Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues
Abstract
1. Introduction
2. Material and Methods
2.1. Material—Reagents, Drugs, and Excipients
2.2. Phase Solubility Study
2.3. Isothermal Titration Calorimetry
2.4. Collapse Temperature
2.5. Preparation of Freeze-Dried HP-β-CD-CAP
2.6. Scanning Electron Microscopy
2.7. Differential Scanning Calorimetry
2.8. Stability
2.9. Powder X-ray Diffraction
2.10. Nuclear Magnetic Resonance
2.11. In Vitro Release Experiments
2.12. Evaluation of Analgesia in Inflamed Tissue
3. Results and Discussion
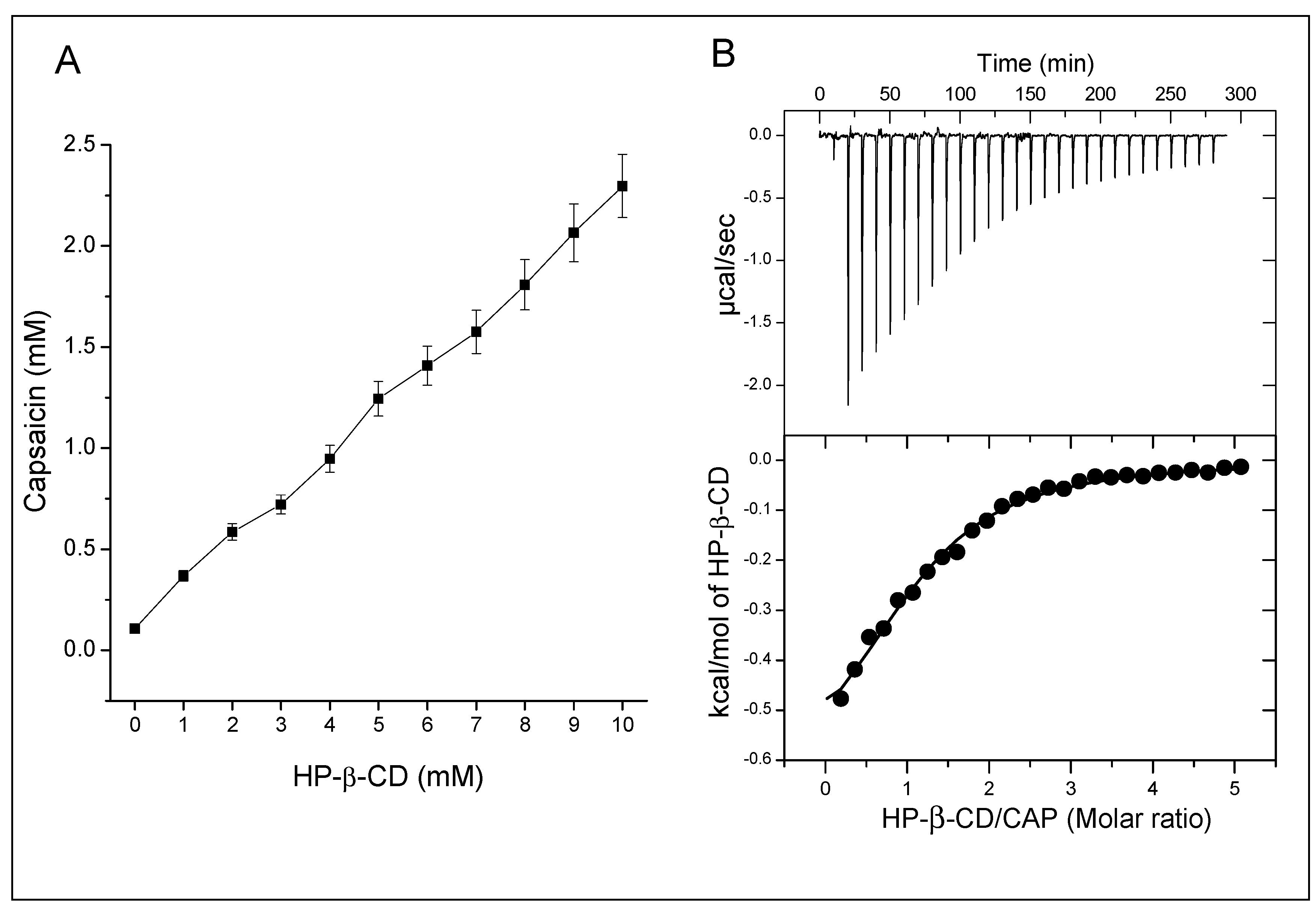
3.1. Phase Solubility Profile
3.2. Isothermal Titration Calorimetry
3.3. Development of Freeze-Dried HP-β-CD-CAP
3.4. Freeze-Dried CAP Complex
3.5. Characterization of the HP-β-CD-CAP Complex
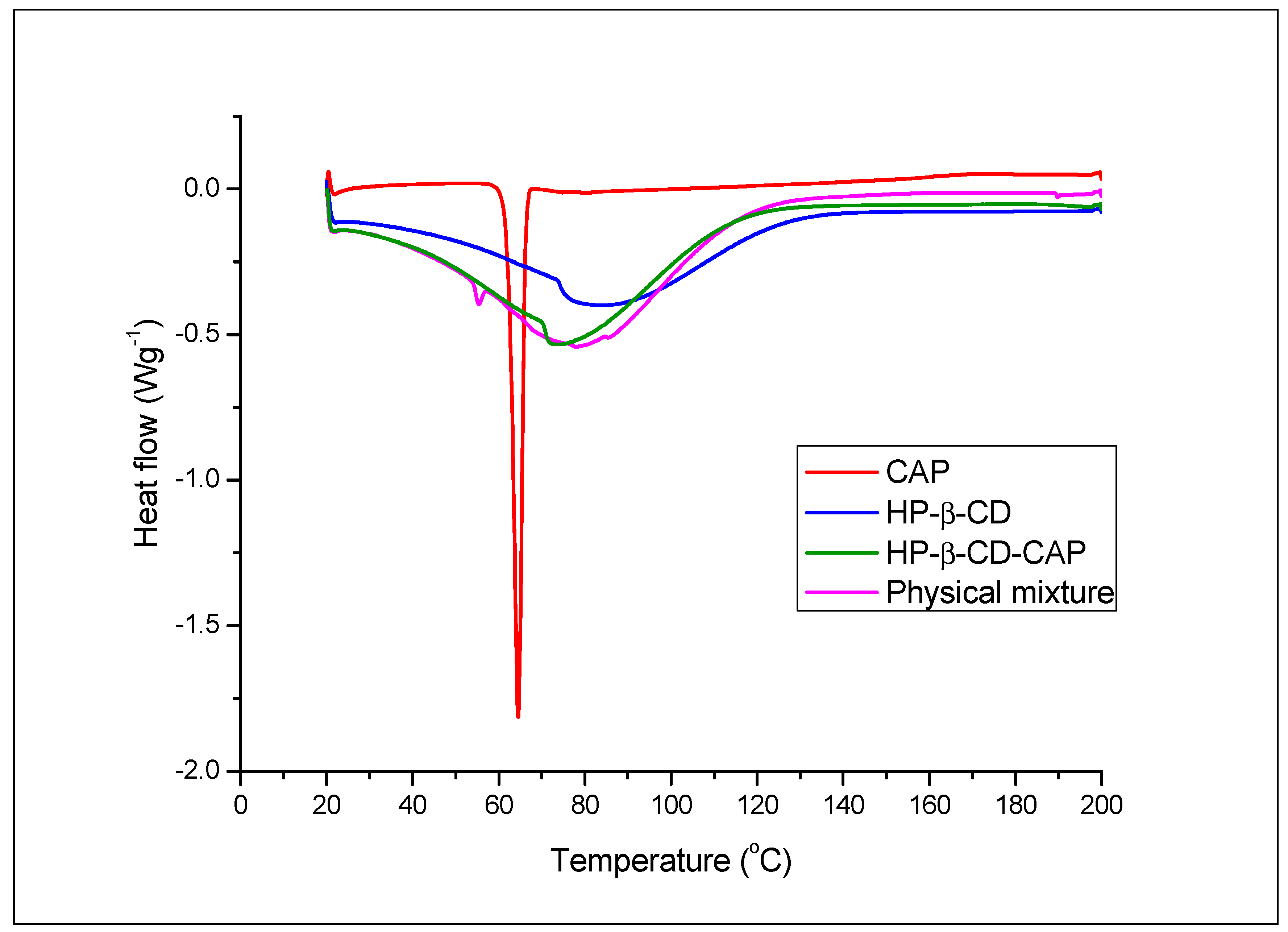
3.5.1. Differential Scanning Calorimetry
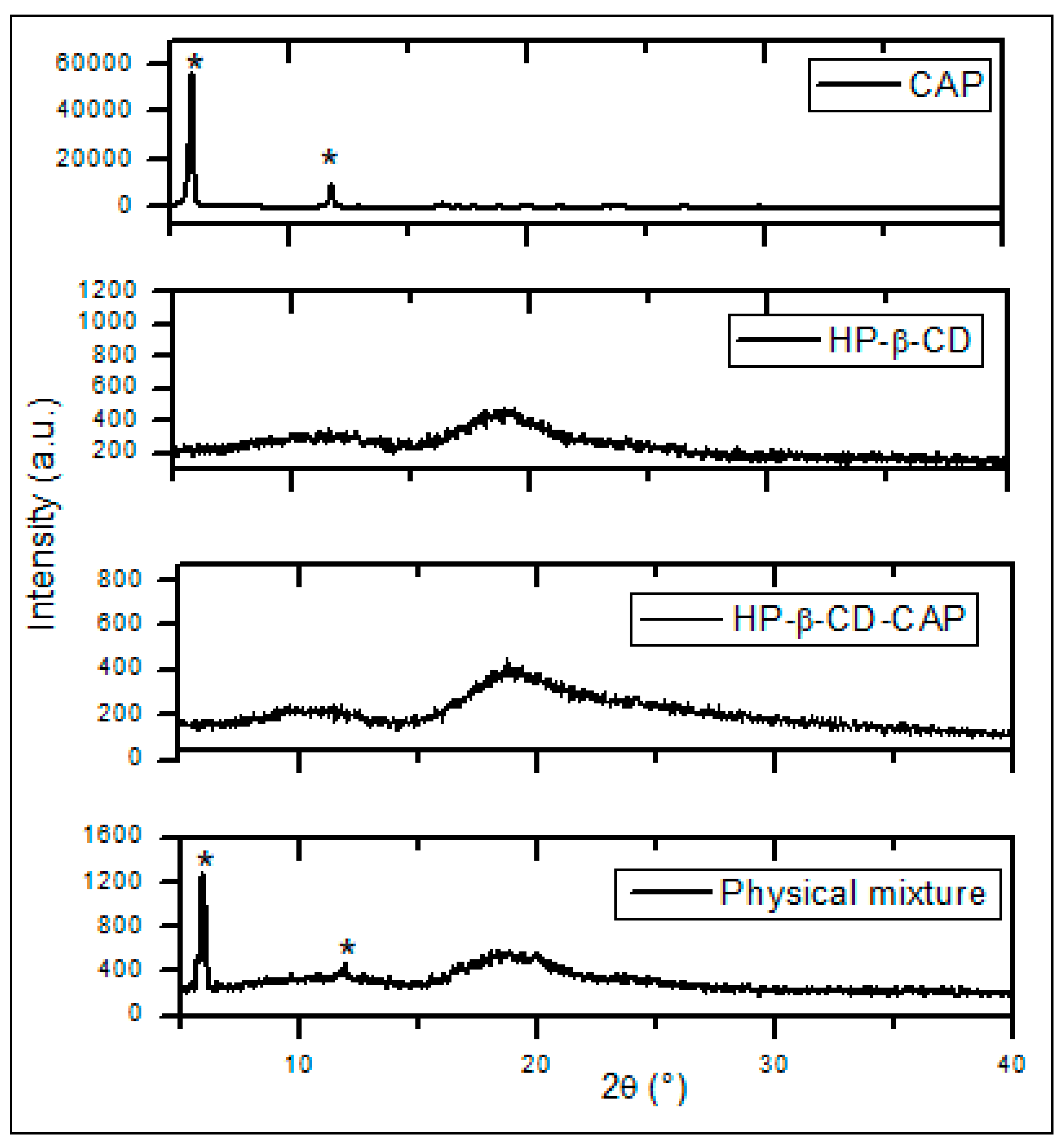
3.5.2. Powder X-ray Diffraction
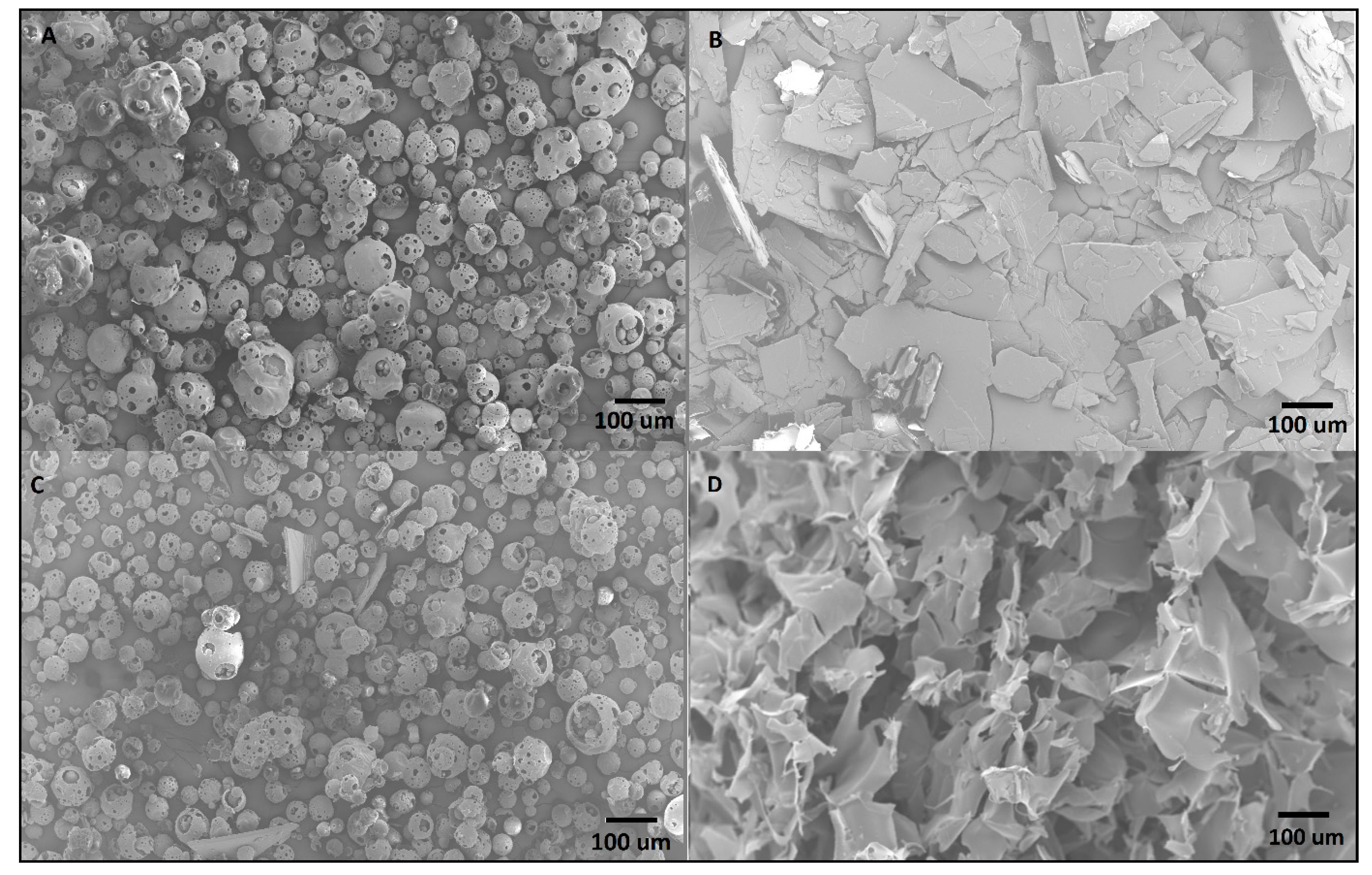
3.5.3. Scanning Electronic Microscopy
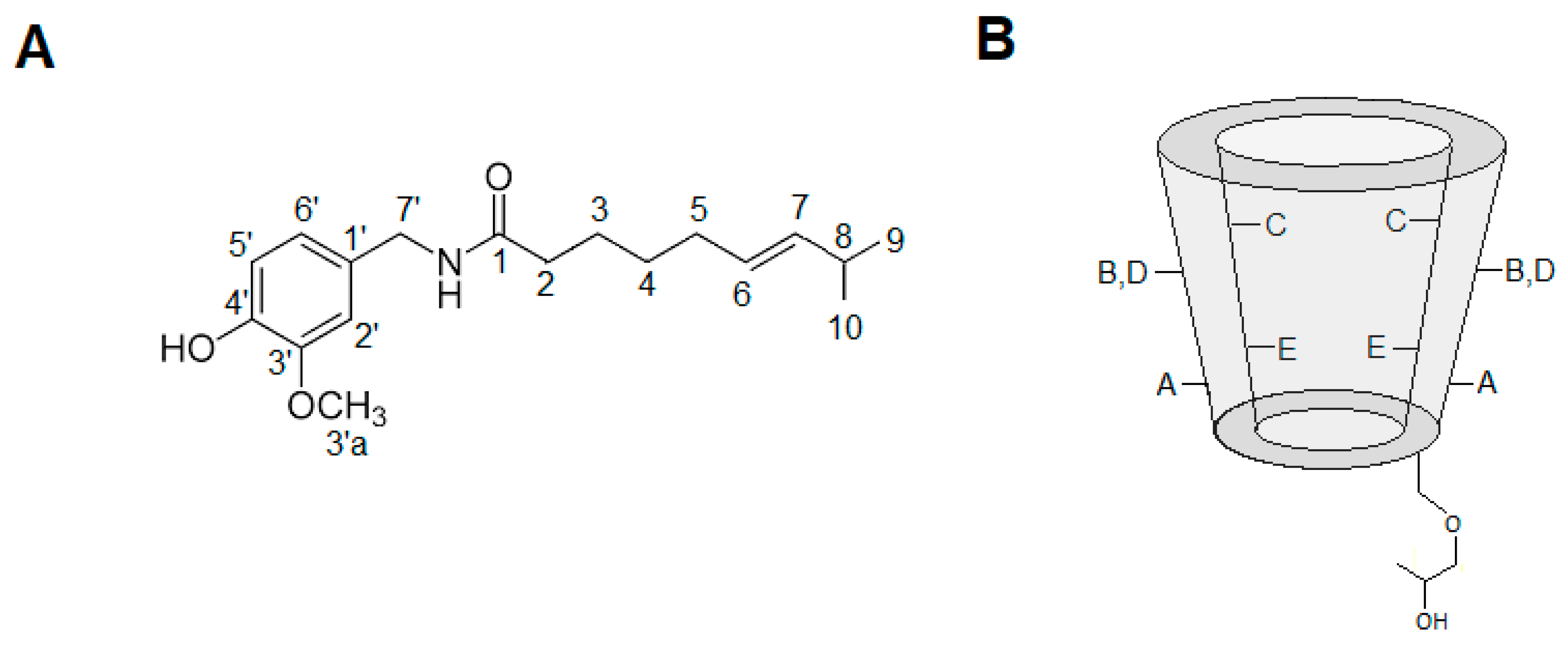
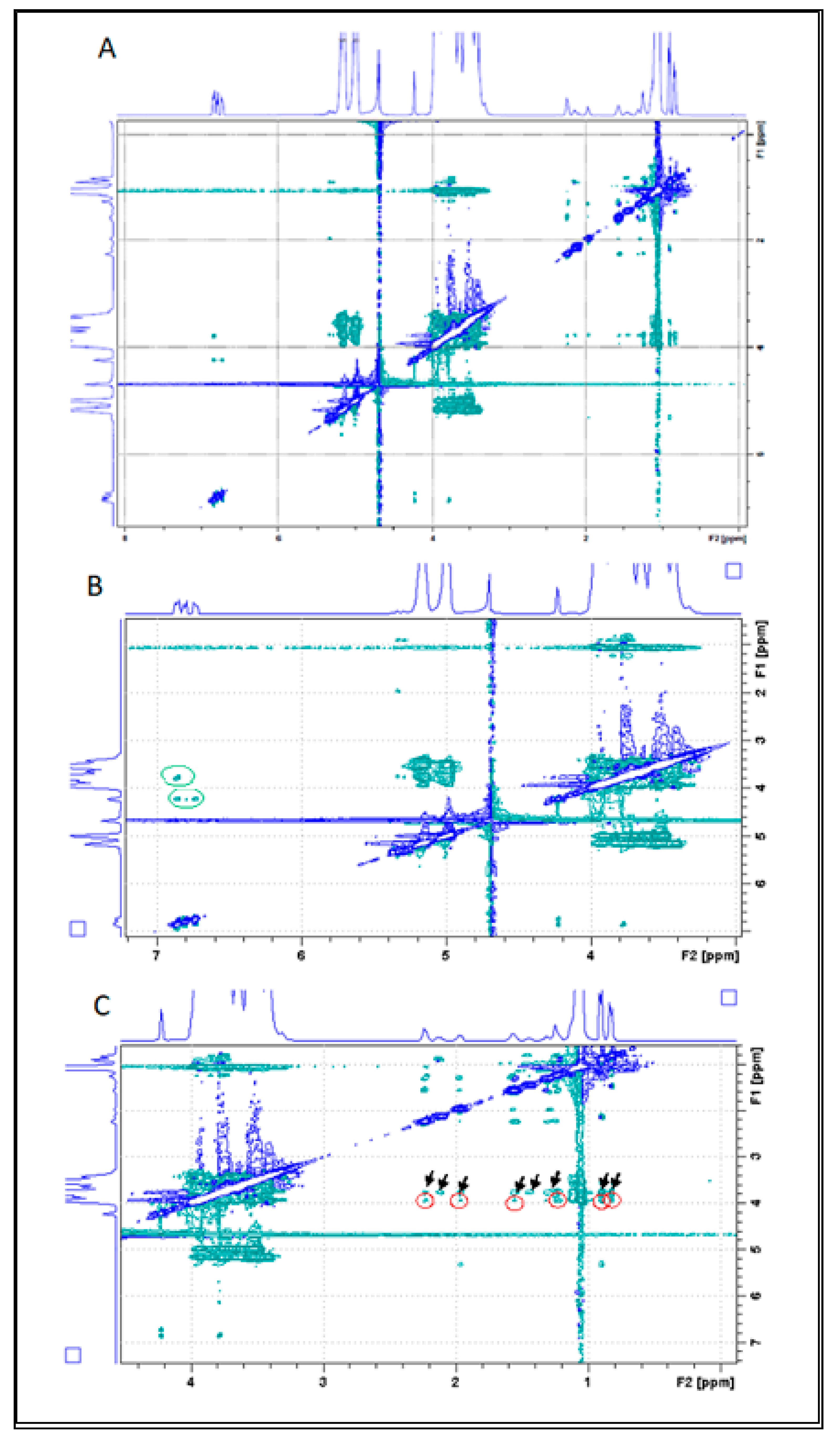
3.5.4. Hydrogen Nuclear Magnetic Resonance
3.5.5. Rotating Frame Overhauser Enhancement Spectroscopy (ROESY)
3.5.6. Diffusion Ordered Spectroscopy—DOSY
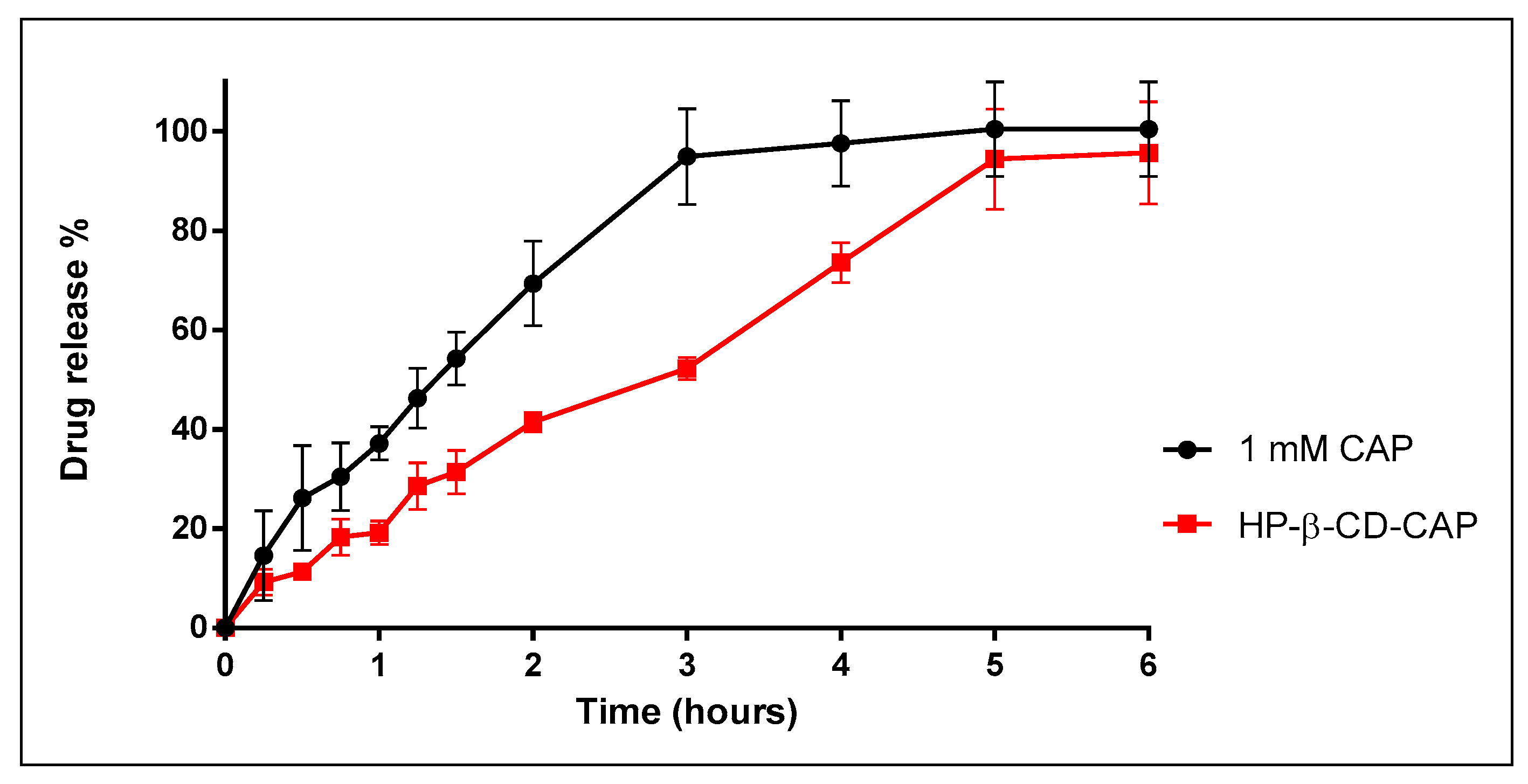
3.6. In Vitro Release Kinetics
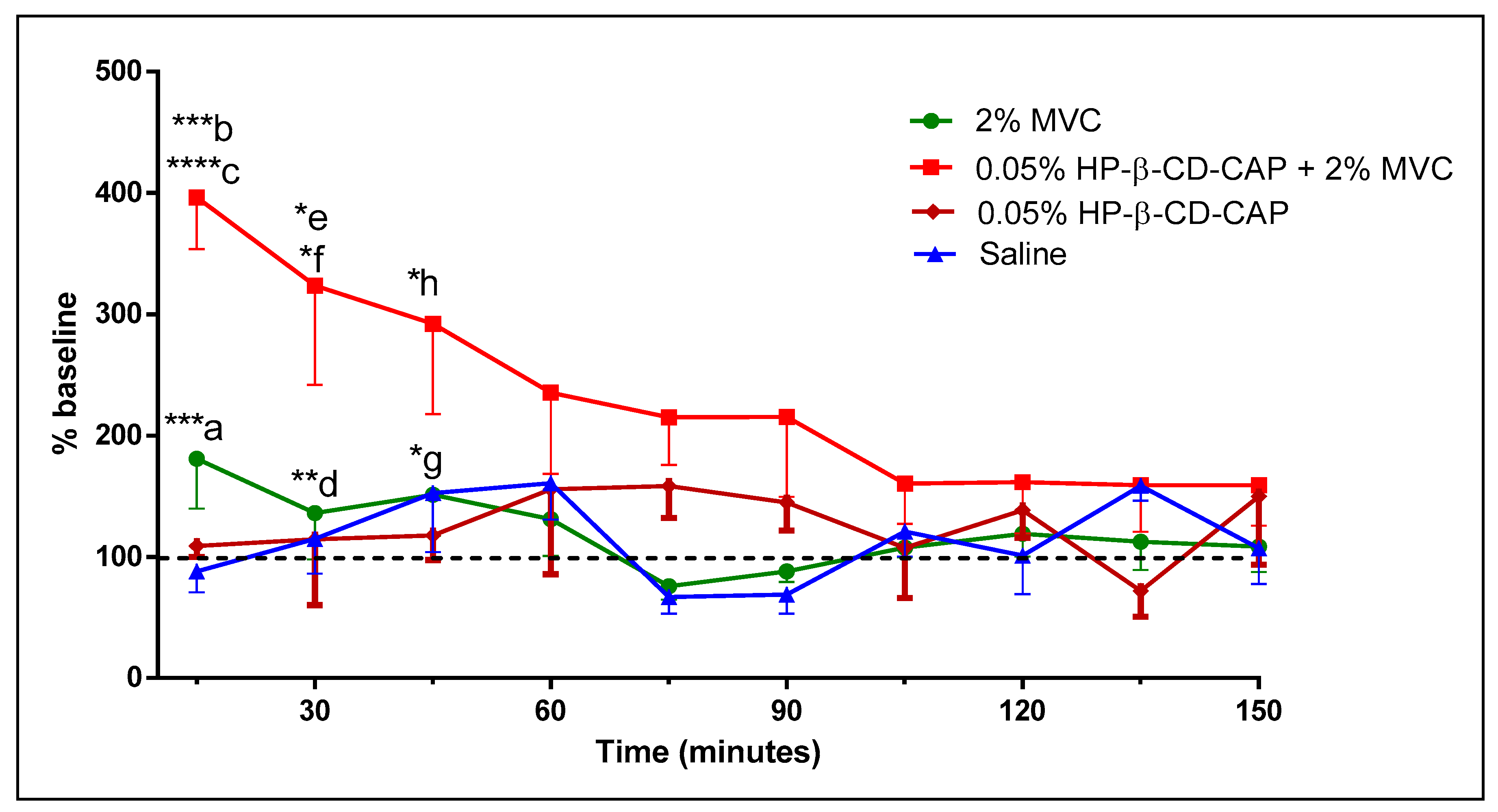
3.7. Evaluation of Analgesia in Inflamed Tissue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, M.H. The use of local anesthesia in the presence of inflammation. Oral Surg. Oral Med. Oral Pathol. 1964, 18, 16–23. [Google Scholar] [CrossRef]
