Aberrant CXCR4 Signaling at Crossroad of WHIM Syndrome and Waldenstrom’s Macroglobulinemia
Abstract
1. Introduction
1.1. WHIM Syndrome
1.2. Waldenstrom’s Macroglobulinaemia
2. Genetic Barcode of WHIM Mutations
3. Signaling of WHIM Mutations
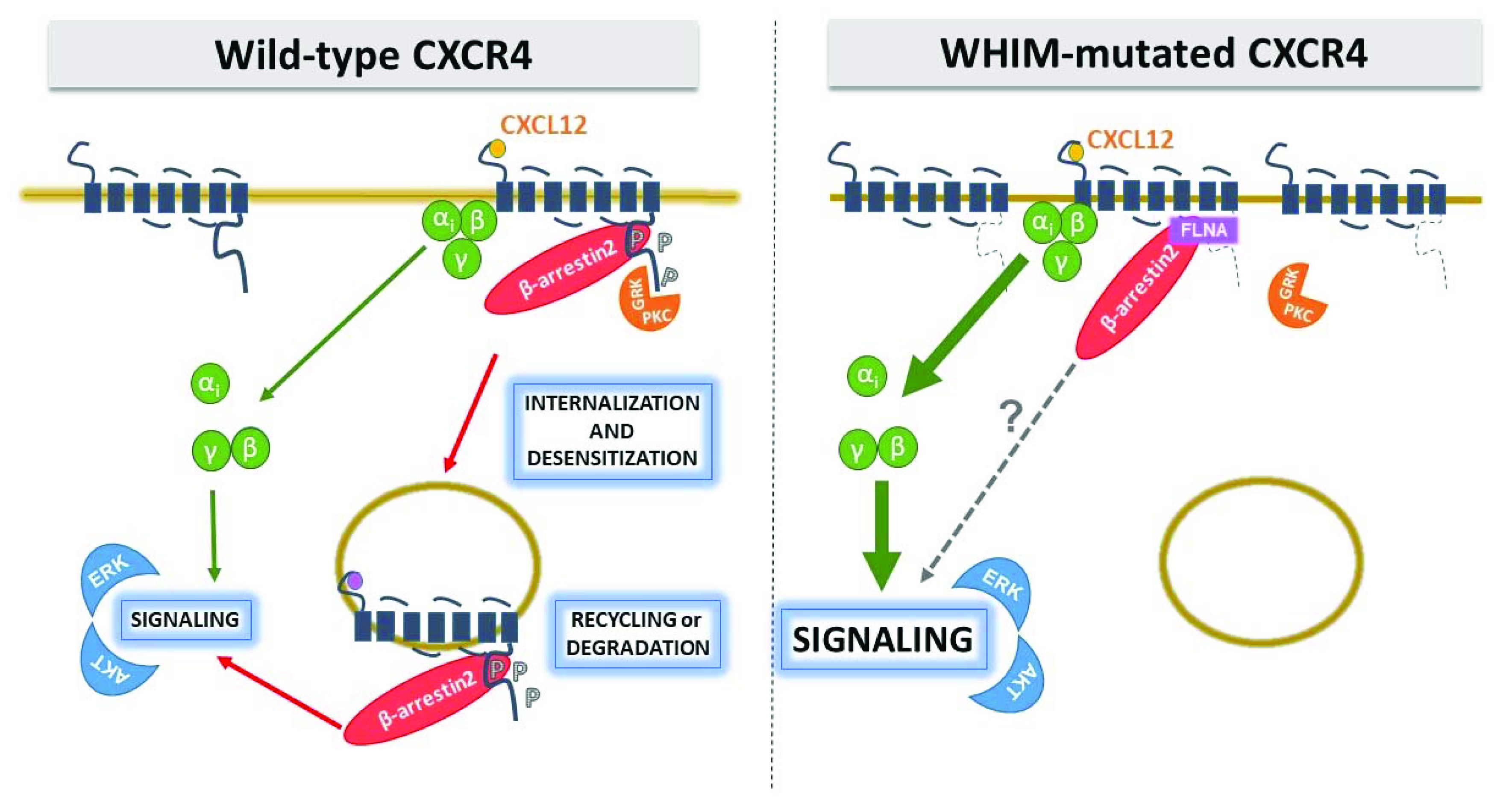
3.1. The Gain-of-Function Nature of WHIM Mutations
3.2. Hyperactivity of WHIM-Mutated CXCR4: A Question of TAIL or deTAILs?
3.3. Effect of Homo or Heterodimerization on WHIM-Mutated CXCR4 Signaling: A Still Unanswered Question
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Loetscher, M.; Geiser, T.; O’Reilly, T.; Zwahlen, R.; Baggiolini, M.; Moser, B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994, 269, 232–237. [Google Scholar] [PubMed]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Teixido, J.; Martinez-Moreno, M.; Diaz-Martinez, M.; Sevilla-Movilla, S. The good and bad faces of the CXCR4 chemokine receptor. Int. J. Biochem. Cell Biol. 2018, 95, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pawig, L.; Klasen, C.; Weber, C.; Bernhagen, J.; Noels, H. Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front. Immunol. 2015, 6, 429. [Google Scholar] [CrossRef]
- McDermott, D.H.; Murphy, P.M. WHIM syndrome: Immunopathogenesis, treatment and cure strategies. Immunol. Rev. 2019, 287, 91–102. [Google Scholar] [CrossRef]
- Kapoor, P.; Ansell, S.M.; Braggio, E. Waldenstrom Macroglobulinemia: Genomic Aberrations and Treatment. Cancer Treat. Res. 2016, 169, 321–361. [Google Scholar] [CrossRef]
- Pozzobon, T.; Goldoni, G.; Viola, A.; Molon, B. CXCR4 signaling in health and disease. Immunol. Lett. 2016, 177, 6–15. [Google Scholar] [CrossRef]
- Tsou, L.K.; Huang, Y.H.; Song, J.S.; Ke, Y.Y.; Huang, J.K.; Shia, K.S. Harnessing CXCR4 antagonists in stem cell mobilization, HIV infection, ischemic diseases, and oncology. Med. Res. Rev. 2018, 38, 1188–1234. [Google Scholar] [CrossRef]
- Scala, S.; D’Alterio, C.; Milanesi, S.; Castagna, A.; Carriero, R.; Farina, F.M.; Locati, M.; Borroni, E.M. New Insights on the Emerging Genomic Landscape of CXCR4 in Cancer: A Lesson from WHIM. Vaccines 2020, 8, 164. [Google Scholar] [CrossRef]
- Vater, A.; Klussmann, S. Toward third-generation aptamers: Spiegelmers and their therapeutic prospects. Curr. Opin. Drug Discov. Dev. 2003, 6, 253–261. [Google Scholar]
