Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of RA Patients
2.2. Comparison of Lipid Profiles, QRISK-2 Scores, and CVD Events between RA Patients with High L5% and Normal L5%
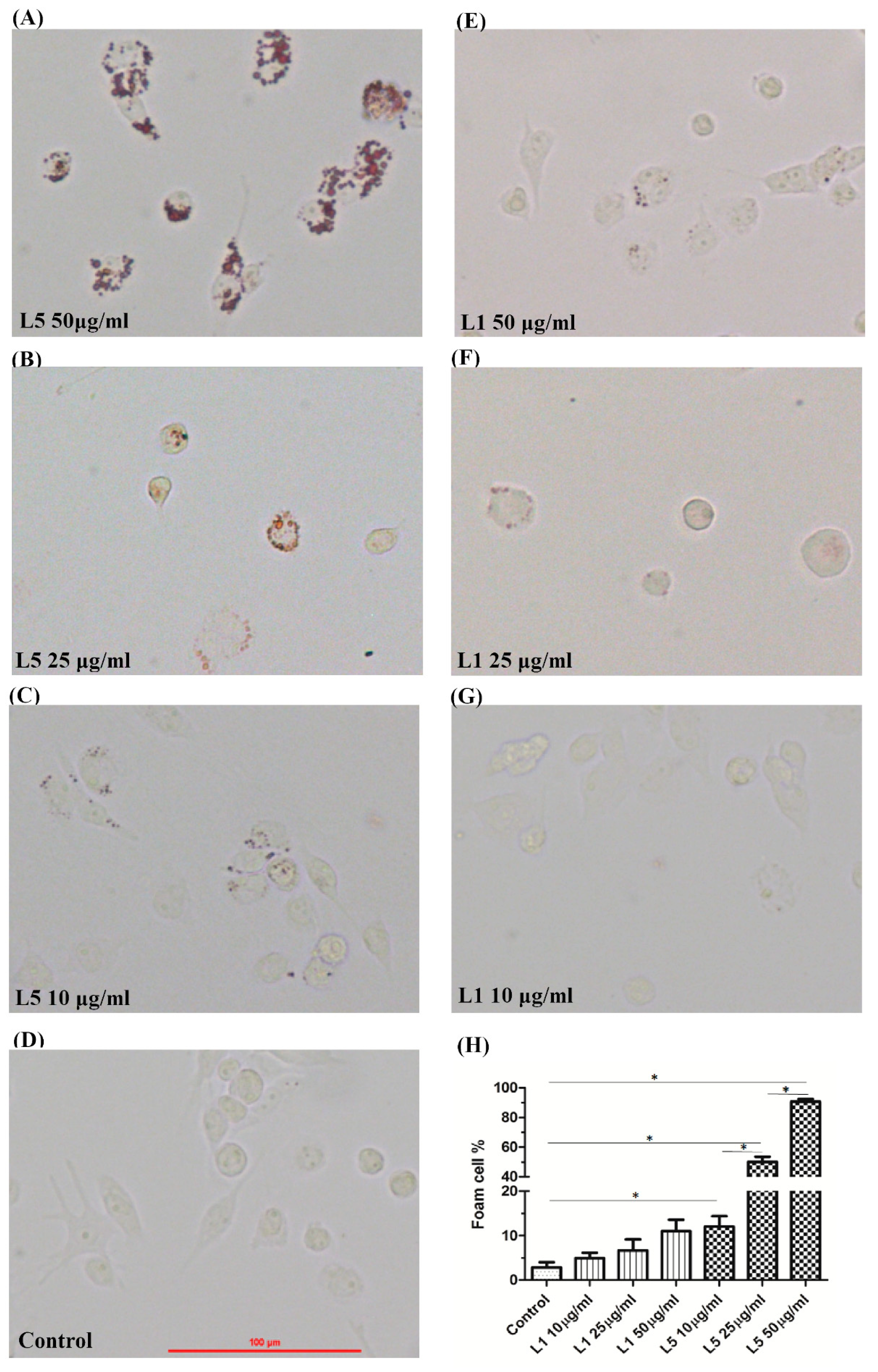
2.3. The Effects of L5 on Macrophage Foam Cell Formation
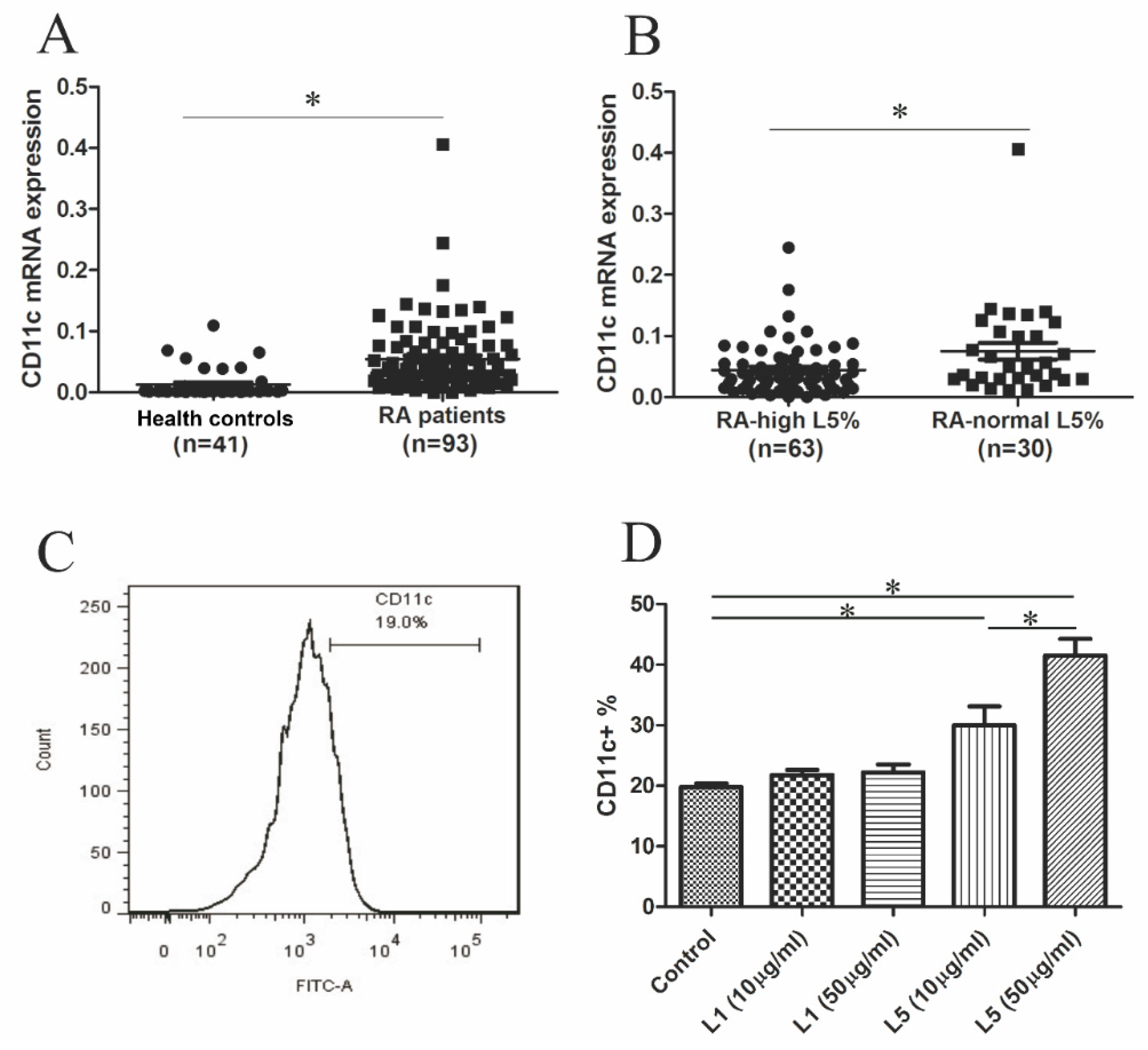
2.4. Comparison of CD11c mRNA Expression Levels between RA Patients with High L5% and Normal L5%
2.5. The Effects of L5 on CD11c Expression in THP-1 Cell
2.6. Correlation between CD11c Expression Levels and Plasma Levels of Inflammatory Mediators in RA Patients
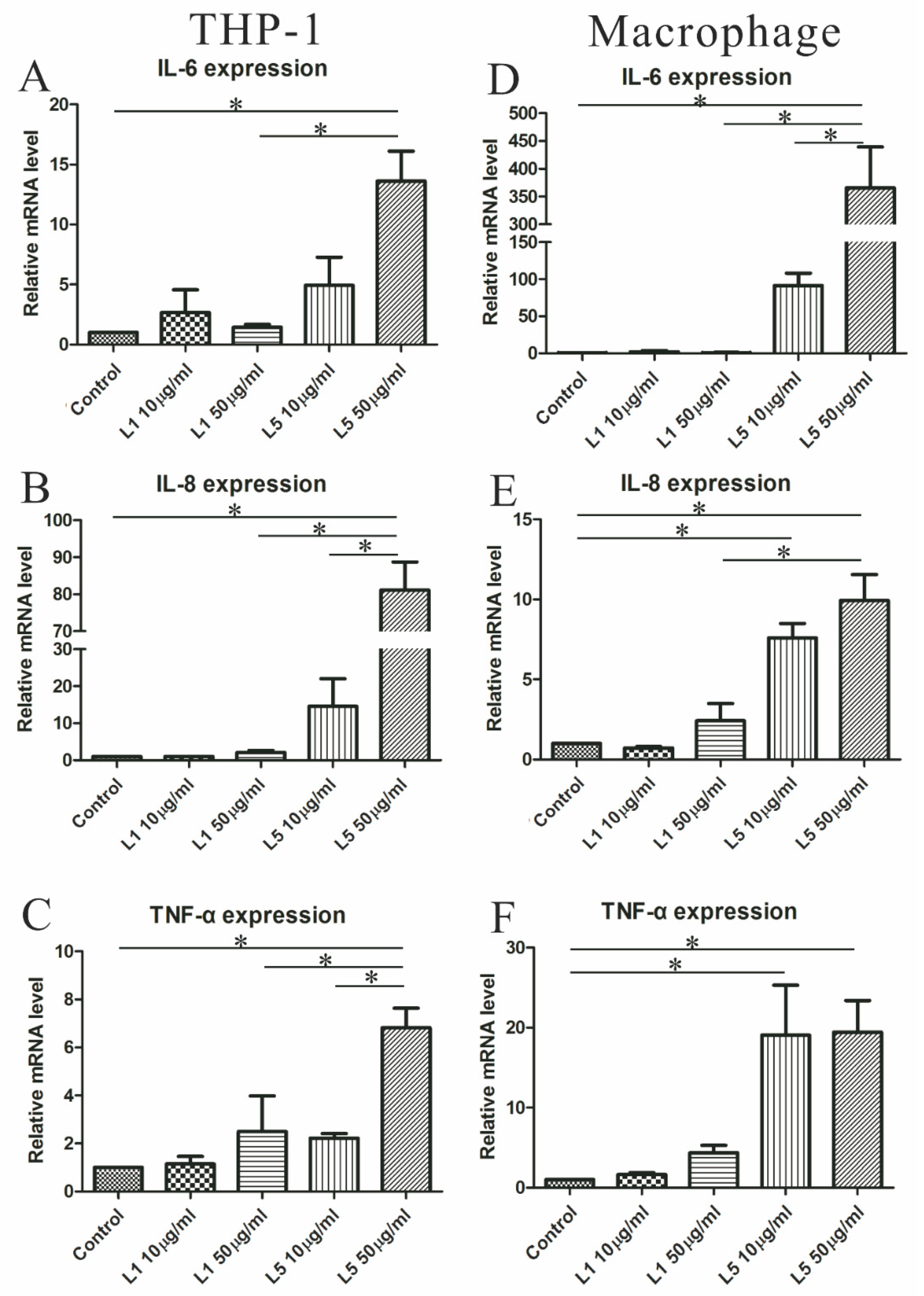
2.7. The Effects of L5 on Cytokine Expression in THP-1 Cells
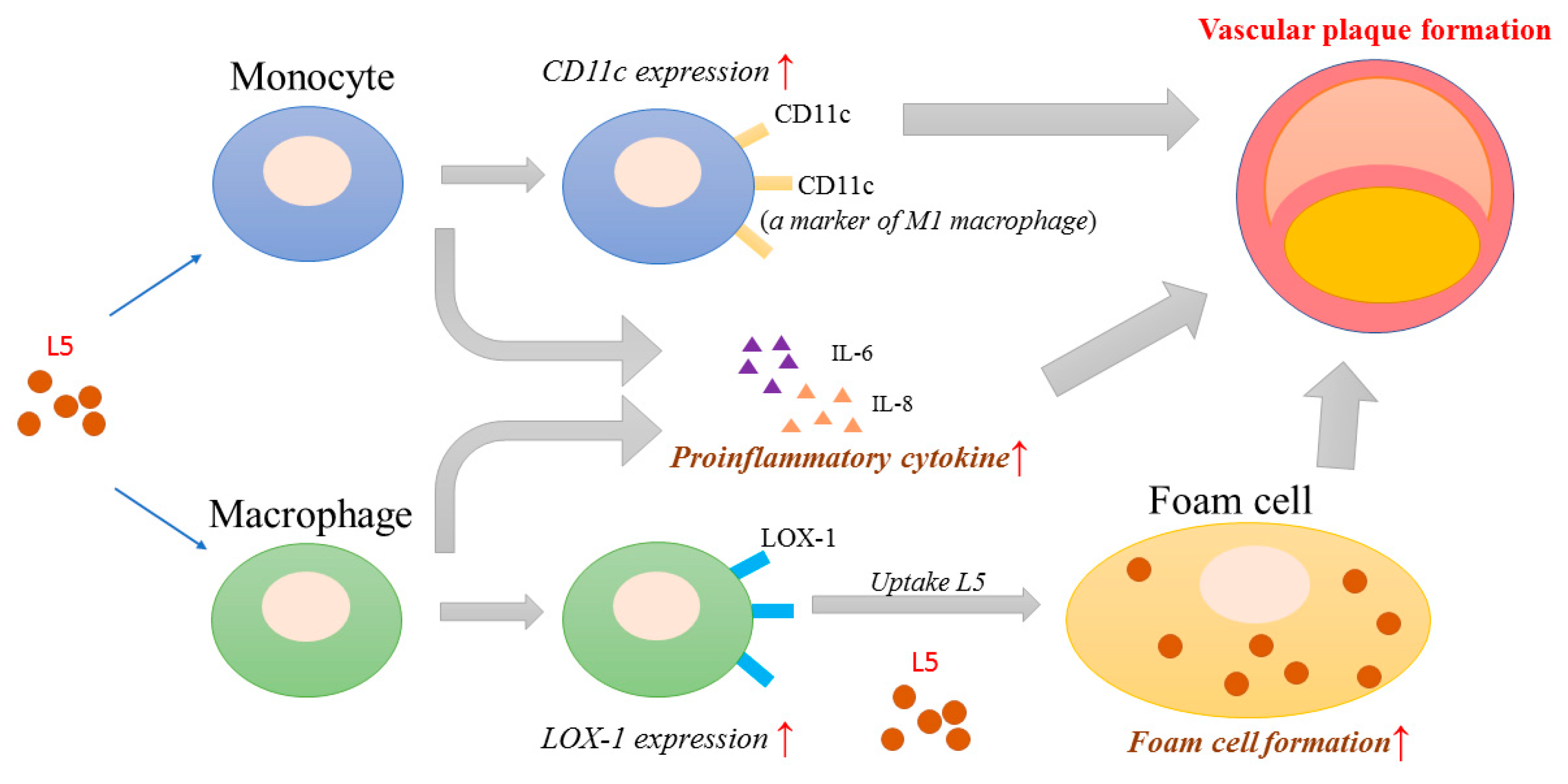
2.8. Proposed Model for the Potential Role of L5 in RA-Related Atherogenesis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Determination of Plasma Lipid Profiles and Atherogenic Index (AI)
4.3. Measurement of 10-Year Risk of CVD Including QRISK-2 Score
4.4. Isolation and Fractionation of LDL-C
4.5. Anion-Exchange Chromatography Purification of LDL-C Subfractions
4.6. Examination of Foam Cells Formation in Monocyte-Derived Macrophages Treated with L1 or L5
4.7. Database Search, RNA Extraction, and Quantitative Real-Time PCR for Their mRNA Expression Levels
4.8. Determination of CD11c Expression in THP-1 Cells Treated with Different Doses of L5 by Flow Cytometry Analysis
4.9. Measurement of Plasma Levels of Inflammatory Mediators
4.10. Measurement of mRNA Expression Levels of Inflammatory Mediators
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette, sub-family A, member 1 |
| ACPA | Anti-citrullinated peptide antibodies |
| AI | Atherogenic index |
| Apo | Apolipoprotein |
| CRP | C-reactive protein |
| csDMARD | conventional synthetic disease-modifying anti-rheumatic drug |
| CVD | Cardiovascular/cerebrovascular disease |
| DAS28 | 28-joint disease activity score |
