The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans
Abstract
1. Introduction
2. Results
2.1. When Perfused at A Concentration Equivalent to Human Cmax, Linagliptin Enhances the Contractile Recovery of Mouse Hearts after Ischemia Reperfusion
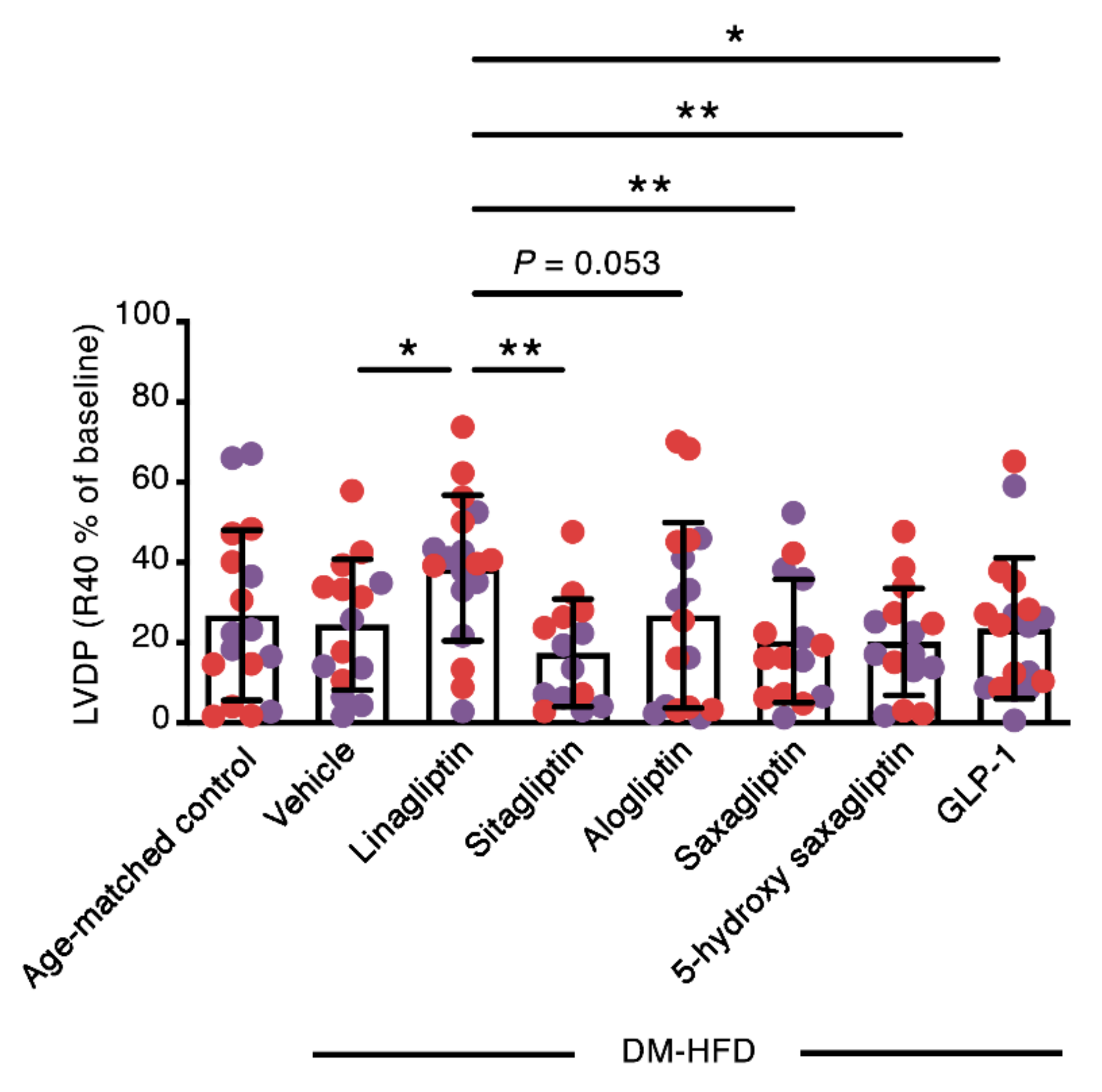
2.2. Linagliptin Enhances the Contractile Recovery of The Hearts of Aged, Diabetic High Fat Diet-Fed Mice
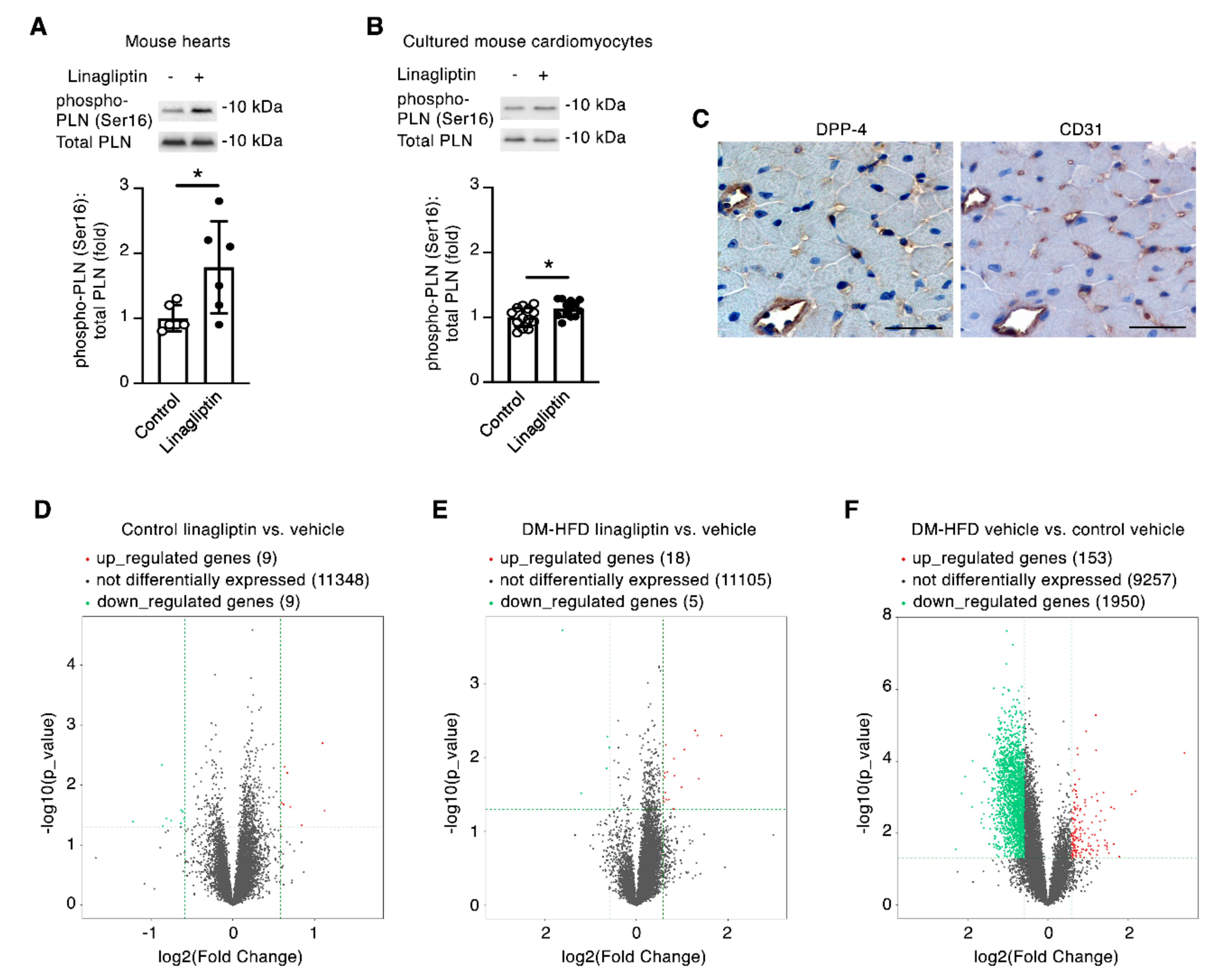
2.3. Linagliptin Induces Phosphorylation of the Cardiomyocyte Ca2+ Handling Protein Phospholamban
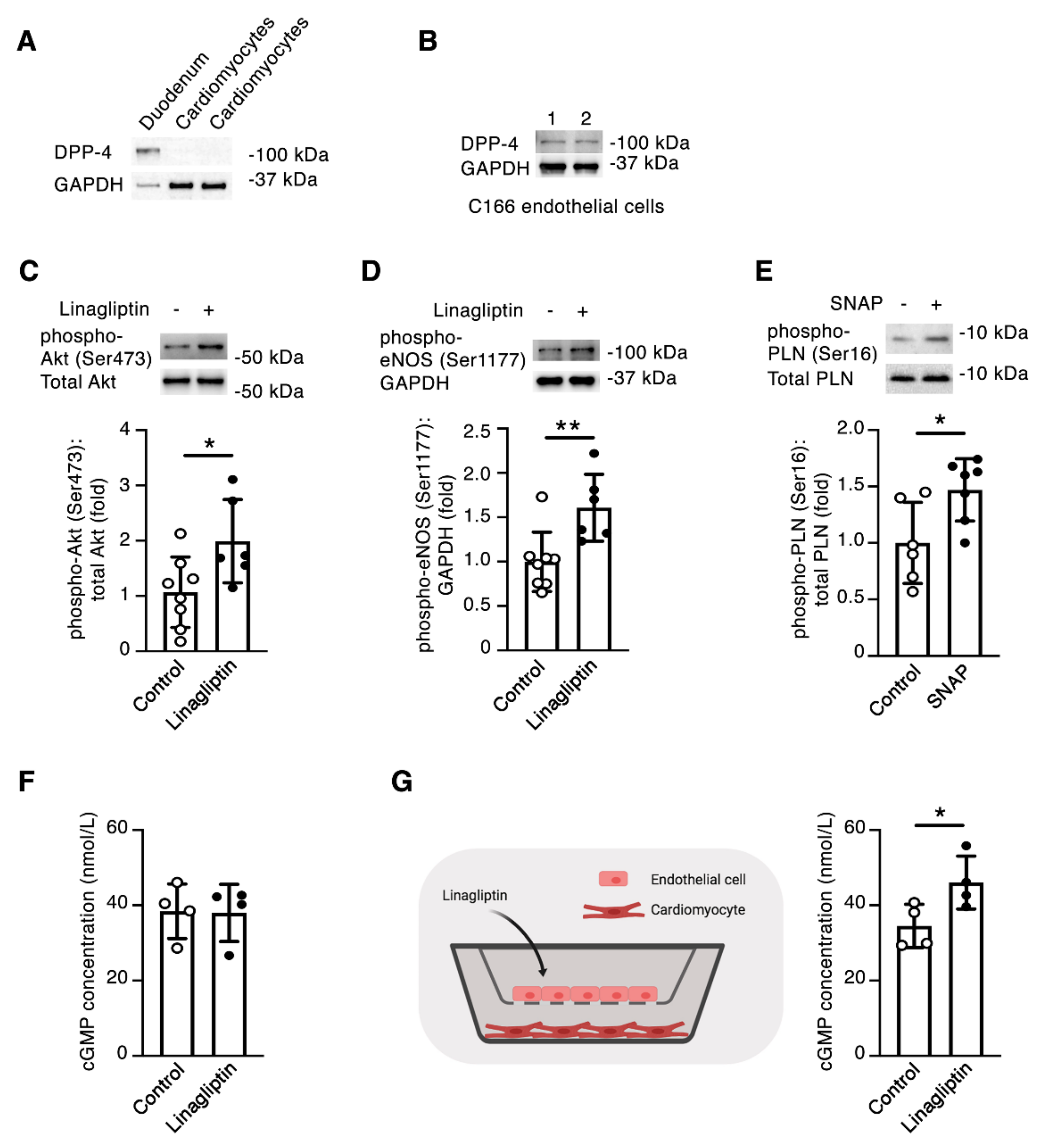
2.4. Linagliptin Directly Induces Intracellular Signaling in Endothelial Cells and Indirectly Induces Intracellular Signaling in Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Isolated Heart Perfusion Study 1
4.2. Isolated Heart Perfusion Study 2
4.3. Cell Culture
4.4. Immunoblotting
4.5. Immunohistochemistry
4.6. RNA Sequencing
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisman, E.Z.; Tenenbaum, A. Antidiabetic treatment with gliptins: Focus on cardiovascular effects and outcomes. Cardiovasc. Diabetol. 2015, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goldenberg, R.M.; Bhatt, D.L.; Farkouh, M.E.; Quan, A.; Teoh, H.; Connelly, K.A.; Leiter, L.A.; Friedrich, J.O. Dipeptidyl peptidase-4 inhibitors and the risk of heart failure: A systematic review and meta-analysis. CMAJ Open 2017, 5, E152–E177. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Alexander, J.H.; Johansen, O.E.; Perkovic, V.; Rosenstock, J.; Cooper, M.E.; Wanner, C.; Kahn, S.E.; Toto, R.D.; Zinman, B.; et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation 2019, 139, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef]
