Abstract
The past two decades have seen extensive research done to pinpoint the role of microRNAs (miRNAs) that have led to discovering thousands of miRNAs in humans. It is not, therefore, surprising to see many of them implicated in a number of common as well as rare human diseases. In this review article, we summarize the progress in our understanding of miRNA-related research in conjunction with different types of cancers and neurodegenerative diseases, as well as their potential in generating more reliable diagnostic and therapeutic approaches.
1. Introduction
The discovery of noncoding RNAs (ncRNAs) [1] has not only challenged the central dogma but also has brought RNA biology to the forefront in the understanding of almost all cellular processes within a cell [2,3,4,5,6]. ncRNAs are divided into two groups based on their transcript length, namely, small (sncRNA; < 200 nucleotides) and long (lncRNA; > 200 nucleotides) noncoding RNA. These ncRNAs form the RNA-infrastructure [7] that are involved not only in the processing of other RNAs such as mRNAs, tRNAs, and rRNAs but also in gene regulation by targeting mRNAs and chromatin [8,9,10,11].
The first tiny regulatory RNAs discovered to play an important role in the gene expression were lin-4 RNA and let-7 RNA, which control the cell fate transition through the larval development in worms [1,12,13,14]. These two short molecules are now the fundamental members of a class of noncoding RNA termed microRNAs (miRNAs), which are endogenous single-stranded, 18–25 nucleotides (nt) long RNA molecules [15]. Since then, miRNAs have been shown to have regulatory functions in most eukaryotes affecting cell growth, development, and differentiation [16,17,18].
2. MiRNAs Biogenesis and Function
Canonical biogenesis of miRNAs starts in the nucleus with the transcription of DNA sequences into primary miRNAs (pri-miRNAs) by RNA Polymerase II/III [3]. Next, these pri-miRNAs (characterized by stem-loop structures with a 3′ poly-A-tail and 5′ methylated cap) are processed by the Microprocessor complex. The Microprocessor complex composed of Drosha (an RNase III endonuclease) and DiGeorge Syndrome Critical Region 8 (DGCR-8) cleaves pri-miRNA to produce 60–70 nt precursor miRNAs (pre-miRNAs) [19].
Pre-miRNA may also be produced by the noncanonical process, where small RNAs are produced from short intronic hairpins termed “mirtrons,” and unlike the canonical mechanism, it does not require Drosha for pre-miRNA production [20,21].
Once pri-miRNAs are generated, they are exported to the cytoplasm in an Exportin5-/RanGTP-dependent manner where they are processed by another RNase III enzyme—Dicer, to produce mature miRNA [22]. The functional strand of mature miRNA is subsequently loaded into the Argonaut (AGO) family proteins to form a miRNA-induced silencing complex (miRISC) [23]. Mature miRNA usually binds to the “seed” region (5–8 nt long) in the 3′ UTR of the target mRNA [24]. However, other sequences such as 5′ UTR, coding region, or gene promoters have also been reported as miRNA-binding sites [25,26,27]. The sequence complementarity via miRNA works leads to degradation, destabilization, or translational repression [28,29].
The majority of miRNA sequences are located in introns of noncoding or coding transcripts, although some miRNAs may overlap with exons [30]. Interestingly, up to 2000 miRNAs have been identified in Homo sapiens alone, which are involved in direct regulation of more than 60% of protein-coding genes [31], suggesting that a single miRNA can regulate expression more than a hundred mRNAs [32]. Therefore, any aberrant regulation and misfunction of miRNAs eventually cause various disease conditions [33], which we would like to discuss in more detail in the following sections.
3. miRNA Located in Genomic Regions Prone to Rearrangements
Numerous studies indicate that miRNAs distribution is not randomly organized in the human genome. It has been shown that some of the chromosomes have higher numbers of miRNAs than the others. Some of the earliest chromosomes discovered first with higher numbers of miRNA are chromosomes (chr.) 1, 2, 19, and X. [34,35]. However, since the number of known miRNAs is expanding continuously, recent data indicates that chr. 14, 16, 17, 22, and X are also abundant with miRNAs with chr. 19 having the highest number in comparison to others [36]. Interestingly, chromosomes that are abundant with miRNAs also have the highest gene densities [37,38], high minisatellites number [39], as well as high expression level [40]. Furthermore, most of the chromosomes abundant with miRNAs are also prone to a higher rate of mutations and are linked to a variety of diseases [41,42,43,44]. Chromosomal fragile sites (CFS) and cancer-associated genomic regions (CAGR) are widely studied examples of such regions.
3.1. miRNA Located at Chromosomal Fragile Sites (CFSs)
Chromosomal fragile sites (CFSs) are specific chromosomal regions (cover 26.38% of human chromosomes [45]) prone to breakage and rearrangements when cells are exposed to DNA replication inhibitors [46]. CFSs are highly transcribed sequences, conserved across the genomes of different eukaryotes such as yeast S. cerevisiae, mouse, rat, and many mammals, including humans [47,48,49]. These specific sites are defined as “rare” and “common” based on their frequency [50,51]. Most “rare” fragile sites can be induced by bromodeoxyuridine (BrdU) or by the removal of folic acid, whereas most “common” fragile sites are induced by aphidicolin or 5-azacytidine [52,53]. CFSs are often characterized by the presence of repetitive sequences. “Rare” CFSs are mostly associated with CCG/CGG trinucleotide repeat sequences adjacent to a CpG island [54], whereas “common” CFSs are located at AT-rich minisatellite repeats [55]. Nevertheless, CFSs may also embody other repetitive elements such as LINE1 and LINE2, Alu, MIR, and MER, as well as endogenous retroviral sequences [56]. Interestingly, some mammalian miRNAs are derived from genomic repeats. For instance, some of them show perfect complementarity to the MIR/LINE-2 class of repeat elements [57].
The abundance of miRNA on fragile sites differs among chromosomes. Lagana et al. have shown that chromosomes 16, 19, and X are abounding in miRNAs at the fragile sites. Unlike these chromosomes, chr. 14 shows the opposite results (e.g., less abundant miRNAs in fragile regions) [58].
The Human Database currently documents 125 fragile sites (containing 4921 protein-coding genes) lying in both somatic chromosomes and the sex chromosome X. Analysis performed by Kumar et al. [59] indicate that 34.51% of human protein-coding genes lie within the CFSs showing the importance of stability of fragile sites in proper gene expression.
3.2. miRNA Located at the Cancer-Associated Genomic Regions (CAGRs)
Another region prone to rearrangements where miRNA is frequently present is cancer-associated genomic regions (CAGR) [34,60,61]. CAGRs are characterized by (i) minimal regions of loss of heterozygosity (LOH), suggestive of the presence of tumour suppressor genes; (ii) minimal regions of amplification, suggestive of the presence of oncogenes; and (iii) common breakpoint regions in or near possible oncogenes or tumour suppressor genes. The frequency of miRNAs localized in these regions is 52.5% [34]. For instance, miR-21, miR155, and miR17-92 cluster are amplified CAGRs [62] expressed at a much higher level in tumour cells [63].
3.3. Relationship between Higher-Order Chromosomal Structure and miRNAs
It has been previously shown that the three-dimensional (3D) organization of the genome contributes to the genome rearrangements and translocations genome-wide [64]. However, the relationship between miRNA and genomic structure has not yet been fully explored. Recent data indicate that the 3D architecture of chromatin influences the transcription of microRNA genes (MIRs) [65]. Chen et al. [65] have shown that miRNAs possess features similar to protein-coding genes; both undergo coordinated expression through their chromosomal loci interactions. It has been shown that a substantial number of miRNAs are controlled by cis genetic regulatory elements, such as CpG islands (2%), promoters (9%), enhancers (35%), and transcription factor (TF) binding regions (15%), which may affect miRNAs expression level [66]. Additionally, the analysis performed on a large number of breast cancer samples has shown that, to some extent, miRNAs and their neighbouring genes may have a positive correlative expression [67].
Beside small noncoding RNAs, long intergenic noncoding RNAs (lincRNAs), class of long noncoding RNAs, have also been shown to influence transcriptional regulation through their long-range chromatin interactions [68]. Like microRNA, most of the lincRNAs interacts with protein-coding genes (two or more) [68]. Furthermore, studies performed by Cai et al. [68] have shown that numerous lincRNA promoters were linked with a higher state of enhancer-like chromatin with a higher level of H3K4me1 compared to H3K4me3, corroborating with previous studies [69,70]. Interestingly, most recent data have shown that AGO1, an RNA interference component, strongly associates with active enhancers as well as RNA produced at those sites (enhancer RNA, eRNA) [71]. Taken together, these data suggest that enhancer-associated AGO1 contributes to chromatin architecture and gene expression in human cells [71]. Moreover, these studies also revealed that AGO1, in association with NEAT1 lncRNA, contributes to nuclear and 3D chromatin architecture in human cells [71]. Additionally, it has been shown that lncRNA associated with RNA-binding proteins (RBPs) in the nucleus is involved in transcriptional regulation via modulation of 3D chromatin architecture [72,73]. For more data related to long noncoding RNA and 3D chromatin structure see Begolli et al. [74].
3.4. Chromosome Fragile Sites in Diseases
CFSs are often involved in chromosomal abnormalities such as deletions, duplications, translocations, and loss of heterozygosity in a number of tumour cells [75,76]. As mentioned earlier, CFSs, in general, are also frequently occupied by miRNAs genes (for more detail see [34]). This was first demonstrated by Calin et al. [34], who showed that over half of the 186 miRNAs studies map to the chromosome regions containing fragile sites. In addition, to confirming Calin et al.’s findings by studying over 700 miRNAs, Lagana et al. [58] demonstrated that the fragile sites are also dense in proteins coding genes. Recently, Kumar et al. [59] have shown that 35.04% of human mature miRNA genes lie within the fragile sites. For instance, fragile sites such as FRA4D (aphidicolin type, common) contain miR-218-1 and FRA5G (folic acid type, rare) contains miR-218-2 [58]. miRNA have also been found to map to the integration sites of human papillomavirus (HPV) [34,77,78]. Additionally, Wang et al. indicated that retrovirus infection induces the expression of the oncogenic miR-17-92 miRNA cluster.
Fragile sites are often associated with multiple neurological diseases and cancers. The most common example of the disease associated with fragile sites is Fragile X syndrome (FXS). FXS is linked to the expansion of the CGG trinucleotide repeats, r(CGG), which is associated with transcriptional silencing of either FMR1 or FMR2 (Fragile X mental retardation genes 1 and 2) on chromosome X [79,80]. Neuronal stem cells are indeed the hotspots for defective DSB repair, especially in the longer genes [81] leading to many neurodegenerative and neurodevelopmental diseases. The first studied link between neurodegeneration and CFS genes was reported in Alzheimer’s disease (AD) where Sze et al. showed that when downregulated, WWOX induces Tau phosphorylation, thus implicating its association to AD [82].
Most studies of miRNAs in cancer have been focused on FRA3B and FRA16D; the two best characterized common fragile sites, which lie within the large tumour suppressor genes. The fragile histidine triad (FHIT) gene was isolated from the region encompassing the most active fragile FRA3B locus [83]. The tumour-suppressor gene WWOX, located within the fragile site FRA16D in chromosome 16q23.3-24.1, is correlated to multiple cancers, especially breast, prostate, and ovary [84,85]. Interestingly, previously mentioned FMR1 also correlates with breast cancer (overexpression of the protein enhances, whereas its downregulation inhibits breast cancer metastasis) [86]. Nevertheless, FMR1 is not only linked to breast cancer since its discovery but also in conjugation with other types of cancer [87].
