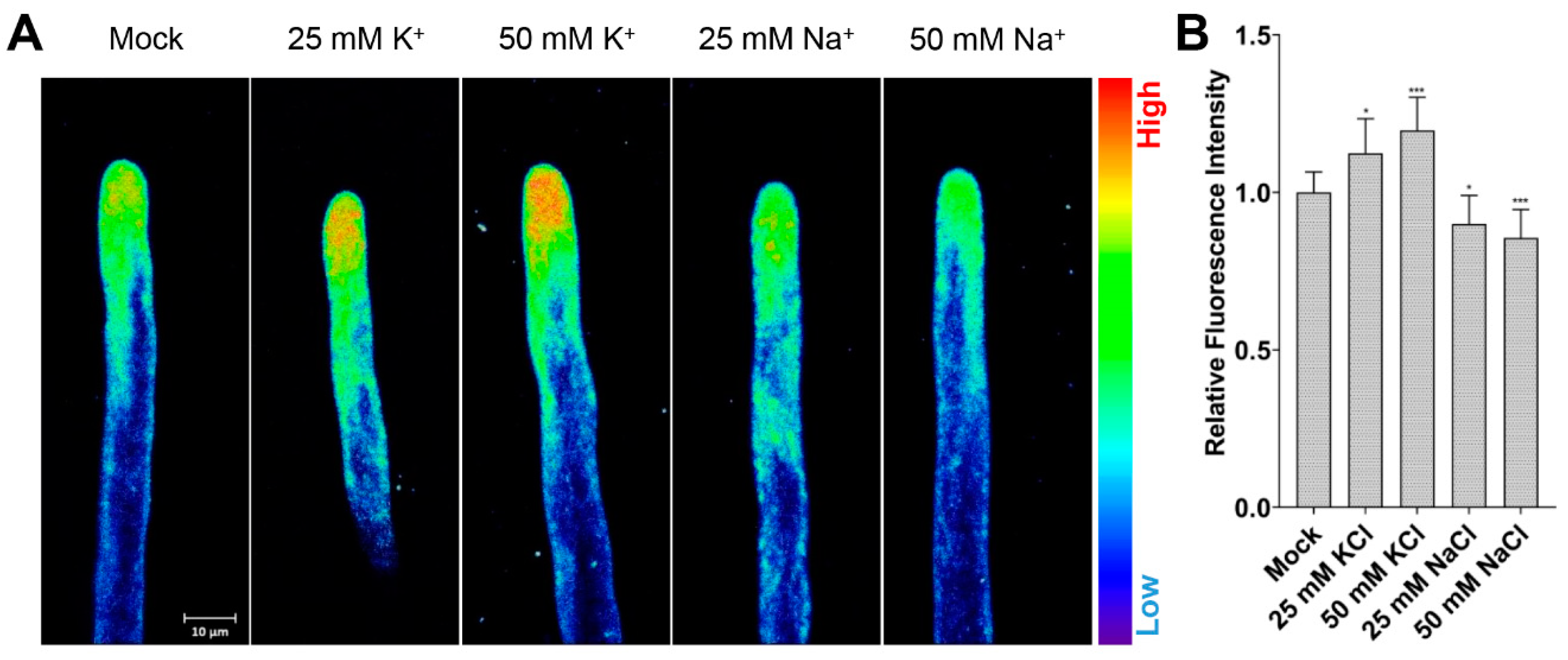
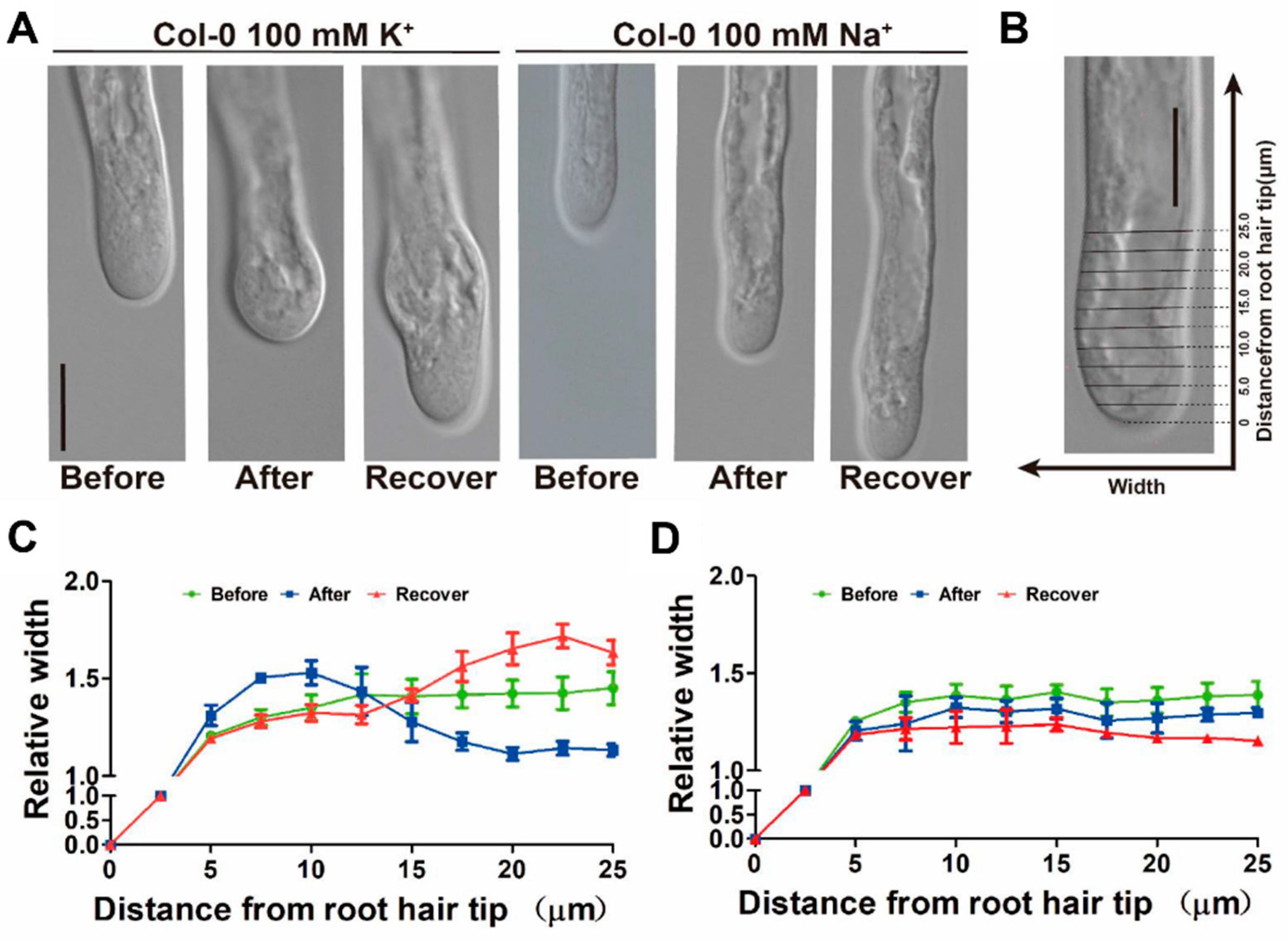
2.4. Manipulating Cytosolic K+ Level Affects Root Hair Growth
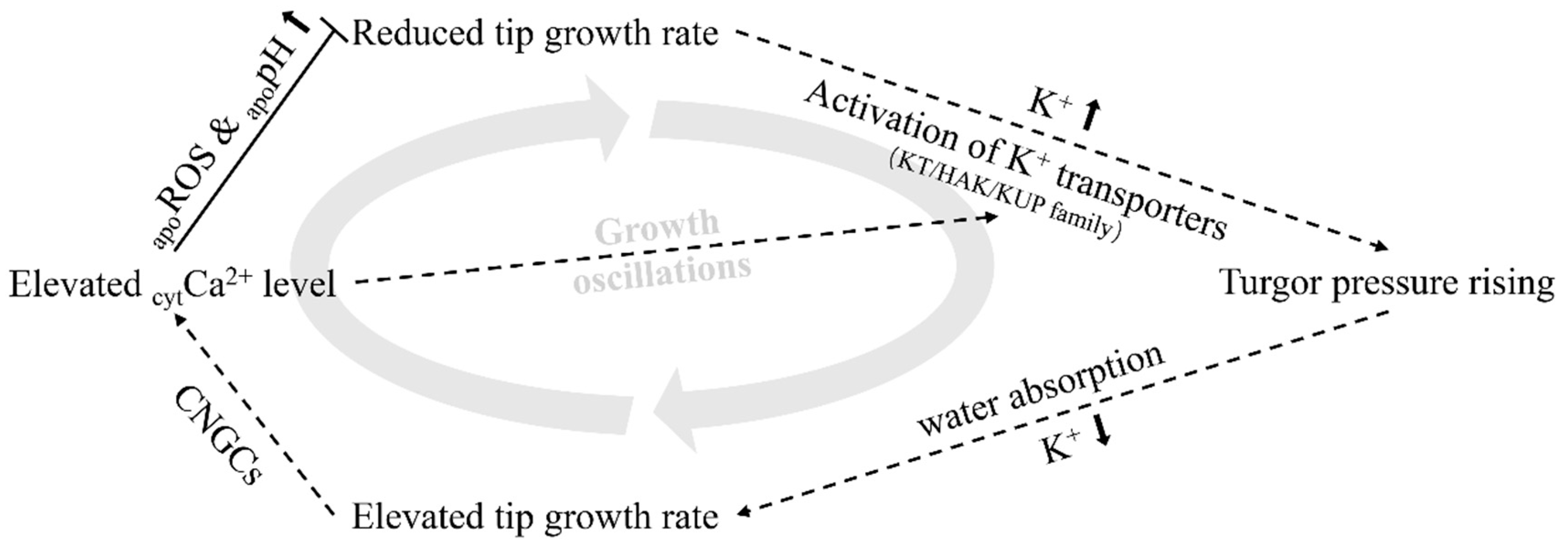
3. Discussion
3.1. Biological Significance of the Application of Fluorescent K+ Sensor in Arabidopsis
3.2. K+ Dynamics and K+ Transporters/Channels May Play Vital Roles in Root Hair Tip Growth
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Sensor Solution
4.3. Confocal Microscopy
4.4. Measurement of Root Hair Growth
4.5. Root Elongation Kinetic Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| K | Potassium |
| Ca | Calcium |
| ROS | Reactive oxygen species |
| TEA | Tetraethylammonium |
| TRH1 | Tiny root hair |
| KUP4 | K+ uptake 4 |
| AtKC1 | Arabidopsis thaliana K+ channel 1 |
| AKT1 | Arabidopsis K+ transporter 1 |
| HAK5 | High affinity K+ transporter 5 |
| YC3.6 | Yellow cameleon 3.6 |
| CNGCs | Cyclic nucleotide-gated channels |
| CBL | Calcineurin B-like protein |
| CIPKs | CBL-interacting protein kinases |
References
- Gilroy, S.; Jones, D.L. Through form to function: Root hair development and nutrient uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root hairs. Arab. Book 2014, 12, e0172. [Google Scholar] [CrossRef] [PubMed]
- Mendrinna, A.; Persson, S. Root hair growth: It’s a one way street. F1000prime Rep. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kurata, T.; Okada, K.; Wada, T. A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 2008, 59, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Bibikova, T.; Gilroy, S. Calcium in root hair growth. In Root Hairs; Emons, A.M.C., Ketelaar, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 145–170. [Google Scholar]
- Libault, M.; Brechenmacher, L.; Cheng, J.; Xu, D.; Stacey, G. Root hair systems biology. Trends Plant Sci. 2010, 15, 641–650. [Google Scholar] [CrossRef]
- Bibikova, T.N.; Zhigilei, A.; Gilroy, S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 1997, 203, 495–505. [Google Scholar] [CrossRef]
- Wymer, C.L.; Bibikova, T.N.; Gilroy, S. Cytoplasmic free calcium distributions during the development of root hairs of Arab. Thaliana Plant J. 1997, 12, 427–439. [Google Scholar] [CrossRef]