- Batchu, S.N.; Thieme, K.; Zadeh, F.H.; Alghamdi, T.A.; Yerra, V.G.; Hadden, M.J.; Majumder, S.; Kabir, M.G.; Bowskill, B.B.; Ladha, D.; et al. The Dipeptidyl Peptidase 4 Substrate CXCL12 Has Opposing Cardiac Effects in Young Mice and Aged Diabetic Mice Mediated by Ca(2+) Flux and Phosphoinositide 3-Kinase gamma. Diabetes 2018, 67, 2443–2455. [Google Scholar] [CrossRef]
- Blenck, C.L.; Harvey, P.A.; Reckelhoff, J.F.; Leinwand, L.A. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ. Res. 2016, 118, 1294–1312. [Google Scholar] [CrossRef]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef]
- Marks, A.R. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J. Clin. Investig. 2013, 123, 46–52. [Google Scholar] [CrossRef]
- Shigeta, T.; Aoyama, M.; Bando, Y.K.; Monji, A.; Mitsui, T.; Takatsu, M.; Cheng, X.W.; Okumura, T.; Hirashiki, A.; Nagata, K.; et al. Dipeptidyl Peptidase-4 Modulates Left Ventricular Dysfunction in Chronic Heart Failure via Angiogenesis-Dependent and -Independent Actions. Circulation 2012, 126, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Otani, H. The role of nitric oxide in myocardial repair and remodeling. Antioxid. Redox Signal 2009, 11, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Koss, K.L.; Kranias, E.G. Phospholamban: A prominent regulator of myocardial contractility. Circ. Res. 1996, 79, 1059–1063. [Google Scholar] [CrossRef]