4. miRNAs and Diseases
It is well known that miRNA expression is highly tissue-specific. Some of the miRNAs are even exclusively expressed in a certain cell or tissue types. Therefore, it is not surprising that specific miRNA expression profiles can be identified in different diseases. The deregulation of miRNAs has also been associated with a number of diseases such as hepatitis C virus (HCV) [88,89], immune-related diseases like multiple sclerosis (MS) [90,91,92] and systemic lupus (SL) [93,94], different cancers, and several neurodegenerative disorders. However, miRNA dysfunction has been widely reported in different types of cancers, followed by several neurodevelopmental and neurodegenerative diseases (NDs). Consequently, studies related to miRNAs and their association with cancer and neurodegenerative diseases are discussed in more detail in the following sections.
4.1. miRNAs Associated to Cancer
As mentioned, more than 50% of miRNA genes are located at the fragile sites where chromosomal rearrangements associated with cancer occur [95]. Moreover, it has been shown that almost half of the miRNAs are located near or within genes translocated in cancer [58]. Recent studies report that in most cancers, miRNAs are apparently deregulated and under certain circumstances can function as oncogenes (oncomirs) or tumour suppressors [96,97,98]. Aberrant expression of miRNA is directed by different mechanisms. These mechanisms include the miRNA biogenesis pathway, epigenetic silencing as well as genetic alterations, and single nucleotide polymorphism (SNP) [99,100,101,102,103,104,105,106].
The first study that directly suggested miRNA’s dysregulation as an important feature of tumourigenesis came from Calin et al. [107]. They were looking for a gene/genes that could be associated with B cell chronic lymphocytic leukaemia (CLL); however, they failed to identify any protein-coding genes; instead, they found a cluster of two miRNAs, miR-15a and miR-16-1, located at the frequently deleted region in CLL (13q14.3). The expression of these miRNAs was diminished or completely deleted in ≈ 68% of CLL examined cases. Furthermore, researchers identified a germline C-to-T mutation located only 7 base pairs (bp) downstream of the miR-16-1 precursor in two out of 75 CLL patients (mutation not found in 160 control individuals), which correlated with the diminished expression of this miRNA [108].
One of the factors that cause miRNA deregulation is through RNA editing. RNA editing is done by two classes of enzymes. Adenosine deaminase acting on RNAs (ADARs) are responsible for the deamination of adenosine (A) to inosine (I). On the other hand, activation-induced deamination (AID), also known as Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC; [109]) deaminates cytidine (C) to uridine (U). These RNA-editing enzymes have a significant role in immunity as well as neural plasticity. Moreover, RNA-editing enzymes also act upon miRNA-editing events reported on miR-140, miR-301a, and miR-455 and frequently occur in the seed sequences and in consequence, impact miRNA regulatory functions [110,111]. miRNA editing of miR-376a-1 has been linked to the formation of human gliomas [112]. Similarly, studies done on the samples from patients with bladder, kidney, and testicular cancer also suggest a crucial role in the downregulation of miRNA editing [113,114]. Further studies are necessary to elucidate the importance of miRNA editing in the context of human diseases.
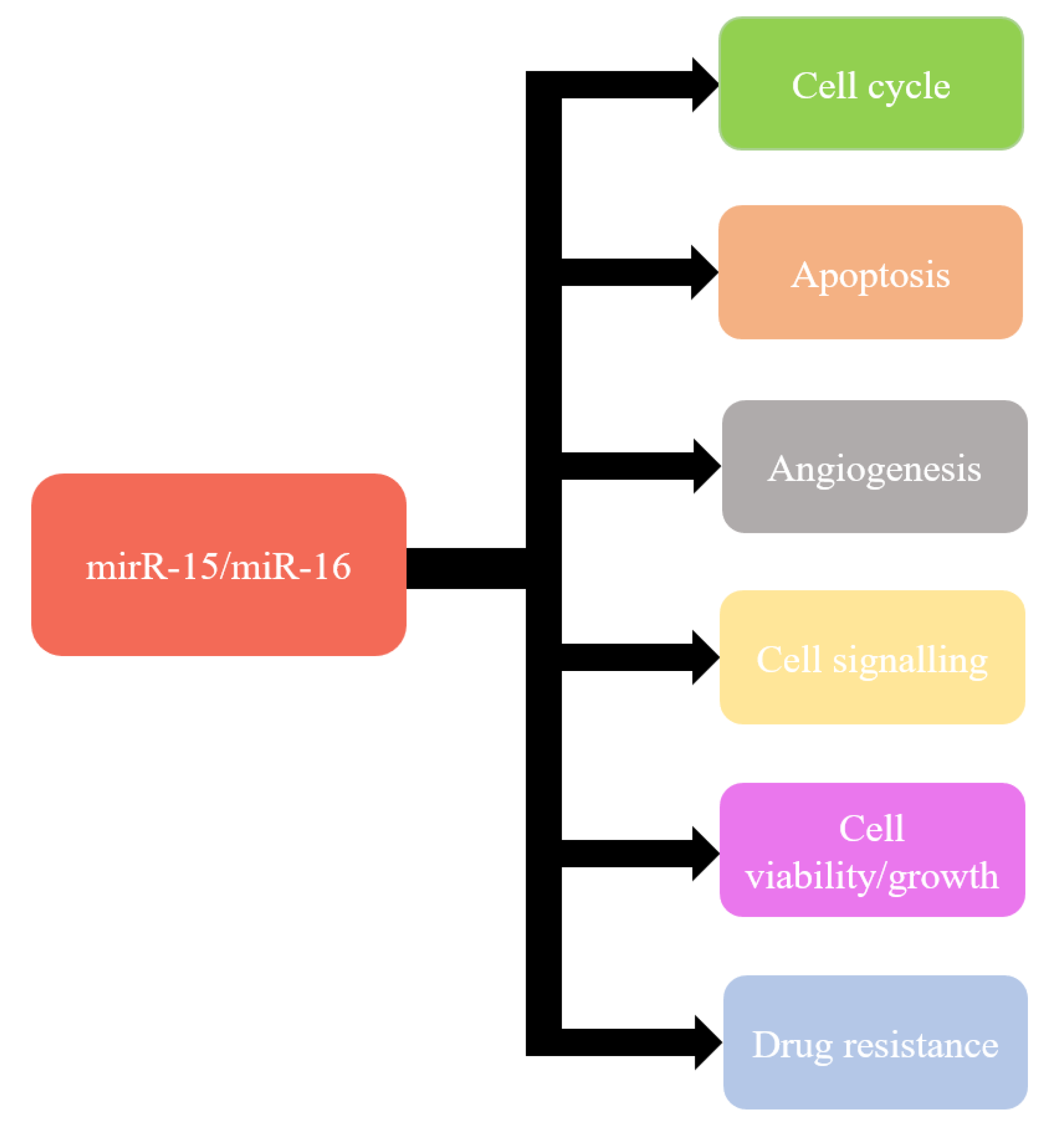
Recent knowledge indicates that miR-15a and miR-16-1 can modulate cell cycle, inhibit cell proliferation, suppress tumourigenicity, and induce apoptosis both in vitro and in vivo [115] (Figure 1). These effects are obtained by targeting the key genes such as BCL2, MCL1, CCND1, WNT3A, and genes involved in G1-S transition [97,116,117,118,119,120]. Moreover, miR-15a and miR-16 have also been shown frequently downregulated and/or deleted in other forms of cancer, such as lung cancer, prostate cancer, stomach cancer, pituitary adenoma, multiple myeloma, osteosarcoma, liver cancer, breast cancer, and ovarian cancer [120,121,122,123,124,125].
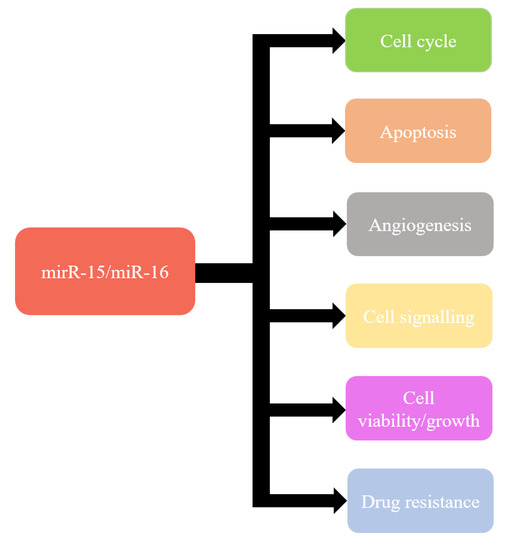
Figure 1.
Functions of miR-15/miR-16.
Through the years, miRNA’s involvement and role were indicated in many types of cancers [126,127,128,129,130,131,132,133,134,135]. The best-characterized tumours and their association with miRNA are listed in Table 1. Since miRNA possesses good stability, high sensitivity, and specificity, it becomes an interesting factor that could be exploited as potential biomarkers. Moreover, inhibition of oncogenic miRNAs or substitution of tumour-suppressive miRNAs serves a potential way for the development of novel treatment strategies.

Table 1.
Cancers and their association with microRNAs (miRNA).
4.2. miRNAs Associated to Neurodegeneration
Similar to their roles in cancer, miRNA editing also leads to neurological disorders [247]. A to I RNA editing can potentially impact miRNA specificity and, consequently, their biological functions in a neuronal cell. For example, Eichler et al. [248] reported that APOBEC-mediated RNA editing is essential in the progression of temporal lobe epilepsy. Similarly, the role of C to U mutations has also been implicated in schizophrenia patients [249]. Deregulation of miR-175 associated with X-linked mental retardation, which also coincides with the early onset of PD [250]. RNA editing and their association with ncRNAs and neurodegeneration are discussed in great detail in the review by Singh [251] and more recently by Lerner et al. [252].
miRNA dysregulation has been reported in a number of neurodegenerative diseases (ND) such as AD, multiple sclerosis (MS), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) (Table 2). Several other neurological disorders, including schizophrenia, autism, dementia, and epilepsy, have also been associated with miRNA dysfunction.

Table 2.
Neurodegenerative diseases and their association with miRNA.
The hallmarks of the neurodegenerative diseases are neuronal degradation and neuronal death. miRNAs play significant functional roles in several pathways that are critical to neuronal differentiation and survival, making miRNA signatures apparent in several NDs. There have been approximately 600 differentially expressed miRNAs reported in 72 different studies (see Brennan et al. for details) on ND patients with 346 miRNAs identified as unique. The study done by Brenan et al. showed that although many miRNAs were present in at least two ND patient samples, each ND has at least one unique miRNA deregulation [279]. Interestingly, the miRNA hsa-miR-30b-5p overlaps with all four widely studied NDs, i.e., AD, PD, ALS, and MS.
Both overexpression and the downregulation of miRNAs have been implicated with NDs. For example, miR-9 that targets several proteins associated with AD pathogenesis (e.g., BACE1, PSEN1, SIRT1, and CAMKK2) is downregulated in human AD brain samples, as well as in mouse and neuronal cell culture models. On the other hand, the upregulation of miRNAs such as brain-miR-112 and brain-miR-161 has also been reported in the brain samples [271]. Additionally, hsa-miR-30b-5p demonstrated an example where it has been reported to be downregulated in ALS, AD, and PD but upregulated in the case of MS. These reports suggest a profound functional relevance of miRNAs in ND that could only be revealed with extensive studies in the future. Nevertheless, miRNAs prove to have great potential to be targeted for developing biomarkers and therapeutics.
