Increased miR-7641 Levels in Peritoneal Hyalinizing Vasculopathy in Long-Term Peritoneal Dialysis Patients
Abstract
1. Introduction
2. Results
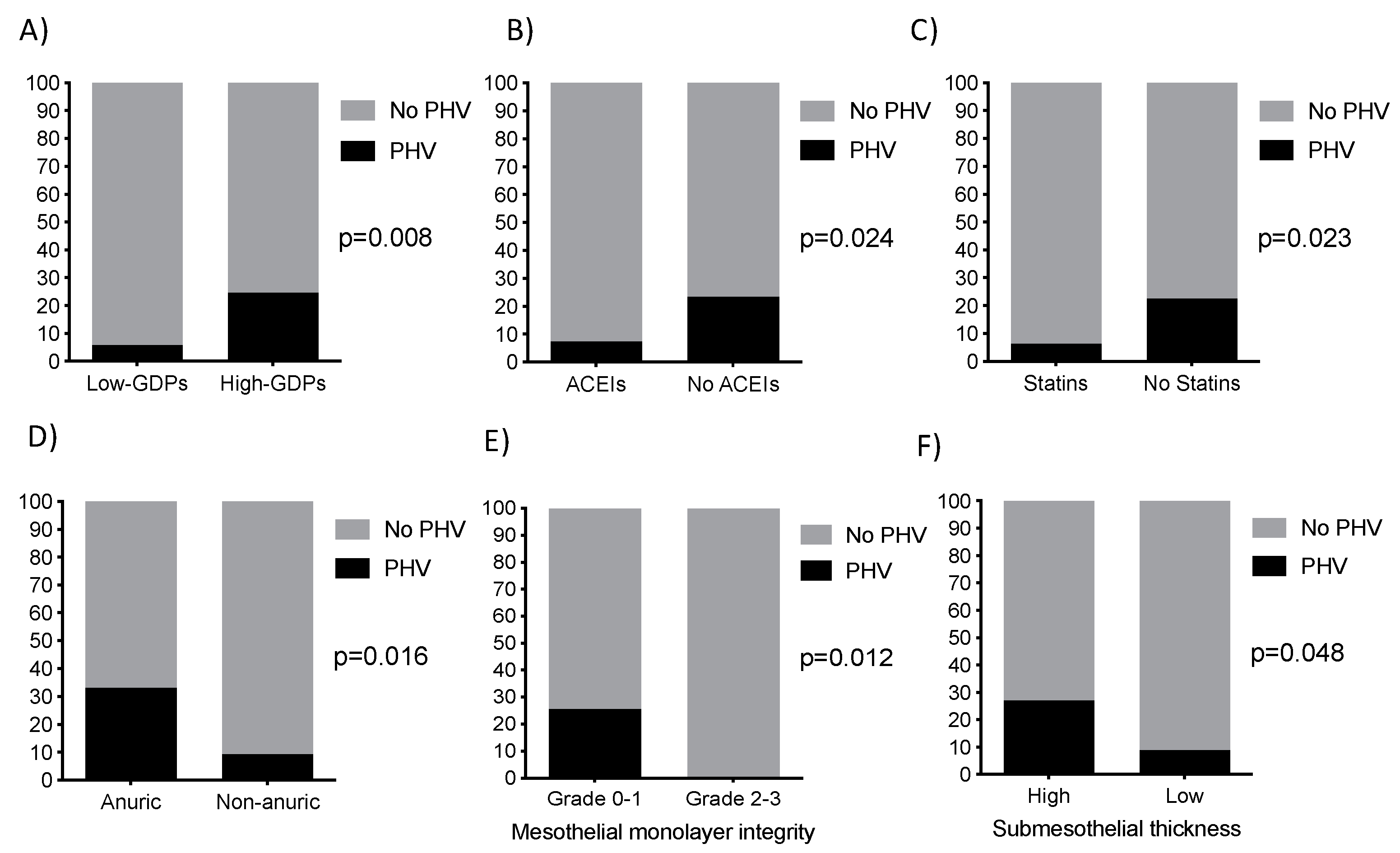
2.1. Clinical Characteristics of Patients
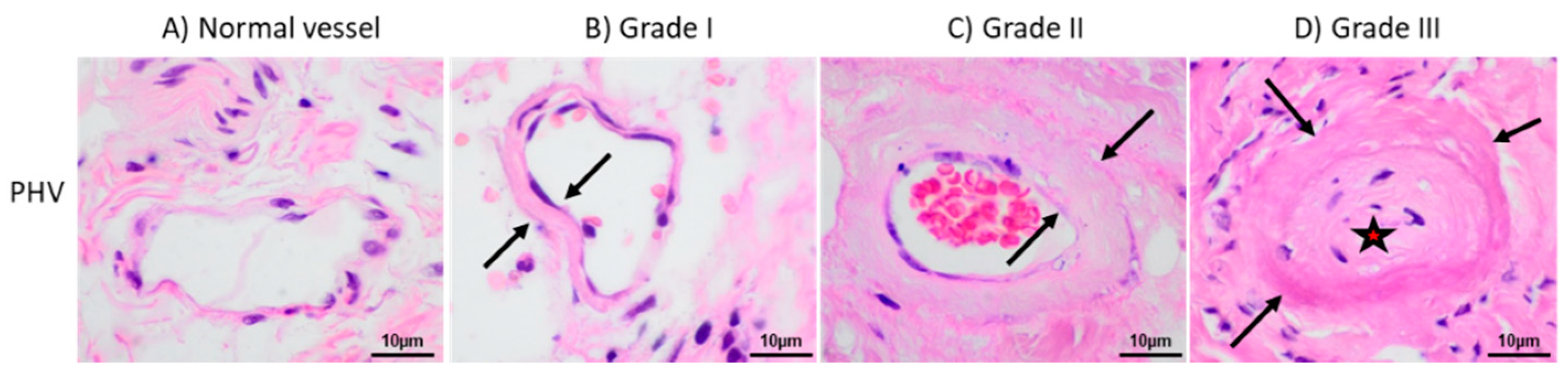
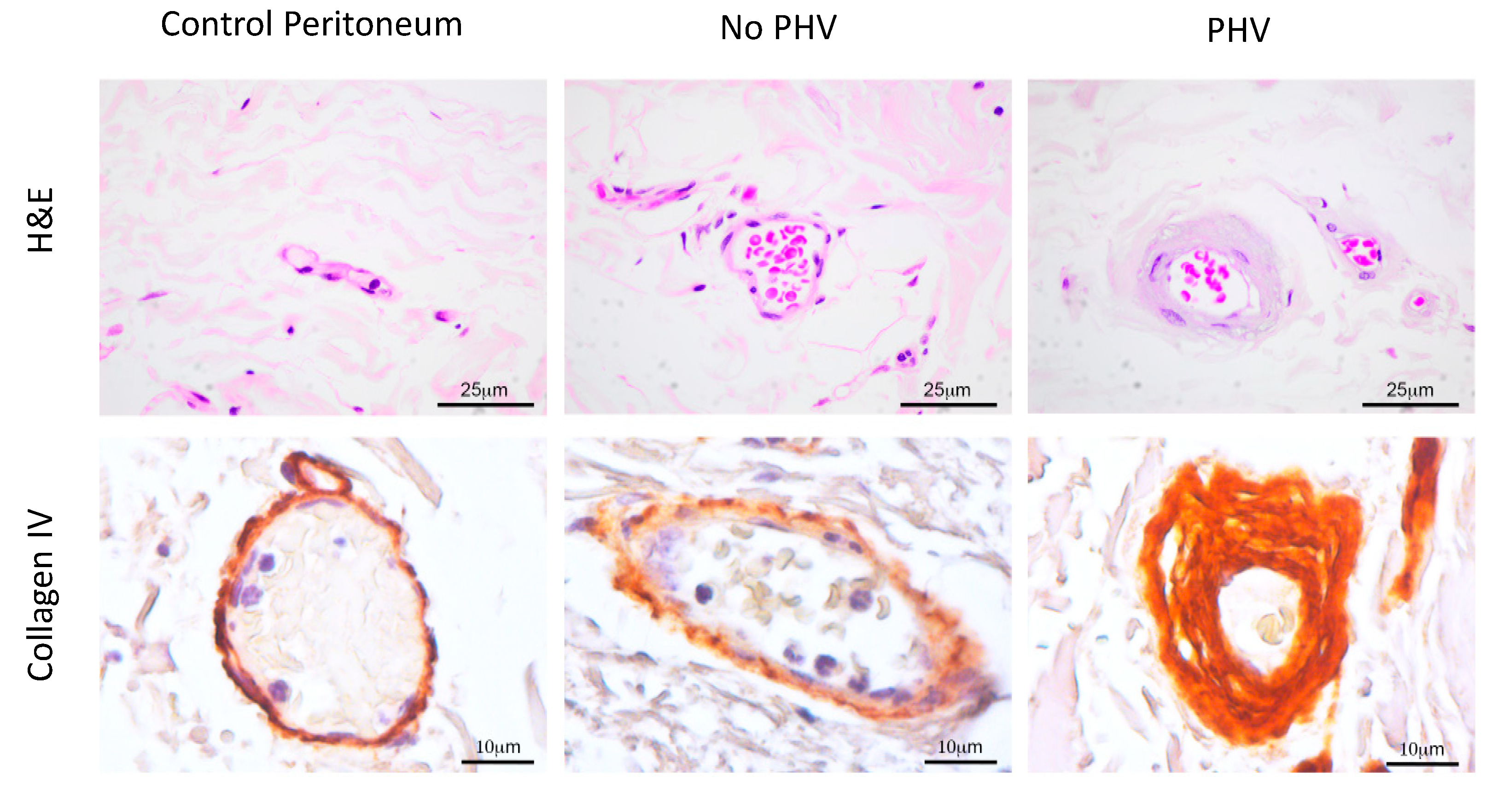
2.2. Aberrant Perivascular Accumulation of Collagen IV in Peritoneal Hyalinizing Vasculopathy
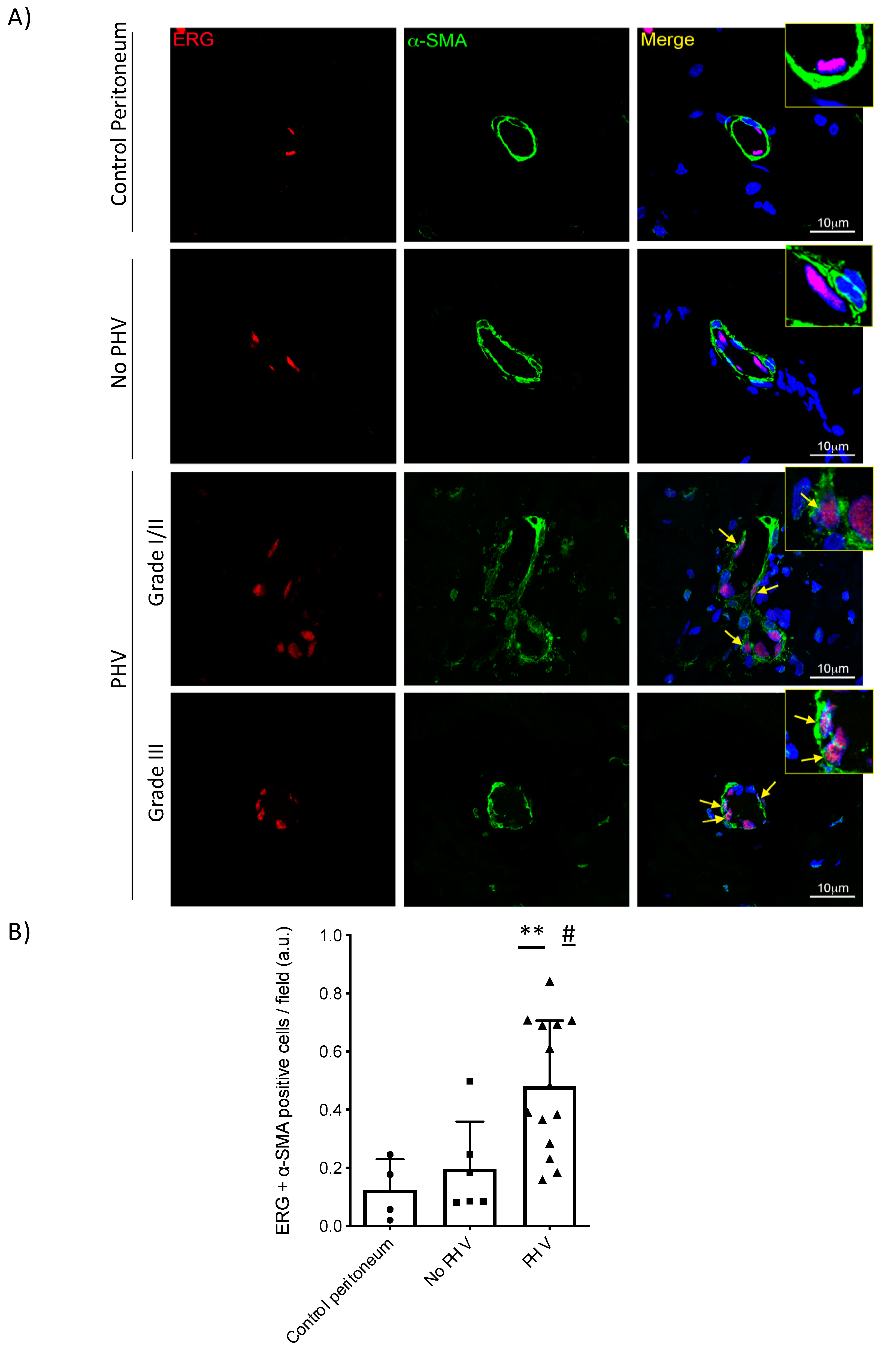
2.3. Endothelial-to-Mesenchymal Transition in Peritoneal Hyalinizing Vasculopathy
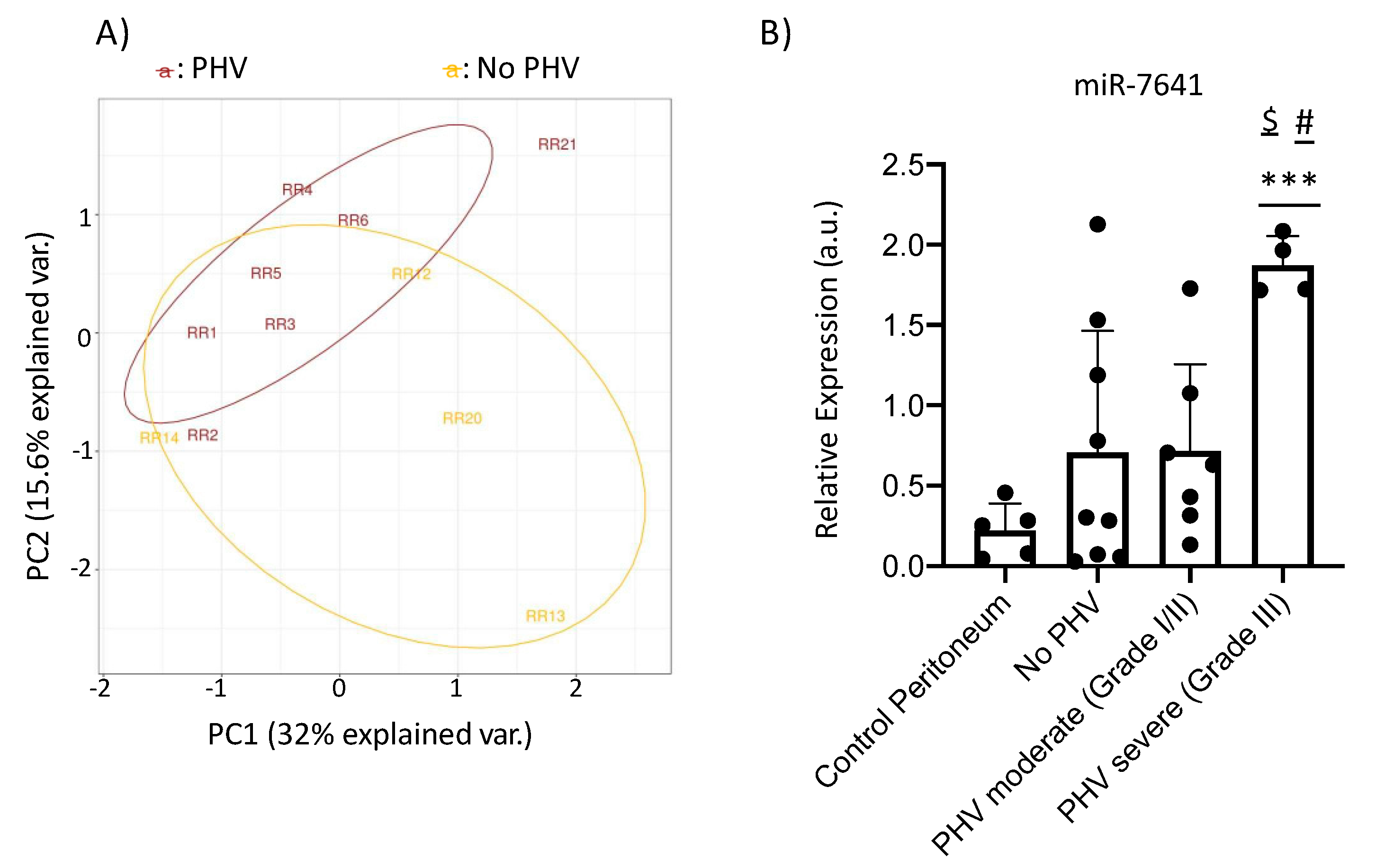
2.4. MicroRNA-Sequencing and qRT-PCR Results
3. Discussion
4. Materials and Methods
4.1. Patients and Peritoneal Samples
4.2. Immunohistochemistry
4.3. Morphological Parameters Analyzed
4.4. Dual-Immunofluorescence
4.5. miRNA Extraction
4.6. miRNA-Sequencing Analysis
4.7. miRNA Validation by Real Time-Quantitative PCR (RT-qPCR)
4.8. Statistics
4.9. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adams, B.D.; Kasinski, A.L.; Slack, F. Aberrant regulation and function of microRNAs in cancer. Curr. Boil. 2014, 24, 762–776. [Google Scholar] [CrossRef]
- Aguilera, A.; Yáñez-Mo, M.; Selgas, R.; Sánchez-Madrid, F.; López-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 2005, 6, 262–268. [Google Scholar]
- Ayuzawa, N.; Ishibashi, Y.; Takazawa, Y.; Kume, H.; Fujita, T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: Recent clinical practice in Japan. Perit. Dial. Int. 2012, 32, 159–167. [Google Scholar] [CrossRef]
- Bartosova, M.; Schaefer, B.; Bermejo, J.L.; Tarantino, S.; Lasitschka, F.; Macher-Goeppinger, S.; Sinn, P.; Warady, B.A.; Zaloszyc, A.; Parapatics, K.; et al. Complement activation in peritoneal dialysis–induced arteriolopathy. J. Am. Soc. Nephrol. 2017, 29, 268–282. [Google Scholar] [CrossRef]
- Borceux, P.; Morelle, J.; Goffin, E. Complement system activation and peritoneal membrane alterations: Culprit or innocent bystander? Perit. Dial. Int. 2020, 40, 115–123. [Google Scholar] [CrossRef]
- Chang, T.I.; Kang, H.-Y.; Kim, K.S.; Lee, S.H.; Nam, B.Y.; Paeng, J.; Kim, S.; Park, J.T.; Yoo, T.-H.; Kang, S.-W.; et al. The effect of statin on epithelial-mesenchymal transition in peritoneal mesothelial cells. PLoS ONE 2014, 9, e109628. [Google Scholar] [CrossRef]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Bárány, P.; Jaminon, A.M.; Schurgers, L.J.; Heimburger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Calore, M. MicroRNAs in cardiac diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Remuzzi, G.; Ruggenenti, P. Targeting the renin angiotensin system in dialysis patients. Semin. Dial. 2011, 24, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Del Peso, G.; Jiménez-Heffernan, J.; Bajo, M.; Aroeira, L.S.; Aguilera, A.; Fernández-Perpén, A.; Cirugeda, A.; Castro, M.; De Gracia, R.; Sánchez-Villanueva, R.; et al. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int. 2008, 73, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; Remón, C.; Ossorio, M.; Fernández-Perpén, A.; Sánchez-Tomero, J.A.; Cirugeda, A.; De Sousa, E.; Sandoval, P.; et al. Biocompatible dialysis solutions preserve peritoneal mesothelial cell and vessel wall integrity. A case-control study on human biopsies. Perit. Dial. Int. 2016, 36, 129–134. [Google Scholar] [CrossRef]
