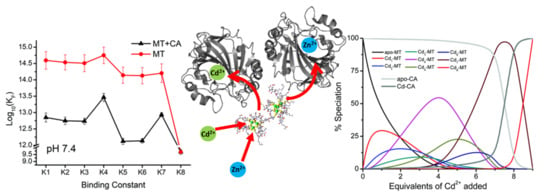
Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation
Abstract
1. Introduction
1.1. Carbonic Anhydrases
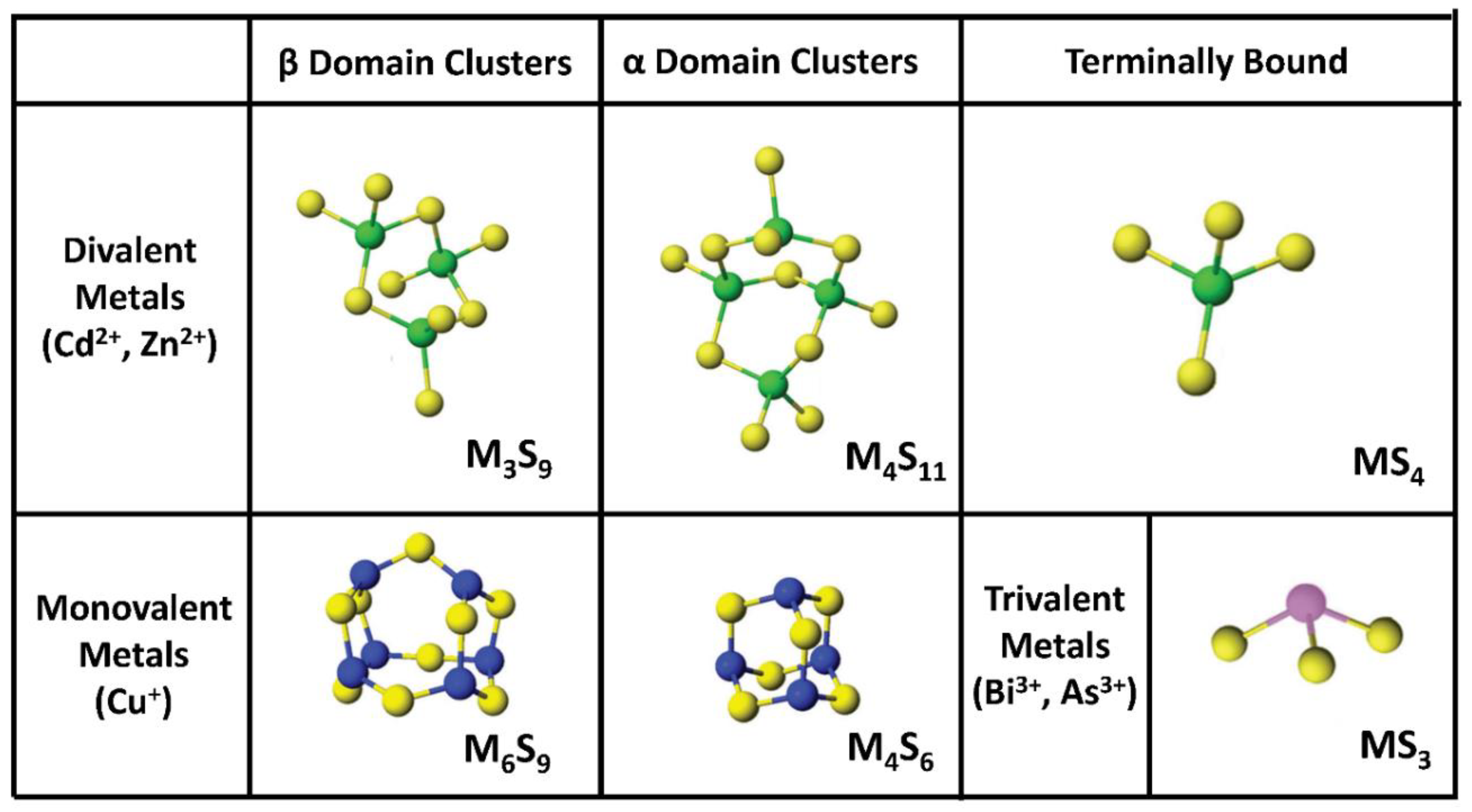
1.2. Metallothioneins
2. Mammalian Isoforms Associated with Dysregulation in Cancer
2.1. Carbonic Anhydrase IX and XII
2.2. Mammalian Metallothioneins
3. Cellular Control and Dysfunction
3.1. pH Effect on Protein Activity
3.2. The Neccessity of Zinc
3.3. Interactions with Toxic Cd2+ and Xenobiotic Metals
4. MT-CA Studies
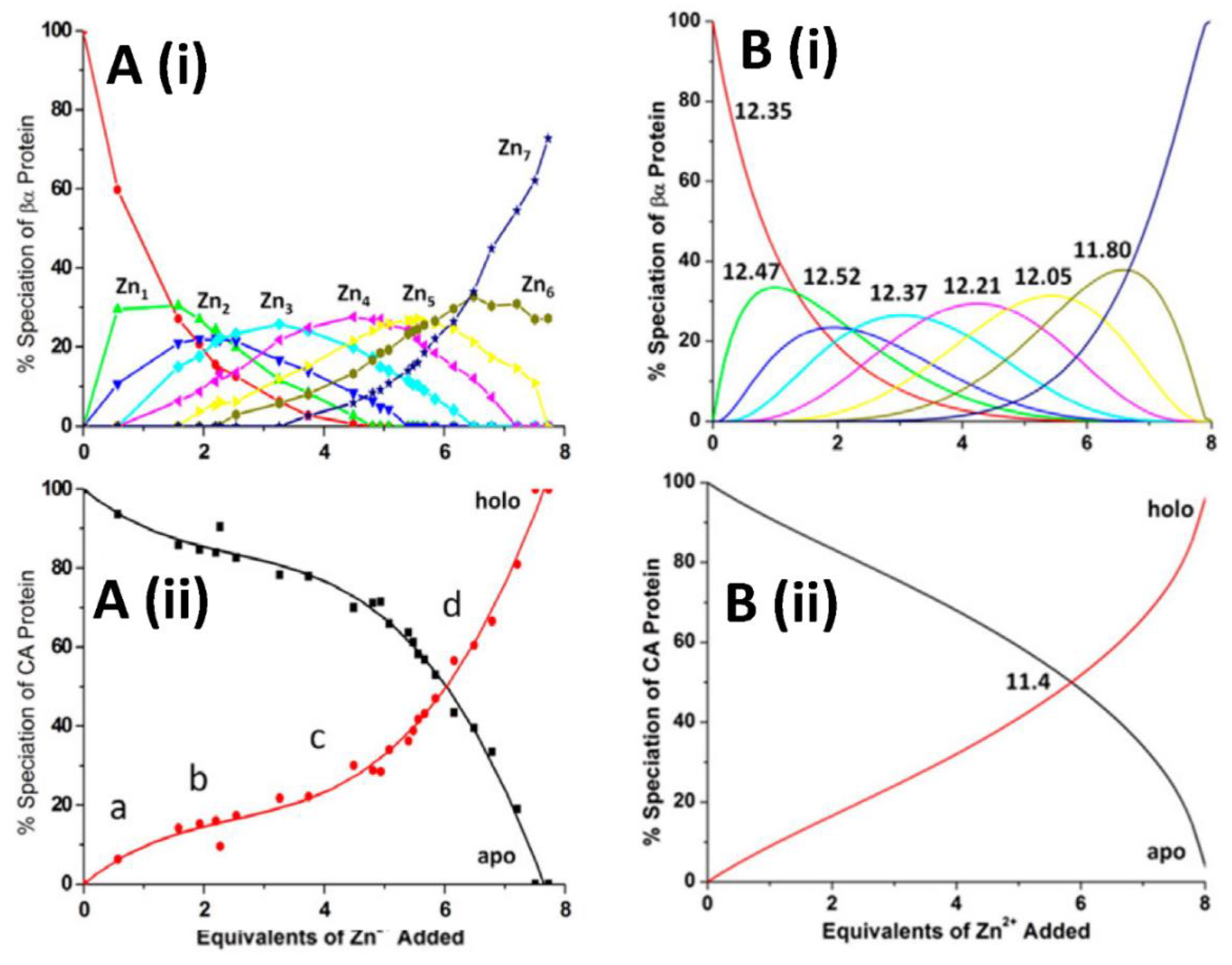
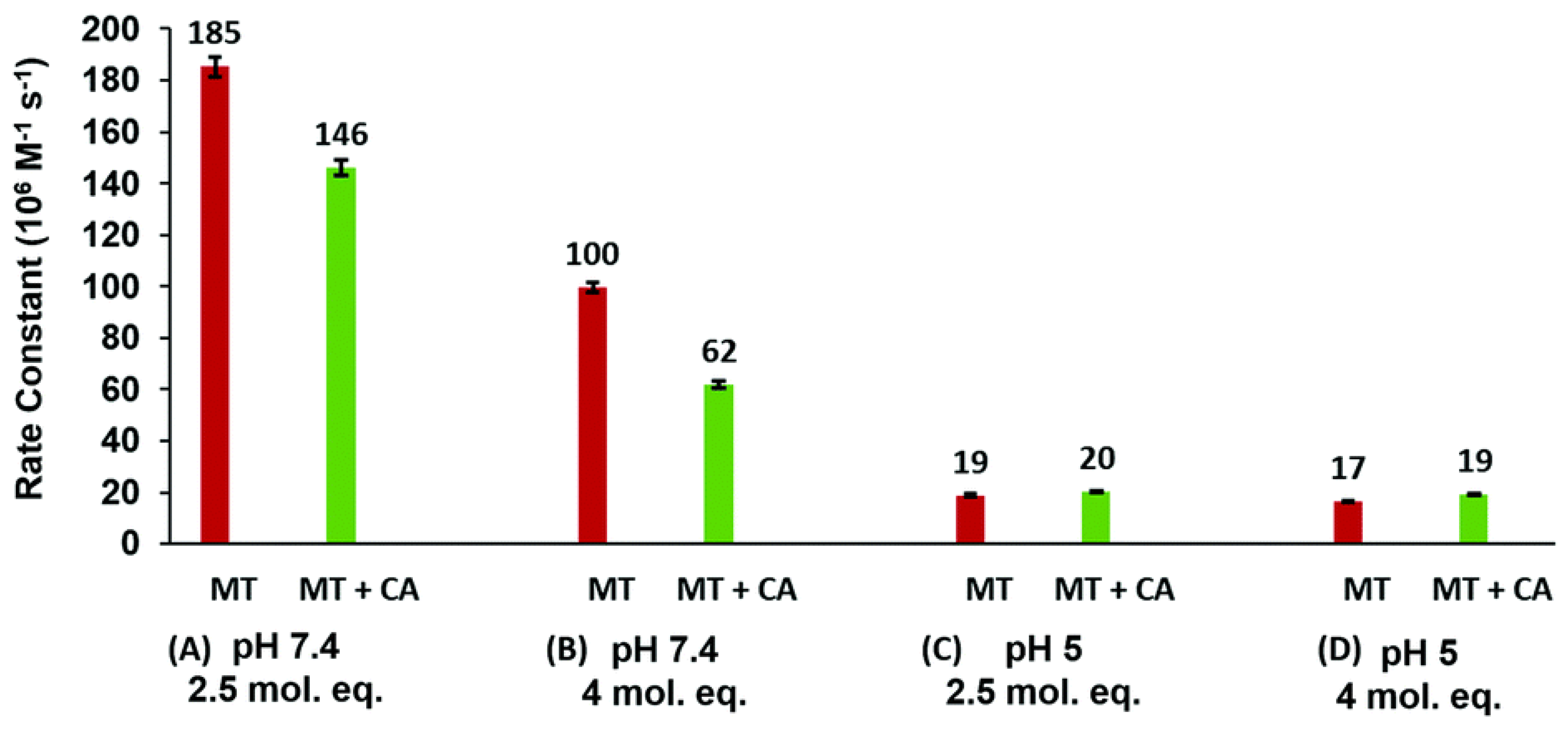
4.1. Kinetic Analysis of Zn2+ Reactivation of Apo-CA
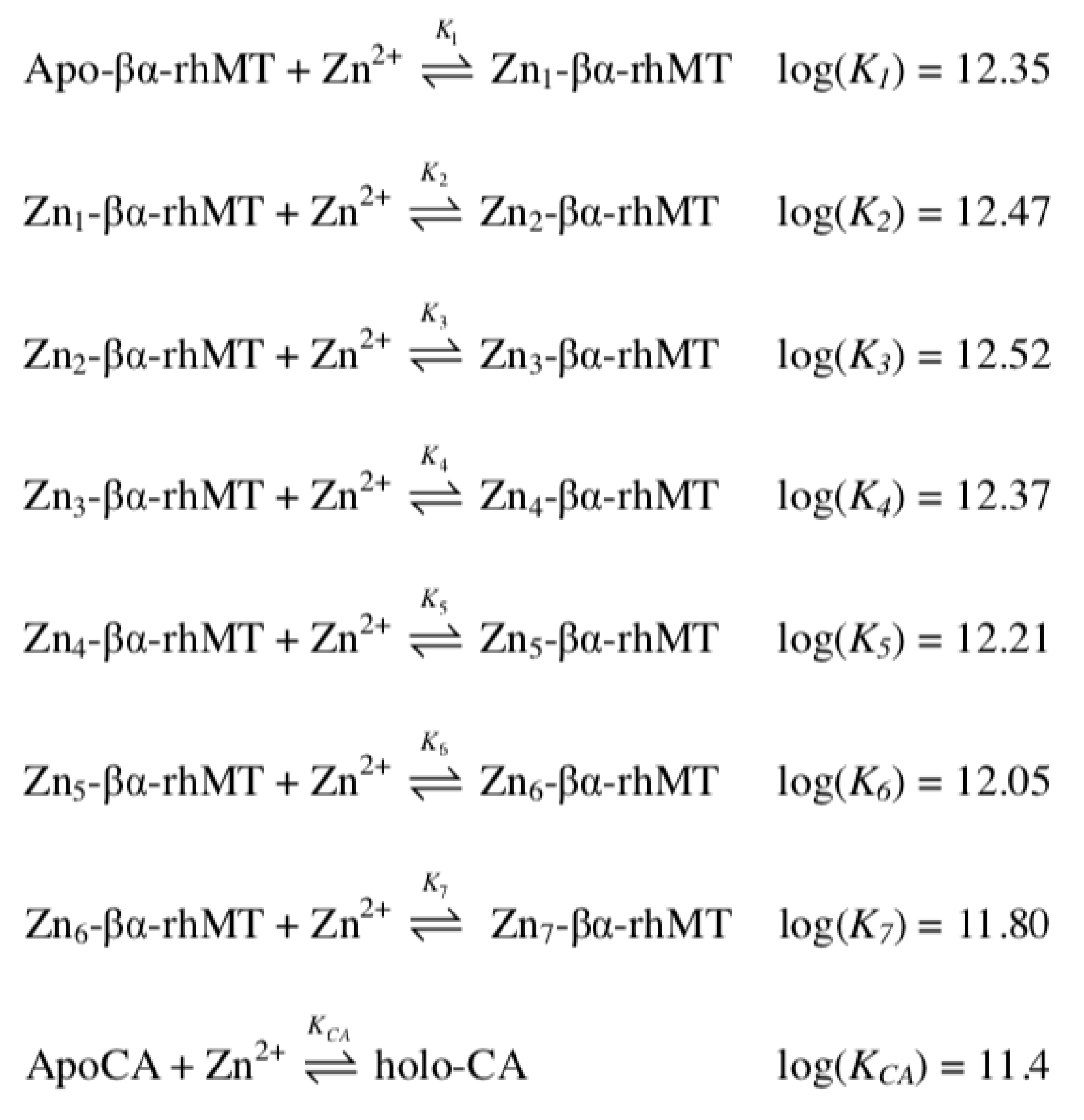
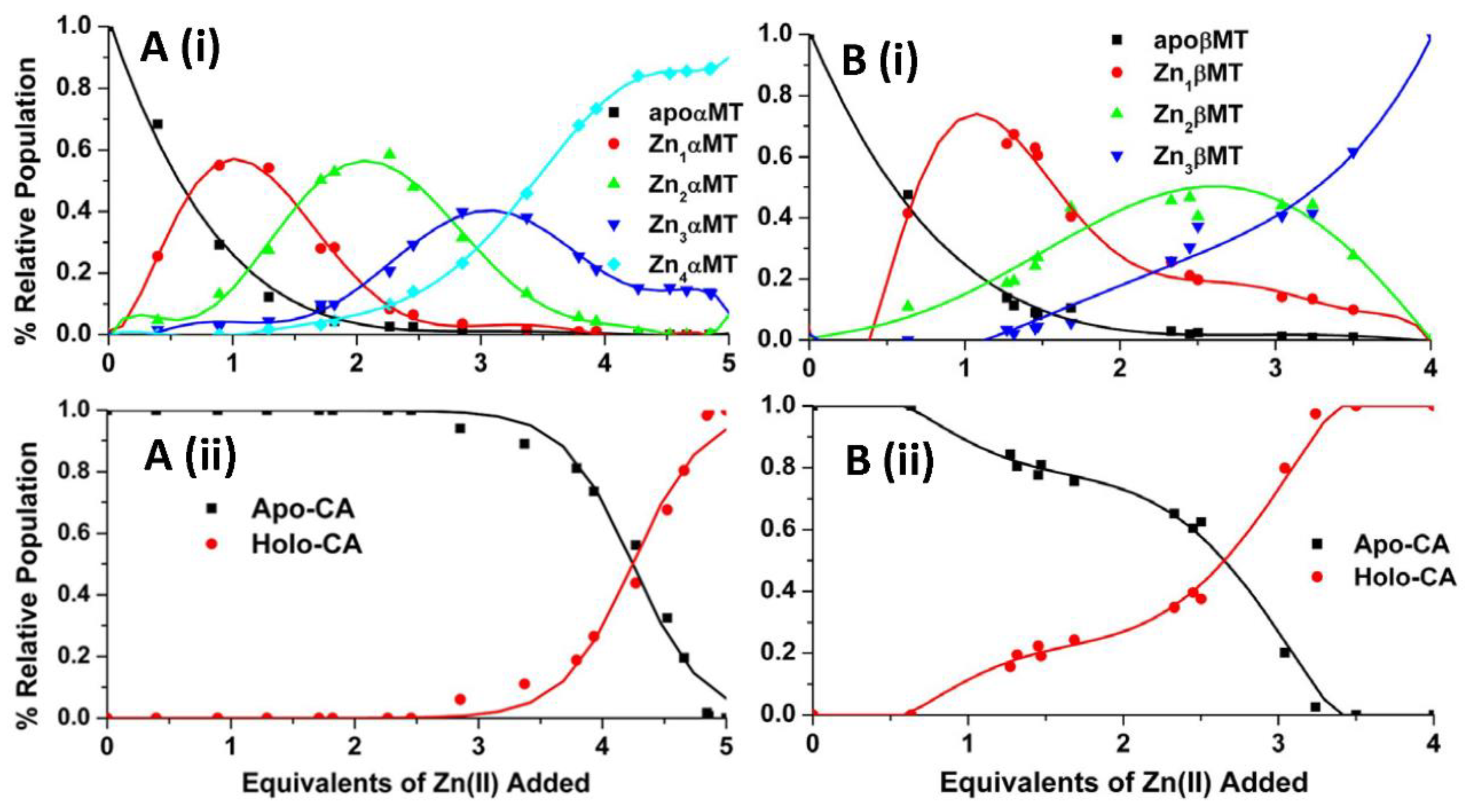
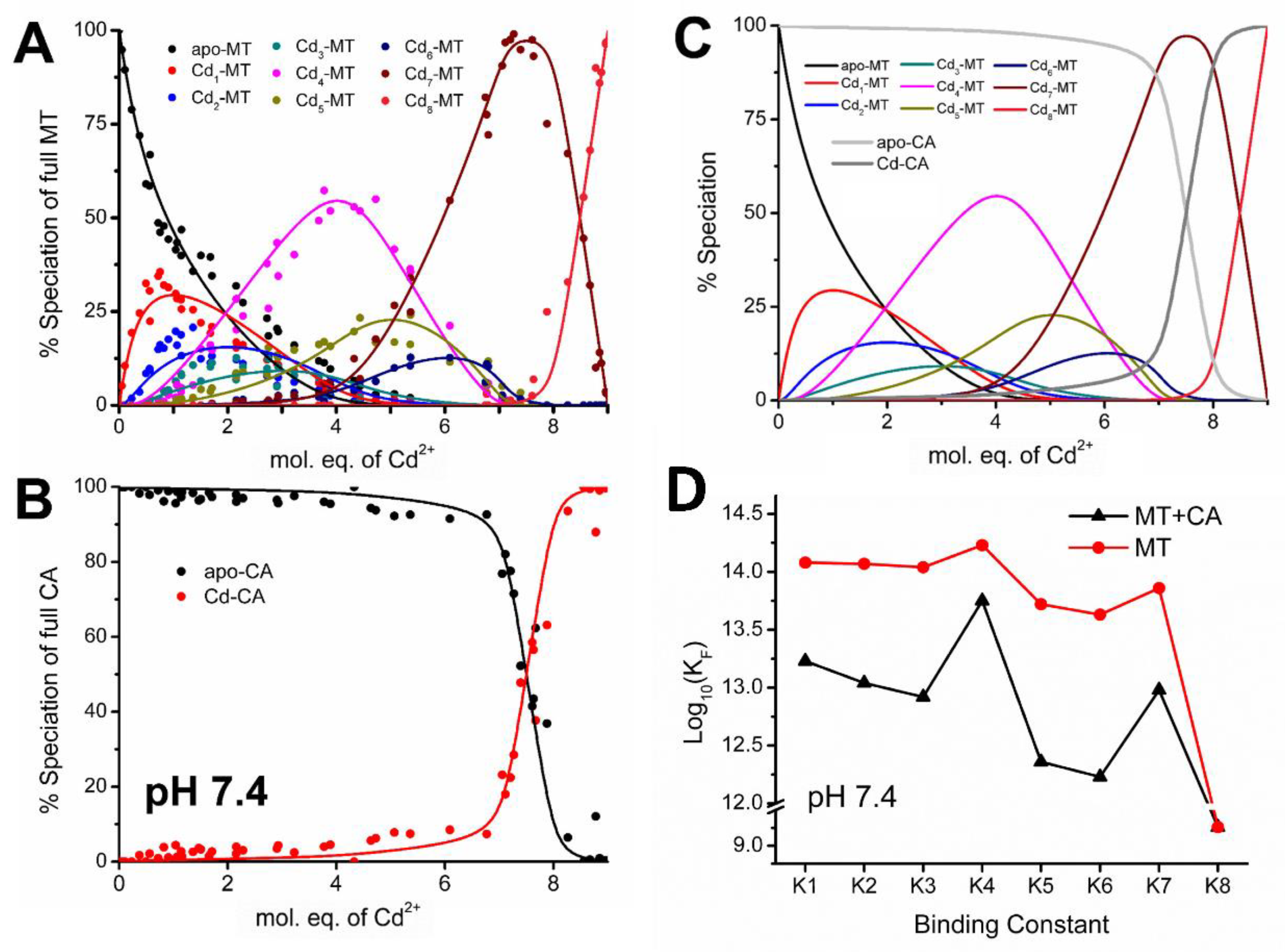
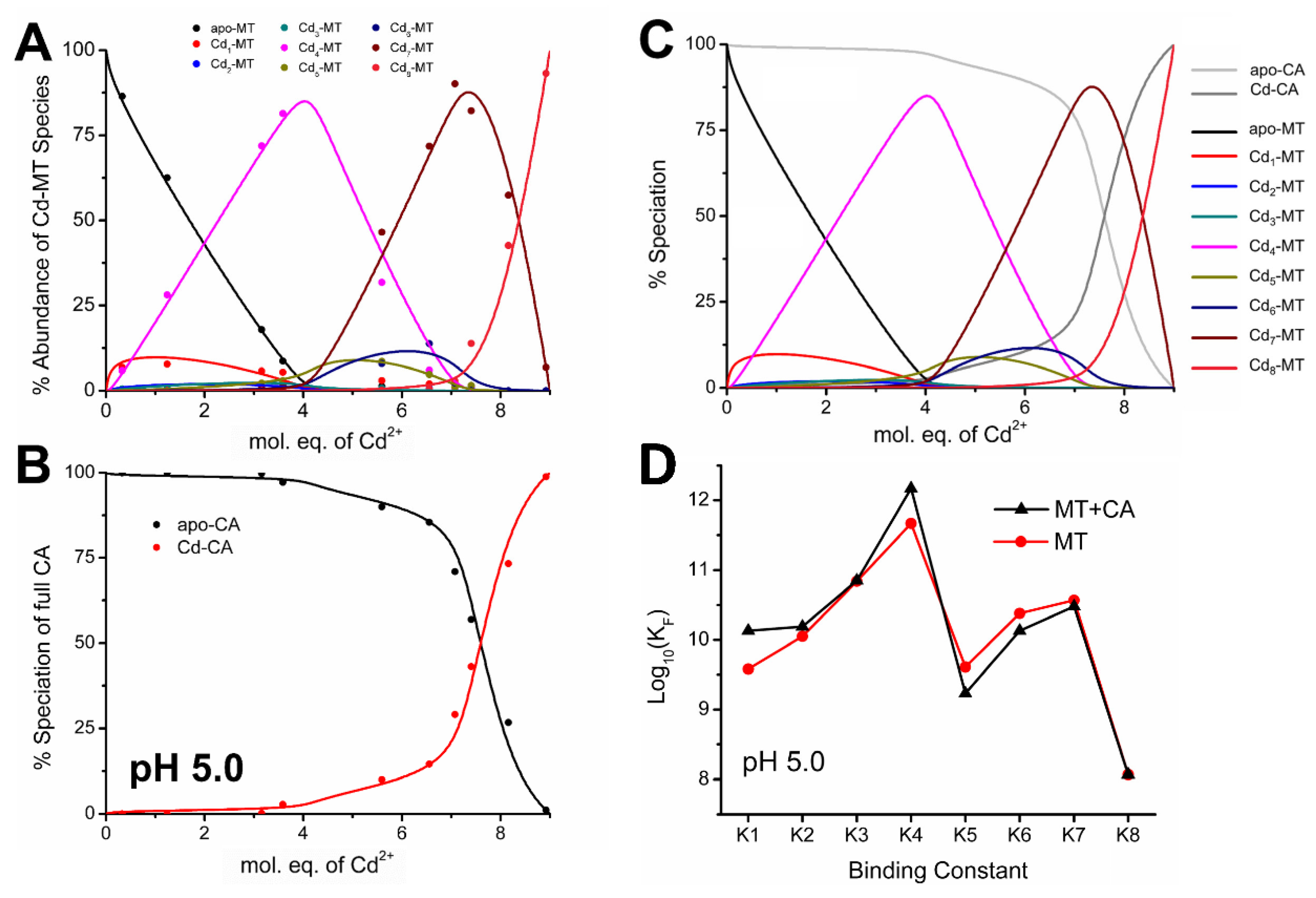
4.2. Equilibrium Competition Studies of Zn2+ Binding between Apo-βα-Metallothionein and Apo-Carbonic Anhydrase Using ESI-MS Methods and Affinity Constant Simulations
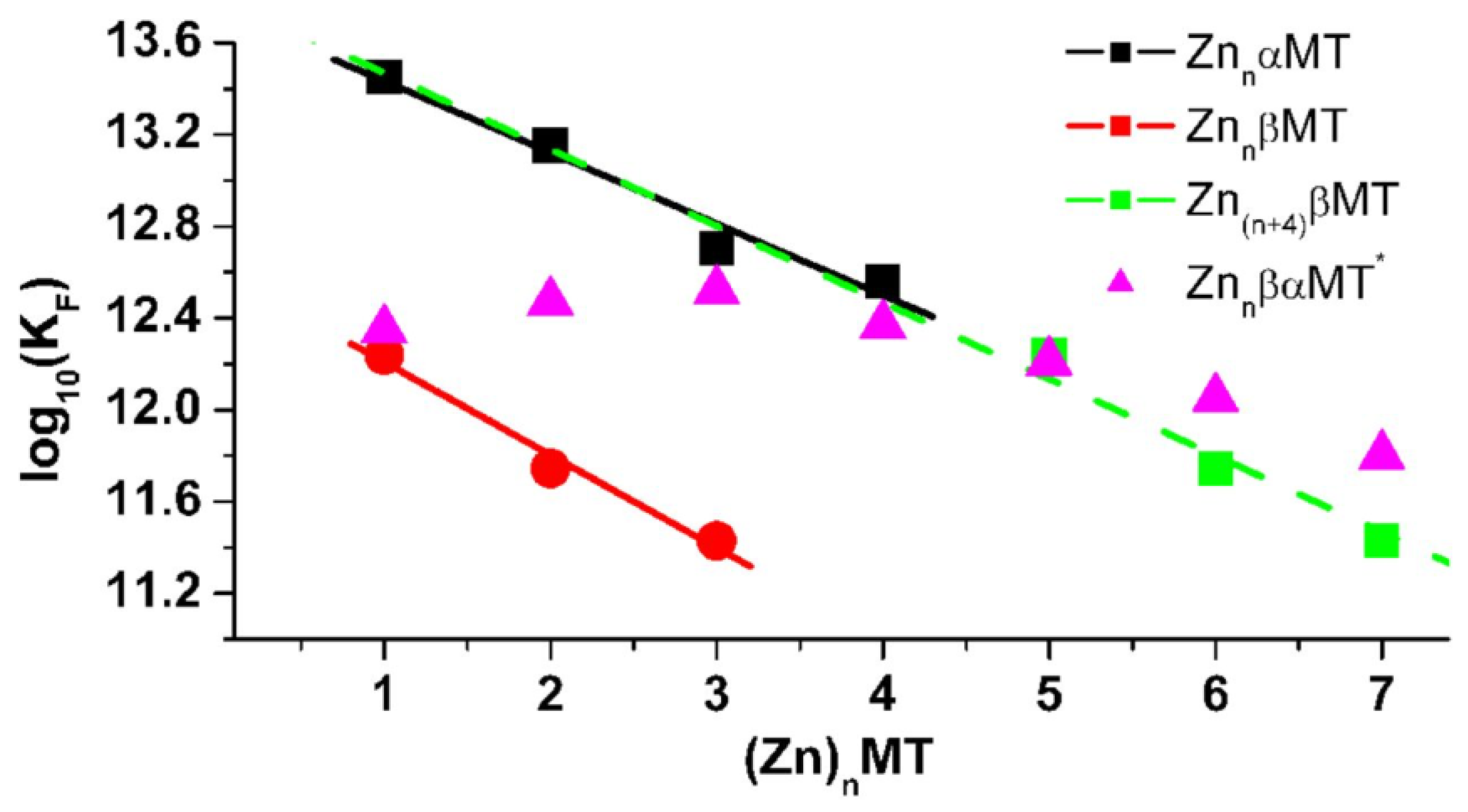
4.3. Equilibrium Competition Studies of Zn2+ Binding between α-MT and β-MT Fragments and Apo-Carbonic Anhydrase Using ESI-MS Methods and Affinity Constant Simulations
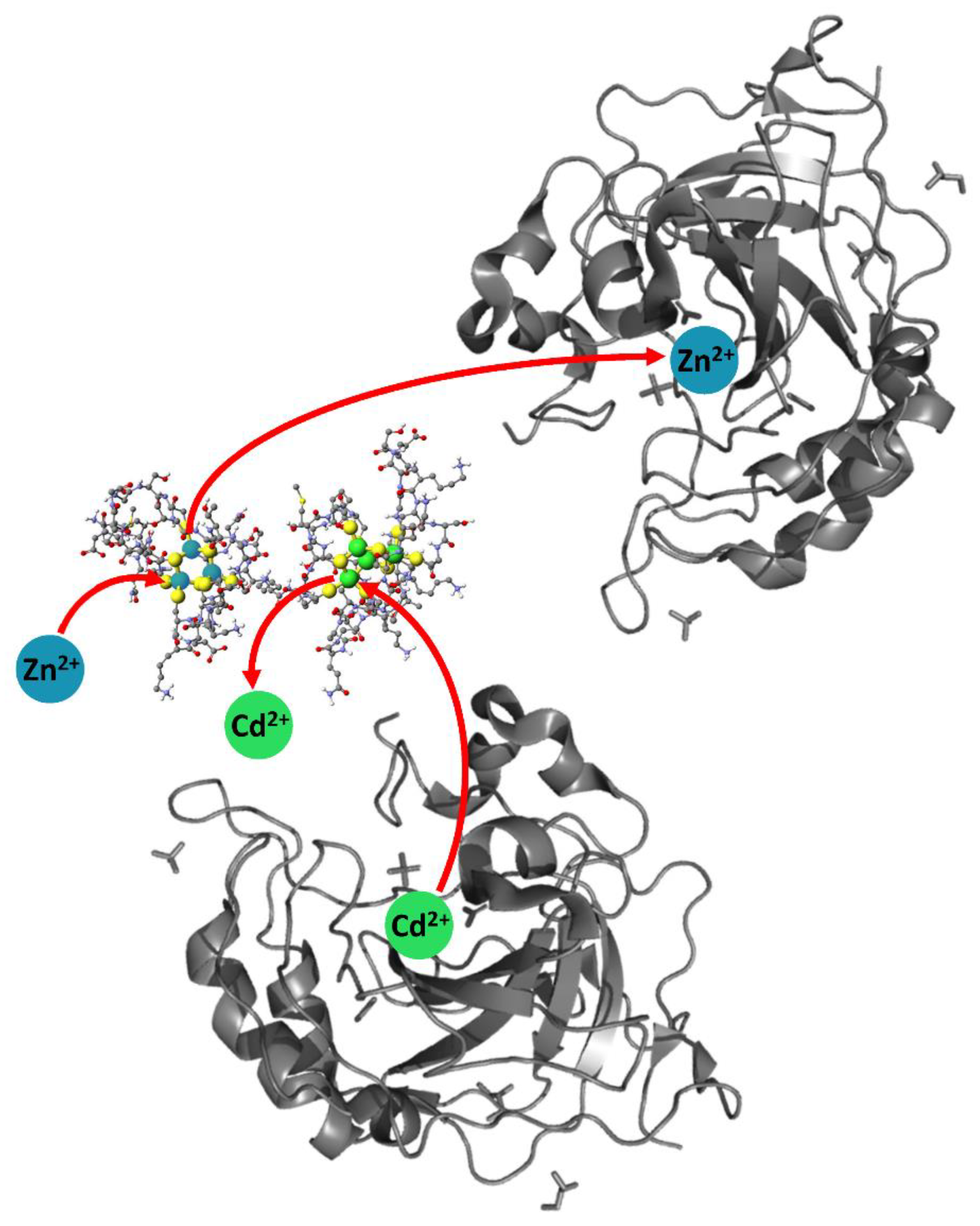
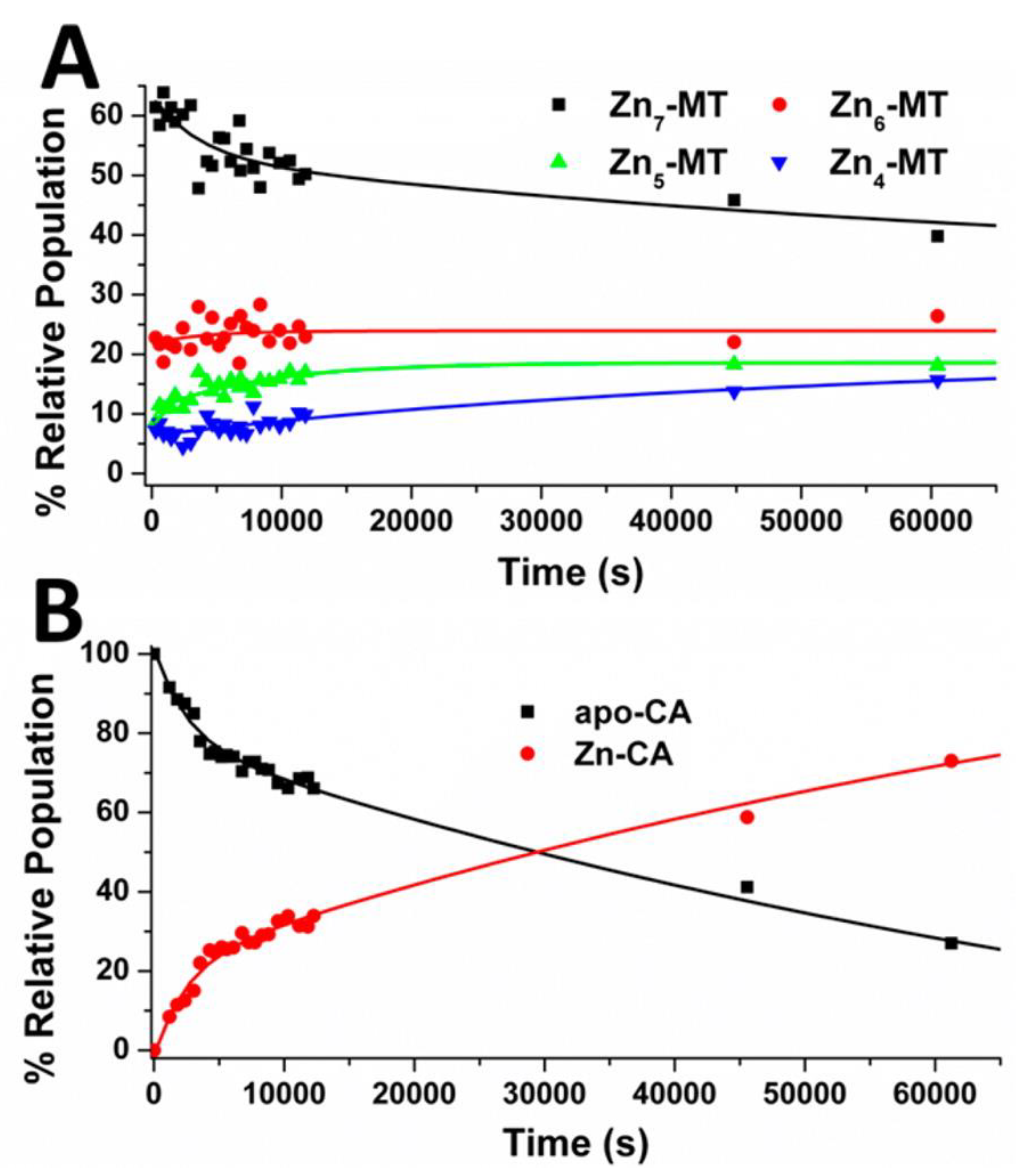
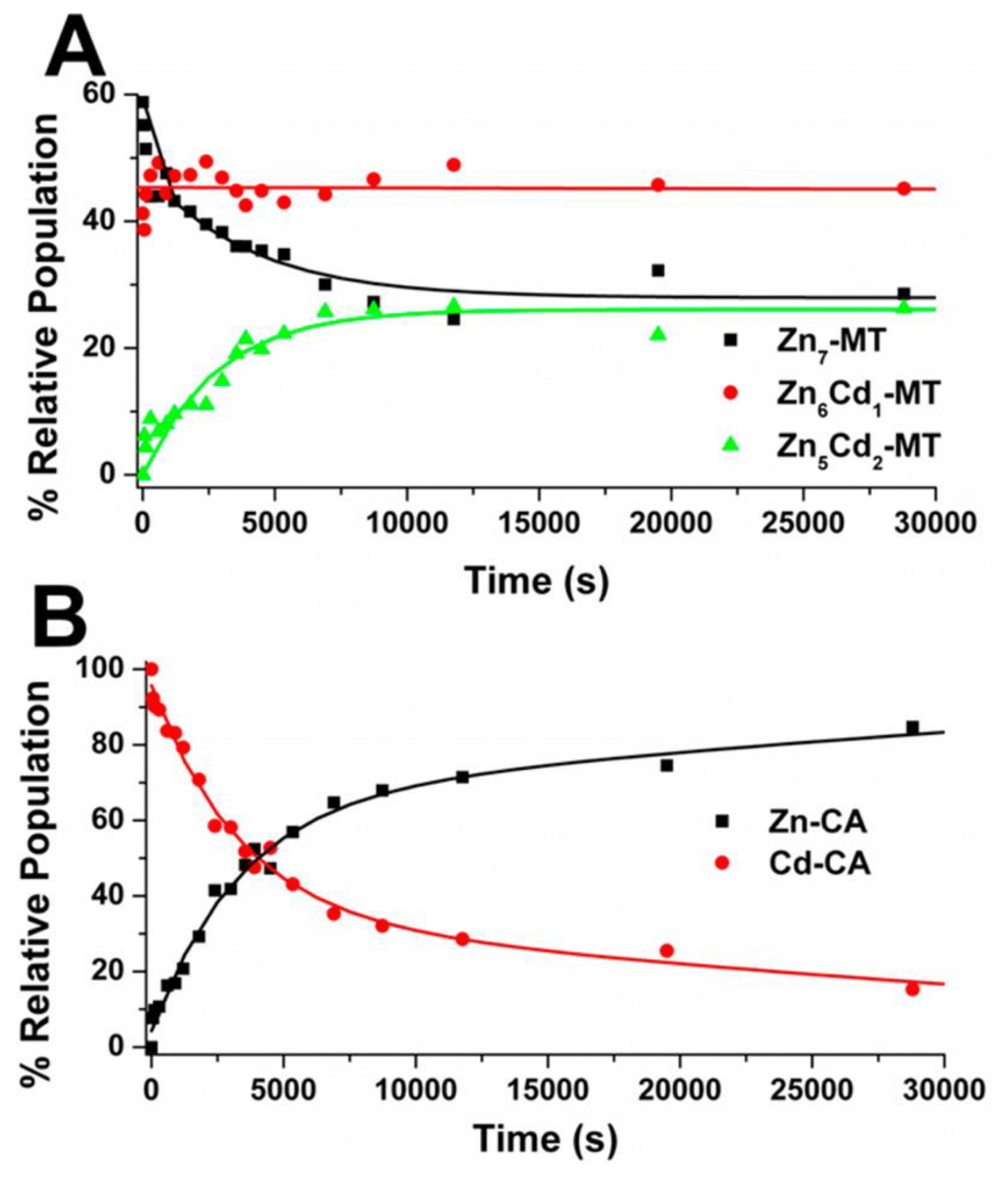
4.4. Kinetic Analysis of CA-MT: Zn2+ and Cd2+ Exchange Show Indirect Evidence of Protein–Protein Interactions
4.5. Electrospray Ionization Mass Spectrometry Shows Indirect Evidence of MT-CA Interactions from Cd2+ Metalation Studies
4.6. Changes in the Apo-MT Metalation Kinetics in the Presence of Apo-CA
4.7. Significance of MT–CA Interactions at Physiological pH and at Tumorigenic Extracellular Acidosis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphophate |
| CA | Carbonic Anhydrase |
| CNS | Central Nervous System |
| ECM | Extracellular Matrix |
| EDTAH4 | Ethylenediaminetetraacetic acid |
| ESI-MS | Electrospray Ionization Mass Spectrometry |
| IARC | International Agency for Research on Cancer |
| MRE | Metal Response Element |
| MT | Metallothionein |
| PG | Proteoglycan |
References
- Hu, Y. Metalloproteins; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Barnett, J.P.; Scanlan, D.J.; Blindauer, C.A. Protein fractionation and detection for metalloproteomics: Challenges and approaches. Anal. Bioanal. Chem. 2012, 402, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.E.; Bridwell-Rabb, J.; Drennan, C.L. Metalloprotein crystallography: More than a structure. Acc. Chem. Res. 2016, 49, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Rittle, J.; Field, M.J.; Green, M.T.; Tezcan, F.A. An efficient, step-economical strategy for the design of functional metalloproteins. Nat. Chem. 2019, 11, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Nastri, F.; D’Alonzo, D.; Leone, L.; Zambrano, G.; Pavone, V.; Lombardi, A. Engineering Metalloprotein Functions in Designed and Native Scaffolds. Trends Biochem. Sci. 2019, 44, 1022–1040. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc metabolism and metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein− ligand binding. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef]
- Forster, R.E. Remarks on the discovery of carbonic anhydrase. In The Carbonic Anhydrases; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–11. [Google Scholar]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Hulikova, A.; Aveyard, N.; Harris, A.L.; Vaughan-Jones, R.D.; Swietach, P. Intracellular carbonic anhydrase activity sensitizes cancer cell pH signaling to dynamic changes in CO2 partial pressure. J. Biol. Chem. 2014, 289, 25418–25430. [Google Scholar] [CrossRef]
- Di Fiore, A.; D’Ambrosio, K.; Ayoub, J.; Alterio, V.; De Simone, G. Carbonic Anhydrases. In Carbonic Anhydrases: Biochemistry and Pharmacology of an Evergreen Pharmaceutical Target; Academic Press: London, UK; Elsevier: London, UK, 2019; Volume 19, pp. 19–54. [Google Scholar]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ-δ-, ζ-and η-class of carbonic anhydrases with amines and amino acids: A review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; Andring, J.T.; McKenna, R. Crystallography and its impact on carbonic anhydrase research. Int. J. Med. Chem. 2018, 2018, 9419521. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Donald, W.A.; Supuran, C.T. Chapter 8-Human carbonic anhydrases: Tissue distribution, physiological role, and druggability. In Carbonic Anhydrases; Supuran, C.T., Nocentini, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 151–185. [Google Scholar]
- Baranauskienė, L.; Matulis, D. Overview of Human Carbonic Anhydrases. In Carbonic Anhydrase as Drug Target; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–14. [Google Scholar]
- Sutherland, D.E.; Stillman, M.J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Gillies, R.J. The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic anhydrases: Role in pH control and cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef]
- DiMario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant carbonic anhydrases: Structures, locations, evolution, and physiological roles. Mol. Plant. 2017, 10, 30–46. [Google Scholar] [CrossRef]