- Hoellenriegel, J.; Zboralski, D.; Maasch, C.; Rosin, N.Y.; Wierda, W.G.; Keating, M.J.; Kruschinski, A.; Burger, J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood 2014, 123, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.; Montillo, M.; Scarfo, L.; Mauro, F.R.; Andel, J.; Wildner, S.; Trentin, L.; Janssens, A.; Burgstaller, S.; Fromming, A.; et al. Olaptesed pegol (NOX-A12) with bendamustine and rituximab: A phase IIa study in patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica 2019, 104, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.L.; Sattler, M.; Azab, A.K.; Eulberg, D.; Kruschinski, A.; Manley, P.W.; Stone, R.; Griffin, J.D. Inhibition of SDF-1-induced migration of oncogene-driven myeloid leukemia by the L-RNA aptamer (Spiegelmer), NOX-A12, and potentiation of tyrosine kinase inhibition. Oncotarget 2017, 8, 109973–109984. [Google Scholar] [CrossRef]
- Karpova, D.; Bonig, H. Concise Review: CXCR4/CXCL12 Signaling in Immature Hematopoiesis--Lessons From Pharmacological and Genetic Models. Stem Cells 2015, 33, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Zuelzer, W.W. “Myelokathexis”—A New Form of Chronic Granulocytopenia. Report of a Case. N. Engl. J. Med. 1964, 270, 699–704. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Gorlin, R.J.; Lukens, J.N.; Taniuchi, S.; Bohinjec, J.; Francois, F.; Klotman, M.E.; Diaz, G.A. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003, 34, 70–74. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Mozobil(R) (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir. Chem. Chemother. 2019, 27, 2040206619829382. [Google Scholar] [CrossRef]
- De Clercq, E. AMD3100/CXCR4 Inhibitor. Front. Immunol. 2015, 6, 276. [Google Scholar] [CrossRef]
- Scala, S. Molecular Pathways: Targeting the CXCR4-CXCL12 Axis—Untapped Potential in the Tumor Microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4278–4285. [Google Scholar] [CrossRef]
- Lim, V.Y.; Zehentmeier, S.; Fistonich, C.; Pereira, J.P. A Chemoattractant-Guided Walk Through Lymphopoiesis: From Hematopoietic Stem Cells to Mature B Lymphocytes. Adv. Immunol. 2017, 134, 47–88. [Google Scholar] [CrossRef]
- Du, H.; Gao, L.; Luan, J.; Zhang, H.; Xiao, T. C-X-C Chemokine Receptor 4 in Diffuse Large B Cell Lymphoma: Achievements and Challenges. Acta Haematol. 2019, 142, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, K.; Gomez, F.; White, B.S.; Matlock, M.; Miller, C.A.; Trani, L.; Fronick, C.C.; Fulton, R.S.; Kreisel, F.; Cashen, A.F.; et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood 2017, 129, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Manning, R.J.; Tripsas, C.; Patterson, C.J.; Sheehy, P.; et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014, 123, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.Y.; Radojcic, V.; Maillard, I. Oncogenic Notch signaling in T-cell and B-cell lymphoproliferative disorders. Curr. Opin. Hematol. 2016, 23, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Passaro, D.; Irigoyen, M.; Catherinet, C.; Gachet, S.; Da Costa De Jesus, C.; Lasgi, C.; Tran Quang, C.; Ghysdael, J. CXCR4 Is Required for Leukemia-Initiating Cell Activity in T Cell Acute Lymphoblastic Leukemia. Cancer Cell 2015, 27, 769–779. [Google Scholar] [CrossRef]