- Di Paolo, N.; Sacchi, G. Atlas of peritoneal histology. Perit. Dial. Int. 2000, 20, 5–96. [Google Scholar]
- Duman, S.; Günal, A.I.; Sen, S.; Asçi, G.; Özkahya, M.; Terzioglu, E.; Akçiçek, F.; Atabay, G. Does Enalapril Prevent Peritoneal Fibrosis Induced by Hypertonic (3.86%) Peritoneal Dialysis Solution? Perit. Dial. Int. 2001, 21, 219–225. [Google Scholar] [CrossRef]
- Duman, S.; Şen, S.; Duman, C.; Oreopoulos, D. Effect of valsartan versus lisinopril on peritoneal sclerosis in rats. Int. J. Artif. Organs 2005, 28, 156–163. [Google Scholar] [CrossRef]
- Duman, S.; Şen, S.; Sözmen, E.; Oreopoulos, D. Atorvastatin improves peritoneal sclerosis induced by hypertonic pd solution in rats. Int. J. Artif. Organs 2005, 28, 170–176. [Google Scholar] [CrossRef]
- Dumas, S.J.; Meta, E.; Borri, M.; Goveia, J.; Rohlenova, K.; Conchinha, N.V.; Falkenberg, K.; Teuwen, L.-A.; De Rooij, L.; Kalucka, J.; et al. Single-Cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol. 2019, 31, 118–138. [Google Scholar] [CrossRef]
- Frontiers Production Office Erratum: MicroRna crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front. Pharmacol. 2020, 11, 11. [CrossRef]
- Fierro-Fernández, M.; Busnadiego, Ó.; Sandoval, P.; Espinosa-Díez, C.; Blanco-Ruiz, E.; Rodríguez, M.; Pian, H.; Ramos-Ruiz, R.; Cabrera, M.L.; García-Bermejo, M.L.; et al. MiR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX 4 and TGFBR 2. EMBO Rep. 2015, 16, 1358–1377. [Google Scholar] [CrossRef]
- Futami, R.; Muñoz-Pomer, L.; Viu, J.M.; Dominguez-Escriba, L.; Covelli, L.; Bernet, G.P.; Sempere, J.M.; Moya, A.; Llorens, C. GPRO: The professional tool for management, functional analysis and annotation of omic sequences and databases. Software article. Biotechvana Bioinform. 2011, 1, 1–5. [Google Scholar]
- Geng, H.; Guan, J. MiR-18a-5p inhibits endothelial–mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem. Biophys. Res. Commun. 2017, 491, 329–336. [Google Scholar] [CrossRef]
- Hashimoto, N.; Phan, S.; Imaizumi, K.; Matsuo, M.; Nakashima, H.; Kawabe, T.; Shimokata, K.; Hasegawa, Y. Endothelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Boil. 2009, 43, 161–172. [Google Scholar] [CrossRef]
- Henry, T.W.; Mendoza, F.A.; Jimenez, S. Role of microRNA in the pathogenesis of systemic sclerosis tissue fibrosis and vasculopathy. Autoimmun. Rev. 2019, 18, 102396. [Google Scholar] [CrossRef]
- Honda, K.; Nitta, K.; Horita, S.; Yumura, W.; Nihei, H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996, 72, 171–176. [Google Scholar] [CrossRef]
