Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency
Abstract
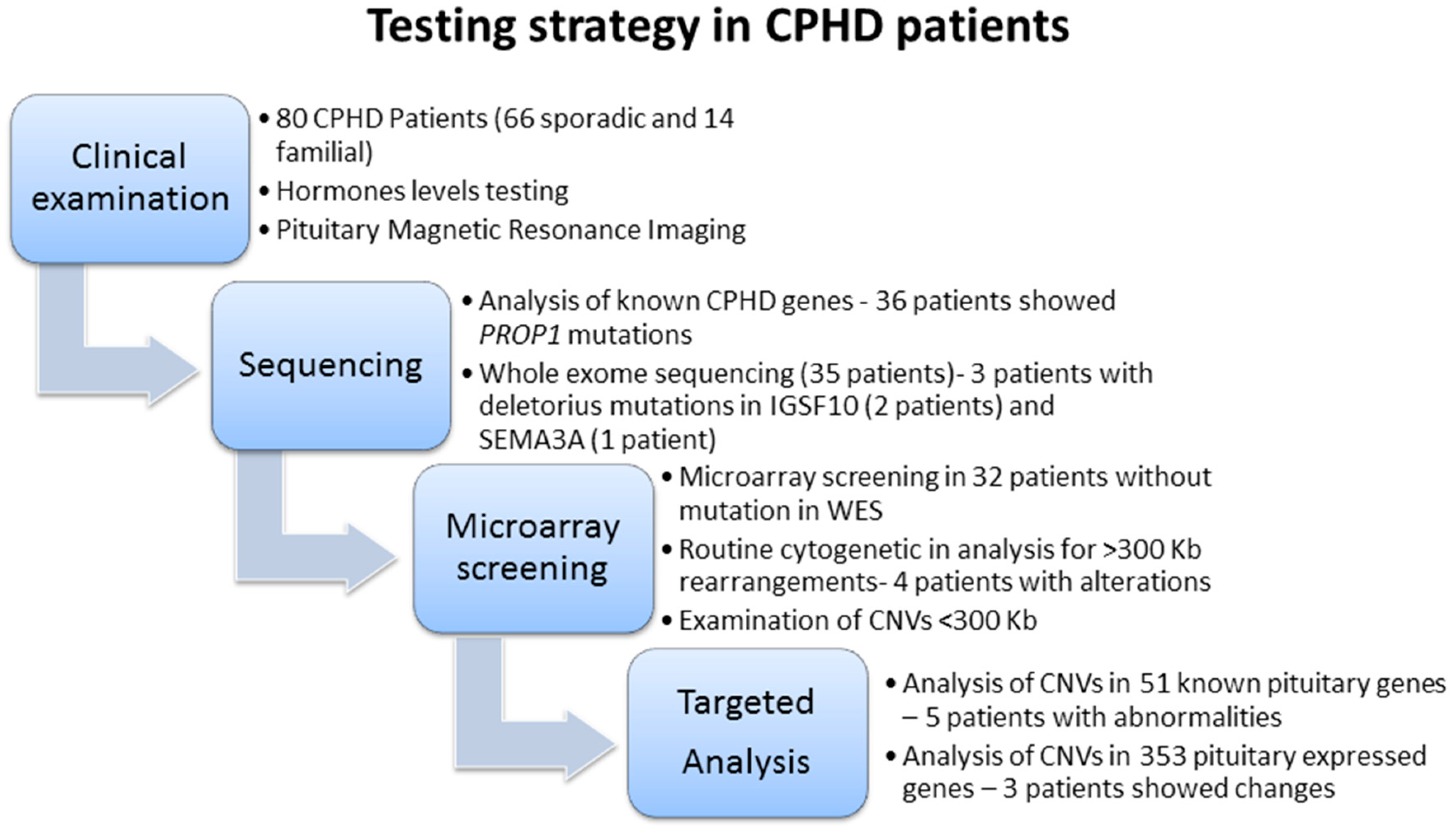
1. Introduction
2. Patients
3. Methods
Filtering Genomic Data
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duquesnoy, P.; Roy, A.; Dastot, F.; Ghali, I.; Teinturier, C.; Netchine, I.; Cacheux, V.; Hafez, M.; Salah, N.; Chaussain, J.L.; et al. Human prop-1: Cloning, mapping, genomic structure. Mutations in familial combined pituitary hormone deficiency. FEBS Lett. 1998, 437, 216–220. [Google Scholar] [CrossRef]
- Fluck, C.; Deladoey, J.; Rutishauser, K.; Eble, A.; Marti, U.; Wu, W.; Mullis, P.E. Phenotypic variability in familial combined pituitary hormone deficiency caused by a prop1 gene mutation resulting in the substitution of arg→cys at codon 120 (r120c). J. Clin. Endocrinol. Metab. 1998, 83, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cogan, J.D.; Pfaffle, R.W.; Dasen, J.S.; Frisch, H.; O’Connell, S.M.; Flynn, S.E.; Brown, M.R.; Mullis, P.E.; Parks, J.S.; et al. Mutations in prop1 cause familial combined pituitary hormone deficiency. Nat. Genet. 1998, 18, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, Y.; Shin, Y.L.; Kim, G.H.; Kim, T.U.; Yoo, H.W. Clinical characteristics and molecular analysis of pit1, prop1,lhx3, and hesx1 in combined pituitary hormone deficiency patients with abnormal pituitary mr imaging. Horm. Res. 2003, 60, 277–283. [Google Scholar] [CrossRef]
- Davis, S.W.; Ellsworth, B.S.; Perez Millan, M.I.; Gergics, P.; Schade, V.; Foyouzi, N.; Brinkmeier, M.L.; Mortensen, A.H.; Camper, S.A. Pituitary gland development and disease: From stem cell to hormone production. Curr. Top. Dev. Biol. 2013, 106, 1–47. [Google Scholar]
- Ellsworth, B.S.; Stallings, C.E. Molecular mechanisms governing embryonic differentiation of pituitary somatotropes. Trends Endocrinol. Metab. TEM 2018, 29, 510–523. [Google Scholar] [CrossRef]
- Perez Millan, M.I.; Vishnopolska, S.A.; Daly, A.Z.; Bustamante, J.P.; Seilicovich, A.; Bergada, I.; Braslavsky, D.; Keselman, A.C.; Lemons, R.M.; Mortensen, A.H.; et al. Next generation sequencing panel based on single molecule molecular inversion probes for detecting genetic variants in children with hypopituitarism. Mol. Genet. Genom. Med. 2018, 6, 514–525. [Google Scholar] [CrossRef]