- Paulus, A.; Akhtar, S.; Yousaf, H.; Manna, A.; Paulus, S.M.; Bashir, Y.; Caulfield, T.R.; Kuranz-Blake, M.; Chitta, K.; Wang, X.; et al. Waldenstrom macroglobulinemia cells devoid of BTK(C481S) or CXCR4(WHIM-like) mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J. 2017, 7, e565. [Google Scholar] [CrossRef]
- Castillo, J.J.; Xu, L.; Gustine, J.N.; Keezer, A.; Meid, K.; Dubeau, T.E.; Liu, X.; Demos, M.G.; Kofides, A.; Tsakmaklis, N.; et al. CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br. J. Haematol. 2019, 187, 356–363. [Google Scholar] [CrossRef]
- Castillo, J.J.; Moreno, D.F.; Arbelaez, M.I.; Hunter, Z.R.; Treon, S.P. CXCR4 mutations affect presentation and outcomes in patients with Waldenstrom macroglobulinemia: A systematic review. Expert Rev. Hematol. 2019, 12, 873–881. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Yang, G.; Xu, L.; Liu, X.; Castillo, J.J.; Treon, S.P. Genomics, Signaling, and Treatment of Waldenstrom Macroglobulinemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 994–1001. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zeng, D.F.; Ma, Y.Y.; Zhang, X.; Zhang, C.; Kong, P.Y. Are there any new insights for G-CSF and/or AMD3100 in chemotherapy of haematological malignants? Med. Oncol. 2015, 32, 262. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zeng, D.F.; Kong, P.Y.; Ma, Y.Y.; Zhang, X. AMD3100 and G-CSF disrupt the cross-talk between leukemia cells and the endosteal niche and enhance their sensitivity to chemotherapeutic drugs in biomimetic polystyrene scaffolds. Blood Cells Mol. Dis. 2016, 59, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, H.; Ojode, T.; Gao, X.; Anaya-O’Brien, S.; Turner, N.A.; Ulrick, J.; DeCastro, R.; Kelly, C.; Cardones, A.R.; et al. WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4. Blood 2012, 120, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Balabanian, K.; Lagane, B.; Pablos, J.L.; Laurent, L.; Planchenault, T.; Verola, O.; Lebbe, C.; Kerob, D.; Dupuy, A.; Hermine, O.; et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 2005, 105, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Venet-Caillault, A.; Figeac, M.; Herbaux, C.; Marot, G.; Doye, E.; Bertrand, E.; Geffroy, S.; Lepretre, F.; et al. Genomic Landscape of CXCR4 Mutations in Waldenstrom Macroglobulinemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1480–1488. [Google Scholar] [CrossRef]