5. Therapeutic Potentials of miRNAs
Targeting miRNAs have been gaining attention as a potential tool for the treatment of a number of diseases including cancer and neurodegenerative diseases [280]. miRNA mimics and anti-miRNAs are two popular strategies that are being explored extensively. miRNA mimics are miRNA precursor-like small RNAs currently being developed to regulate the expression of target proteins. In contrast to miRNA mimics, anti-miRNAs are molecules that can interfere and create a loss-of-function for miRNAs of interest [281]. However, like other strategies, miRNAs therapeutic potentials have their share of challenges. More research is required to improve target specificity, efficacy, drug delivery, optimizing off-target effects, etc. One possible and widely researched area of studies in miRNAs’ association with diseases is their potential usage as biomarkers to improve disease diagnosis or prognosis. Using genomic tools for identifying novel miRNAs would more likely give researchers an edge over other currently used methods. Furthermore, FDA-approved clinical tests using real-time quantitative PCRs (qPCR) could be used to amplify low abundant miRNAs for detection. This could prove advantageous, especially because such techniques are not available to measure low abundant proteins or other molecules currently. However, to ensure reliable miRNA measurement, selection of appropriate normalization techniques is equally important [282,283,284].
6. Conclusions
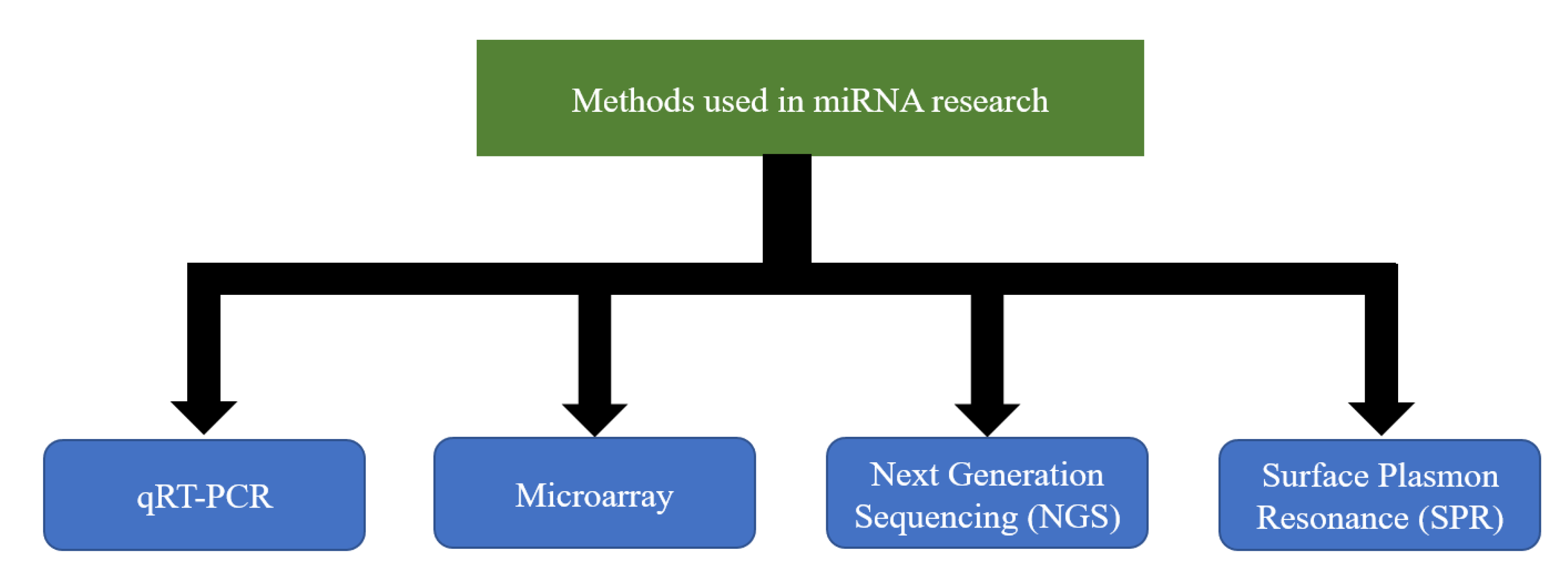
Extensive studies done in the past decades have helped to elucidate the importance of miRNA regulation in the context of a number of diseases and the potential to exploit its use in therapeutics, especially for the so-called incurable diseases. Despite the early success of SPC3649 [285] and the fact that multiple miRNAs have been proposed as potential biomarkers, their use in clinical practice has not been sufficiently materialized. One of the main reasons is the technical challenge of accurately measuring miRNA expression. So far, there does not exist an easy, fast, and inexpensive method that could overcome it. Nevertheless, a number of techniques are currently being used to allow the assessment of the expression levels of the number of miRNAs in a variety of cell types [286,287,288,289,290,291,292,293] (Figure 2). Each technique has its strengths and weaknesses [294]. A strong collaboration between clinicians and researchers with expertise in different techniques would undoubtedly bring different perspectives on the same table that could give the boost required for the steady development of clinical applications.
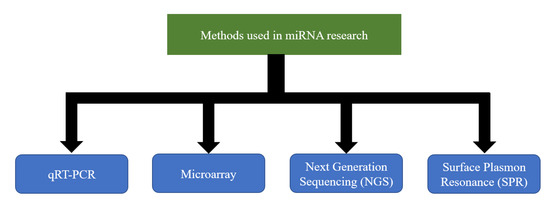
Figure 2.
Methods currently used in microRNAs (miRNA) research.
Funding
The research received no external funding.
Acknowledgments
We would like to thank the reviewers for valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Czech, B.; Hannon, G.J. One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends Biochem. Sci. 2016, 41, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Rzeszutek, I.; Maurer-Alcalá, X.X.; Nowacki, M. Programmed genome rearrangements in ciliates. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Danielsen, J.M.R.; Yang, Y.G.; Qi, Y. A role for small RNAs in DNA double-strand break repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef]
- Francia, S.; Michelini, F.; Saxena, A.; Tang, D.; De Hoon, M.; Anelli, V.; Mione, M.; Carninci, P.; D’adda Di Fagagna, F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 2012, 488, 231–235. [Google Scholar] [CrossRef]
- Collins, L.J. The RNA infrastructure: An introduction to ncRNA networks. Adv. Exp. Med. Biol. 2011, 1–19. [Google Scholar]
- Brosnan, C.A.; Voinnet, O. The long and the short of noncoding RNAs. Curr. Opin. Cell Biol. 2009, 21, 416–425. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Chen, L.L.; Carmichael, G.G. Long noncoding RNAs in mammalian cells: What, where, and why? Wiley Interdiscip. Rev. RNA 2010, 1, 2–21. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquienelll, A.E.; Bettlnger, J.C.; Rougvle, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Basson, M.; Liu, Z.; Ambros, V.; Horvitz, H.R.; Ruvkun, G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol. Cell 2000, 5, 659–669. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, K.; Cao, Y. MicroRNA-944 affects cell growth by targeting EPHA7 in non-small cell lung cancer. Int. J. Mol. Sci. 2016, 17, 1493. [Google Scholar] [CrossRef]
- Kwon, C.; Han, Z.; Olson, E.N.; Srivastava, D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc. Natl. Acad. Sci. USA 2005, 52, 18986–18991. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.H.; Nam, J.W.; Heo, I.; Rhee, J.K.; Sohn, S.Y.; Cho, Y.; Zhang, B.T.; Kim, V.N. Molecular Basis for the Recognition of Primary microRNAs by the Drosha-DGCR8 Complex. Cell 2006, 125, 887–901. [Google Scholar] [CrossRef]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The Mirtron Pathway Generates microRNA-Class Regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A high-Resolution structure of the pre-microrna nuclear export machinery. Science. 2009, 326, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Smith, S.K.; Hannon, G.J.; Joshua-Tor, L. Crystal structure of argonaute and its implications for RISC slicer activity. Science. 2004, 305, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Kasinski, A.L.; Slack, F.J. Aberrant regulation and function of microRNAs in cancer. Curr. Biol. 2014, 24, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.; Ghosh, U. miRNA gene counts in chromosomes vary widely in a species and biogenesis of miRNA largely depends on transcription or post-transcriptional processing of coding genes. Front. Genet. 2014, 5, 100. [Google Scholar] [CrossRef]
- Boroumand, F.; Saadat, I.; Saadat, M. Non-randomness distribution of micro-RNAs on human chromosomes. Egypt. J. Med. Hum. Genet. 2019, 20, 1–5. [Google Scholar] [CrossRef]
- Grimwood, J.; Gordon, L.A.; Olsen, A.; Terry, A.; Schmutz, J.; Lamerdin, J.; Hellsten, U.; Goodstein, D.; Couronne, O.; Tran-Gyamil, M.; et al. The DNA sequence and biology of human chromosome 19. Nature 2004, 428, 529–535. [Google Scholar] [CrossRef]
- Dehal, P.; Predki, P.; Olsen, A.S.; Kobayashi, A.; Folta, P.; Lucas, S.; Land, M.; Terry, A.; Ecale Zhou, C.L.; Rash, S.; et al. Human chromosome 19 and related regions in mouse: Conservative and lineage-specific evolution. Science 2001, 293, 104–111. [Google Scholar] [CrossRef]
- Wright, F.A.; Lemon, W.J.; Zhao, W.D.; Sears, R.; Zhuo, D.; Wang, J.P.; Yang, H.Y.; Baer, T.; Stredney, D.; Spitzner, J.; et al. A draft annotation and overview of the human genome. Genome Biol. 2001, 2, research0025-1. [Google Scholar]
- Caron, H.; Van Schaik, B.; Van der Mee, M.; Baas, F.; Riggins, G.; Van Sluis, P.; Hermus, M.C.; Van Asperen, R.; Boon, K.; Voûte, P.A.; et al. The human transcriptome map: Clustering of highly expressed genes in chromosomal domains. Science 2001, 291, 1289–1292. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Sharp, P.M.; Li, W.H. Mutation rates differ among regions of the mammalian genome. Nature 1989, 337, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Nachman, M.W.; Crowell, S.L. Estimate of the mutation rate per nucleotide in humans. Genetics 2000, 156, 297–304. [Google Scholar] [PubMed]
- Smith, N.G.C.; Webster, M.T.; Ellegren, H. Deterministic mutation rate variation in the human genome. Genome Res. 2002, 12, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M. Rate, molecular spectrum, and consequences of human mutation. Proc. Natl. Acad. Sci. USA 2010, 107, 961–968. [Google Scholar] [CrossRef]
- Mathelier, A.; Carbone, A. Large scale chromosomal mapping of human microRNA structural clusters. Nucleic Acids Res. 2013, 41, 4392–4408. [Google Scholar] [CrossRef]
- Franchitto, A. Genome instability at common fragile sites: Searching for the cause of their instability. Biomed Res. Int. 2013, 2013, 730714. [Google Scholar] [CrossRef]
- Helmrich, A.; Stout-Weider, K.; Hermann, K.; Schrock, E.; Heiden, T. Common fragile sites are conserved features of human and mouse chromosomes and relate to large active genes. Genome Res. 2006, 16, 1222–1230. [Google Scholar] [CrossRef]
- Elder, F.F.B.; Robinson, T.J. Rodent common fragile sites: Are they conserved? Evidence from mouse and rat. Chromosoma 1989, 97, 459–464. [Google Scholar] [CrossRef]
- Raveendranathan, M.; Chattopadhyay, S.; Bolon, Y.T.; Haworth, J.; Clarke, D.J.; Bielinsky, A.K. Genome-wide replication profiles of S-phase checkpoint mutants reveal fragile sites in yeast. EMBO J. 2006, 25, 3627–3639. [Google Scholar] [CrossRef]
- Lukusa, T.; Fryns, J.P. Human chromosome fragility. Biochim. Biophys. Acta Gene Regul. Mech. 2008, 1779, 3–16. [Google Scholar] [CrossRef]
- Durkin, S.G.; Glover, T.W. Chromosome Fragile Sites. Annu. Rev. Genet. 2007, 41, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Zlotorynski, E.; Rahat, A.; Skaug, J.; Ben-Porat, N.; Ozeri, E.; Hershberg, R.; Levi, A.; Scherer, S.W.; Margalit, H.; Kerem, B. Molecular Basis for Expression of Common and Rare Fragile Sites. Mol. Cell. Biol. 2003, 23, 7143–7151. [Google Scholar] [CrossRef] [PubMed]
- Glover, T.W.; Arlt, M.F.; Casper, A.M.; Durkin, S.G. Mechanisms of common fragile site instability. Hum. Mol. Genet. 2005, 14, 197–205. [Google Scholar] [CrossRef]
- Sutherland, G.R. Rare fragile sites. Cytogenet. Genome Res. 2003, 100, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Mangelsdorf, M.; Hewett, D.; Hobson, L.; Baker, E.; Eyre, H.J.; Lapsys, N.; Le Paslier, D.; Doggett, N.A.; Sutherland, G.R.; et al. Human chromosomal fragile site FRA16B is an amplified AT-rich minisatellite repeat. Cell 1997, 88, 367–374. [Google Scholar] [CrossRef]