- Grant, G.J.; Lax, J.; Susser, L.; Zakowski, M.; Weissman, E. Wound infiltration with liposomal bupivacaine prolongs analgesia in rats. Acta Anaesthesiol. Scand. 1997, 41, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.; Pateromichelakis, S. Local anaesthetic failures due to an increase in sensory nerve impulses from inflammatory sensitization. J. Dent. 1982, 10, 201–206. [Google Scholar] [CrossRef]
- Rood, J.; Pateromichelakis, S. Inflammation and peripheral nerve sensitisation. Br. J. Oral Surg. 1981, 19, 67–72. [Google Scholar] [CrossRef]
- Tsuchiya, H. Dental Anesthesia in the Presence of Inflammation: Pharmacological Mechanisms for the Reduced Efficacy of Local Anesthetics. Int. J. Clin. Anesthesiol. 2016, 4, 1–16. [Google Scholar]
- Nelson, D.L.; Cox, M.M.; Lehninger, A.L. Lehninger Principles of Biochemistry; Macmillan Higher Education: Basingstoke, UK, 2017. [Google Scholar]
- De Araujo, D.R.; De Paula, E.; Fraceto, L.F. Anestésicos locais: Interação com membranas biológicas e com o canal de sódio voltagem-dependente. Química Nova 2008, 31, 1775–1783. [Google Scholar] [CrossRef][Green Version]
- Hargreaves, K.M.; Keiser, K. Local anesthetic failure in endodontics: Mechanisms and Management. Endod. Top. 2002, 1, 26–39. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Bean, B.P.; Woolf, C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 2007, 449, 607–610. [Google Scholar] [CrossRef]