- Fumagalli, A.; Zarca, A.; Neves, M.; Caspar, B.; Hill, S.J.; Mayor, F., Jr.; Smit, M.J.; Marin, P. CXCR4/ACKR3 Phosphorylation and Recruitment of Interacting Proteins: Key Mechanisms Regulating Their Functional Status. Mol. Pharmacol. 2019, 96, 794–808. [Google Scholar] [CrossRef]
- Busillo, J.M.; Benovic, J.L. Regulation of CXCR4 signaling. Biochim. Et Biophys. Acta 2007, 1768, 952–963. [Google Scholar] [CrossRef]
- Schwartz, V.; Lue, H.; Kraemer, S.; Korbiel, J.; Krohn, R.; Ohl, K.; Bucala, R.; Weber, C.; Bernhagen, J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009, 583, 2749–2757. [Google Scholar] [CrossRef]
- Forde, S.; Tye, B.J.; Newey, S.E.; Roubelakis, M.; Smythe, J.; McGuckin, C.P.; Pettengell, R.; Watt, S.M. Endolyn (CD164) modulates the CXCL12-mediated migration of umbilical cord blood CD133+ cells. Blood 2007, 109, 1825–1833. [Google Scholar] [CrossRef]
- DeWire, S.M.; Ahn, S.; Lefkowitz, R.J.; Shenoy, S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007, 69, 483–510. [Google Scholar] [CrossRef]
- Moore, C.A.; Milano, S.K.; Benovic, J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007, 69, 451–482. [Google Scholar] [CrossRef]
- Gomez-Mouton, C.; Fischer, T.; Peregil, R.M.; Jimenez-Baranda, S.; Stossel, T.P.; Nakamura, F.; Manes, S. Filamin A interaction with the CXCR4 third intracellular loop regulates endocytosis and signaling of WT and WHIM-like receptors. Blood 2015, 125, 1116–1125. [Google Scholar] [CrossRef]
- Bhandari, D.; Robia, S.L.; Marchese, A. The E3 ubiquitin ligase atrophin interacting protein 4 binds directly to the chemokine receptor CXCR4 via a novel WW domain-mediated interaction. Mol. Biol. Cell 2009, 20, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, M.; Gordon-Alonso, M.; Cabrero, J.R.; Barrero-Villar, M.; Rey, M.; Mittelbrunn, M.; Lamana, A.; Morlino, G.; Calabia, C.; Yamazaki, H.; et al. F-actin-binding protein drebrin regulates CXCR4 recruitment to the immune synapse. J. Cell Sci. 2010, 123, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Wyse, M.M.; Goicoechea, S.; Garcia-Mata, R.; Nestor-Kalinoski, A.L.; Eisenmann, K.M. mDia2 and CXCL12/CXCR4 chemokine signaling intersect to drive tumor cell amoeboid morphological transitions. Biochem. Biophys. Res. Commun. 2017, 484, 255–261. [Google Scholar] [CrossRef]