| GEO | Gene Expression Omnibus |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HC | Healthy controls |
| HDL-C | High-density lipoprotein cholesterol |
| IL | Interleukin |
| IP-10 | Interferon gamma-induced protein 10 |
| ITGAX | Integrin Subunit Alpha X |
| L5% | Percentage of L5 in LDL |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NPC1 | Niemann-Pick disease, type C1 |
| PMA | Phorbol myristate acetate |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RA | Rheumatoid arthritis |
| RF | Rheumatoid factor |
| sCD40L | Soluble CD40 ligand |
| SD | Standard deviation |
| SEM | Standard error of mean |
| SLE | Systemic lupus erythematosus |
| TC | Total cholesterol |
| TNF-α | Tumor necrosis factor-α |
References
- Libby, P. Inflammation in atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cvetkovic, J.T.; Wallberg-Jonsson, S.; Ahmed, E.; Rantapaa-Dahlqvist, S.; Lefvert, A.K. Increased levels of autoantibodies against copper-oxidized low density lipoprotein, malondialdehyde-modified low density lipoprotein and cardiolipin in patients with rheumatoid arthritis. Rheumatology 2002, 41, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Symmons, D.P.; Gabriel, S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 2011, 7, 399–408. [Google Scholar] [CrossRef]
- Im, C.H.; Kim, N.R.; Kang, J.W.; Kim, J.H.; Kang, J.Y.; Bae, G.B.; Nam, E.J.; Kang, Y.M. Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology 2015, 54, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014, 53, 2143–2154. [Google Scholar] [CrossRef] [Green Version]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Rho, Y.H.; Chung, C.P.; Oeser, A.; Solus, J.; Asanuma, Y.; Sokka, T.; Pincus, T.; Raggi, P.; Gebretsadik, T.; Shintani, A.; et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Boisvert, W.A.; Curtiss, L.K.; Terkeltaub, R.A. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol. Res. 2000, 21, 129–137. [Google Scholar] [CrossRef]