- Sayhan, H.; Beyaz, S.G.; Çeliktaş, A. The Local Anesthetic and Pain Relief Activity of Alkaloids. In Alkaloids: Alternatives in Synthesis, Modification and Application; IntechOpen: London, UK, 2017. [Google Scholar]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharm. Sci. Tech. 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–210. [Google Scholar]
- Loftsson, T.; Hreinsdôttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Meister, E.; Šaši, S.; Gieseler, H. Freeze-Dry Microscopy: Impact of Nucleation Temperature and Excipient Concentration on Collapse Temperature Data. AAPS Pharm. Sci. Tech. 2009, 10, 582–588. [Google Scholar] [CrossRef][Green Version]
- Cui, L.; Zhang, Z.-H.; Sun, E.; Jia, X. Effect of β-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Cabeça, L.F.; Marsaioli, A.J.; De Paula, E. Investigation of tetracaine complexation with beta-cyclodextrins and p-sulphonic acid calix[6]arenes by nOe and PGSE NMR. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 395–401. [Google Scholar] [CrossRef]
- Morris, K.F.; Johnson, C.S. Diffusion-ordered two-dimensional nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1992, 114, 3139–3141. [Google Scholar] [CrossRef]
- Gounarides, J.S.; Chen, A.; Shapiro, M.J. Nuclear magnetic resonance chromatography: Applications of pulse field gradient diffusion NMR to mixture analysis and ligand-receptor interactions. J. Chromatogr. B Biomed. Sci. Appl. 1999, 725, 79–90. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Aulton, M.E.; Taylor, K.M.G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Churchill Livingstone: London, UK, 2013. [Google Scholar]