- Schmid, M.C.; Avraamides, C.J.; Dippold, H.C.; Franco, I.; Foubert, P.; Ellies, L.G.; Acevedo, L.M.; Manglicmot, J.R.; Song, X.; Wrasidlo, W.; et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell 2011, 19, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Vicente-Manzanares, M.; Viedma, F.; Yanez-Mo, M.; Urzainqui, A.; Barreiro, O.; Vazquez, J.; Sanchez-Madrid, F. Cutting edge: Association of the motor protein nonmuscle myosin heavy chain-IIA with the C terminus of the chemokine receptor CXCR4 in T lymphocytes. J Immunol. 2002, 169, 5410–5414. [Google Scholar] [CrossRef]
- Heusinkveld, L.E.; Majumdar, S.; Gao, J.L.; McDermott, D.H.; Murphy, P.M. WHIM Syndrome: From Pathogenesis Towards Personalized Medicine and Cure. J. Clin. Immunol. 2019, 39, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.M.; Ertle, J.O.; Tharp, M.D. B-cell lymphoma in a patient with WHIM syndrome. J. Am. Acad. Dermatol. 2001, 44, 124–128. [Google Scholar] [CrossRef]
- Imashuku, S.; Miyagawa, A.; Chiyonobu, T.; Ishida, H.; Yoshihara, T.; Teramura, T.; Kuriyama, K.; Imamura, T.; Hibi, S.; Morimoto, A.; et al. Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Ann. Hematol. 2002, 81, 470–473. [Google Scholar] [CrossRef]
- Ditzel Santos, D.; Ho, A.W.; Tournilhac, O.; Hatjiharissi, E.; Leleu, X.; Xu, L.; Tassone, P.; Neri, P.; Hunter, Z.R.; Chemaly, M.A.; et al. Establishment of BCWM.1 cell line for Waldenstrom’s macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp. Hematol. 2007, 35, 1366–1375. [Google Scholar] [CrossRef]
- Hodge, L.S.; Novak, A.J.; Grote, D.M.; Braggio, E.; Ketterling, R.P.; Manske, M.K.; Price Troska, T.L.; Ziesmer, S.C.; Fonseca, R.; Witzig, T.E.; et al. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL-1. Blood 2011, 117, e190–e197. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.H.; Lopez, J.; Deng, F.; Liu, Q.; Ojode, T.; Chen, H.; Ulrick, J.; Kwatemaa, N.; Kelly, C.; Anaya-O’Brien, S.; et al. AMD3100 is a potent antagonist at CXCR4(R334X), a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J. Cell. Mol. Med. 2011, 15, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Choi, U.; Whiting-Theobald, N.L.; Linton, G.F.; Brenner, S.; Sechler, J.M.; Murphy, P.M.; Malech, H.L. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp. Hematol. 2005, 33, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection With Gain-of-Function Variant CXCR4(R334X). Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.J.; Segarra, M.; Gasperini, P.; Gulino, A.V.; Tosato, G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS ONE 2009, 4, e8102. [Google Scholar] [CrossRef] [PubMed]