- Honda, K. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol. Dial. Transplant. 1999, 14, 1541–1549. [Google Scholar] [CrossRef]
- Honda, K.; Hamada, C.; Nakayama, M.; Miyazaki, M.; Sherif, A.M.; Harada, T.; Hirano, H. Peritoneal biopsy study group of the Japanese society for peritoneal dialysis impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin. J. Am. Soc. Nephrol. 2008, 3, 720–728. [Google Scholar] [CrossRef]
- Jiménez-Heffernan, J.A.; Perna, C.; Bajo, M.A.; Picazo, M.L.; Del Peso, G.; Aroeira, L.S.; Aguilera, A.; Tejerina, E.; Cabrera, M.L.; Selgas, R. Tissue distribution of hyalinazing vasculopathy lesions in peritoneal dialysis patients. Pathol. Res. Pr. 2008, 204, 563–567. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. Review series The basics o epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Kawanishi, K.; Honda, K.; Hamada, C. Recommendations for pathological diagnosis on biopsy samples from peritoneal dialysis patients. Pleura Peritoneum 2017, 2, 3–15. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Boil. 2013, 14, R36. [Google Scholar] [CrossRef]
- Kiribayashi, K.; Masaki, T.; Naito, T.; Ogawa, T.; Ito, T.; Yorioka, N.; Kohno, N. Angiotensin II induces fibronectin expression in human peritoneal mesothelial cells via ERK1/2 and p38 MAPK. Kidney Int. 2005, 67, 1126–1135. [Google Scholar] [CrossRef]
- Krediet, R.T.; Van Diepen, A.T.; Coester, A.M.; Struijk, D.G. Peritoneal vasculopathy in the pathophysiology of long-term ultrafiltration failure: A hypothesis based on clinical observations. Clin. Nephrol. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, K.-H.; Choi, K.H.; Kim, S.B.; Kim, Y.-S.; Do, J.Y.; Kim, D.J.; Kim, Y.S.; Kim, Y.-L. Statin therapy is associated with improved survival in incident peritoneal dialysis patients: Propensity-matched comparison. Nephrol. Dial. Transplant. 2011, 26, 4090–4094. [Google Scholar] [CrossRef][Green Version]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef]
- Liu, T.; Zou, X.-Z.; Huang, N.; Ge, X.-Y.; Yao, M.-Z.; Liu, H.; Zhang, Z.; Hu, C.-P. Down-regulation of miR-204 attenuates endothelial-mesenchymal transition by enhancing autophagy in hypoxia-induced pulmonary hypertension. Eur. J. Pharmacol. 2019, 863, 172673. [Google Scholar] [CrossRef]
- Lopez-Anton, M.; Bowen, T.; Jenkins, R.H. MicroRNA regulation of peritoneal cavity homeostasis in peritoneal dialysis. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Lopez-Anton, M.; Lambie, M.; López-Cabrera, M.; Schmitt, C.P.; Ruiz-Carpio, V.; Bartosova, M.; Schaefer, B.; Davies, S.; Stone, T.; Jenkins, R.H.; et al. MiR-21 promotes fibrogenesis in peritoneal dialysis. Am. J. Pathol. 2017, 187, 1537–1550. [Google Scholar] [CrossRef]
- Cabrera, M.L. Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv. Med. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Perez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodriguez-Pascual, F.; et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef]