- Tripp, B.C.; Smith, K.; Ferry, J.G. Carbonic anhydrase: New insights for an ancient enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase activators. Future Med. Chem. 2018, 10, 561–573. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Carta, F.; Monti, S.M.; De Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G. Regulation of metallothionein gene expression. Prog. Food Nutr. Sci. 1990, 14, 193–258. [Google Scholar] [PubMed]
- Drozd, A.; Wojewska, D.; Peris-Díaz, M.D.; Jakimowicz, P.; Krężel, A. Crosstalk of the structural and zinc buffering properties of mammalian metallothionein-2. Metallomics 2018, 10, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Heffron, G.; Hill, H.A.O.; Djuricic, D.; Jiang, L.-J.; Vallee, B.L. The ATP/metallothionein interaction: NMR and STM. Biochemistry 2002, 41, 1689–1694. [Google Scholar] [CrossRef]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- Orihuela, R.; Fernández, B.; Palacios, Ò.; Valero, E.; Atrian, S.; Watt, R.K.; Domínguez-Vera, J.M.; Capdevila, M. Ferritin and metallothionein: Dangerous liaisons. Chem. Commun. 2011, 47, 12155–12157. [Google Scholar] [CrossRef]
- Atrian, S.; Capdevila, M. Metallothionein-protein interactions. Biomol. Concepts 2013, 4, 143–160. [Google Scholar] [CrossRef]
- Quiming, N.S.; Vergel, R.B.; Nicolas, M.G.; Villanueva, J.A. Interaction of bovine serum albumin and metallothionein. J. Health Sci. 2005, 51, 8–15. [Google Scholar] [CrossRef][Green Version]
- Endresen, L.; Bakka, A.; Rugstad, H.E. Increased resistance to chlorambucil in cultured cells with a high concentration of cytoplasmic metallothionein. Cancer Res. 1983, 43, 2918–2926. [Google Scholar]
- Rodrigo, M.A.M.; Jimemez, A.M.J.; Haddad, Y.; Bodoor, K.; Adam, P.; Krizkova, S.; Heger, Z.; Adam, V. Metallothionein Isoforms as Double Agents-Their Roles in Carcinogenesis, Cancer Progression and Chemoresistance. Drug Resist. Updat. 2020, 52, 100691. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, P.; Materna, V.; Maciejczyk, A.; Pudełko, M.; Markwitz, E.; Spaczyński, M.; Dietel, M.; Zabel, M.; Lage, H. Nuclear metallothionein expression correlates with cisplatin resistance of ovarian cancer cells and poor clinical outcome. Virchows Arch. 2007, 450, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Żelazowski, A.J.; Garvey, J.S.; Hoeschele, J.D. In vivo and in vitro binding of platinum to metallothionein. Arch. Biochem. Biophys. 1984, 229, 246–252. [Google Scholar] [CrossRef]