- Gulino, A.V.; Moratto, D.; Sozzani, S.; Cavadini, P.; Otero, K.; Tassone, L.; Imberti, L.; Pirovano, S.; Notarangelo, L.D.; Soresina, R.; et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 2004, 104, 444–452. [Google Scholar] [CrossRef]
- Cao, Y.; Hunter, Z.R.; Liu, X.; Xu, L.; Yang, G.; Chen, J.; Patterson, C.J.; Tsakmaklis, N.; Kanan, S.; Rodig, S.; et al. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom’s Macroglobulinemia. Leukemia 2015, 29, 169–176. [Google Scholar] [CrossRef]
- Cao, Y.; Hunter, Z.R.; Liu, X.; Xu, L.; Yang, G.; Chen, J.; Tsakmaklis, N.; Kanan, S.; Castillo, J.J.; Treon, S.P. CXCR4 WHIM-like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88(L265P)-directed survival signalling in Waldenstrom macroglobulinaemia cells. Br. J. Haematol. 2015, 168, 701–707. [Google Scholar] [CrossRef]
- Gustine, J.N.; Xu, L.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Chen, J.G.; Liu, X.; Munshi, M.; Guerrera, M.L.; Chan, G.G.; et al. CXCR4 (S338X) clonality is an important determinant of ibrutinib outcomes in patients with Waldenstrom macroglobulinemia. Blood Adv. 2019, 3, 2800–2803. [Google Scholar] [CrossRef]
- Castellani, F.; Visentin, A.; Campagnolo, M.; Salvalaggio, A.; Cacciavillani, M.; Candiotto, C.; Bertorelle, R.; Trentin, L.; Briani, C. The Bruton tyrosine kinase inhibitor ibrutinib improves anti-MAG antibody polyneuropathy. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef]
- Lagane, B.; Chow, K.Y.; Balabanian, K.; Levoye, A.; Harriague, J.; Planchenault, T.; Baleux, F.; Gunera-Saad, N.; Arenzana-Seisdedos, F.; Bachelerie, F. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood 2008, 112, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Balabanian, K.; Levoye, A.; Klemm, L.; Lagane, B.; Hermine, O.; Harriague, J.; Baleux, F.; Arenzana-Seisdedos, F.; Bachelerie, F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J. Clin. Investig. 2008, 118, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, C.; Lopez, L.; Gao, J.; Velez, D.; Anaya-O’Brien, S.; Ulrick, J.; Littel, P.; Corns, J.S.; Ellenburg, D.T.; et al. WHIM Syndrome Caused by Waldenstrom’s Macroglobulinemia-Associated Mutation CXCR4 (L329fs). J. Clin. Immunol. 2016, 36, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.P.; Gong, J.H.; Loetscher, P.; Rajarathnam, K.; Amara, A.; Arenzana-Seisdedos, F.; Virelizier, J.L.; Baggiolini, M.; Sykes, B.D.; Clark-Lewis, I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997, 16, 6996–7007. [Google Scholar] [CrossRef] [PubMed]
- Kehrl, J.H. The impact of RGS and other G-protein regulatory proteins on Galphai-mediated signaling in immunity. Biochem. Pharmacol. 2016, 114, 40–52. [Google Scholar] [CrossRef]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef]