- Morelli, C.; Karayianni, E.; Magnanini, C.; Mungall, A.J.; Thorland, E.; Negrini, M.; Smith, D.I.; Barbanti-Brodano, G. Cloning and characterization of the common fragile site FRA6F harboring a replicative senescence gene and frequently deleted in human tumors. Oncogene 2002, 21, 7266–7276. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Torvik, V.I. Mammalian microRNAs derived from genomic repeats. Trends Genet. 2005, 21, 322–326. [Google Scholar] [CrossRef]
- Laganà, A.; Russo, F.; Sismeiro, C.; Giugno, R.; Pulvirenti, A.; Ferro, A. Variability in the incidence of miRNAs and genes in fragile sites and the role of repeats and CpG islands in the distribution of genetic material. PLoS ONE 2010, 5, e11166. [Google Scholar] [CrossRef]
- Kumar, R.; Nagpal, G.; Kumar, V.; Usmani, S.S.; Agrawal, P.; Raghava, G.P.S. HumCFS: A database of fragile sites in human chromosomes. BMC Genomics 2019, 19, 985. [Google Scholar] [CrossRef]
- Gaur, A.; Jewell, D.A.; Liang, Y.; Ridzon, D.; Moore, J.H.; Chen, C.; Ambros, V.R.; Israel, M.A. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007, 67, 2456–2468. [Google Scholar] [CrossRef]
- Oulas, A.; Boutla, A.; Gkirtzou, K.; Reczko, M.; Kalantidis, K.; Poirazi, P. Prediction of novel microRNA genes in cancer-associated genomic regions - A combined computational and experimental approach. Nucleic Acids Res. 2009, 37, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Tsuchiya, S.; Meltzer, S.J.; Shimizu, K. MicroRNAs and epigenetics. FEBS J. 2011, 278, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Murai, N.; Yanaihara, N.; Saito, M.; Saito, M.; Urashima, M.; Murakami, Y.; Matsufuji, S.; Okamoto, A. MicroRNA-21 is a candidate driver gene for 17q23-25 amplification in ovarian clear cell carcinoma. BMC Cancer 2014, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McCord, R.P.; Ho, Y.-J.; Lajoie, B.R.; Hildebrand, D.G.; Simon, A.C.; Becker, M.S.; Alt, F.W.; Dekker, J. Chromosomal translocations are guided by the spatial organization of the genome. Cell 2012, 148, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fu, L.Y.; Zhang, Z.; Li, G.; Zhang, H.; Jiang, L.; Harrison, A.P.; Shanahan, H.P.; Klukas, C.; Zhang, H.Y.; et al. Dissecting the chromatin interactome of microRNA genes. Nucleic Acids Res. 2014, 42, 3028–3043. [Google Scholar] [CrossRef]
- Huan, T.; Rong, J.; Liu, C.; Zhang, X.; Tanriverdi, K.; Joehanes, R.; Chen, B.H.; Murabito, J.M.; Yao, C.; Courchesne, P.; et al. Genome-wide identification of microRNA expression quantitative trait loci. Nat. Commun. 2015, 6, 6601. [Google Scholar] [CrossRef]
- Dvinge, H.; Git, A.; Gräf, S.; Salmon-Divon, M.; Curtis, C.; Sottoriva, A.; Zhao, Y.; Hirst, M.; Armisen, J.; Miska, E.A.; et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 2013, 497, 378–382. [Google Scholar] [CrossRef]
- Cai, L.; Chang, H.; Fang, Y.; Li, G. A Comprehensive Characterization of the Function of LincRNAs in Transcriptional Regulation through Long-Range Chromatin Interactions. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Li, G.; Ruan, X.; Auerbach, R.K.; Sandhu, K.S.; Peh, S.Q.; Mulawadi, F.H.; Ong, C.T.; Orlov, Y.L.; Hong, S.; Zhang, Z.; et al. Extensive Promoter-centered Chromatin Interactions Provide a Topological Basis for Transcription Regulation. Cell 2013, 148, 84–98. [Google Scholar] [CrossRef]
- Marques, A.C.; Hughes, J.; Graham, B.; Kowalczyk, M.S.; Higgs, D.R.; Ponting, C.P. Chromatin signatures at transcriptional start sites separate two equally populated yet distinct classes of intergenic long noncoding RNAs. Genome Biol. 2013, 14, R131. [Google Scholar] [CrossRef]
- Shuaib, M.; Parsi, K.M.; Thimma, M.; Adroub, S.A.; Kawaji, H.; Seridi, L.; Ghosheh, Y.; Fort, A.; Fallatah, B.; Ravasi, T.; et al. Nuclear AGO1 Regulates Gene Expression by Affecting Chromatin Architecture in Human Cells. Cell Syst. 2019, 9, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Hafner, M.; Tuschl, T. A census of human RNA-binding proteins. Nat. Rev. Genet. 2014, 15, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Kuwano, Y.; Nishikawa, T.; Masuda, K.; Rokutan, K. RNA binding proteins and genome integrity. Int. J. Mol. Sci. 2017, 18, 1341. [Google Scholar] [CrossRef] [PubMed]
- Begolli, R.; Sideris, N.; Giakountis, A. Lncrnas as chromatin regulators in cancer: From molecular function to clinical potential. Cancers 2019, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Huebner, K.; Croce, C.M. FRA3B and other common fragile sites: The weakest links. Nat. Rev. Cancer 2001, 1, 214–221. [Google Scholar] [CrossRef]
- O’Keefe, L.V.; Richards, R.I. Common chromosomal fragile sites and cancer: Focus on FRA16D. Proc. Cancer Lett. 2006, 232, 37–47. [Google Scholar] [CrossRef]
- Thorland, E.C.; Myers, S.L.; Gostout, B.S.; Smith, D.I. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene 2003, 22, 1225–1237. [Google Scholar] [CrossRef]
- Jiménez-Wences, H.; Peralta-Zaragoza, O.; Fernández-Tilapa, G. Human papilloma virus, DNA methylation and microRNA expression in cervical cancer (Review). Oncol. Rep. 2014, 35, 2297–2305. [Google Scholar] [CrossRef]
- D’Hulst, C.; Kooy, R.F. Fragile X syndrome: From molecular genetics to therapy. J. Med. Genet. 2009, 2, 242–255. [Google Scholar] [CrossRef]
- Fry, M.; Loeb, L.A. The fragile X syndrome d(CGG)(n) nucleotide repeats form a stable tetrahelical structure. Proc. Natl. Acad. Sci. USA 1994, 91, 4950–4954. [Google Scholar] [CrossRef]
- Wei, P.C.; Chang, A.N.; Kao, J.; Du, Z.; Meyers, R.M.; Alt, F.W.; Schwer, B. Long Neural Genes Harbor Recurrent DNA Break Clusters in Neural Stem/Progenitor Cells. Cell 2016, 164, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Hsu, L.J.; Heath, J.; Schultz, L.; Chang, N.S. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro: A potential role in Alzheimer’s disease. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Inoue, H.; Cotticelli, M.G.; Kastury, K.; Baffa, R.; Palazzo, J.; Siprashvili, Z.; Mori, M.; McCue, P.; Druck, T.; et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell 1996, 84, 587–597. [Google Scholar] [CrossRef]
- Bednarek, A.K.; Keck-Waggoner, C.L.; Daniel, R.L.; Laflin, K.J.; Bergsagel, P.L.; Kiguchi, K.; Brenner, A.J.; Aldaz, C.M. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001, 61, 8068–8073. [Google Scholar]
- Ludes-Meyers, J.H.; Bednarek, A.K.; Popescu, N.C.; Bedford, M.; Aldaz, C.M. WWOX, the common chromosomal fragile site, FRA16D, cancer gene. Cytogenet. Genome Res. 2003, 240, 338–344. [Google Scholar] [CrossRef]
- Lucá, R.; Averna, M.; Zalfa, F.; Vecchi, M.; Bianchi, F.; Fata, G.L.; Del Nonno, F.; Nardacci, R.; Bianchi, M.; Nuciforo, P.; et al. The Fragile X Protein binds mRNAs involved in cancer progression and modulates metastasis formation. EMBO Mol. Med. 2013, 5, 1523–1536. [Google Scholar] [CrossRef]
- Zalfa, F.; Panasiti, V.; Carotti, S.; Zingariello, M.; Perrone, G.; Sancillo, L.; Pacini, L.; Luciani, F.; Roberti, V.; D’Amico, S.; et al. The fragile X mental retardation protein regulates tumor invasiveness-related pathways in melanoma cells. Cell Death Dis. 2017, 8, e3169. [Google Scholar] [CrossRef]
- Jopling, C.L.; Yi, M.K.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Molecular biology: Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef]
- Randall, G.; Panis, M.; Cooper, J.D.; Tellinghuisen, T.L.; Sukhodolets, K.E.; Pfeffer, S.; Landthaler, M.; Landgraf, P.; Kan, S.; Lindenbach, B.D.; et al. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. USA 2007, 104, 12884–12889. [Google Scholar] [CrossRef]
- Keller, A.; Leidinger, P.; Lange, J.; Borries, A.; Schroers, H.; Scheffler, M.; Lenhof, H.P.; Ruprecht, K.; Meese, E. Multiple Sclerosis: MicroRNA Expression Profiles Accurately Differentiate Patients with Relapsing-Remitting Disease from Healthy Controls. PLoS ONE 2009, 4, e7440. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009, 132, 3342–3352. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, C.; Kang, J.; Zhao, G.; Ye, Z.; Huang, S.; Li, Z.; Wu, Z.; Pei, G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009, 1, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, W.; Ye, D.Q.; Cui, H.; Zhang, Y.; Hirankarn, N.; Qian, X.; Tang, Y.; Lau, Y.L.; de Vries, N.; et al. A functional variant in microRNA-146a promoter modulates its expression and confers disease risk for systemic lupus erythematosus. PLoS Genet. 2011, 7, e1002128. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, S.E.; Frostegård, J.; Truedsson, L.; Pons-Estel, B.A.; D’Alfonso, S.; Witte, T.; Lauwerys, B.R.; Endreffy, E.; Kovács, L.; Vasconcelos, C.; et al. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Genes Immun. 2012, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Calin, G.A.; Croce, C.M.; Coukos, G.; Zhang, L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle 2008, 7, 2643–2646. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Linsley, P.S.; Schelter, J.; Burchard, J.; Kibukawa, M.; Martin, M.M.; Bartz, S.R.; Johnson, J.M.; Cummins, J.M.; Raymond, C.K.; Dai, H.; et al. Transcripts Targeted by the MicroRNA-16 Family Cooperatively Regulate Cell Cycle Progression. Mol. Cell. Biol. 2007, 27, 2240–2252. [Google Scholar] [CrossRef]
- Schembri, F.; Sridhar, S.; Perdomo, C.; Gustafson, A.M.; Zhang, X.; Ergun, A.; Lu, J.; Liu, G.; Zhang, X.; Bowers, J.; et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 2319–2324. [Google Scholar] [CrossRef]
- Wynendaele, J.; Böhnke, A.; Leucci, E.; Nielsen, S.J.; Lambertz, I.; Hammer, S.; Sbrzesny, N.; Kubitza, D.; Wolf, A.; Gradhand, E.; et al. An illegitimate microRNA target site within the 3′ UTR of MDM4 affects ovarian cancer progression and chemosensitivity. Cancer Res. 2010, 70, 9641–9649. [Google Scholar] [CrossRef]
- Mishra, P.J.; Mishra, P.J.; Banerjee, D.; Bertino, J.R. MiRSNPs or MiR-polymorphisms, new players in microRNA mediated regulation of the cell: Introducing microRNA pharmacogenomics. Cell Cycle 2008, 7, 853–858. [Google Scholar] [CrossRef]
- Chin, L.J.; Ratner, E.; Leng, S.; Zhai, R.; Nallur, S.; Babar, I.; Muller, R.U.; Straka, E.; Su, L.; Burki, E.A.; et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008, 68, 8535–8540. [Google Scholar] [CrossRef]