- Fehrenbacher, J.C.; Vasko, M.R.; Duarte, D.B. Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr. Protoc. Pharmacol. 2012, 56, 4. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Le Corre, P.; Guilbaud, G.; Le Verge, R. Antinociceptive effect of bupivacaine encapsulated in poly (D, L)-lactide-co-glycolide microspheres in the acute inflammatory pain model of carrageenin-injected rats. Anesth. Analg. 1997, 84, 90–94. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, C.; Shi, F.; Firempong, C.K.; Yu, J.; Xu, X.; Zhang, W. Preparation, characterization, and pharmacokinetics study of capsaicin via hydroxypropyl-beta-cyclodextrin encapsulation. Pharm. Biol. 2015, 54, 1–9. [Google Scholar] [CrossRef]
- Zi, P.; Yang, X.; Kuang, H.; Yang, Y.; Yu, L. Effect of HPβCD on solubility and transdermal delivery of capsaicin through rat skin. Int. J. Pharm. 2008, 358, 151–158. [Google Scholar] [CrossRef]
- Do, T.T.; Van Hooghten, R.; Mooter, G.V.D.; Thi, T.D. A study of the aggregation of cyclodextrins: Determination of the critical aggregation concentration, size of aggregates and thermodynamics using isodesmic and K2–K models. Int. J. Pharm. 2017, 521, 318–326. [Google Scholar] [CrossRef]
- Loftsson, T.; Magnúsdóttir, A.; Másson, M.; Sigurjónsdóttir, J.F. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, X.; Wang, Y.; Zhou, J.; Chen, C. Complexation of capsaicin with β-cyclodextrins to improve pesticide formulations: Effect on aqueous solubility, dissolution rate, stability and soil adsorption. J. Incl. Phenom. Macrocycl. Chem. 2011, 72, 263–274. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K. New challenges for pharmaceutical formulations and drug delivery systems characterization using isothermal titration calorimetry. Drug Discov. Today 2008, 13, 960–972. [Google Scholar] [CrossRef]