- Shah, Z.; Pineda, C.; Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Racoma, I.; Deiuliis, J.; Xu, X.; Sun, Q.; Moffatt-Bruce, S.; et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul. Pharmacol. 2011, 55, 2–9. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, R.; Kondo, K.; Kambara, T.; Shimizu, Y.; Tanigawa, T.; Bando, Y.K.; Nishimura, M.; Ouchi, N.; Murohara, T. Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 2014, 289, 27235–27245. [Google Scholar] [CrossRef]
- Vellecco, V.; Mitidieri, E.; Gargiulo, A.; Brancaleone, V.; Matassa, D.; Klein, T.; Esposito, F.; Cirino, G.; Bucci, M. Vascular effects of linagliptin in non-obese diabetic mice are glucose-independent and involve positive modulation of the endothelial nitric oxide synthase (eNOS)/caveolin-1 (CAV-1) pathway. Diabetes Obes. Metab. 2016, 18, 1236–1243. [Google Scholar] [CrossRef]
- Schulz, R.; Kelm, M.; Heusch, G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 402–413. [Google Scholar] [CrossRef]
- Colyer, J. Phosphorylation states of phospholamban. Ann. N. Y. Acad. Sci. 1998, 853, 79–91. [Google Scholar] [CrossRef]
- Kojda, G.; Kottenberg, K. Regulation of basal myocardial function by NO. Cardiovasc. Res. 1999, 41, 514–523. [Google Scholar] [CrossRef]
- Massion, P.B.; Balligand, J.L. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): Lessons from genetically modified mice. J. Physiol. 2003, 546, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Spurgeon, H.A.; Sollott, S.J.; Talo, A.; Lakatta, E.G. 8-bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ. Res. 1994, 74, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Flogel, U.; Decking, U.K.; Godecke, A.; Schrader, J. Contribution of NO to ischemia-reperfusion injury in the saline-perfused heart: A study in endothelial NO synthase knockout mice. J. Mol. Cell Cardiol. 1999, 31, 827–836. [Google Scholar] [CrossRef]