- Bibikova, T.N.; Blancaflor, E.B.; Gilroy, S. Microtubules regulate tip growth and orientation in root hairs of Arab. Thaliana. Plant J. 1999, 17, 657–665. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Messerli, M.A.; Shi, C.; Gilroy, S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root haris. Proc. Natl. Acad. Sci. USA 2007, 104, 20996–21001. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Messerli, M.A.; Gilroy, S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008, 147, 1690–1698. [Google Scholar] [CrossRef]
- Lew, R.R. Electrogenic transport properties of growing Arabidopsis root hairs. Plant Physiol. 1991, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rigas, S.; Debrosses, G.; Haralampidis, K.; Vicente-Agullo, F.; Feldmann, K.A.; Grabov, A.; Dolan, L.; Hatzopoulos, P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 2001, 13, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Gaymard, F.; Cerutti, M.; Horeau, C.; Lemaillet, G.; Urbach, S.; Ravallec, M.; Devauchelle, G.; Sentenac, H.; Thibaud, J.B. The baculovirus/insect cell system as an alternative to Xenopus oocytes - First characterization of the AKT1 K+ channel from Arab. Thaliana. J. Biol. Chem. 1996, 271, 22863–22870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Desbrosses, G.; Josefsson, C.; Rigas, S.; Hatzopoulos, P.; Dolan, L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J. Exp. Bot. 2003, 54, 781–788. [Google Scholar] [CrossRef]
- Liu, H.; Ning, J.; Song, G.; Sun, X.; Su, F.; Li, P.; Tian, Y. Tricolor dual sensor for ratiometrically analyzing potassium ions and dissolved oxygen. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118155. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Feng, S.; Wang, Z.; Zhang, J.; Zhang, J.; Wang, D.; Gan, Y. AtHKT1 gene regulating K+ state in whole plant improves salt tolerance in transgenic tobacco plants. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Jeanguenin, L.; Alcon, C.; Duby, G.; Boeglin, M.; Cherel, I.; Gaillard, I.; Zimmermann, S.; Sentenac, H.; Very, A.