- Zwaveling-Soonawala, N.; Alders, M.; Jongejan, A.; Kovacic, L.; Duijkers, F.A.; Maas, S.M.; Fliers, E.; van Trotsenburg, A.S.P.; Hennekam, R.C. Clues for polygenic inheritance of pituitary stalk interruption syndrome from exome sequencing in 20 patients. J. Clin. Endocrinol. Metab. 2018, 103, 415–428. [Google Scholar] [CrossRef]
- Guo, Q.H.; Wang, C.Z.; Wu, Z.Q.; Qin, Y.; Han, B.Y.; Wang, A.P.; Wang, B.A.; Dou, J.T.; Wu, X.S.; Mu, Y.M. Multi-genic pattern found in rare type of hypopituitarism: A whole-exome sequencing study of han chinese with pituitary stalk interruption syndrome. J. Cell. Mol. Med. 2017, 21, 3626–3632. [Google Scholar] [CrossRef]
- Fang, Q.; George, A.S.; Brinkmeier, M.L.; Mortensen, A.H.; Gergics, P.; Cheung, L.Y.; Daly, A.Z.; Ajmal, A.; Perez Millan, M.I.; Ozel, A.B.; et al. Genetics of combined pituitary hormone deficiency: Roadmap into the genome era. Endocr. Rev. 2016, 37, 636–675. [Google Scholar] [CrossRef]
- Vetro, A.; Pagani, S.; Silengo, M.; Severino, M.; Bozzola, E.; Meazza, C.; Zuffardi, O.; Bozzola, M. Severe growth hormone deficiency and pituitary malformation in a patient with chromosome 2p25 duplication and 2q37 deletion. Mol. Cytogenet. 2014, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- El Chehadeh-Djebbar, S.; Callier, P.; Masurel-Paulet, A.; Bensignor, C.; Mejean, N.; Payet, M.; Ragon, C.; Durand, C.; Marle, N.; Mosca-Boidron, A.L.; et al. 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur. J. Med. Genet. 2011, 54, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Bauters, M.; Frints, S.G.; Van Esch, H.; Spruijt, L.; Baldewijns, M.M.; de Die-Smulders, C.E.; Fryns, J.P.; Marynen, P.; Froyen, G. Evidence for increased sox3 dosage as a risk factor for x-linked hypopituitarism and neural tube defects. Am. J. Med. Genet. A 2014, 164A, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.S.; Cundall, M.; Turton, J.; Rizotti, K.; Mehta, A.; Palmer, R.; Wong, J.; Chong, W.K.; Al-Zyoud, M.; El-Ali, M.; et al. Over- and underdosage of sox3 is associated with infundibular hypoplasia and hypopituitarism. Am. J. Hum. Genet. 2005, 76, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.A.; Jorge, A.A.; Nakaguma, M.; Canton, A.P.; Costa, S.S.; Funari, M.F.; Lerario, A.M.; Franca, M.M.; Carvalho, L.R.; Krepischi, A.C.; et al. Pathogenic copy number variants in patients with congenital hypopituitarism associated with complex phenotypes. Clin. Endocrinol. 2018, 88, 425–431. [Google Scholar] [CrossRef]