- Wong, D.L.; Stillman, M.J. Capturing platinum in cisplatin: Kinetic reactions with recombinant human apo-metallothionein 1a. Metallomics 2018, 10, 713–721. [Google Scholar] [CrossRef]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF-1. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1416–1425. [Google Scholar] [CrossRef]
- Lazo, J.S.; Kuo, S.-M.; Woo, E.S.; Pitt, B.R. The protein thiol metallothionein as an antioxidant and protectant against antineoplastic drugs. Chem. Biol. Interact. 1998, 111, 255–262. [Google Scholar] [CrossRef]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef]
- Capdevila, M.; Bofill, R.; Palacios, Ò.; Atrian, S. State-of-the-art of metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Freisinger, E. Structural features specific to plant metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1035–1045. [Google Scholar] [CrossRef]
- Freisinger, E. The metal-thiolate clusters of plant metallothioneins. Chimia 2010, 64, 217–224. [Google Scholar] [CrossRef]
- Freisinger, E. Metallothioneins in plants. Met. Ions Life Sci. 2009, 5, 107–153. [Google Scholar]
- Freisinger, E. Plant MTs—Long neglected members of the metallothionein superfamily. Dalton Trans. 2008, 21, 6663–6675. [Google Scholar] [CrossRef] [PubMed]
- Domènech, J.; Mir, G.; Huguet, G.; Capdevila, M.; Molinas, M.; Atrian, S. Plant metallothionein domains: Functional insight into physiological metal binding and protein folding. Biochimie 2006, 88, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, M.A.; Nicosia, A.; Costa, S.; Cuttitta, A.; Gianguzza, F. Metallothionein Gene Family in the Sea Urchin Paracentrotus lividus: Gene Structure, Differential Expression and Phylogenetic Analysis. Int. J. Mol. Sci. 2017, 18, 812. [Google Scholar] [CrossRef]
- Tomas, M.; Domènech, J.; Capdevila, M.; Bofill, R.; Atrian, S. The sea urchin metallothionein system: Comparative evaluation of the SpMTA and SpMTB metal-binding preferences. FEBS Open Bio 2013, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Nemer, M.; Wilkinson, D.G.; Travaglini, E.C.; Sternberg, E.J.; Butt, T.R. Sea urchin metallothionein sequence: Key to an evolutionary diversity. Proc. Natl. Acad. Sci. USA 1985, 82, 4992–4994. [Google Scholar] [CrossRef]
- Ziller, A.; Fraissinet-Tachet, L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics 2018, 10, 1549–1559. [Google Scholar] [CrossRef]
- Ziller, A.; Yadav, R.K.; Capdevila, M.; Reddy, M.S.; Vallon, L.; Marmeisse, R.; Atrian, S.; Palacios, Ò.; Fraissinet-Tachet, L. Metagenomics analysis reveals a new metallothionein family: Sequence and metal-binding features of new environmental cysteine-rich proteins. J. Inorg. Biochem. 2017, 167, 1–11. [Google Scholar] [CrossRef]