- Bertaut, E.; Landy, D. Improving ITC studies of cyclodextrin inclusion compounds by global analysis of conventional and non-conventional experiments. Beilstein J. Org. Chem. 2014, 10, 2630–2641. [Google Scholar] [CrossRef]
- Costanzo, M.T.; Yost, R.; Davenport, P.W. Standardized method for solubility and storage of capsaicin-based solutions for cough induction. Cough 2014, 10, 6. [Google Scholar] [CrossRef]
- Franks, F. Freeze-drying of bioproducts: Putting principles into practice. Eur. J. Pharm. Biopharm. 1998, 45, 221–229. [Google Scholar] [CrossRef]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of End Point of Primary Drying in Freeze-Drying Process Control. AAPS Pharm. Sci. Tech. 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Suihko, E.; Poso, A.; Korhonen, O.; Gynther, J.; Ketolainen, J.; Paronen, P. Deformation Behaviors of Tolbutamide, Hydroxypropyl-β-Cyclodextrin, and Their Dispersions. Pharm. Res. 2000, 17, 942–948. [Google Scholar] [CrossRef]
- Ma, D.Q.; Rajewski, R.A.; Stella, V. New injectable melphalan formulations utilizing (SBE)7m-β-CD or HP-β-CD. Int. J. Pharm. 1999, 189, 227–234. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Novák, C.; Moyano, J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Braga, M.; Martini, M.F.; Pickholz, M.; Yokaichiya, F.; Franco, M.K.; Cabeça, L.; Guilherme, V.; Silva, C.; Limia, C.; De Paula, E. Clonidine complexation with hydroxypropyl-beta-cyclodextrin: From