- Loureiro, J.; Schilte, M.; Aguilera, A.; Albar-Vizcaíno, P.; Ramírez-Huesca, M.; Perez-Lozano, M.L.; González-Mateo, G.T.; Aroeira, L.S.; Selgas, R.; Mendoza, L.; et al. BMP-7 blocks mesenchymal conversion of mesothelial cells and prevents peritoneal damage induced by dialysis fluid exposure. Nephrol. Dial. Transplant. 2010, 25, 1098–1108. [Google Scholar] [CrossRef]
- Lozier, M.R.; Sanchez, A.M.; Lee, J.J.; Tamariz, L.J.; Valle, G.A. Comparison of cardiovascular outcomes by dialysis modality: A systematic review and meta-analysis. Perit. Dial. Int. 2019, 39, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Yoshizawa, H.; Watanabe, M.; Imai, R.; Imai, T.; Hirahara, I.; Akimoto, T.; Ookawara, S.; Muto, S.; Nagata, D. MicroRNA expression profiling in peritoneal fibrosis. Transl. Res. 2016, 169, 47–66. [Google Scholar] [CrossRef]
- Nessim, S.J.; Perl, J.; Bargman, J.M. The renin–angiotensin–aldosterone system in peritoneal dialysis: Is what is good for the kidney also good for the peritoneum? Kidney Int. 2010, 78, 23–28. [Google Scholar] [CrossRef]
- Noh, H.; Ha, H.; Yu, M.R.; Kim, Y.O.; Kim, J.H.; Lee, H.B. Angiotensin II mediates high glucose-induced TGF-beta1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit. Dial. Int. 2005, 25, 38–47. [Google Scholar] [CrossRef]
- Ossorio, M.; Martínez, V.; Bajo, M.-A.; Del Peso, G.; Castro, M.-J.; Romero, S.; Selgas, R.; Bellón, T. Prominent levels of the profibrotic chemokine CCL18 during peritonitis: In vitro downregulation by vitamin D receptor agonists. BioMed Res. Int. 2018, 1–12. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, C.; Dijke, P.T. TGF-β-Induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- Peng, W.; Dou, X.; Hao, W.; Zhou, Q.; Tang, R.; Nie, J.; Lan, H.; Yu, X. Smad7 gene transfer attenuates angiogenesis in peritoneal dialysis rats. Nephrology 2013, 18, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Mendoza, F.A.; Jimenez, S.A. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J. Clin. Med. 2016, 5, 45. [Google Scholar] [CrossRef]
- Plum, J.; Hermann, S.; Fusshöller, A.; Schoenicke, G.; Donner, A.; Rohrborn, A.; Grabensee, B. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int. 2001, 59, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Poppelaars, F.; Faria, B.; Da Costa, M.G.; Franssen, C.F.M.; Van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The complement system in dialysis: a forgotten story? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.M.M.T.; Choi, Y.-J.; Yuan, Y.-G.; Das, J.; Yasuda, H.; Kim, J.-H. MicroRNA-7641 is a regulator of ribosomal proteins and a promising targeting factor to improve the efficacy of cancer therapy. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; De La Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vita, J.; Sánchez-López, E.; Esteban, V.; Rupérez, M.; Egido, J.; Ruiz-Ortega, M. Angiotensin II activates the smad pathway in vascular smooth muscle cells by a transforming growth factor-β–independent mechanism. Circulation 2005, 111, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carpio, V.; Sandoval, P.; Aguilera, A.; Albar-Vizcaíno, P.; Perez-Lozano, M.L.; González-Mateo, G.T.; Acuña-Ruiz, A.; García-Cantalejo, J.M.; Botías, P.; Bajo, M.A.; et al. Genomic reprograming analysis of the mesothelial to mesenchymal transition identifies biomarkers in peritoneal dialysis patients. Sci. Rep. 2017, 7, 44941. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 1–20. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rodriguez-Vita, J.; Sánchez-López, E.; Carvajal, G.; Egido, J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef]
- Rynne-Vidal, A.; Au-Yeung, C.L.; A Jiménez-Heffernan, J.; Perez-Lozano, M.L.; Cremades-Jimeno, L.; Bárcena, C.; Cristóbal-García, I.; Fernández-Chacón, C.; Yeung, T.L.; Mok, S.C.; et al. Mesothelial-to-mesenchymal transition as a possible therapeutic target in peritoneal metastasis of ovarian cancer. J. Pathol. 2017, 242, 140–151. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; De Vinuesa, A.G.; Dijke, P.T. Endothelial-to-mesenchymal transition in cardiovascular diseases: Developmental signaling pathways gone awry. Dev. Dyn. 2017, 247, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Guerra-Azcona, G.; Perez-Lozano, M.L.; Rynne-Vidal, A.; Albar-Vizcaíno, P.; Gil-Vera, F.; Martin, P.; Coronado, M.J.; Bárcena, C.; et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 2016, 239, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, P.; Loureiro, J.; González-Mateo, G.T.; Perez-Lozano, M.L.; Maldonado-Rodríguez, A.; Sánchez-Tomero, J.; Mendoza, L.; Santamaría, B.; Ortiz, A.; Ruiz-Ortega, M.; et al. PPAR-γ agonist rosiglitazone protects peritoneal membrane from dialysis fluid-induced damage. Lab. Investig. 2010, 90, 1517–1532. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Sniegon, I.; Prieß, M.; Heger, J.; Schulz, R.; Euler, G. Endothelial mesenchymal transition in hypoxic microvascular endothelial cells and paracrine induction of cardiomyocyte apoptosis are mediated via TGFβ1/SMAD signaling. Int. J. Mol. Sci. 2017, 18, 2290. [Google Scholar] [CrossRef]
- Sternberg, M.; Grigorova-Borsos, A.M.; Guillot, R.; Kassab, J.P.; Bakillah, A.; Urios, P.; Cohen-Forterre, L.; Mozere, G.; Andre, J.; Leblond, V. Changes in collagen type IV metabolism in diabetes. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales 1993, 187, 247–257. [Google Scholar]
- Tang, R.; Li, Q.; Lv, L.-L.; Dai, H.; Zheng, M.; Ma, K.-L.; Liu, B.-C. Angiotensin II mediates the high-glucose-induced endothelial-to-mesenchymal transition in human aortic endothelial cells. Cardiovasc. Diabetol. 2010, 9, 31. [Google Scholar] [CrossRef]
- Tăranu, T.; Florea, L.; Păduraru, D.; Georgescu, S.O.; Frâncu, L.L.; Stan, C.I. Morphological changes of the peritoneal membrane in patients with long-term dialysis. Romanian, J. Morphol. Embryol.Rev. Roum. de Morphol. Embryol. 2014, 55, 927–932. [Google Scholar]