- Kanno, S.; Lee, P.C.; Zhang, Y.; Ho, C.; Griffith, B.P.; Shears, L.L., 2nd; Billiar, T.R. Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 2000, 101, 2742–2748. [Google Scholar] [CrossRef]
- Nabeno, M.; Akahoshi, F.; Kishida, H.; Miyaguchi, I.; Tanaka, Y.; Ishii, S.; Kadowaki, T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Commun. 2013, 434, 191–196. [Google Scholar]
- Berger, J.P.; SinhaRoy, R.; Pocai, A.; Kelly, T.M.; Scapin, G.; Gao, Y.D.; Pryor, K.A.D.; Wu, J.K.; Eiermann, G.J.; Xu, S.S.; et al. A comparative study of the binding properties, dipeptidyl peptidase-4 (DPP-4) inhibitory activity and glucose-lowering efficacy of the DPP-4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol. Diabetes Metab. 2018, 1, e00002. [Google Scholar] [CrossRef]
- Schnapp, G.; Klein, T.; Hoevels, Y.; Bakker, R.A.; Nar, H. Comparative Analysis of Binding Kinetics and Thermodynamics of Dipeptidyl Peptidase-4 Inhibitors and Their Relationship to Structure. J. Med. Chem. 2016, 59, 7466–7477. [Google Scholar]
- Ju, H.; Zou, R.; Venema, V.J.; Venema, R.C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997, 272, 18522–18525. [Google Scholar] [CrossRef]
- Batchu, S.N.; Law, E.; Brocks, D.R.; Falck, J.R.; Seubert, J.M. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J. Mol. Cell Cardiol. 2009, 46, 67–74. [Google Scholar] [CrossRef]
- Golightly, L.K.; Drayna, C.C.; McDermott, M.T. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin. Pharm. 2012, 51, 501–514. [Google Scholar] [CrossRef]