- Brueckner, B.; Stresemann, C.; Kuner, R.; Mund, C.; Musch, T.; Meister, M.; Sültmann, H.; Lyko, F. The human let-7a-3 locus contains an epigenetically regulated microRNA gene with oncogenic function. Cancer Res. 2007, 67, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Chen, G.; Gallegos, M.; Lin, L.; Ferrer-Torres, D.; Truini, A.; Wang, Z.; Lin, J.; Reddy, R.M.; Llatjos, R.; et al. Epigenetic inactivation of microRNA-34b/c predicts poor disease-free survival in early-stage lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Hasemeier, B.; Christgen, M.; Müller, M.; Römermann, D.; Länger, F.; Kreipe, H. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J. Pathol. 2008, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Liang, G.; Egger, G.; Friedman, J.M.; Chuang, J.C.; Coetzee, G.A.; Jones, P.A. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006, 9, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.P.; Wang, L.; Han, D.; Wang, E.S.; Huskey, N.E.; Lim, L.; Truitt, M.; McManus, M.T.; Ruggero, D.; Goga, A.; et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic caspase-2. Science 2012, 338, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef]
- Teng, B.B.; Burant, C.F.; Davidson, N.O. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science (80-.) 1993, 260, 1816–1819. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, T.; Zhang, X. RNA editing of microRNA prevents RNA-induced silencing complex recognition of target mRNA. Open Biol. 2015, 5, 150126. [Google Scholar] [CrossRef]
- Warnefors, M.; Liechti, A.; Halbert, J.; Valloton, D.; Kaessmann, H. Conserved microRNA editing in Mammalian evolution, development and disease. Genome Biol. 2014, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Y.; Ang, B.; Wang, S.; Choudhury, Y.; Tay, F.C.; Lam, D.H.; Sandanaraj, E. Attenuated adenosine-to-inosine editing of microRNA-376a * promotes invasiveness of glioblastoma cells Find the latest version: Attenuated adenosine-to-inosine editing of microRNA-376a * promotes invasiveness of glioblastoma cells. J. Clin. Investig. 2012, 122, 4059–4076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of MicroRNAs and mRNAs: MicroRNAs Located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, J.; Hu, X.; Huang, Y.; Li, Z.; Zhou, L.; Tian, Z.; Ma, H.; Wu, Z.; Chen, M.; et al. Comparative mRNA and microrna expression profiling of three genitourinary cancers reveals common hallmarks and Cancer-Specific molecular events. PLoS ONE 2011, 6, e22570. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Calin, G.A.; Croce, C.M. MiR-15a and miR-16-1 in cancer: Discovery, function and future perspectives. Cell Death Differ. 2010, 17, 215–220. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, H.; Sun, F.; Zhang, H.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008, 36, 5391–5404. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Feng, L.; Zhang, W.; Pelicano, H.; Wang, F.; Ogasawara, M.A.; Lu, W.; Amin, H.M.; Croce, C.M.; et al. Loss of p53 and altered miR15-a/16-1→MCL-1 pathway in CLL: Insights from TCL1-Tg:p53-/- mouse model and primary human leukemia cells. Leukemia 2014, 28, 118–128. [Google Scholar] [CrossRef][Green Version]
- Cai, C.K.; Zhao, G.Y.; Tian, L.Y.; Liu, L.; Yan, K.; Ma, Y.L.; Ji, Z.W.; Li, X.X.; Han, K.; Gao, J.; et al. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol. Rep. 2012, 28, 1764–1770. [Google Scholar] [CrossRef]
- Bonci, D.; Coppola, V.; Musumeci, M.; Addario, A.; Giuffrida, R.; Memeo, L.; D’Urso, L.; Pagliuca, A.; Biffoni, M.; Labbaye, C.; et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008, 14, 1271–1277. [Google Scholar] [CrossRef]
- Bandi, N.; Zbinden, S.; Gugger, M.; Arnold, M.; Kocher, V.; Hasan, L.; Kappeler, A.; Brunner, T.; Vassella, E. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009, 69, 5553–5559. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Pan, Q.; Jiang, B.; Chen, G.Y.; Li, D.G. Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis 2009, 14, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, X.; Lei, Y.; Liu, X.; Liu, Z.; Tong, T.; Wang, W. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. J. Cell. Biochem. 2010, 111, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Nicoloso, M.; Arvizo, R.; Wang, E.; Cortez, A.; Rossi, S.; Calin, G.A.; Mukherjee, P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009, 69, 9090–9095. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Shi, M.; Kuang, Y.; Fang, L. Downregulation of miRNA-15a and miRNA-16 promote tumor proliferation in multiple myeloma by increasing CABIN1 expression. Oncol. Lett. 2018, 15, 1287–1296. [Google Scholar] [CrossRef]
- Ambs, S.; Prueitt, R.L.; Yi, M.; Hudson, R.S.; Howe, T.M.; Petrocca, F.; Wallace, T.A.; Liu, C.G.; Volinia, S.; Calin, G.A.; et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008, 68, 6162–6170. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Thompson, B.; Leleu, X.; Azab, A.K.; Azab, F.; Runnels, J.; Jia, X.; Ngo, H.T.; Melhem, M.R.; et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood 2009, 113, 6669–6680. [Google Scholar] [CrossRef]
- Musumeci, M.; Coppola, V.; Addario, A.; Patrizii, M.; Maugeri-Saccá, M.; Memeo, L.; Colarossi, C.; Francescangeli, F.; Biffoni, M.; Collura, D.; et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 2011, 30, 4231–4242. [Google Scholar] [CrossRef]
- Cummins, J.M.; Velculescu, V.E. Implications of micro-RNA profiling for cancer diagnosis. Oncogene 2006, 25, 6220–6227. [Google Scholar] [CrossRef]
- He, H.; Jazdzewski, K.; Li, W.; Liyanarachchi, S.; Nagy, R.; Volinia, S.; Calin, G.A.; Liu, C.G.; Franssila, K.; Suster, S.; et al. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA 2005, 102, 19075–19080. [Google Scholar] [CrossRef] [PubMed]
- De La Chapelle, A.; Jazdzewski, K. MicroRNAs in thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Ferracin, M.; Liu, C.G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.Z.; O’Connor, S.M.; Van Holst Pellekaan, N.G.; Young, G.P.; James, R.J. Reduced Accumulation of Specific MicroRNAs in Colorectal Neoplasia. Mol. Cancer Res. 2003, 1, 882–891. [Google Scholar]
- Hibino, Y.; Sakamoto, N.; Naito, Y.; Goto, K.; Oo, H.Z.; Sentani, K.; Hinoi, T.; Ohdan, H.; Oue, N.; Yasui, W. Significance of miR-148a in colorectal neoplasia: Downregulation of miR-148a contributes to the carcinogenesis and cell invasion of colorectal cancer. Pathobiology 2015, 82, 233–241. [Google Scholar] [CrossRef]
- Machová Polaková, K.; Lopotová, T.; Klamová, H.; Burda, P.; Trněný, M.; Stopka, T.; Moravcová, J. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol. Cancer 2011, 10, 41. [Google Scholar] [CrossRef]
- Fulci, V.; Chiaretti, S.; Goldoni, M.; Azzalin, G.; Carucci, N.; Tavolaro, S.; Castellano, L.; Magrelli, A.; Citarella, F.; Messina, M.; et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007, 109, 4944–4951. [Google Scholar] [CrossRef]
- Carabia, J.; Carpio, C.; Abrisqueta, P.; Jiménez, I.; Purroy, N.; Calpe, E.; Palacio, C.; Bosch, F.; Crespo, M. Microenvironment regulates the expression of MIR-21 and tumor suppressor genes PTEN, PIAS3 and PDCD4 through ZAP-70 in chronic lymphocytic leukemia. Sci. Rep. 2017, 7, 12262. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Croce, C.M. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget 2010, 1, 224–227. [Google Scholar] [CrossRef]
- Nateghi, B.; Behshood, P.; Fathullahzadeh, S.; Mardanshah, O. Circulating miR-95 Is a Potential Biomarker of Chronic Lymphocytic Leukemia. Res. Mol. Med. 2019, 6. [Google Scholar] [CrossRef]
- Li, Y.; Mao, M.; Liu, H.; Wang, X.; Kou, Z.; Nie, Y.; Wang, Y.; Wang, Z.; Huang, Q.; Lang, T.; et al. miR-34a and miR-29b as indicators for prognosis of treatment-free survival of chronic lymphocytic leukemia patients in Chinese Uygur and Han populations. Mol. Cell. Probes 2019, 47, 101436. [Google Scholar] [CrossRef]
- Raeisi, F.; Mahmoudi, E.; Dehghani-Samani, M.; Hosseini, S.S.E.; Ghahfarrokhi, A.M.; Arshi, A.; Forghanparast, K.; Ghazanfari, S. Differential Expression Profile of miR-27b, miR-29a, and miR-155 in Chronic Lymphocytic Leukemia and Breast Cancer Patients. Mol. Ther. Oncolytics 2020, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pekarsky, Y.; Santanam, U.; Cimmino, A.; Palamarchuk, A.; Efanov, A.; Maximov, V.; Volinia, S.; Alder, H.; Liu, C.G.; Rassenti, L.; et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006, 66, 11590–11593. [Google Scholar] [CrossRef] [PubMed]
- Klein, U.; Lia, M.; Crespo, M.; Siegel, R.; Shen, Q.; Mo, T.; Ambesi-Impiombato, A.; Califano, A.; Migliazza, A.; Bhagat, G.; et al. The DLEU2/miR-15a/16-1 Cluster Controls B Cell Proliferation and Its Deletion Leads to Chronic Lymphocytic Leukemia. Cancer Cell 2010, 17, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Kovalchuk, I.; Apel, I.J.; Chinnaiyan, A.M.; Wóycicki, R.K.; Cantor, C.R.; Kovalchuk, O. miR-34a directly targets tRNAiMet precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc. Natl. Acad. Sci. USA 2018, 119, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Chang, E.S.; Lee, E.J.; Lee, H.W.; Kang, H.G.; Chun, K.H.; Woo, Y.M.; Kong, H.K.; Ko, J.Y.; Suzuki, H.; et al. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res. 2014, 74, 7573–7582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, S.; Mao, X. miR-145-5p affects the differentiation of gastric cancer by targeting KLF5 directly. J. Cell. Physiol. 2019, 234, 7634–7644. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, Z.; Guo, J.; Ding, X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol. 2010, 119, 586–593. [Google Scholar] [CrossRef]
- Asaga, S.; Kuo, C.; Nguyen, T.; Terpenning, M.; Giuliano, A.E.; Hoon, D.S.B. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin. Chem. 2011, 57, 84–91. [Google Scholar] [CrossRef]