- Ejnik, J.; Shaw, C.F., III; Petering, D.H. Mechanism of cadmium ion substitution in mammalian zinc metallothionein and metallothionein α domain: Kinetic and structural studies. Inorg. Chem. 2010, 49, 6525–6534. [Google Scholar] [CrossRef]
- Winge, D.R. Limited proteolysis of metallothioneins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1991; Volume 205, pp. 438–447. [Google Scholar]
- Wong, D.L.; Korkola, N.C.; Stillman, M.J. Kinetics of competitive Cd 2+ binding pathways: The realistic structure of intrinsically disordered, partially metallated metallothioneins. Metallomics 2019, 11, 894–905. [Google Scholar] [CrossRef]
- Scheller, J.S.; Irvine, G.W.; Wong, D.L.; Hartwig, A.; Stillman, M.J. Stepwise copper (I) binding to metallothionein: A mixed cooperative and non-cooperative mechanism for all 20 copper ions. Metallomics 2017, 9, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.W.; Pinter, T.B.; Stillman, M.J. Defining the metal binding pathways of human metallothionein 1a: Balancing zinc availability and cadmium seclusion. Metallomics 2016, 8, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ngu, T.T.; Stillman, M.J. Arsenic binding to human metallothionein. J. Am. Chem. Soc. 2006, 128, 12473–12483. [Google Scholar] [CrossRef] [PubMed]
- Ngu, T.T.; Krecisz, S.; Stillman, M.J. Bismuth binding studies to the human metallothionein using electrospray mass spectrometry. Biochem. Biophys. Res. Commun. 2010, 396, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Korkola, N.C.; Scarrow, P.M.; Stillman, M.J. pH dependence of the non-cooperative binding of Bi3+ to human apo-metallothionein 1A: Kinetics, speciation, and stoichiometry. Metallomics 2020, 12, 435–448. [Google Scholar] [CrossRef]
- Udom, A.O.; Brady, F.O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem. J. 1980, 187, 329–335. [Google Scholar] [CrossRef]
- Li, T.-Y.; Kraker, A.J.; Shaw, C.F.; Petering, D.H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc. Natl. Acad. Sci. USA 1980, 77, 6334–6338. [Google Scholar] [CrossRef]
- Ejnik, J.; Muñoz, A.; Gan, T.; Shaw, C.F., III; Petering, D. Interprotein metal ion exchange between cadmium-carbonic anhydrase and apo-or zinc-metallothionein. J. Biol. Inorg. Chem. 1999, 4, 784–790. [Google Scholar] [CrossRef]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- De Simone, G.; Supuran, C.T. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 404–409. [Google Scholar] [CrossRef]