- Sarashina, A.; Sesoko, S.; Nakashima, M.; Hayashi, N.; Taniguchi, A.; Horie, Y.; Graefe-Mody, E.U.; Woerle, H.J.; Dugi, K.A. Linagliptin a dipeptidyl peptidase-4 inhibitor in development for the treatment of type 2 diabetes mellitus: A Phase I, randomized, double-blind, placebo-controlled trial of single and multiple escalating doses in healthy adult male Japanese subjects. Clin. Ther. 2010, 32, 1188–1204. [Google Scholar] [CrossRef]
- Bergman, A.; Ebel, D.; Liu, F.; Stone, J.; Wang, A.; Zeng, W.; Chen, L.; Dilzer, S.; Lasseter, K.; Herman, G.; et al. Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm. Drug Dispos. 2007, 28, 315–322. [Google Scholar] [CrossRef]
- Christopher, R.; Covington, P.; Davenport, M.; Fleck, P.; Mekki, Q.A.; Wann, E.R.; Karim, A. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin. Ther. 2008, 30, 513–527. [Google Scholar] [CrossRef]
- Christopher, R.; Karim, A. Clinical pharmacology of alogliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of Type 2 diabetes. Expert. Rev. Clin. Pharmacol. 2009, 2, 589–600. [Google Scholar] [CrossRef]
- Wang, Q.D.; Tokuno, S.; Valen, G.; Sjoquist, P.O.; Thoren, P. Cyclic fluctuations in the cardiac performance of the isolated Langendorff-perfused mouse heart: Pyruvate abolishes the fluctuations and has an anti-ischaemic effect. Acta Physiol. Scand. 2002, 175, 279–287. [Google Scholar] [CrossRef]
- Vakkalagadda, B.; Vetter, M.L.; Rana, J.; Smith, C.H.; Huang, J.; Karkas, J.; Boulton, D.W.; LaCreta, F. Bioequivalence of saxagliptin/dapagliflozin fixed-dose combination tablets compared with coadministration of the individual tablets to healthy subjects. Pharmacol. Res. Perspect 2015, 3, e00201. [Google Scholar]