- Roth, C.; Rack, B.; Müller, V.; Janni, W.; Pantel, K.; Schwarzenbach, H. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 2010, 12, 90. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, Z.; Li, H.; Lu, J.; Guo, L.; Ge, Q. Next-generation sequencing of microRNAs for breast cancer detection. J. Biomed. Biotechnol. 2011, 2011, 597145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, J.; Medico, L.; Wang, D.; Ambrosone, C.B.; Liu, S. A pilot study of circulating miRNAs as potential biomarkers of early stage breast cancer. PLoS ONE 2010, 5, e13735. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Wu, R.C.; Fu, J. MicroRNA-34 family in breast cancer: From research to therapeutic potential. J. Cancer 2018, 9, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, X.; Hu, X.L.; Cheng, L.Z.; Yu, J.Y.; Wei, Z.B. Downregulation of MIR-145-5p correlates with poor prognosis in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3026–3030. [Google Scholar] [PubMed]
- Shi, H.; Chen, X.; Jiang, H.; Wang, X.; Yu, H.; Sun, P.; Sui, X. miR-148a suppresses cell invasion and migration in gastric cancer by targeting DNA methyltransferase 1. Oncol. Lett. 2018, 15, 4944–4950. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Yan, S.; Gao, F. Effect of miR-145 on gastric cancer cells. Mol. Med. Rep. 2019, 19, 3403–3410. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, Y.; Xie, Y.; Ren, Y.; Zhang, H.; Zhou, Y.; Gao, N.; Ding, S.; Han, S. miR-200a-3p plays tumor suppressor roles in gastric cancer cells by targeting KLF12. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3697–3703. [Google Scholar] [CrossRef]
- Hwang, J.; Min, B.H.; Jang, J.; Kang, S.Y.; Bae, H.; Jang, S.S.; Kim, J.I.; Kim, K.M. MicroRNA Expression Profiles in Gastric Carcinogenesis. Sci. Rep. 2018, 8, 14393. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Q.; Wu, X. Hif1α/mir-520a-3p/akt1/mtor feedback promotes the proliferation and glycolysis of gastric cancer cells. Cancer Manag. Res. 2019, 11, 10145–10156. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, Y.; Sun, D.; Wang, S.; Ding, K.; Liu, M.; Zhang, Y.; Miao, Y.; Liu, H.; Zhou, F. MicroRNA-181a functions as an oncogene in gastric cancer by targeting caprin-1. Front. Pharmacol. 2019, 9, 1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jeong, S.; Jung, W.Y.; Choi, J.W.; Hwang, K.C.; Kim, S.W.; Lee, Y.C. Mirnas as potential biomarkers for the progression of gastric cancer inhibit crebzf and regulate migration of gastric adenocarcinoma cells. Int. J. Med. Sci. 2020, 17, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, W.S.; Kim, S.W.; Jung, W.Y. Regulation of gastric carcinoma development in gastric adenoma/dysplasia by crebzf inhibition via miRNA-421. J. Clin. Oncol. 2020, 38, 410. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, K.C.; Kim, S.W.; Lee, Y.C. Potential miRNA-target interactions for the screening of gastric carcinoma development in gastric adenoma/dysplasia. Int. J. Med. Sci. 2018, 15, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, L.; Sun, Y.; Yin, Q.; Chen, X.; Liang, S.; Meng, Q.; Long, H.; Li, F.; Luo, C.; et al. MIR-421 in plasma as a potential diagnostic biomarker for precancerous gastric lesions and early gastric cancer. PeerJ 2019, 7, e7002. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, X.; Xia, J.; Shan, T.; Gu, C.; Liang, Z.; Zhao, W.; Jin, S. MiR-148a regulates MEG3 in gastric cancer by targeting DNA methyltransferase 1. Med. Oncol. 2014, 31, 879. [Google Scholar] [CrossRef]
- Mercatelli, N.; Coppola, V.; Bonci, D.; Miele, F.; Costantini, A.; Guadagnoli, M.; Bonanno, E.; Muto, G.; Frajese, G.V.; De Maria, R.; et al. The inhibition of the highly expressed mir-221 and mir-222 impairs the growth of prostate carcinoma xenografts in mice. PLoS ONE 2008, 3, 4029. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Wang, L.; Zhang, D.; Luo, Q.; Wang, B. Overexpressing miR-335 inhibits DU145 cell proliferation by targeting early growth response 3 in prostate cancer. Int. J. Oncol. 2019, 54, 1981–1994. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Gan, R.; Zhao, L.; Li, W.; Zhou, H.; Wang, X.; Lu, J.; Meng, Q.H. Down-regulation of miR-221 and miR-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS ONE 2014, 9, e98833. [Google Scholar] [CrossRef]
- Richardsen, E.; Andersen, S.; Melbø-Jørgensen, C.; Rakaee, M.; Ness, N.; Al-Saad, S.; Nordby, Y.; Pedersen, M.I.; Dønnem, T.; Bremnes, R.M.; et al. MicroRNA 141 is associated to outcome and aggressive tumor characteristics in prostate cancer. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T.F. Circulating miR-141 and miR-375 are associated with treatment outcome in metastatic castration resistant prostate cancer. Sci. Rep. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Osther, P.J.S.; Assenholt, J.; Madsen, J.S.; Hansen, T. Association between circulating miRNA-141 and miRNA-375 and treatment outcome in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2019, 37, e16507. [Google Scholar] [CrossRef]
- Yan, X.; Tang, B.; Chen, B.; Shan, Y.; Yang, H.; Iorns, E.; Tsui, R.; Denis, A.; Perfito, N.; Errington, T.M. Replication study: The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Elife 2019, 17, 211–215. [Google Scholar]
- Zhao, H.; Zhang, W.; Lai, X.; Zhu, H.; Zhang, S.; Wu, W.; Wang, S.; Tang, M.; Deng, Z.; Tan, J. MiR-30a-5p frequently downregulated in prostate cancer inhibits cell proliferation via targeting PCLAF. Artif. Cells Nanomed. Biotechnol. 2019, 47, 278–289. [Google Scholar] [CrossRef]
- Jin, W.; Chen, F.; Wang, K.; Song, Y.; Fei, X.; Wu, B. miR-15a/miR-16 cluster inhibits invasion of prostate cancer cells by suppressing TGF-β signaling pathway. Biomed. Pharmacother. 2018, 104, 637–644. [Google Scholar] [CrossRef]
- Galardi, S.; Mercatelli, N.; Giorda, E.; Massalini, S.; Frajese, G.V.; Ciafrè, S.A.; Farace, M.G. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 2007, 282, 23716–23724. [Google Scholar] [CrossRef]
- Le Sage, C.; Nagel, R.; Egan, D.A.; Schrier, M.; Mesman, E.; Mangiola, A.; Anile, C.; Maira, G.; Mercatelli, N.; Ciafrè, S.A.; et al. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007, 26, 3699–3708. [Google Scholar] [CrossRef]
- Zhang, H.L.; Qin, X.J.; Cao, D.L.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Ye, D.W. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J. Androl. 2013, 15, 231–235. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Zhu, C.; Hou, X.; Zhu, J.; Jiang, C.; Wei, W. Expression of miR-30c and miR-29b in prostate cancer and its diagnostic significance. Oncol. Lett. 2018, 16, 3140–3144. [Google Scholar] [CrossRef]
- Xiong, S.W.; Lin, T.X.; Xu, K.W.; Dong, W.; Ling, X.H.; Jiang, F.N.; Chen, G.; Zhong, W.D.; Huang, J. MicroRNA-335 acts as a candidate tumor suppressor in prostate cancer. Pathol. Oncol. Res. 2013, 19, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, Y.; Daniels, G.; Sfanos, K.; De Marzo, A.; Wei, J.; Li, X.; Chen, W.; Wang, J.; Zhong, X.; et al. LEF1 targeting EMT in prostate cancer invasion is regulated by miR-34a. Mol. Cancer Res. 2015, 13, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.L.; Sun, B.F.; Huang, L.R.; Yuan, H.B.; Zhang, S.; Chen, J.; Yu, Z.J.; Luo, H. Potent inhibition of miR-34b on migration and invasion in metastatic prostate cancer cells by regulating the TGF-β pathway. Int. J. Mol. Sci. 2017, 18, 2762. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Zhu, A.; Jiao, X.; Ge, J.; Xu, X. Combined low miR-34s are associated with unfavorable prognosis in children with hepatoblastoma: A Chinese population-based study. J. Pediatr. Surg. 2016, 51, 1355–1361. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali-Eldin, Z.A.; Elbedewy, T.A.; El-Serafy, M.; Ali-Eldin, F.A.; AbdelAziz, H. MicroRNAs and clinical implications in hepatocellular carcinoma. World J. Hepatol. 2017, 9, 1001–1007. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Erconi, V.; Valenti, L.; Gatti, S.; Fracanzani, A.L.; Dongiovanni, P. Mir-101-3p downregulation promotes fibrogenesis by facilitating hepatic stellate cell transdifferentiation during insulin resistance. Nutrients 2019, 11, 2597. [Google Scholar] [CrossRef]
- Chang, J.; Nicolas, E.; Marks, D.; Sander, C.; Lerro, A.; Buendia, M.A.; Xu, C.; Mason, W.S.; Moloshok, T.; Bort, R.; et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004, 1, 106–113. [Google Scholar] [CrossRef]
- Ha, S.Y.; Yu, J.I.; Choi, C.; Kang, S.Y.; Joh, J.W.; Paik, S.W.; Kim, S.; Kim, M.; Park, H.C.; Park, C.K. Prognostic significance of miR-122 expression after curative resection in patients with hepatocellular carcinoma. Sci. Rep. 2019, 9, 14738. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, X.; Fan, Y.; Yang, X.; Li, A.; Qian, J. The contribution of miR-122 to the innate immunity by regulating toll-like receptor 4 in hepatoma cells. BMC Gastroenterol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, M.; Zhao, Y.; Pan, Y.; Kan, M.; Li, J.; He, K.; Zhang, X. HNF-4α inhibits hepatocellular carcinoma cell proliferation through mir-122-adam17 pathway. PLoS ONE 2020, 15, e0230450. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Wang, Y.; Wen, S.; Wang, J.; Chen, Z.; He, Q.; Feng, D. MicroRNA-145 inhibits cell proliferation by directly targeting ADAM17 in hepatocellular carcinoma. Oncol. Rep. 2014, 32, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Qin, L.; Huang, J.; Huang, H. Lower miR-145-5p expression and its potential pathways in hepatocellular carcinoma: A bioinformatics analysis with RNA-seq and microarray data. Int. J. Clin. Exp. Med. 2018, 11, 3060–3307. [Google Scholar]
- Men, R.; Wen, M.; Zhao, M.; Dan, X.; Yang, Z.; Wu, W.; Wang, M.H.; Liu, X.; Yang, L. MircoRNA-145 promotes activation of hepatic stellate cells via targeting krüppel-like factor 4. Sci. Rep. 2017, 7, 40468. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Jiang, H.X.; Shi, Q.Y.; Qiu, X.; Wei, X.; Zhang, X.L.; Qin, S.Y. MiR-145 inhibits Th9 cell differentiation by suppressing activation of the PI3K/Akt/mTOR/p70S6K/HIF-1α pathway in malignant ascites from liver cancer. Onco. Targets. Ther. 2020, 13, 3789–3800. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Tong, L.; Ma, X.; Qiu, X. miR-195 is a key negative regulator of hepatocellular carcinoma metastasis by targeting FGF2 and VEGFA. Int. J. Clin. Exp. Pathol. 2015, 8, 14110–14120. [Google Scholar]