-A. AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J. 2011, 67, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, Y.; Fu, Y.; Guo, H. Ethylene-induced microtubule reorientation is essential for fast inhibition of root elongation in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pan, Y.; Tian, W.; Dong, M.; Zhu, H.; Luan, S.; Li, L. Arabidopsis CNGC14 Mediates Calcium Influx Required for Tip Growth in Root Hairs. Mol. Plant 2017, 10, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Kim, C.M.; Pernas, M.; Pires, N.D.; Proust, H.; Tam, T.; Vijayakumar, P.; Dolan, L. Root hairs: Development, growth and evolution at the plant-soil interface. Plant Soil 2011, 346, 1–14. [Google Scholar] [CrossRef]
- Xu, H.; Martinoia, E.; Szabo, I. Organellar channels and transporters. Cell Calcium 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef]
- Johnson, I. Review: Fluorescent probes for living cells. Histochem. J. 1998, 30, 123–140. [Google Scholar] [CrossRef]
- Minta, A.; Tsien, R.Y. Fluorescent indicators for cytosolic sodium. J. Biol. Chem. 1989, 264, 19449–19457. [Google Scholar]
- Rimmele, T.S.; Chatton, J.-Y. A novel optical intracellular imaging approach for potassium dynamics in astrocytes. PLoS ONE 2014, 9, e109243. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Boens, N.; Meuwis, K.; Ameloot, M. Potential misevaluation of the ground-state dissociation constant from fluorimetric titrations: Application to the ion indicators SBFI, PBFI, and Fura-2. Anal. Biochem. 1997, 245, 28–37. [Google Scholar] [CrossRef]
- Depauw, A.; Dossi, E.; Kumar, N.; Fiorini-Debuisschert, C.; Huberfeld, G.; Ha-Thi, M.-H.; Rouach, N.; Leray, I. A highly selective potassium sensor for the detection of potassium in living tissues. Chem. A Eur. J. 2016, 22, 14902–14911. [Google Scholar] [CrossRef]
- Namkung, W.; Padmawar, P.; Mills, A.D.; Verkman, A.S. Cell-based fluorescence screen for K+ channels and transporters using an extracellular triazacryptand-based K+ sensor. J. Am. Chem. Soc. 2008, 130, 7794–7795. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S. In-situ determination of intracellular concentrations of K+ in barley (Hordeum vulgare L. cv. Kara) using the K+-binding fluorescent dye benzofuran isophthalate. Planta 1995, 195, 525–529. [Google Scholar] [CrossRef]
- Halperin, S.J.; Lynch, J.P. Effects of salinity on cytosolic Na+ and K+ in root hairs of Arabidopsis thaliana: in vivo measurements using the fluorescent dyes SBFI and PBFI. J. Exp. Bot. 2003, 54, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Zhou, X.; Su, F.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A new highly selective fluorescent K+ sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef]