- Barnabe, C.; Martin, B.J.; Ghali, W.A. Systematic review and meta-analysis: Anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. 2011, 63, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juanatey, C.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Gomez-Acebo, I.; Testa, A.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J.; Gonzalez-Gay, M.A. Anti-TNF-alpha-adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima-media wall thickness in patients with rheumatoid arthritis refractory to conventional therapy. Mediat. Inflamm. 2012, 2012, 674265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niccoli, G.; Baca, M.; De Spirito, M.; Parasassi, T.; Cosentino, N.; Greco, G.; Conte, M.; Montone, R.A.; Arcovito, G.; Crea, F. Impact of electronegative low-density lipoprotein on angiographic coronary atherosclerotic burden. Atherosclerosis 2012, 223, 166–170. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, C.H.; Chen, Y.M.; Hsieh, T.Y.; Li, J.P.; Shen, M.Y.; Lan, J.L.; Chen, D.Y. Association between Negatively Charged Low-Density Lipoprotein L5 and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. J. Clin. Med. 2019, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Chan, H.C.; Chan, H.C.; Liang, C.J.; Lee, H.C.; Su, H.; Lee, A.S.; Shiea, J.; Tsai, W.C.; Ou, T.T.; Wu, C.C.; et al. Role of Low-Density Lipoprotein in Early Vascular Aging Associated with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 972–984. [Google Scholar] [CrossRef]
- Kathiresan, S.; Srivastava, D. Genetics of human cardiovascular disease. Cell 2012, 148, 1242–1257. [Google Scholar] [CrossRef] [Green Version]
- Abbate, R.; Sticchi, E.; Fatini, C. Genetics of cardiovascular disease. Clin. Cases Min. Bone Metab. 2008, 5, 63–66. [Google Scholar]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Yu, X.H.; Jiang, N.; Yao, P.B.; Zheng, X.L.; Cayabyab, F.S.; Tang, C.K. NPC1, intracellular cholesterol trafficking and atherosclerosis. Clin. Chim. Acta 2014, 429, 69–75. [Google Scholar] [CrossRef]