- Xiong, Y.; Fang, J.H.; Yun, J.P.; Yang, J.; Zhang, Y.; Jia, W.H.; Zhuang, S.M. Effects of microrna-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef]
- Shi, K.Q.; Lin, Z.; Chen, X.J.; Song, M.; Wang, Y.Q.; Cai, Y.J.; Yang, N.B.; Zheng, M.H.; Dong, J.Z.; Zhang, L.; et al. Hepatocellular carcinoma associated microRNA expression signature: Integrated bioinformatics analysis, experimental validation and clinical significance. Oncotarget 2015, 6, 25093–25108. [Google Scholar] [CrossRef]
- Yu, S.; Jing, L.; Yin, X.R.; Wang, M.C.; Chen, Y.M.; Guo, Y.; Nan, K.J.; Han, L.L. MiR-195 suppresses the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by inhibiting YAP. Oncotarget 2017, 15, 99757–99771. [Google Scholar] [CrossRef][Green Version]
- Amer, M.; Elhefnawi, M.; El-Ahwany, E.; Awad, A.F.; Gawad, N.A.; Zada, S.; Tawab, F.M.A. Hsa-miR-195 targets PCMT1 in hepatocellular carcinoma that increases tumor life span. Tumor Biol. 2014, 35, 11301–11309. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, A.T.C.; Ma, J.Z.I.; Wang, J.; Ren, J.; Yang, Y.; Tantoso, E.; Li, K.B.; Ooi, L.L.P.J.; Tan, P.; et al. Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J. Biol. Chem. 2008, 283, 13205–13215. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chen, Y.; Xie, Q.; Dong, N.; Gao, Y.; Deng, H.; Lu, C.; Wang, S. MicroRNA-214-3p inhibits proliferation and cell cycle progression by targeting MELK in hepatocellular carcinoma and correlates cancer prognosis. Cancer Cell Int. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.P.; Yi, M.; Li, Q.Q.; Zhou, W.P.; Cong, W.M.; Yang, Y.; Ning, B.F.; Yin, C.; Huang, Z.W.; Wang, J.; et al. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology 2013, 58, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Hou, Y.B.; Jia, H.Y.; Bi, X.H.; Yu, L.; Chen, D.J. MiR-370 promotes cell death of liver cancer cells by Akt/FoxO3a signalling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2011–2019. [Google Scholar] [PubMed]
- Liu, Z.; Ma, M.; Yan, L.; Chen, S.; Li, S.; Yang, D.; Wang, X.; Xiao, H.; Deng, H.; Zhu, H.; et al. MiR-370 regulates ISG15 expression and influences IFN-α sensitivity in hepatocellular carcinoma cells. Cancer Biomarkers 2018, 22, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Jung, K.H.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Kim, S.J.; Park, W.S.; et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene 2014, 33, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wei, L.; Law, C.T.; Ho, D.W.H.; Tsang, F.H.C.; Au, S.L.K.; Sze, K.M.F.; Lee, J.M.F.; Wong, C.C.L.; Ng, I.O.L. Up-regulation of histone methyltransferase SETDB1 by multiple mechanisms in hepatocellular carcinoma promotes cancer metastasis. Hepatology 2016, 63, 474–487. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Ding, H. XPD suppresses cell proliferation and migration via miR-29a-3p-Mdm2/PDGF-B axis in HCC. Cell Biosci. 2019, 9, 6. [Google Scholar] [CrossRef]
- Loosen, S.H.; Lurje, G.; Wiltberger, G.; Vucur, M.; Koch, A.; Kather, J.N.; Paffenholz, P.; Tacke, F.; Ulmer, F.T.; Trautwein, C.; et al. Serum levels of miR-29, miR-122, miR-155 and miR-192 are elevated in patients with cholangiocarcinoma. PLoS ONE 2019, 14, e0210944. [Google Scholar] [CrossRef]
- Su, H.; Yang, J.R.; Xu, T.; Huang, J.; Xu, L.; Yuan, Y.; Zhuang, S.M. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009, 69, 1135–1142. [Google Scholar] [CrossRef]
- Chai, Z.; Yin, X.; Chen, J.; Shi, J.; Sun, J.; Liu, C.; Liu, F.; Cheng, S. MicroRNA-101 modulates cisplatin chemoresistance in liver cancer cells via the DNA-PKcs signaling pathway. Oncol. Lett. 2019, 18, 3655–3663. [Google Scholar] [CrossRef]
- Meng, X.; Shi, Y.; Xiang, X.; Li, C.; Ge, X.; Pan, K.; Liang, Y. Influence of miR-101 on proliferation of liver cancer cells through the MAPK/ERK signaling pathway. Oncol. Lett. 2020, 19, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.F.; Wu, J.; Xu, S.J.; Xu, Y.Y.; Li, D.; Lou, J.T.; Liu, M.F. IL-1b-mediated repression of microRNA-101 Is Crucial for Inflammation-promoted lung Tumorigenesis. Cancer Res. 2014, 74, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Nikota, J.; Wu, D.; Williams, A.; Yauk, C.L.; Stampfli, M. IL-1 Receptor Regulates microRNA-135b Expression in a Negative Feedback Mechanism during Cigarette Smoke–Induced Inflammation. J. Immunol. 2013, 190, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.S.; Yin, M.H.; Zhang, X.; Wang, Z.; Feng, S.P.; Wang, G.X.; Luo, Y.J.; Liang, P.Z.; Yang, X.Q.; He, J.X.; et al. Tumor-suppressive microRNA-449a induces growth arrest and senescence by targeting E2F3 in human lung cancer cells. Cancer Lett. 2014, 344, 195–203. [Google Scholar] [CrossRef]
- Luo, W.; Huang, B.; Li, Z.; Li, H.; Sun, L.; Zhang, Q.; Qiu, X.; Wang, E. MicroRNA-449a Is Downregulated in Non-Small Cell Lung Cancer and Inhibits Migration and Invasion by Targeting c-Met. PLoS ONE 2013, 8, e64759. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, O.; Boeri, M.; Moro, M.; Verri, C.; Mensah, M.; Conte, D.; Caleca, L.; Roz, L.; Pastorino, U.; Sozzi, G. Mir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2-p53 interaction. Cell Death Dis. 2014, 5, e1564. [Google Scholar] [CrossRef]
- Kong, Q.; Shu, N.; Li, J.; Xu, N. miR-641 functions as a tumor suppressor by targeting MDM2 in human lung cancer. Oncol. Res. 2018, 26, 735–741. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef]
- Barshack, I.; Lithwick-Yanai, G.; Afek, A.; Rosenblatt, K.; Tabibian-Keissar, H.; Zepeniuk, M.; Cohen, L.; Dan, H.; Zion, O.; Strenov, Y.; et al. MicroRNA expression differentiates between primary lung tumors and metastases to the lung. Pathol. Res. Pract. 2010, 206, 578–584. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, Y.; Zhou, X.; Chen, Y. Low expression of let-7 predicts poor prognosis in patients with multiple cancers: A meta-analysis. Tumor Biol. 2014, 35, 5143–5148. [Google Scholar] [CrossRef]
- Aharonov, R.; Lebanony, D.; Benjamin, H.; Gilad, S.; Ezagouri, M.; Dov, A.; Ashkenazi, K.; Gefen, N.; Izraeli, S.; Rechavi, G.; et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009, 27, 2030–2037. [Google Scholar]
- Nadal, E.; Zhong, J.; Lin, J.; Reddy, R.M.; Ramnath, N.; Orringer, M.B.; Chang, A.C.; Beer, D.G.; Chen, G. A microRNA cluster at 14q32 drives aggressive lung adenocarcinoma. Clin. Cancer Res. 2014, 20, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wei, F.; Zhang, J.; Wang, X.; Li, B. miR-134 inhibits non-small cell lung cancer growth by targeting the epidermal growth factor receptor. J. Cell. Mol. Med. 2016, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Ding, H.; Wang, W.; Liao, Z.; Fu, Z.; Hong, Y.; Zhou, Y.; Zhang, C.Y.; Chen, X. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget 2016, 7, 28075–28085. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, W.; Shi, X.; Zheng, J.; Jin, X.; Huo, J. MiR-760 suppresses non-small cell lung cancer proliferation and metastasis by targeting ROS1. Environ. Sci. Pollut. Res. 2018, 25, 18385–18391. [Google Scholar] [CrossRef]
- Zou, J.G.; Ma, L.F.; Li, X.; Xu, F.L.; Fei, X.Z.; Liu, Q.; Bai, Q.L.; Dong, Y.L. Circulating microRNA array (miR-182, 200b and 205) for the early diagnosis and poor prognosis predictor of non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1108–1115. [Google Scholar]
- Wang, Y.Y.; Ren, T.; Cai, Y.Y.; He, X.Y. MicroRNA let-7a inhibits the proliferation and invasion of nonsmall cell lung cancer cell line 95D by regulating K-RAS and HMGA2 gene expression. Cancer Biother. Radiopharm. 2013, 28, 131–137. [Google Scholar] [CrossRef]
- He, X.Y.; Chen, J.X.; Zhang, Z.; Li, C.L.; Peng, Q.L.; Peng, H.M. The let-7a microRNA protects from growth of lung carcinoma by suppression of k-Ras and c-Myc in nude mice. J. Cancer Res. Clin. Oncol. 2010, 136, 1023–1028. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, K.; Pan, C.; Li, H.; Cao, J.; Han, X.; Tang, Y.; Zhu, S.; Yuan, W.; He, Y.; et al. MicroRNA-1258 suppresses tumour progression via GRB2/Ras/Erk pathway in non-small-cell lung cancer. Cell Prolif. 2018, 51, e12502. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, L.; Ni, R.; Zhang, H.; Li, L.; Wang, X.; Li, X.; Wang, J. MicroRNA-520a-3p inhibits proliferation and cancer stem cell phenotype by targeting HOXD8 in non-small cell lung cancer. Oncol. Rep. 2016, 36, 3529–3535. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tan, Q.; Deng, B.; Fang, C.; Qi, D.; Wang, R. The microRNA-520a-3p inhibits proliferation, apoptosis and metastasis by targeting MAP3K2 in non-small cell lung cancer. Am J Cancer Res 2015, 5, 802–811. [Google Scholar] [PubMed]
- Kim, J.S.; Kim, E.J.; Lee, S.; Tan, X.; Liu, X.; Park, S.; Kang, K.; Yoon, J.S.; Ko, Y.H.; Kurie, J.M.; et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, Y.; Jing, R. MicroRNA-29a functions as a potential tumor suppressor through directly targeting CDC42 in non-small cell lung cancer. Oncol. Lett. 2017, 13, 3896–3904. [Google Scholar] [CrossRef]
- Tan, M.; Wu, J.; Cai, Y. Suppression of Wnt signaling by the miR-29 family is mediated by demethylation of WIF-1 in non-small-cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 438, 673–679. [Google Scholar] [CrossRef]
- Zou, P.; Zhu, M.; Lian, C.; Wang, J.; Chen, Z.; Zhang, X.; Yang, Y.; Chen, X.; Cui, X.; Liu, J.; et al. miR-192-5p suppresses the progression of lung cancer bone metastasis by targeting TRIM44. Sci. Rep. 2019, 9, 19619. [Google Scholar] [CrossRef]
- Hirono, T.; Jingushi, K.; Nagata, T.; Sato, M.; Minami, K.; Aoki, M.; Takeda, A.H.; Umehara, T.; Egawa, H.; Nakatsuji, Y.; et al. MicroRNA-130b functions as an oncomiRNA in non-small cell lung cancer by targeting tissue inhibitor of metalloproteinase-2. Sci. Rep. 2019, 9, 6956. [Google Scholar] [CrossRef]
- Li, L.; Feng, T.; Zhang, W.; Gao, S.; Wang, R.; Lv, W.; Zhu, T.; Yu, H.; Qian, B. MicroRNA Biomarker hsa-miR-195-5p for Detecting the Risk of Lung Cancer. Int. J. Genomics 2020, 2020, 7415909. [Google Scholar] [CrossRef]