- Nagai, K.; Tsuchiya, K.; Ezaki, T.; Tsuchiya, M.; Ohgawara, H. Effect of GLP-1 (glucagon-like peptide 1:7-36 amide) on porcine pancreatic endocrine cell proliferation and insulin secretion. Pancreas 2004, 28, 138–145. [Google Scholar] [CrossRef]
- Petroff, M.G.V.; Egan, J.M.; Wang, X.; Sollott, S.J. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ. Res. 2001, 89, 445–452. [Google Scholar] [CrossRef]
- Ackers-Johnson, M.; Li, P.Y.; Holmes, A.P.; O’Brien, S.M.; Pavlovic, D.; Foo, R.S.; Simplified, A. Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ. Res. 2016, 119, 909–920. [Google Scholar] [CrossRef]
- Gu, M.; Brecher, P. Nitric oxide-induced increase in p21(Sdi1/Cip1/Waf1) expression during the cell cycle in aortic adventitial fibroblasts. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 27–34. [Google Scholar] [CrossRef][Green Version]
- Mayer, E.J.; Huckle, W.; Johnson, R.G., Jr.; McKenna, E. Characterization and quantitation of phospholamban and its phosphorylation state using antibodies. Biochem. Biophys. Res. Commun. 2000, 267, 40–48. [Google Scholar] [CrossRef]
- Asahi, M.; Otsu, K.; Nakayama, H.; Hikoso, S.; Takeda, T.; Gramolini, A.O.; Trivieri, M.G.; Oudit, G.Y.; Morita, T.; Kusakari, Y.; et al. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 9199–9204. [Google Scholar] [CrossRef]
- Advani, A.; Kelly, D.J.; Cox, A.J.; White, K.E.; Advani, S.L.; Thai, K.; Connelly, K.A.; Yuen, D.; Trogadis, J.; Herzenberg, A.M.; et al. The (Pro)renin receptor: Site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 2009, 54, 261–269. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