- Budny, B.; Zemojtel, T.; Kaluzna, M.; Gut, P.; Niedziela, M.; Obara-Moszynska, M.; Rabska-Pietrzak, B.; Karmelita-Katulska, K.; Stajgis, M.; Ambroziak, U.; et al. Sema3a and igsf10 are novel contributors to combined pituitary hormone deficiency (cphd). Front. Endocrinol. 2020, 11, 368. [Google Scholar] [CrossRef]
- Ziemnicka, K.; Gut, P.; Golab, M.; Dworacki, G.; Wrotkowska, E.; Stajgis, M.; Katulska, K.; Rabska-Pietrzak, B.; Obara-Moszynska, M.; Niedziela, M.; et al. Pituitary microsomal autoantibodies in patients with childhood-onset combined pituitary hormone deficiency: An antigen identification attempt. Arch. Immunol. Ther. Exp. 2016, 64, 485–495. [Google Scholar] [CrossRef]
- Davis, S.W.; Castinetti, F.; Carvalho, L.R.; Ellsworth, B.S.; Potok, M.A.; Lyons, R.H.; Brinkmeier, M.L.; Raetzman, L.T.; Carninci, P.; Mortensen, A.H.; et al. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol. Cell. Endocrinol. 2010, 323, 4–19. [Google Scholar] [CrossRef]
- Budny, B.; Szczepanek-Parulska, E.; Zemojtel, T.; Szaflarski, W.; Rydzanicz, M.; Wesoly, J.; Handschuh, L.; Wolinski, K.; Piatek, K.; Niedziela, M.; et al. Mutations in proteasome-related genes are associated with thyroid hemiagenesis. Endocrine 2017, 56, 279–285. [Google Scholar] [CrossRef]
- Rogers, K.W.; Schier, A.F. Morphogen gradients: From generation to interpretation. Annu. Rev. Cell Dev. Biol. 2011, 27, 377–407. [Google Scholar] [CrossRef]
- Hirt, N.; Eggermann, K.; Hyrenbach, S.; Lambeck, J.; Busche, A.; Fischer, J.; Rudnik-Schoneborn, S.; Gaspar, H. Genetic dosage compensation via co-occurrence of pmp22 duplication and pmp22 deletion. Neurology 2015, 84, 1605–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zevallos, S.E.; Rizzoti, K.; Jeong, Y.; Lovell-Badge, R.; Epstein, D.J. Disruption of soxb1-dependent sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Dev. Cell 2012, 22, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Camper, S.A.; Daly, A.Z.; Stallings, C.E.; Ellsworth, B.S. Hypothalamic beta-catenin is essential for fgf8-mediated anterior pituitary growth: Links to human disease. Endocrinology 2017, 158, 3322–3324. [Google Scholar] [CrossRef] [PubMed]
- Osmundsen, A.M.; Keisler, J.L.; Taketo, M.M.; Davis, S.W. Canonical wnt signaling regulates the pituitary organizer and pituitary gland formation. Endocrinology 2017, 158, 3339–3353. [Google Scholar] [CrossRef] [PubMed]