- Waheed, A.; Sly, W.S. Carbonic anhydrase XII functions in health and disease. Gene 2017, 623, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Shajee, B.; Waheed, A.; Ahmad, F.; Sly, W.S. Structure, function and applications of carbonic anhydrase isozymes. Bioorgan. Med. Chem. 2013, 21, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Hilvo, M.; Di Fiore, A.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Pastorekova, S.; Pedone, C.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef] [PubMed]
- Švastová, E.; Hulíková, A.; Rafajová, M.; Zat’ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.R. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Thiry, A.; Dogne, J.M.; Masereel, B.; Supuran, C.T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol. Sci. 2006, 27, 566–573. [Google Scholar] [CrossRef]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef]
- Kawata, T.; Nakamura, S.; Nakayama, A.; Fukuda, H.; Ebara, M.; Nagamine, T.; Minami, T.; Sakurai, H. An improved diagnostic method for chronic hepatic disorder: Analyses of metallothionein isoforms and trace metals in the liver of patients with hepatocellular carcinoma as determined by capillary zone electrophoresis and inductively coupled plasma-mass spectrometry. Biol. Pharm. Bull. 2006, 29, 403–409. [Google Scholar]
- Krizkova, S.; Kepinska, M.; Emri, G.; Eckschlager, T.; Stiborova, M.; Pokorna, P.; Heger, Z.; Adam, V. An insight into the complex roles of metallothioneins in malignant diseases with emphasis on (sub) isoforms/isoforms and epigenetics phenomena. Pharmacol. Ther. 2018, 183, 90–117. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Pedersen, M.Ø.; Larsen, A.; Stoltenberg, M.; Penkowa, M. The role of metallothionein in oncogenesis and cancer prognosis. Prog. Histochem. Cytochem. 2009, 44, 29–64. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Sunder, A.S.; Kumar, K.M.; Ganesh, G.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.J.-K.; Bay, B.-H.; Chow, V.T.-K. Differential expression of metallothionein isoforms in nasopharyngeal cancer and inhibition of cell growth by antisense down-regulation of metallothionein-2A. Oncol. Rep. 2005, 13, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Sens, M.A.; Somji, S.; Lamm, D.L.; Garrett, S.H.; Slovinsky, F.; Todd, J.H.; Sens, D.A. Metallothionein isoform 3 as a potential biomarker for human bladder cancer. Environ. Health Perspect. 2000, 108, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, Q.; Wu, L.; Gao, F.; Xie, H.; Zhou, L.; Zheng, S.; Xu, X. Metallothionein 1 family profiling identifies MT1X as a tumor suppressor involved in the progression and metastastatic capacity of hepatocellular carcinoma. Mol. Carcinogen. 2018, 57, 1435–1444. [Google Scholar] [CrossRef]