- Kong, X.; Su, F.; Zhang, L.; Yaron, J.; Lee, F.; Shi, Z.; Tian, Y.; Meldrum, D.R. A highly selective mitochondria-targeting fluorescent K+ sensor. Angew. Chem. Int. Ed. 2015, 54, 12053–12057. [Google Scholar] [CrossRef]
- Yaron, J.R.; Gangaraju, S.; Rao, M.Y.; Kong, X.; Zhang, L.; Su, F.; Tian, Y.; Glenn, H.L.; Meldrum, D.R. K+ regulates Ca2+ to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death Dis. 2015, 6, e1954. [Google Scholar] [CrossRef]
- Ning, J.; Tian, Y. Development of a new simple mitochondria-targeted fluorescent K+ sensor and the application in high-throughput monitoring K+ fluxes. Sens. Actuators B Chem. 2020, 307, 127659. [Google Scholar] [CrossRef]
- Song, G.; Jiang, D.; Wang, L.; Ning, J.; Sun, X.; Su, F.; Chen, M.; Tian, Y. A mitochondria-targeting NIR fluorescent potassium ion sensor: Real-time investigation of the mitochondrial K+ regulation of apoptosis in situ. Chem. Commun. 2020, 56, 5405–5408. [Google Scholar] [CrossRef]
- Mangano, S.; Denita Juarez, S.P.; Estevez, J.M. ROS regulation of polar growth in plant cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef]
- Peremyslov, V.V.; Prokhnevsky, A.I.; Avisar, D.; Dolja, V.V. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 2008, 146, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Braidwood, L.; Breuer, C.; Sugimoto, K. My body is a cage: Mechanisms and modulation of plant cell growth. New Phytol. 2014, 201, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Hoefte, H. Growth control: A saga of cell walls, ROS, and peptide receptors. Plant Cell 2014, 26, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, L.; Zhu, B.; Jiang, Z. Plant calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2018, 19, 3456. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.-D.; Chen, L.-Q.; Wang, Y.; Liu, L.-L.; He, L.; Wu, W.-H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Brost, C.; Studtrucker, T.; Reimann, R.; Denninger, P.; Czekalla, J.; Krebs, M.; Fabry, B.; Schumacher, K.; Grossmann, G.; Dietrich, P. Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 2019, 99, 910–923. [Google Scholar] [CrossRef]