- Sobrier, M.L.; Attie-Bitach, T.; Netchine, I.; Encha-Razavi, F.; Vekemans, M.; Amselem, S. Pathophysiology of syndromic combined pituitary hormone deficiency due to a lhx3 defect in light of lhx3 and lhx4 expression during early human development. Gene Expr. Patterns GEP 2004, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Machinis, K.; Pantel, J.; Netchine, I.; Leger, J.; Camand, O.J.; Sobrier, M.L.; Dastot-Le Moal, F.; Duquesnoy, P.; Abitbol, M.; Czernichow, P.; et al. Syndromic short stature in patients with a germline mutation in the lim homeobox lhx4. Am. J. Hum. Genet. 2001, 69, 961–968. [Google Scholar] [CrossRef]
- Pfaeffle, R.W.; Hunter, C.S.; Savage, J.J.; Duran-Prado, M.; Mullen, R.D.; Neeb, Z.P.; Eiholzer, U.; Hesse, V.; Haddad, N.G.; Stobbe, H.M.; et al. Three novel missense mutations within the lhx4 gene are associated with variable pituitary hormone deficiencies. J. Clin. Endocrinol. Metab. 2008, 93, 1062–1071. [Google Scholar] [CrossRef]
- Castinetti, F.; Saveanu, A.; Reynaud, R.; Quentien, M.H.; Buffin, A.; Brauner, R.; Kaffel, N.; Albarel, F.; Guedj, A.M.; El Kholy, M.; et al. A novel dysfunctional lhx4 mutation with high phenotypical variability in patients with hypopituitarism. J. Clin. Endocrinol. Metab. 2008, 93, 2790–2799. [Google Scholar] [CrossRef]
- Tajima, T.; Ohtake, A.; Hoshino, M.; Amemiya, S.; Sasaki, N.; Ishizu, K.; Fujieda, K. Otx2 loss of function mutation causes anophthalmia and combined pituitary hormone deficiency with a small anterior and ectopic posterior pituitary. J. Clin. Endocrinol. Metab. 2009, 94, 314–319. [Google Scholar] [CrossRef]
- Dateki, S.; Kosaka, K.; Hasegawa, K.; Tanaka, H.; Azuma, N.; Yokoya, S.; Muroya, K.; Adachi, M.; Tajima, T.; Motomura, K.; et al. Heterozygous orthodenticle homeobox 2 mutations are associated with variable pituitary phenotype. J. Clin. Endocrinol. Metab. 2010, 95, 756–764. [Google Scholar] [CrossRef]
- Oliver, G.; Mailhos, A.; Wehr, R.; Copeland, N.G.; Jenkins, N.A.; Gruss, P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 1995, 121, 4045–4055. [Google Scholar] [PubMed]
- Wallis, D.E.; Roessler, E.; Hehr, U.; Nanni, L.; Wiltshire, T.; Richieri-Costa, A.; Gillessen-Kaesbach, G.; Zackai, E.H.; Rommens, J.; Muenke, M. Mutations in the homeodomain of the human six3 gene cause holoprosencephaly. Nat. Genet. 1999, 22, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Anchan, R.M.; Lachke, S.A.; Gerami-Naini, B.; Lindsey, J.; Ng, N.; Naber, C.; Nickerson, M.; Cavallesco, R.; Rowan, S.; Eaton, J.L.; et al. Pax6- and six3-mediated induction of lens cell fate in mouse and human es cells. PLoS ONE 2014, 9, e115106. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hoffmann, H.M.; Meadows, J.D.; Mayo, S.L.; Trang, C.; Leming, S.S.; Maruggi, C.; Davis, S.W.; Larder, R.; Mellon, P.L. Homeodomain proteins six3 and six6 regulate gonadotrope-specific genes during pituitary development. Mol. Endocrinol. 2015, 29, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Gaston-Massuet, C.; Andoniadou, C.L.; Signore, M.; Sajedi, E.; Bird, S.; Turner, J.M.; Martinez-Barbera, J.P. Genetic interaction between the homeobox transcription factors hesx1 and six3 is required for normal pituitary development. Dev. Biol. 2008, 324, 322–333. [Google Scholar] [CrossRef]
- Sajedi, E.; Gaston-Massuet, C.; Signore, M.; Andoniadou, C.L.; Kelberman, D.; Castro, S.; Etchevers, H.C.; Gerrelli, D.; Dattani, M.T.; Martinez-Barbera, J.P. Analysis of mouse models carrying the i26t and r160c substitutions in the transcriptional repressor hesx1 as models for septo-optic dysplasia and hypopituitarism. Dis. Models Mech. 2008, 1, 241–254. [Google Scholar] [CrossRef]
- Gaston-Massuet, C.; Kelberman, D.; Dattani, M.; Martinez-Barbera, J.P. Absence of six3 mutations in patients with congenital hypopituitarism. Am. J. Med. Genet. A 2009, 149A, 2874–2876. [Google Scholar] [CrossRef]

| Patient | Type | Cytoband | Size (Kbp) | Gene Number | Gene Name | HGVS |
|---|---|---|---|---|---|---|
| 1 | Loss | 1q22 | 54.284 | 2 | ASH1L, POU5F1P4 | arr[hg19] 1q22(155,394,484-155,448,768)x1 |
| 2 | Gain | 1q25.2 | 95.535 | 4 | QSOX1, FLJ23867, LHX4, LOC100527964 | arr[hg19] 1q25.2(180,143,701-180,239,236)x3 |
| 3 | Gain | 2p21 | 23.746 | 1 | SIX3 | arr[hg19] 2p21(45,151,117-45,174,863)x3 |
| 4 | Gain | 2p21 | 27.832 | 1 | SIX3 | arr[hg19] 2p21(45,151,117-45,178,949)x3 |
| 5 | Gain | 2p25.3 | 536.643 | 8 | TSSC1, TRAPPC12, ADI1, RNASEH1, LOC100506054, RPS7, COLEC11, ALLC | arr[hg19] 2p25.3(3,232,368-3,769,011)x3 |
| 6 | Gain | 2p25.3 | 475.292 | 8 | TSSC1, TRAPPC12, ADI1, RNASEH1, LOC100506054, RPS7, COLEC11, ALLC | arr[hg19] 2p25.3(3,339,014-3,814,306)x3 |
| 7 | Gain | 3p14.3 | 131.223 | 3 | IL17RD, HESX1, APPL1 | arr[hg19] 3p14.3(57,149,424-57,280,647)x3 |
| 8 | Gain | 14q22.3 | 46.766 | 2 | OTX2 | arr[hg19] 14q22.3(57251373-57298139)x3 |
| 9 | Gain | 14q22.3 | 47.781 | 2 | OTX2 | arr[hg19] 14q22.3(5763675-57311456)x3 |
| 10 | Gain | 14q22.3 | 24.896 | 2 | OTX2 | arr[hg19] 14q22.3(57263675-57288571)x3 |