- Demidenko, R.; Daniunaite, K.; Bakavicius, A.; Sabaliauskaite, R.; Skeberdyte, A.; Petroska, D.; Laurinavicius, A.; Jankevicius, F.; Lazutka, J.R.; Jarmalaite, S. Decreased expression of MT1E is a potential biomarker of prostate cancer progression. Oncotarget 2017, 8, 61709–61718. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Maupin, C.M.; Voth, G.A. Preferred orientations of His64 in human carbonic anhydrase II. Biochemistry 2007, 46, 2938–2947. [Google Scholar] [CrossRef]
- Murphy, B.J.; Andrews, G.K.; Bittel, D.; Discher, D.J.; McCue, J.; Green, C.J.; Yanovsky, M.; Giaccia, A.; Sutherland, R.M.; Laderoute, K.R. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor-1. Cancer Res. 1999, 59, 1315–1322. [Google Scholar]
- Li, Z.; Ding, J.; Chen, C.; Chang, J.; Huang, B.; Geng, Z.; Wang, Z. Dual-target cancer theranostic for glutathione S-transferase and hypoxia-inducible factor-1α inhibition. Chem. Commun. 2017, 53, 12406–12409. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. Zinc in evolution. J. Inorg. Biochem. 2012, 111, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C.A. Lessons on the critical interplay between zinc binding and protein structure and dynamics. J. Inorg. Biochem. 2013, 121, 145–155. [Google Scholar] [CrossRef]
- Ackland, M.L.; Michalczyk, A. Zinc deficiency and its inherited disorders-a review. Genes Nutr. 2006, 1, 41–49. [Google Scholar] [CrossRef]
- Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Heger, Z.; Krizkova, S.; Adam, V. Zinc and zinc-containing biomolecules in childhood brain tumors. J. Mol. Med. 2016, 94, 1199–1215. [Google Scholar] [CrossRef]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Choudhary, M.; Bailey, L.D.; Grant, C. Effect of zinc on cadmium concentration in the tissue of durum wheat. Can. J. Plant Sci. 1994, 74, 549–552. [Google Scholar] [CrossRef]
- Honma, Y.; Hirata, H. Noticeable increase in cadmium absorption by zinc deficient rice plants. J. Soil Sci. Plant. Nutr. 1978, 24, 295–297. [Google Scholar] [CrossRef]
- Dabin, P.; Marafante, E.; Mousny, J.; Myttenaere, C. Absorption, distribution and binding of cadmium and zinc in irrigated rice plants. Plant. Soil 1978, 50, 329–341. [Google Scholar] [CrossRef]
- Cadmium and cadmium compounds. IARC Monogr. Eval. Carcinog. Risk Chem. Man 1976, 11, 39–74.