- Tiwari, J.; Gupta, G.; Pinto, T.D.J.A.; Sharma, R.; Pabreja, K.; Matta, Y.; Arora, N.; Mishra, A.; Sharma, R.; Dua, K. Role of microRNAs (miRNAs) in the pathophysiology of diabetes mellitus. Panminerva Medica 2017, 60, 25–28. [Google Scholar]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Tsilibary, E.C. Microvascular basement membranes in diabetes mellitus. J. Pathol. 2003, 200, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An overview. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; Volume 1509, pp. 1–10. [Google Scholar]
- Walther, C.P.; Richardson, P.; Virani, S.S.; Winkelmayer, W.C.; Navaneethan, S.D. Association between intensity of statin therapy and mortality in persons with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 35, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Liu, P.-Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; MacKenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [PubMed]
- Xu, L.; Fu, M.; Chen, D.; Han, W.; Ostrowski, M.C.; Grossfeld, P.; Gao, P.; Ye, M. Endothelial-specific deletion of Ets-1 attenuates Angiotensin II-induced cardiac fibrosis via suppression of endothelial-to-mesenchymal transition. BMB Rep. 2019, 52, 595–600. [Google Scholar] [CrossRef]
- Yanai, K.; Ishii, H.; Aomatsu, A.; Ishibashi, K.; Morishita, Y. MicroRNAs in peritoneal fibrosis: A systematic review. Discov. Med. 2018, 26, 271–280. [Google Scholar]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. New Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Yoo, J.K.; Jung, H.Y.; Kim, C.-H.; Son, W.-S.; Kim, J.K. MiR-7641 modulates the expression of CXCL1 during endothelial differentiation derived from human embryonic stem cells. Arch. Pharmacal Res. 2013, 36, 353–358. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Watabe, T. Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. Int. J. Inflamm. 2011, 1–8. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediat. Inflamm. 2012, 1–21. [Google Scholar] [CrossRef]
- Zavvos, V.; Buxton, A.T.; Evans, C.; Lambie, M.; Davies, S.J.; Topley, N.; Wilkie, M.; Summers, A.; Brenchley, P.; Goumenos, D.S.; et al. A prospective, proteomics study identified potential biomarkers of encapsulating peritoneal sclerosis in peritoneal effluent. Kidney Int. 2017, 92, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Kalluri, R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 2012, 304, C216–C225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, Y.; Chen, J.; Cai, D.; Chen, C.; Zhang, S.; Chen, Z. MiR-29a/b cluster suppresses high glucose-induced endothelial-mesenchymal transition in human retinal microvascular endothelial cells by targeting Notch2. Exp. Ther. Med. 2019, 17, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Liu, Y.; Xu, Y.; Zhao, X.; Qian, J.; Sun, B.; Xing, C. Fluvastatin inhibits the expression of fibronectin in human peritoneal mesothelial cells induced by high-glucose peritoneal dialysis solution via SGK1 pathway. Clin. Exp. Nephrol. 2014, 19, 336–342. [Google Scholar] [CrossRef]