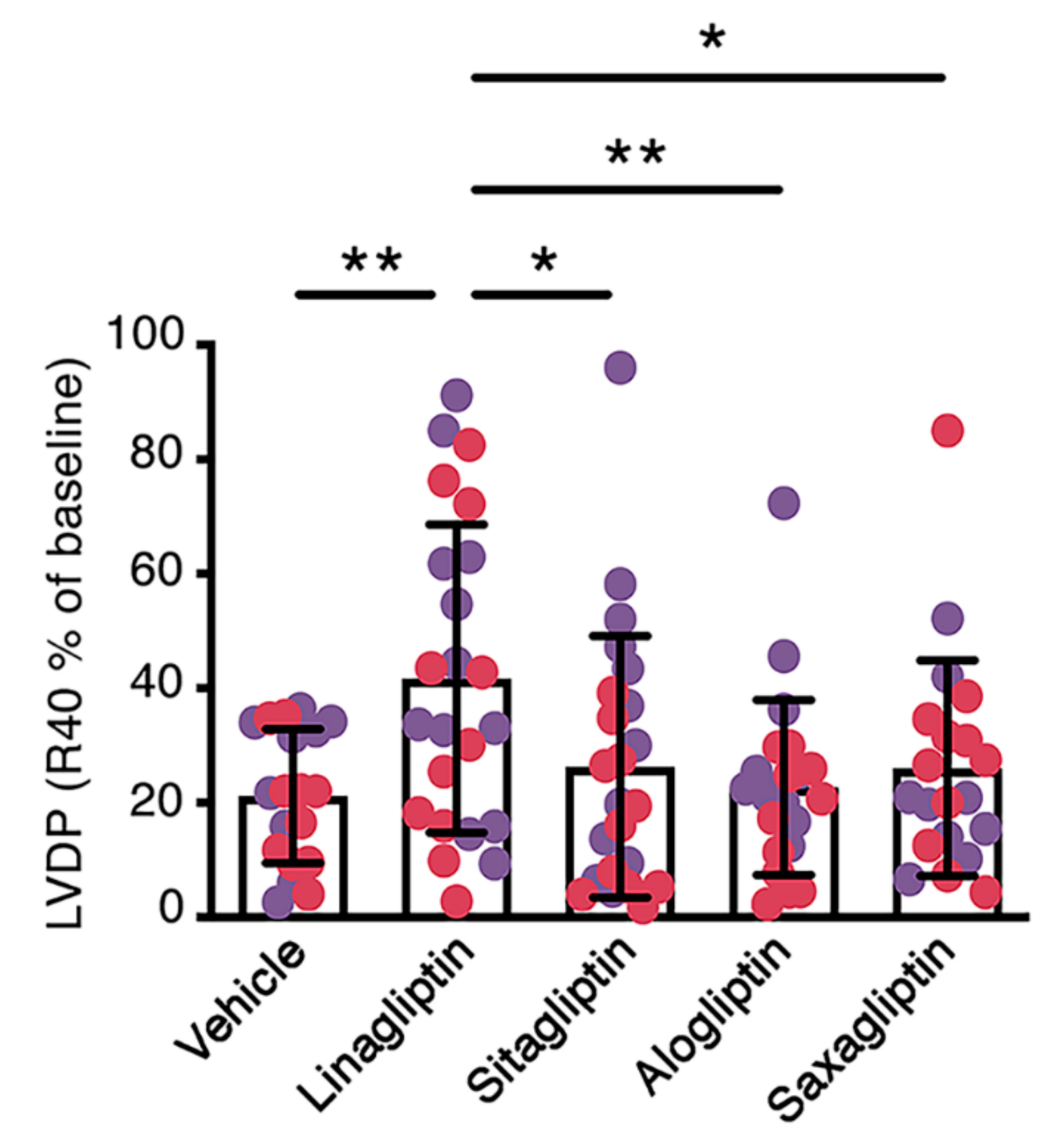
| LVDP (cmH2O) | dP/dtmax (cmH2O × ms−1) | dP/dtmin (cmH2O × ms−1) | Heart Rate (bpm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | R40 | Baseline | R40 | Baseline | R40 | Baseline | R40 | |
| Vehicle | 19 | 94 ± 32 | 21 ± 16 | 3493 ± 1527 | 1268 ± 904 | −2821 ± 979 | −1127 ± 746 | 278 ± 86 | 348 ± 89 |
| Linagliptin | 23 | 95 ± 35 | 42 ± 32 a | 3957 ± 1957 | 2426 ± 2025 | −2762 ± 1080 | −1782 ± 1207 | 306 ± 77 | 377 ± 90 |
| Sitagliptin | 23 | 84 ± 29 | 21 ± 16 b | 4058 ± 2317 | 1667 ± 1188 | −2706 ± 1063 | −1335 ± 840 | 277 ± 95 | 340 ± 89 |
| Alogliptin | 22 | 88 ± 43 | 20 ± 18 b | 4134 ± 2722 | 1492 ± 1077 | −2930 ± 1486 | −1289 ± 908 | 294 ± 97 | 331 ± 87 |
| Saxagliptin | 20 | 90 ± 36 | 23 ± 18 c | 3898 ± 2522 | 1765 ± 1632 | −2765 ± 1158 | −1297 ± 894 | 300 ± 109 | 332 ± 121 |
| LVDP (cmH2O) | dP/dtmax (cmH2O × ms−1) | dP/dtmin (cmH2O × ms−1) | Heart Rate (bpm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | R40 | Baseline | R40 | Baseline | R40 | Baseline | R40 | |
| Age-matched control + Vehicle | 17 | 101 ± 30 | 25 ± 19 | 3777 ± 1913 | 1274 ± 1011 | −2600 ± 960 | −1295 ± 1240 | 275 ± 101 | 337 ± 98 |
| DM-HFD + Vehicle | 15 | 90 ± 27 | 21 ± 14 | 3433 ± 1021 | 1266 ± 796 | −2541 ± 604 | −1152 ± 692 | 295 ± 83 | 293 ± 139 |
| DM-HFD + Linagliptin | 18 | 103 ± 22 | 38 ± 16 ab | 3951 ± 1856 | 1920 ± 1295 | −2763 ± 946 | −1639 ± 1521 | 288 ± 87 | 327 ± 86 |