| 11 | Loss | 18q12.3 | 373.468 | 1 | SLC14A2 | arr[hg19] 18q12.3(42,689,695-43,063,163)x1 |
| 12 | Loss | 18q12.3 | 390.92 | 1 | SLC14A2 | arr[hg19] 18q12.3(42,681,091-43,072,011)x1 |
| Case | Gene(s) | Gender | Age at Diagnosis | Pituitary Hormone Deficiency | MRI of Pituitary | ||||
|---|---|---|---|---|---|---|---|---|---|
| GH | Gn | TSH | PRL | ACTH | |||||
| 1 | ASH1L, POU5F1P4 | M | 13 y.o. | + | + | + | − | + | Pituitary hypoplasia |
| 2 | QSOX1, FLJ23867, LHX4, LOC100527964 | F | 9 y.o. | + | + | + | − | − | PSIS |
| 3 | SIX3 | M | 12 y.o | + | + | + | − | + | Pituitary hypoplasia |
| 4 | SIX3 | M | 15 y.o | + | + | + | + | + | Pituitary hypoplasia |
| 5 | TSSC1, TRAPPC12, ADI1, RNASEH1, LOC100506054, RPS7, COLEC11, ALLC | M | 12 y.o | + | + | + | − | − | Pituitary hypoplasia |
| 6 | TSSC1, TRAPPC12, ADI1, RNASEH1, LOC100506054, RPS7, COLEC11, ALLC | F | 24 y.o. | + | + | + | − | − | PSIS |
| 7 | IL17RD, HESX1, APPL1 | M | 12 y.o. | + | + | + | − | + | Pituitary hypoplasia |
| 8 | OTX2 | F | 10 y.o. | + | + | + | − | + | PSIS |
| 9 | OTX2 | F | 6 y.o. | + | + | − | − | − | Pituitary hypoplasia |
| 10 | OTX2 | M | 15 y.o. | + | + | + | − | − | Pituitary hypoplasia |
| 11 | SLC14A2 | F | 8 y.o. | + | + | + | − | − | Pituitary hypoplasia |
| 12 | SLC14A2 | F | 8 y.o. | + | + | + | − | − | Pituitary hypoplasia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budny, B.; Karmelita-Katulska, K.; Stajgis, M.; Żemojtel, T.; Ruchała, M.; Ziemnicka, K. Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency. Int. J. Mol. Sci. 2020, 21, 5757. https://doi.org/10.3390/ijms21165757
Budny B, Karmelita-Katulska K, Stajgis M, Żemojtel T, Ruchała M, Ziemnicka K. Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency. International Journal of Molecular Sciences. 2020; 21(16):5757. https://doi.org/10.3390/ijms21165757
Chicago/Turabian StyleBudny, Bartłomiej, Katarzyna Karmelita-Katulska, Marek Stajgis, Tomasz Żemojtel, Marek Ruchała, and Katarzyna Ziemnicka. 2020. "Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency" International Journal of Molecular Sciences 21, no. 16: 5757. https://doi.org/10.3390/ijms21165757
APA StyleBudny, B., Karmelita-Katulska, K., Stajgis, M., Żemojtel, T., Ruchała, M., & Ziemnicka, K. (2020). Copy Number Variants Contributing to Combined Pituitary Hormone Deficiency. International Journal of Molecular Sciences, 21(16), 5757. https://doi.org/10.3390/ijms21165757