- Busillo, J.M.; Armando, S.; Sengupta, R.; Meucci, O.; Bouvier, M.; Benovic, J.L. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 2010, 285, 7805–7817. [Google Scholar] [CrossRef]
- Mueller, W.; Schutz, D.; Nagel, F.; Schulz, S.; Stumm, R. Hierarchical organization of multi-site phosphorylation at the CXCR4 C terminus. PLoS ONE 2013, 8, e64975. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Zhao, J.; Sun, Y.; Hu, W.; Wu, Y.L.; Cen, B.; Wu, G.X.; Pei, G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J. Biol. Chem. 2000, 275, 2479–2485. [Google Scholar] [CrossRef]
- Luo, J.; Busillo, J.M.; Stumm, R.; Benovic, J.L. G Protein-Coupled Receptor Kinase 3 and Protein Kinase C Phosphorylate the Distal C-Terminal Tail of the Chemokine Receptor CXCR4 and Mediate Recruitment of beta-Arrestin. Mol. Pharmacol. 2017, 91, 554–566. [Google Scholar] [CrossRef]
- Yang, P.; Hu, Y.; Zhou, Q. The CXCL12-CXCR4 signaling axis plays a key role in cancer metastasis and is a potential target for developing novel therapeutics against metastatic cancer. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Razinia, Z.; Makela, T.; Ylanne, J.; Calderwood, D.A. Filamins in mechanosensing and signaling. Annu. Rev. Biophys. 2012, 41, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Salahpour, A.; Bouvier, M. Dimerization: An emerging concept for G protein-coupled receptor ontogeny and function. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Doijen, J.; Van Loy, T.; De Haes, W.; Landuyt, B.; Luyten, W.; Schoofs, L.; Schols, D. Signaling properties of the human chemokine receptors CXCR4 and CXCR7 by cellular electric impedance measurements. PLoS ONE 2017, 12, e0185354. [Google Scholar] [CrossRef] [PubMed]
- Levoye, A.; Balabanian, K.; Baleux, F.; Bachelerie, F.; Lagane, B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 2009, 113, 6085–6093. [Google Scholar] [CrossRef]
- Luker, K.E.; Gupta, M.; Luker, G.D. Imaging chemokine receptor dimerization with firefly luciferase complementation. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 823–834. [Google Scholar] [CrossRef]
- Sierro, F.; Biben, C.; Martinez-Munoz, L.; Mellado, M.; Ransohoff, R.M.; Li, M.; Woehl, B.; Leung, H.; Groom, J.; Batten, M.; et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. USA 2007, 104, 14759–14764. [Google Scholar] [CrossRef]
- Mellado, M.; Rodriguez-Frade, J.M.; Vila-Coro, A.J.; Fernandez, S.; Martin de Ana, A.; Jones, D.R.; Toran, J.L.; Martinez, A.C. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001, 20, 2497–2507. [Google Scholar] [CrossRef]