| DM-HFD + Sitagliptin | 14 | 81 ± 39 | 14 ± 11 c | 3139 ± 1894 | 1065 ± 870 | −2156 ± 1143 | −913 ± 760 | 311 ± 91 | 320 ± 108 |
| DM-HFD + Alogliptin | 17 | 83 ± 28 | 20 ± 19 d | 3422 ± 2024 | 1212 ± 949 | −2483 ± 1361 | −1313 ± 1475 | 297 ± 114 | 362 ± 148 |
| DM-HFD + Saxagliptin | 15 | 81 ± 15 | 15 ± 11 c | 3219 ± 1620 | 1156 ± 1099 | −2251 ± 993 | −957 ± 842 | 278 ± 94 | 321 ± 135 |
| DM-HFD + 5-hydroxy saxagliptin | 15 | 69 ± 54 | 14 ± 11 ac | 2578 ± 2275 | 805 ± 871 | −1744 ± 1393 aef | −672 ± 732 | 237 ± 73 | 317 ± 106 |
| DM-HFD + GLP-1 | 18 | 96 ± 39 | 21 ± 17 e | 3929 ± 1486 | 1400 ± 937 | −2981 ± 839 gh | −1287 ± 758 | 311 ± 93 | 346 ± 91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batchu, S.N.; Yerra, V.G.; Liu, Y.; Advani, S.L.; Klein, T.; Advani, A. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans. Int. J. Mol. Sci. 2020, 21, 5756. https://doi.org/10.3390/ijms21165756
Batchu SN, Yerra VG, Liu Y, Advani SL, Klein T, Advani A. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans. International Journal of Molecular Sciences. 2020; 21(16):5756. https://doi.org/10.3390/ijms21165756
Chicago/Turabian StyleBatchu, Sri Nagarjun, Veera Ganesh Yerra, Youan Liu, Suzanne L. Advani, Thomas Klein, and Andrew Advani. 2020. "The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans" International Journal of Molecular Sciences 21, no. 16: 5756. https://doi.org/10.3390/ijms21165756
APA StyleBatchu, S. N., Yerra, V. G., Liu, Y., Advani, S. L., Klein, T., & Advani, A. (2020). The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans. International Journal of Molecular Sciences, 21(16), 5756. https://doi.org/10.3390/ijms21165756