physico-chemical characterization to in vivo adjuvant effect in local anesthesia. J. Pharm. Biomed. Anal. 2016, 119, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O.; Mahaguna, V.; Sriwongjanya, M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Biopharm. 1998, 46, 355–360. [Google Scholar] [CrossRef]
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An Overview about Versatile Molecule Capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar]
- De Paula, E.; de Araújo, D.R.; Fraceto, L.F. Nuclear Magnetic Resonance Spectroscopy Tools for the Physicochemical Characterization of Cyclodextrins Inclusion Complexes. In Cyclodextrins: Chemistry and Physics; Transworld Research Network: Kerala, India, 2010. [Google Scholar]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.R.; Yokaichiya, F.; Franco, M.K.K.D.; Da Silva, C.M.G.; Nascimento, L.D.O.; Franz-Montan, M.; Volpato, M.C.; Cabeça, L.F.; De Paula, E. Complexation of oxethazaine with 2-hydroxypropyl-?-cyclodextrin: Increased drug solubility, decreased cytotoxicity and analgesia at inflamed tissues. J. Pharm. Pharmacol. 2017, 69, 652–662. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Dou, Y.; He, B.; Liu, L.; Wei, Z.; Li, J.; Wang, C.; Mao, C.; Zhang, J.; et al. A pH-responsive cyclodextrin-based hybrid nanosystem as a nonviral vector for gene delivery. Biomaterials 2013, 34, 4159–4172. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Shimpi, S.; Chauhan, B.; Shimpi, P. Cyclodextrins: Application in different routes of drug administration. Acta Pharm. 2005, 55, 139–156. [Google Scholar]