- Kurohori, Y.; Sato, K.; Suzuki, S.; Kashiwazaki, S. Adhesion molecule expression on peripheral blood mononuclear cells in rheumatoid arthritis: Positive correlation between the proportion of L-selectin and disease activity. Clin. Rheumatol. 1995, 14, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sandor, N.; Lukacsi, S.; Ungai-Salanki, R.; Orgovan, N.; Szabo, B.; Horvath, R.; Erdei, A.; Bajtay, Z. CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18. PLoS ONE 2016, 11, e0163120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Gower, R.M.; Wang, H.; Perrard, X.Y.; Ma, R.; Bullard, D.C.; Burns, A.R.; Paul, A.; Smith, C.W.; Simon, S.I.; et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 2009, 119, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianello, E.; Dozio, E.; Arnaboldi, F.; Marazzi, M.G.; Martinelli, C.; Lamont, J.; Tacchini, L.; Sigruner, A.; Schmitz, G.; Corsi Romanelli, M.M. Epicardial adipocyte hypertrophy: Association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 246–253. [Google Scholar] [CrossRef]
- Shu, Q.H.; Ge, Y.S.; Ma, H.X.; Gao, X.Q.; Pan, J.J.; Liu, D.; Xu, G.L.; Ma, J.L.; Jia, W.D. Prognostic value of polarized macrophages in patients with hepatocellular carcinoma after curative resection. J. Cell. Mol. Med. 2016, 20, 1024–1035. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Cho, K.Y.; Miyoshi, H.; Kuroda, S.; Yasuda, H.; Kamiyama, K.; Nakagawara, J.; Takigami, M.; Kondo, T.; Atsumi, T. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J. Stroke Cereb. Dis. 2013, 22, 910–918. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Aslani, S.; Fadaei, R.; Jamshidi, A.R. New insights to the mechanisms underlying atherosclerosis in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; He, M.; Liang, X.; Gao, S.S.; Zhou, J.; Yuan, Z.Y. Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity 2016, 49, 115–123. [Google Scholar] [CrossRef]