- Handel, T.M. The Structure of a CXCR4:Chemokine Complex. Front. Immunol. 2015, 6, 282. [Google Scholar] [CrossRef]
- Wu, B.; Chien, E.Y.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef]
- Martinez-Munoz, L.; Rodriguez-Frade, J.M.; Barroso, R.; Sorzano, C.O.S.; Torreno-Pina, J.A.; Santiago, C.A.; Manzo, C.; Lucas, P.; Garcia-Cuesta, E.M.; Gutierrez, E.; et al. Separating Actin-Dependent Chemokine Receptor Nanoclustering from Dimerization Indicates a Role for Clustering in CXCR4 Signaling and Function. Mol. Cell 2018, 70, 106–119 e110. [Google Scholar] [CrossRef] [PubMed]
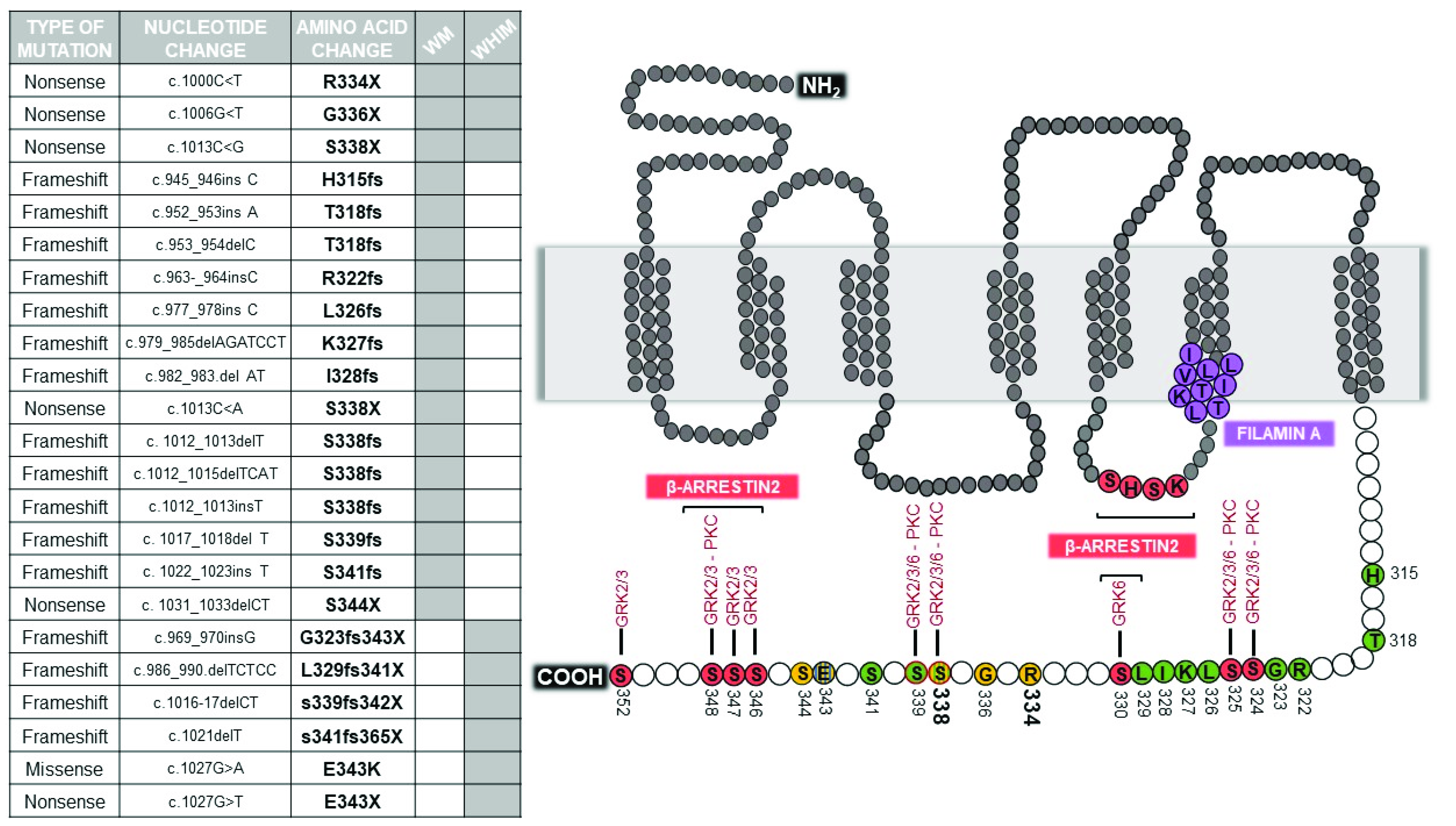
| Type of Mutation | G PROTEIN | β-ARRESTIN | Calcium | ERK | AKT | CXCR4 Internalization | CXCR4 Membrane Expression | CXCR4 Desensitization | CXCR4 Eterodimerization | Cell Migration | Ibrutinib Response | Disease and Cell Type | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R334X | WHIM: K562, CHO | [52] | |||||||||||
| WHIM: CD34+, K562 | [53] | ||||||||||||
| WHIM: EBV | [16] | ||||||||||||
| WHIM: NK | [54] | ||||||||||||
| WHIM: HeLa, HEK293 | [55] | ||||||||||||
| G336X | WHIM: PMBC | [56] | |||||||||||
| S338X | R | WM: BCWM.1, MWCL-1 | [57] | ||||||||||
| R | WM: BCWM.1 | [58] | |||||||||||
| R | WM: Patients (undefined) | [59] | |||||||||||
| R | WM: Patients (undefined) | [60] | |||||||||||
| WHIM: PBMC, HEK293 | [61] | ||||||||||||
| WHIM: PBMC, CHO | [33] | ||||||||||||
| WHIM: EBV | [62] | ||||||||||||
| E343K | WHIM: K562, PBMC | [32] | |||||||||||
| L329fs | WHIM: K562, PBMC, HEK293 | [63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanesi, S.; Locati, M.; Borroni, E.M. Aberrant CXCR4 Signaling at Crossroad of WHIM Syndrome and Waldenstrom’s Macroglobulinemia. Int. J. Mol. Sci. 2020, 21, 5696. https://doi.org/10.3390/ijms21165696
Milanesi S, Locati M, Borroni EM. Aberrant CXCR4 Signaling at Crossroad of WHIM Syndrome and Waldenstrom’s Macroglobulinemia. International Journal of Molecular Sciences. 2020; 21(16):5696. https://doi.org/10.3390/ijms21165696
Chicago/Turabian StyleMilanesi, Samantha, Massimo Locati, and Elena Monica Borroni. 2020. "Aberrant CXCR4 Signaling at Crossroad of WHIM Syndrome and Waldenstrom’s Macroglobulinemia" International Journal of Molecular Sciences 21, no. 16: 5696. https://doi.org/10.3390/ijms21165696
APA StyleMilanesi, S., Locati, M., & Borroni, E. M. (2020). Aberrant CXCR4 Signaling at Crossroad of WHIM Syndrome and Waldenstrom’s Macroglobulinemia. International Journal of Molecular Sciences, 21(16), 5696. https://doi.org/10.3390/ijms21165696