- Stella, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Dollo, G.; Le Corre, P.; Chevanne, F.; Le Verge, R. Inclusion complexation of amide-typed local anaesthetics with β-cyclodextrin and its derivatives. ii. evaluation of affinity constants and in vitro transfer rate constants. Int. J. Pharm. 1996, 136, 165–174. [Google Scholar] [CrossRef]
- Puopolo, M.; Binshtok, A.M.; Yao, G.-L.; Oh, S.B.; Woolf, C.J.; Bean, B.P. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. J. Neurophysiol. 2013, 109, 1704–1712. [Google Scholar] [CrossRef] [PubMed]

| Specification | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|
| Cake appearance * (white, uniform, no collapse) | met the criteria | met the criteria | met the criteria | met the criteria |
| Reconstitution time ** (<10 s) | met the criteria | met the criteria | met the criteria | met the criteria |
| pH (5–8) | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.5 ± 0.1 | 6.1 ± 0.0 |
| CAP content *** (95–105%) | 99.2 ± 1.5 | 102.5 ± 0.6 | 103.2 ± 0.5 | 96.3 ± 3.4 |
| Capsaicin | HP-β-CD | HP-β-CD-CAP | Δ (ppm) | |||
|---|---|---|---|---|---|---|
| H atom | ppm | H atom | ppm | H atom | ppm | |
| 9 | 0.83 | 9 | 0.90 | −0.07 | ||
| 10 | 0.83 | 10 | 0.90 | −0.07 | ||
| 4 | 1.24 | 4 | 1.24 | 0 | ||
| 3 | 1.52 | 3 | 1.57 | −0.05 | ||
| 5 | 1.87 | 5 | 1.97 | −0.1 | ||
| 2 | 2.19 | 2 | 2.24 | −0.05 | ||
| 8 | 2.19 | 8 | 2.24 | −0.05 | ||
| D | 3.41 | 3.42 | −0.01 | |||
| B | 3.65 | 3.65 | 0 | |||
| E | 3.77 | - | - | |||
| 3′a | 3.78 | 3′a | - | - | ||
| C | 3.95 | 3.94 | −0.01 | |||
| 7′ | 4.21 | 7′ | 4.24 | −0.03 | ||