- Zhou, Q.; Bajo, M.-A.; Del Peso, G.; Yu, X.; Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Li, R.; Zeng, Y.; Zhou, T.; Xiong, F.; Zhu, M. MicroRNA-142-3p inhibits high-glucose-induced endothelial-to-mesenchymal transition through targeting TGF-beta1/Smad pathway in primary human aortic endothelial cells. Int. J. Clin. Exp. Pathol. 2018, 11, 1208–1217. [Google Scholar]

| PHV | No PHV | p | ||
|---|---|---|---|---|
| (n = 15) | (n = 85) | |||
| Gender Male/Female | 66.7% (10)/33.3% (5) | 55.3% (47)/44.7% (38) | 0.412 | |
| Age (years) | 51.2 ± 16.2 | 48.8 ±15.3 | 0.668 | |
| Type of dialysis- Automated PD | 80% (12) | 71.8% (61) | 0.508 | |
| Time on PD (months) | 32.6 ± 21.9 | 21.7±14 | 0.076 | |
| Medical record | Diabetes | 6.7% (1) | 9.4% (8) | 0.732 |
| Hypertension | 93.3% (14) | 83.5% (71) | 0.327 | |
| Ischemic cardiopathy | 13.3% (2) | 7.1% (6) | 0.409 | |
| Stroke | 13.3% (2) | 11.8% (10) | 0.863 | |
| Peripheral artery disease | 13.3% (2) | 16.5% (14) | 0.76 | |
| Pharmacological treatment | ACEI | 26.7% (4) | 57.6% (49) | 0.027 |
| ARB | 40% (6) | 31.8% (27) | 0.532 | |
| B-blockers | 53.3% (8) | 47.1% (40) | 0.654 | |
| Steroids | 20% (3) | 14.1% (12) | 0.556 | |
| Statins | 20% (3) | 51.2% (43) | 0.026 | |
| Antiplatelets | 6.7% (1) | 9.5% (8) | 0.723 | |
| Anticoagulants | 6.7% (1) | 2.4% (2) | 0.372 | |
| Other PD-related data | Biocompatible dialysis solutions use | 20% (3) | 56.5% (48) | 0.009 |
| Previous peritonitis episodes | 33.3% (5) | 36.5% (31) | 0.815 | |
| Accumulated days of peritonitis | 9.7 ± 11.3 | 4.6 ± 3.3 | 0.569 | |
| Residual renal function (ml/min/1.73m2) | 2.7±2.1 | 4.8 ± 3.1 | 0.001 |
| Cluster ID | Predicted Targets in Mirbase | Chr | Region Start | Region End | log2 (Fold_Change) | p-Value | q-Value |
|---|---|---|---|---|---|---|---|
| hsa-mir-1185-1-3p | 2269 | chr14 | 101042976 | 101043062 | 3.05 | 7.55 × 10−3 | 4.15 × 10−2 |
| hsa-mir-1193 | no | chr14 | 101030051 | 101030129 | -inf | 5.00 × 10−4 | 5.10 × 10−3 |
| hsa-mir-1246 | no | chr2 | 176600979 | 176601052 | −1.64 | 7.50 × 10−4 | 7.07 × 10−3 |
| hsa-mir-1299 | 5405 | chr9 | 40929009 | 40929092 | −1.49 | 8.25 × 10−3 | 4.43 × 10−2 |
| hsa-miR-154-3p | 83 | chr14 | 101059754 | 101059838 | 2.47 | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-miR-154-5p | 165 | chr14 | 101059754 | 101059838 | 2.47 | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-miR-200a-3p | 110 | chr1 | 1167862 | 1167952 | 2.13 | 2.45 × 10−3 | 1.82 × 10−2 |
| hsa-miR-323b-5p | 998 | chr14 | 101056218 | 101056300 | 4.24 | 1.50 × 10−4 | 1.91 × 10−3 |
| hsa-miR-34a-5p | 319 | chr11 | 111513438 | 111513515 | 1.70 | 0.0072 | 4.03 × 10−2 |
| hsa-miR-34c-5p | 319 | chr11 | 111513438 | 111513515 | 1.70 | 7.20 × 10−3 | 4.03 × 10−2 |