- Lee, A.S.; Wang, Y.C.; Chang, S.S.; Lo, P.H.; Chang, C.M.; Lu, J.; Burns, A.R.; Chen, C.H.; Kakino, A.; Sawamura, T.; et al. Detection of a High Ratio of Soluble to Membrane-Bound LOX-1 in Aspirated Coronary Thrombi From Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014008. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Chang, C.F.; Lu, P.L.; Chu, C.S.; Lai, W.T.; Shin, S.J.; Liu, F.T.; Chen, C.H. Increased APOE glycosylation plays a key role in the atherogenicity of L5 low-density lipoprotein. FASEB J. 2020, 34, 9802–9813. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Aikawa, M.; Libby, P.; Alcaide, P.; Luscinskas, F.W.; Sacks, F.M. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 2006, 113, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.S.; Chan, H.C.; Tsai, M.H.; Stancel, N.; Lee, H.C.; Cheng, K.H.; Tung, Y.C.; Chan, H.C.; Wang, C.Y.; Shin, S.J.; et al. Range of L5 LDL levels in healthy adults and L5’s predictive power in patients with hyperlipidemia or coronary artery disease. Sci. Rep. 2018, 8, 11866. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Chen, F.Y.; Hsu, J.F.; Fu, R.H.; Chang, C.M.; Chang, C.T.; Liu, C.H.; Wu, J.R.; Lee, A.S.; Chan, H.C.; et al. Plasma L5 levels are elevated in ischemic stroke patients and enhance platelet aggregation. Blood 2016, 127, 1336–1345. [Google Scholar] [CrossRef] [Green Version]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef] [Green Version]
- Bancells, C.; Canals, F.; Benitez, S.; Colome, N.; Julve, J.; Ordonez-Llanos, J.; Sanchez-Quesada, J.L. Proteomic analysis of electronegative low-density lipoprotein. J. Lipid. Res. 2010, 51, 3508–3515. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Changes in transcriptome of macrophages in atherosclerosis. J. Cell. Mol. Med. 2015, 19, 1163–1173. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Gonzalez-Gay, M.A.; Kaplan, I.; Boy, M.; Geier, J.; Luo, Z.; Zuckerman, A.; Riese, R. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: Implications for the rheumatologist. Semin. Arthritis Rheum. 2016, 46, 71–80. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Prevoo, M.L.; Van ’t Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van de Putte, L.B.; Van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Hippisley-Cox, J.; Coupland, C.; Vinogradova, Y.; Robson, J.; Minhas, R.; Sheikh, A.; Brindle, P. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ 2008, 336, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, L.Y.; Chan, H.C.; Chan, H.C.; Kalu, F.C.U.; Lee, H.C.; Lin, I.L.; Jhuo, S.J.; Lai, W.T.; Tsao, C.R.; Sawamura, T.; et al. Electronegative Low-Density Lipoprotein L5 Induces Adipose Tissue Inflammation Associated With Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 4615–4625. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halvorsen, B.; Waehre, T.; Scholz, H.; Clausen, O.P.; Von der Thusen, J.H.; Muller, F.; Heimli, H.; Tonstad, S.; Hall, C.; Froland, S.S.; et al. Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J. Lipid. Res. 2005, 46, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, C.S.; Richards, S.J.; Master, P.S.; Kendall, J.; Limbert, H.J.; Roberts, B.E. Flow cytometric analysis of membrane CD11b, CD11c and CD14 expression in acute myeloid leukaemia: Relationships with monocytic subtypes and the concept of relative antigen expression. Eur. J. Haematol. 1990, 44, 24–29. [Google Scholar] [CrossRef]
| RA with High L5% (n = 30) | RA with Normal L5% (n = 63) | |
|---|---|---|
| Age at entry, years | 60.4 ± 10.9 | 58.4 ± 12.1 |
| Women proportion | 25 (83.3%) | 50 (79.4%) |
| RA duration, months | 68.9 ± 22.6 | 74.9 ± 28.4 |
| BMI, kg/m2 | 23.7 ± 2.2 | 23.0 ± 2.3 |
| RF positivity | 20 (66.7%) | 46 (73.0%) |
| ACPA positivity | 18 (60.0%) | 45 (71.4%) |
| ESR, mm/1st hour | 24.9 ± 12.2 | 20.4 ± 15.6 |