- Zeb, Q.; Wang, X.; Hou, C.; Zhang, X.; Dong, M.; Zhang, S.; Zhang, Q.; Ren, Z.; Tian, W.; Zhu, H.; et al. The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 887–896. [Google Scholar] [CrossRef]
- Era, A.; Tominaga, M.; Ebine, K.; Awai, C.; Saito, C.; Ishizaki, K.; Yamato, K.T.; Kohchi, T.; Nakano, A.; Ueda, T. Application of Lifeact Reveals F-Actin Dynamics in Arabidopsis thaliana and the Liverwort, Marchantia polymorpha. Plant Cell Physiol. 2009, 50, 1041–1048. [Google Scholar] [CrossRef]
- Tenorio-Berrio, R.; Perez-Alonso, M.-M.; Vicente-Carbajosa, J.; Martin-Torres, L.; Dreyer, I.; Pollmann, S. Identification of two Auxin-regulated potassium transporters involved in seed maturation. Int. J. Mol. Sci. 2018, 19, 2132. [Google Scholar] [CrossRef] [PubMed]
- Reintanz, B.; Szyroki, A.; Ivashikina, N.; Ache, P.; Godde, M.; Becker, D.; Palme, K.; Hedrich, R. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA 2002, 99, 4079–4084. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Aleman, F.; Martinez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Qiu, Y.; Peng, Y.; Ning, J.; Song, G.; Yang, Y.; Deng, M.; Men, Y.; Zhao, X.; Wang, Y.; et al. Close Temporal Relationship between Oscillating Cytosolic K+ and Growth in Root Hairs of Arabidopsis. Int. J. Mol. Sci. 2020, 21, 6184. https://doi.org/10.3390/ijms21176184
Sun X, Qiu Y, Peng Y, Ning J, Song G, Yang Y, Deng M, Men Y, Zhao X, Wang Y, et al. Close Temporal Relationship between Oscillating Cytosolic K+ and Growth in Root Hairs of Arabidopsis. International Journal of Molecular Sciences. 2020; 21(17):6184. https://doi.org/10.3390/ijms21176184
Chicago/Turabian StyleSun, Xiangzhong, Yuping Qiu, Yang Peng, Juewei Ning, Guangjie Song, Yanzhu Yang, Mengyu Deng, Yongfan Men, Xingzhong Zhao, Yichuan Wang, and et al. 2020. "Close Temporal Relationship between Oscillating Cytosolic K+ and Growth in Root Hairs of Arabidopsis" International Journal of Molecular Sciences 21, no. 17: 6184. https://doi.org/10.3390/ijms21176184
APA StyleSun, X., Qiu, Y., Peng, Y., Ning, J., Song, G., Yang, Y., Deng, M., Men, Y., Zhao, X., Wang, Y., Guo, H., & Tian, Y. (2020). Close Temporal Relationship between Oscillating Cytosolic K+ and Growth in Root Hairs of Arabidopsis. International Journal of Molecular Sciences, 21(17), 6184. https://doi.org/10.3390/ijms21176184