- Hojbjerg, J.A.; Ebert, E.B.F.; Clement, M.S.; Winther-Larsen, A.; Meldgaard, P.; Sorensen, B. Circulating miR-30b and miR-30c predict erlotinib response in EGFR-mutated non-small cell lung cancer patients. Lung Cancer 2019, 135, 92–96. [Google Scholar] [CrossRef]
- Zang, Y.S.; Zhong, Y.F.; Fang, Z.; Li, B.; An, J. MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression. Cancer Gene Ther. 2012, 19, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Du, W.; Wang, Y.; Xu, C.; Wang, J.; Zhang, Y.; Wang, H.; Ju, J.; Zhao, L.; Wang, Z.; et al. Suppression of AKT expression by miR-153 produced anti-tumor activity in lung cancer. Int. J. Cancer 2015, 136, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Shen, L.; Wang, J.; He, D.; Duan, C. MiR-153 inhibits migration and invasion of human non-small-cell lung cancer by targeting ADAM19. Biochem. Biophys. Res. Commun. 2015, 456, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.; Brawner, E.; Batte, K.; Yu, L.; Hunter, M.G.; Otterson, G.A.; Nuovo, G.; Marsh, C.B.; Nana-Sinkam, S.P. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem. Biophys. Res. Commun. 2008, 373, 607–612. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, P.; Li, X.; Chen, Y. Micro ribonucleic acid (RNA)-101 inhibits cell proliferation and invasion of lung cancer by regulating cyclooxygenase-2. Thorac. Cancer 2015, 6, 778–784. [Google Scholar] [CrossRef]
- Yanaihara, N.; Caplen, N.; Bowman, E.; Seike, M.; Kumamoto, K.; Yi, M.; Stephens, R.M.; Okamoto, A.; Yokota, J.; Tanaka, T.; et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006, 9, 189–198. [Google Scholar] [CrossRef]
- Gagnidze, K.; Rayon-Estrada, V.; Harroch, S.; Bulloch, K.; Papavasiliou, F.N. A New Chapter in Genetic Medicine: RNA Editing and its Role in Disease Pathogenesis. Trends Mol. Med. 2018, 24, 294–303. [Google Scholar] [CrossRef]
- Eichler, S.A.; Kirischuk, S.; Jüttner, R.; Schafermeier, P.K.; Legendre, P.; Lehmann, T.N.; Gloveli, T.; Grantyn, R.; Meier, J.C. Glycinergic tonic inhibition of hippocampal neurons with depolarizing GABAergic transmission elicits histopathological signs of temporal lobe epilepsy. J. Cell. Mol. Med. 2008, 12, 2848–2866. [Google Scholar] [CrossRef]
- Grohmann, M.; Hammer, P.; Walther, M.; Paulmann, N.; Büttner, A.; Eisenmenger, W.; Baghai, T.C.; Schüle, C.; Rupprecht, R.; Bader, M.; et al. Alternative splicing and extensive RNA editing of human TPH2 transcripts. PLoS ONE 2010, 5, e8956. [Google Scholar] [CrossRef]
- Dostie, J.; Mourelatos, Z.; Yang, M.; Sharma, A.; Dreyfuss, G. Numerous microRNPs in neuronal cells containing novel microRNAs. Rna 2003, 9, 180–186. [Google Scholar] [CrossRef]
- Singh, M. Dysregulated A to I RNA editing and non-coding rnas in neurodegeneration. Front. Genet. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lerner, T.; Papavasiliou, F.N.; Pecori, R. RNA editors, cofactors, and mRNA targets: An overview of the c-to-U RNA editing machinery and its implication in human disease. Genes 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.H.; Wang, H. Exploring microRNA biomarker for amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2018, 19, 1318. [Google Scholar] [CrossRef] [PubMed]
- Freischmidt, A.; Müller, K.; Ludolph, A.C.; Weishaupt, J.H. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Raheja, R.; Regev, K.; Healy, B.C.; Mazzola, M.A.; Beynon, V.; Von Glehn, F.; Paul, A.; Diaz-Cruz, C.; Gholipour, T.; Glanz, B.I.; et al. Correlating serum micrornas and clinical parameters in amyotrophic lateral sclerosis. Muscle Nerve 2018, 58, 261–269. [Google Scholar] [CrossRef]
- Waller, R.; Goodall, E.F.; Milo, M.; Cooper-Knock, J.; Da Costa, M.; Hobson, E.; Kazoka, M.; Wollff, H.; Heath, P.R.; Shaw, P.J.; et al. Serum miRNAs miR-206, 143-3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS). Neurobiol. Aging 2017, 55, 123–131. [Google Scholar] [CrossRef]
- Junn, E.; Lee, K.W.; Byeong, S.J.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of α-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances α-synuclein expression in Parkinson’s disease. FEBS Lett. 2015, 589, 319–325. [Google Scholar] [CrossRef]
- Doxakis, E. Post-transcriptional regulation of α-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010, 285, 12726–12734. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, Y. MiR-16-1 promotes the aberrant α -synuclein accumulation in parkinson disease via targeting heat shock protein 70. Sci. World J. 2014, 2014, 938348. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Schapira, A.H.V.; Rodriguez-Oroz, M.C.; Obeso, J.A.; Cooper, J.M. Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in parkinson’s disease. Cell Death Dis. 2013, 4, e545. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.B.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A microRNA feedback circuit in midbrain dopamine neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Sethi, P.; Lukiw, W.J. Micro-RNA abundance and stability in human brain: Specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci. Lett. 2009, 459, 100–104. [Google Scholar] [CrossRef]
- Schonrock, N.; Ke, Y.D.; Humphreys, D.; Staufenbiel, M.; Ittner, L.M.; Preiss, T.; Götz, J. Neuronal microrna deregulation in response to Alzheimer’s disease amyloid-β. PLoS ONE 2010, 5, e11070. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, L.H.; Xu, W.U.P.; Jing, P.; Zhan, P.Y. microRNA-9 attenuates amyloidβ-induced synaptotoxicity by targeting calcium/calmodulin-dependent protein kinase kinase 2. Mol. Med. Rep. 2014, 9, 1917–1922. [Google Scholar] [CrossRef]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef]
- Augustin, R.; Endres, K.; Reinhardt, S.; Kuhn, P.H.; Lichtenthaler, S.F.; Hansen, J.; Wurst, W.; Trümbach, D. Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med. Genet. 2012, 13, 35. [Google Scholar] [CrossRef]
- Yao, J.; Hennessey, T.; Flynt, A.; Lai, E.; Flint Beal, M.; Lin, M.T. MicroRNA-Related Cofilin Abnormality in Alzheimer’s Disease. PLoS ONE 2010, 5, e15546. [Google Scholar] [CrossRef]
- Shaik, M.M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, I.; Cichalewska, M.; Namiecinska, M.; Galazka, G.; Horzelski, W.; Selmaj, K.W.; Mycko, M.P. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017, 81, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, W.Z.; Zhang, X.M.; Yue, H.; Li, B.; Lin, L.; Fu, J. MicroRNA expression aberration in chinese patients with relapsing remitting multiple sclerosis. J. Mol. Neurosci. 2014, 52, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.H.; Sheng, K.T.; Zhao, Y.X.; Li, H.; Zhou, J.L.; Yao, H.Y.; Li, X.H. Identifying the biomarkers of multiple sclerosis based on non-coding RNA signature. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3635–3642. [Google Scholar] [PubMed]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J. Neurosci. 2008, 28, 14341–14346. [Google Scholar] [CrossRef]
- Cheng, P.H.; Li, C.L.; Chang, Y.F.; Tsai, S.J.; Lai, Y.Y.; Chan, A.W.S.; Chen, C.M.; Yang, S.H. MiR-196a ameliorates phenotypes of huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am. J. Hum. Genet. 2013, 93, 306–312. [Google Scholar] [CrossRef]
- Hoss, A.G.; Labadorf, A.; Latourelle, J.C.; Kartha, V.K.; Hadzi, T.C.; Gusella, J.F.; MacDonald, M.E.; Chen, J.F.; Akbarian, S.; Weng, Z.; et al. MiR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genomics 2015, 8, 10. [Google Scholar] [CrossRef]
- Hoss, A.G.; Lagomarsino, V.N.; Frank, S.; Hadzi, T.C.; Myers, R.H.; Latourelle, J.C. Study of plasma-derived miRNAs mimic differences in Huntington’s disease brain. Mov. Disord. 2015, 30, 1961–1964. [Google Scholar] [CrossRef]
- Brennan, S.; Keon, M.; Liu, B.; Su, Z.; Saksena, N.K. Panoramic Visualization of Circulating MicroRNAs Across Neurodegenerative Diseases in Humans. Mol. Neurobiol. 2019, 56, 7380–7407. [Google Scholar] [CrossRef]
- Mack, G.S. MicroRNA gets down to business. Nat. Biotechnol. 2007, 25, 631–638. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Dorsett, Y.; Tuschl, T. Sequence-specific inhibition of microRNA-and siRNA-induced RNA silencing. RNA 2004, 10, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Heyn, J.; Limbeck, E.; Kreth, S. Selection of reliable reference genes for quantitative real-time PCR in human T cells and neutrophils. BMC Res. Notes 2011, 4, 427. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Shi, L.; Guo, L. microRNA Profiling: Strategies and Challenges. In microRNAs in Toxicology and Medicine; Wiley: Hoboken, NJ, USA, 2013; ISBN 9781118695999. [Google Scholar]
- Aleksic, J.; Carl, S.H.; Frye, M. Beyond library size: A field guide to NGS normalization. bioRxiv 2014. [Google Scholar] [CrossRef]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Calin, G.A.; Meloon, B.; Gamliel, N.; Sevignani, C.; Ferracin, M.; Dumitru, C.D.; Shimizu, M.; Zupo, S.; Dono, M.; et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 9740–9744. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. microRNA detection comes of age. Nat. Methods 2006, 3, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef]
- Benes, V.; Castoldi, M. Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available. Methods 2010, 50, 244–249. [Google Scholar] [CrossRef]
- Hafner, M.; Landgraf, P.; Ludwig, J.; Rice, A.; Ojo, T.; Lin, C.; Holoch, D.; Lim, C.; Tuschl, T. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods 2008, 44, 3–12. [Google Scholar] [CrossRef]
- Ho, H.P.; Loo, F.C.; Wu, S.Y.; Gu, D.; Yong, K.T.; Kong, S.K. MicroRNA biosensing with two-dimensional surface plasmon resonance imaging. Methods Mol. Biol. 2017, 1571, 117–127. [Google Scholar]
- Matullo, G.; Naccarati, A.; Pardini, B. MicroRNA expression profiling in bladder cancer: The challenge of next-generation sequencing in tissues and biofluids. Int. J. Cancer 2016, 138, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Kappel, A.; Keller, A. MiRNA assays in the clinical laboratory: Workflow, detection technologies and automation aspects. Clin. Chem. Lab. Med. 2017, 55, 636–647. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).