| A | 5.18 | 5.17 | −0.01 | |||
| 6 | 5.32 | 6 | 5.33 | −0.01 | ||
| 7 | 5.32 | 7 | 5.33 | −0.01 | ||
| Aromatic (2′,5′,6′) | 6.89–6.75 | Aromatic (2′,5′,6′) | 6.88–6.73 | −0.01–0.02 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, V.M.; de Oliveira-Nascimento, L.; Cabeça, L.F.; Geraldes, D.C.; Costa, J.S.R.; Riske, K.A.; Franz-Montan, M.; Yokaychiya, F.; Franco, M.K.K.D.; de Paula, E. Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues. Int. J. Mol. Sci. 2020, 21, 5741. https://doi.org/10.3390/ijms21165741
Couto VM, de Oliveira-Nascimento L, Cabeça LF, Geraldes DC, Costa JSR, Riske KA, Franz-Montan M, Yokaychiya F, Franco MKKD, de Paula E. Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues. International Journal of Molecular Sciences. 2020; 21(16):5741. https://doi.org/10.3390/ijms21165741
Chicago/Turabian StyleCouto, Verônica Muniz, Laura de Oliveira-Nascimento, Luiz Fernando Cabeça, Danilo Costa Geraldes, Juliana Souza Ribeiro Costa, Karin A. Riske, Michelle Franz-Montan, Fabiano Yokaychiya, Margareth K. K. Dias Franco, and Eneida de Paula. 2020. "Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues" International Journal of Molecular Sciences 21, no. 16: 5741. https://doi.org/10.3390/ijms21165741
APA StyleCouto, V. M., de Oliveira-Nascimento, L., Cabeça, L. F., Geraldes, D. C., Costa, J. S. R., Riske, K. A., Franz-Montan, M., Yokaychiya, F., Franco, M. K. K. D., & de Paula, E. (2020). Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues. International Journal of Molecular Sciences, 21(16), 5741. https://doi.org/10.3390/ijms21165741