| CRP, mg/dl | 1.08 ± 1.07 b | 0.61 ± 0.69 |
| DAS28 at study entry | 4.25 ± 1.27 | 3.78 ± 1.07 |
| Daily steroid dose (mg) | 4.8 ± 1.7 | 4.2 ± 2.0 |
| csDMARDs alone at entry | 8 (26.7%) | 15 (23.8%) |
| Biologics used at entry | ||
| TNF-α inhibitors | 11 (36.7%) | 19 (30.2%) |
| IL-6R inhibitor | 9 (30.0%) | 17 (27.0%) |
| Rituximab | 2 (6.7%) | 2 (3.2%) |
| Hypertension | 12 (40.0%) | 20 (31.7%) |
| Diabetes mellitus | 5 (16.7%) | 6 (9.5%) |
| Current smoker | 2 (6.7%) | 5 (7.9%) |
| TC, mg/dl | 207 (164-236) | 211 (176–244) |
| HDL-C, mg/dl | 59.5 (44.5–74.8) | 59.6 (49.4–75.0) |
| Triglyceride, mg/dl | 114 (77–151) | 100 (67–138) |
| LDL-C, mg/dl | 129 (87–154) | 129 (105–154) |
| Atherogenic index | 3.4 (2.5–4.4) | 3.3 (2.7–4.3) |
| QRISK-2 score | 8.7 (5.8–14.5) c | 5.7 (2.7–9.1) |
| CVD events | 6 (20.0%) d | 4 (6.3%) e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Chen, P.-K.; Lan, J.-L.; Chang, S.-H.; Hsieh, T.-Y.; Liao, P.-J.; Chen, C.-H.; Chen, D.-Y. Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2020, 21, 5883. https://doi.org/10.3390/ijms21165883
Chang C-K, Chen P-K, Lan J-L, Chang S-H, Hsieh T-Y, Liao P-J, Chen C-H, Chen D-Y. Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences. 2020; 21(16):5883. https://doi.org/10.3390/ijms21165883
Chicago/Turabian StyleChang, Ching-Kun, Po-Ku Chen, Joung-Liang Lan, Shih-Hsin Chang, Tsu-Yi Hsieh, Pei-Jyuan Liao, Chu-Huang Chen, and Der-Yuan Chen. 2020. "Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients" International Journal of Molecular Sciences 21, no. 16: 5883. https://doi.org/10.3390/ijms21165883
APA StyleChang, C.-K., Chen, P.-K., Lan, J.-L., Chang, S.-H., Hsieh, T.-Y., Liao, P.-J., Chen, C.-H., & Chen, D.-Y. (2020). Association of Electronegative LDL with Macrophage Foam Cell Formation and CD11c Expression in Rheumatoid Arthritis Patients. International Journal of Molecular Sciences, 21(16), 5883. https://doi.org/10.3390/ijms21165883