| hsa-miR-369-5p | 294 | chr14 | 101065597 | 1010656,67 | 2.35 | 6.50 × 10−3 | 3.78 × 10−2 |
| hsa-mir-376a-2-5p | no | chr14 | 101040068 | 101040148 | -inf | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-miR-377-3p | 635 | chr14 | 101062049 | 101062118 | 3.20 | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-miR-383-5p.1 | 235 | chr8 | 14853437 | 14853510 | −2.50 | 6.45 × 10−3 | 3.76 × 10−2 |
| hsa-miR-383-5p.2 | 223 | chr8 | 14853437 | 14853510 | −2.50 | 6.45 × 10−3 | 3.76 × 10−2 |
| hsa-miR-412-3p | 2888 | chr14 | 101065446 | 101065537 | 2.70 | 6.50 × 10−3 | 3.78 × 10−2 |
| hsa-miR-494-5p | 712 | chr14 | 101029633 | 101029714 | 4.03 | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-mir-542-5p | 1047 | chrX | 134541340 | 134541437 | 3.21 | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-mir-548ad-3p | 1717 | chr2 | 35471404 | 35471486 | -inf | 8.70 × 10−3 | 4.60 × 10−2 |
| hsa-miR-6507-3p | no | chr10 | 98924498 | 98924568 | -inf | 5.00 × 10−5 | 7.77 × 10−4 |
| hsa-miR-6507-5p | no | chr10 | 98924498 | 98924568 | -inf | 5.00× 10−5 | 7.77 × 10−4 |
| hsa-miR-651-5p | 3719 | chrX | 8126964 | 8127061 | 2.12 | 8.30× 10−3 | 4.44 × 10−2 |
| hsa-mir-7641 | no | chr11 | 10425259 | 104252651 | 1.60 | 5.55 × 10−3 | 3.37 × 10−2 |
| hsa-mir-873-3p | 4301 | chr9 | 28888878 | 28888955 | −2.58 | 7.50 × 10−4 | 7.07 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, R.; Sandoval, P.; Rodrigues-Diez, R.R.; del Peso, G.; Jiménez-Heffernan, J.A.; Ramos-Ruíz, R.; Llorens, C.; Laham, G.; Alvarez-Quiroga, M.; López-Cabrera, M.; et al. Increased miR-7641 Levels in Peritoneal Hyalinizing Vasculopathy in Long-Term Peritoneal Dialysis Patients. Int. J. Mol. Sci. 2020, 21, 5824. https://doi.org/10.3390/ijms21165824
Díaz R, Sandoval P, Rodrigues-Diez RR, del Peso G, Jiménez-Heffernan JA, Ramos-Ruíz R, Llorens C, Laham G, Alvarez-Quiroga M, López-Cabrera M, et al. Increased miR-7641 Levels in Peritoneal Hyalinizing Vasculopathy in Long-Term Peritoneal Dialysis Patients. International Journal of Molecular Sciences. 2020; 21(16):5824. https://doi.org/10.3390/ijms21165824
Chicago/Turabian StyleDíaz, Raquel, Pilar Sandoval, Raul R. Rodrigues-Diez, Gloria del Peso, José A Jiménez-Heffernan, Ricardo Ramos-Ruíz, Carlos Llorens, Gustavo Laham, Mabel Alvarez-Quiroga, Manuel López-Cabrera, and et al. 2020. "Increased miR-7641 Levels in Peritoneal Hyalinizing Vasculopathy in Long-Term Peritoneal Dialysis Patients" International Journal of Molecular Sciences 21, no. 16: 5824. https://doi.org/10.3390/ijms21165824
APA StyleDíaz, R., Sandoval, P., Rodrigues-Diez, R. R., del Peso, G., Jiménez-Heffernan, J. A., Ramos-Ruíz, R., Llorens, C., Laham, G., Alvarez-Quiroga, M., López-Cabrera, M., Ruiz-Ortega, M., Bajo, M. A., & Selgas, R. (2020). Increased miR-7641 Levels in Peritoneal Hyalinizing Vasculopathy in Long-Term Peritoneal Dialysis Patients. International Journal of Molecular Sciences, 21(16), 5824. https://doi.org/10.3390/ijms21165824