- Chen, P.; Duan, X.; Li, M.; Huang, C.; Li, J.; Chu, R.; Ying, H.; Song, H.; Jia, X.; Ba, Q. Systematic network assessment of the carcinogenic activities of cadmium. Toxicol. Appl. Pharm. 2016, 310, 150–158. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Moore, M.R. Chronic exposure to low-level cadmium induced zinc-copper dysregulation. J. Trace Elem. Med. Bio. 2018, 46, 32–38. [Google Scholar] [CrossRef]
- Alterio, V.; Langella, E.; De Simone, G.; Monti, S.M. Cadmium-containing carbonic anhydrase CDCA1 in marine diatom Thalassiosira weissflogii. Mar. Drugs 2015, 13, 1688–1697. [Google Scholar] [CrossRef]
- Håkansson, K.; Wehnert, A. Structure of cobalt carbonic anhydrase complexed with bicarbonate. J. Mol. Biol. 1992, 228, 1212–1218. [Google Scholar] [CrossRef]
- Kajikawa, K.; Nakanishi, I.; Kuroda, K. Morphological changes of the kidney and bone of rats in chronic cadmium poisoning. Exp. Mol. Pathol. 1981, 34, 9–24. [Google Scholar] [CrossRef]
- Kepinska, M.; Kizek, R.; Milnerowicz, H. Metallothionein and Superoxide Dismutase—Antioxidative Protein Status in Fullerene-Doxorubicin Delivery to MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3253. [Google Scholar] [CrossRef]
- Karotki, A.V.; Vašák, M. Reaction of human metallothionein-3 with cisplatin and transplatin. J. Biol. Inorg. Chem. 2009, 14, 1129–1138. [Google Scholar] [CrossRef]
- Hagrman, D.; Goodisman, J.; Dabrowiak, J.C.; Souid, A.-K. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metab. Dispos. 2003, 31, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Jiang, G.; Li, X.F. Direct evidence for co-binding of cisplatin and cadmium to a native zinc-and cadmium-containing metallothionein. Appl. Organomet. Chem. 2003, 17, 675–681. [Google Scholar] [CrossRef]
- Casini, A.; Karotki, A.; Gabbiani, C.; Rugi, F.; Vašák, M.; Messori, L.; Dyson, P.J. Reactivity of an antimetastatic organometallic ruthenium compound with metallothionein-2: Relevance to the mechanism of action. Metallomics 2009, 1, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.L.; Stillman, M.J. Metallothionein: An Aggressive Scavenger—The Metabolism of Rhodium (II) Tetraacetate (Rh2 (CH3CO2) 4). ACS Omega 2018, 3, 16314–16327. [Google Scholar] [CrossRef]
- Wong, D.L.; Stillman, M.J. Destructive interactions of dirhodium (II) tetraacetate with β metallothionein rh1a. Chem. Commun. 2016, 52, 5698–5701. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Hu, H.-Y.; Gu, W.-Q.; Wu, G. Study on model clusters of metallothionein: Structure and its kinetics of zinc transfer reaction to apo-carbonic anhydrase. J. Inorg. Biochem. 1994, 54, 147–155. [Google Scholar] [CrossRef]
- Huang, Z.X.; Hu, H.Y.; Gu, W.Q.; Sun, J.Q. Zinc transfer kinetics between Cd5Zn2-metallothionein and apo-carbonic anhydrase. Chin. J. Chem. 1993, 11, 246–250. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Du, L.; Zheng, W.-J.; Tang, W.-X. Isolation of GIF from porcine brain and studies of its zinc transfer kinetics with apo-carbonic anhydrase. Biometals 2002, 15, 421–427. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Santoro, A.; Pountney, D.L.; Meloni, G.; Hureau, C.; Faller, P. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem. Soc. Rev. 2017, 46, 7683–7693. [Google Scholar] [CrossRef]
- Roschitzki, B.; Vašák, M. Redox Labile Site in a Zn4 Cluster of Cu4, Zn4− Metallothionein-3. Biochemistry 2003, 42, 9822–9828. [Google Scholar] [CrossRef]
- Loo, J.A.; Loo, R.R.O.; Udseth, H.R.; Edmonds, C.G.; Smith, R.D. Solvent-induced conformational changes of polypeptides probed by electrospray-ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1991, 5, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wojciechowski, M.; Fenselau, C. Assessment of metals in reconstituted metallothioneins by electrospray mass spectrometry. Anal. Chem. 1993, 65, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J.; Fabris, D.; Wei, D.; Karpel, R.L.; Fenselau, C. Monitoring metal ion flux in reactions of metallothionein and drug-modified metallothionein by electrospray mass spectrometry. Prot. Sci. 1998, 7, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.B.; Stillman, M.J. The zinc balance: Competitive zinc metalation of carbonic anhydrase and metallothionein 1A. Biochemistry 2014, 53, 6276–6285. [Google Scholar] [CrossRef]
- Pinter, T.B.; Stillman, M.J. Putting the pieces into place: Properties of intact zinc metallothionein 1A determined from interaction of its isolated domains with carbonic anhydrase. Biochem. J. 2015, 471, 347–356. [Google Scholar] [CrossRef]
- Sutherland, D.E.; Summers, K.L.; Stillman, M.J. Noncooperative metalation of metallothionein 1A and its isolated domains with zinc. Biochemistry 2012, 51, 6690–6700. [Google Scholar] [CrossRef]
- Ngu, T.T.; Easton, A.; Stillman, M.J. Kinetic analysis of arsenic− metalation of human metallothionein: Significance of the two-domain structure. J. Am. Chem. Soc. 2008, 130, 17016–17028. [Google Scholar] [CrossRef]
- Rigby, K.E.; Chan, J.; Mackie, J.; Stillman, M.J. Molecular dynamics study on the folding and metallation of the individual domains of metallothionein. Proteins Struct. Funct. Bioinf. 2006, 62, 159–172. [Google Scholar] [CrossRef]
- Pinter, T.B.; Stillman, M.J. Kinetics of zinc and cadmium exchanges between metallothionein and carbonic anhydrase. Biochemistry 2015, 54, 6284–6293. [Google Scholar]
- Sutherland, D.E.; Willans, M.J.; Stillman, M.J. Single domain metallothioneins: Supermetalation of human MT 1a. J. Am. Chem. Soc. 2012, 134, 3290–3299. [Google Scholar] [CrossRef]
- Yuan, A.T.; Korkola, N.C.; Wong, D.L.; Stillman, M.J. Metallothionein Cd 4 S 11 cluster formation dominates in the protection of carbonic anhydrase. Metallomics 2020, 12, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.W.; Duncan, K.E.; Gullons, M.; Stillman, M.J. Metalation kinetics of the human α-metallothionein 1a fragment is dependent on the fluxional structure of the apo-protein. Chem. Eur. J. 2015, 21, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Anzellotti, A.; Farrell, N. Zinc metalloproteins as medicinal targets. Chem. Soc. Rev. 2008, 37, 1629–1651. [Google Scholar] [CrossRef]
- Kogan, S.; Carpizo, D.R. Zinc metallochaperones as mutant p53 reactivators: A new paradigm in cancer therapeutics. Cancers 2018, 10, 166. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, D.L.; Yuan, A.T.; Korkola, N.C.; Stillman, M.J. Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation. Int. J. Mol. Sci. 2020, 21, 5697. https://doi.org/10.3390/ijms21165697
Wong DL, Yuan AT, Korkola NC, Stillman MJ. Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation. International Journal of Molecular Sciences. 2020; 21(16):5697. https://doi.org/10.3390/ijms21165697
Chicago/Turabian StyleWong, Daisy L., Amelia T. Yuan, Natalie C. Korkola, and Martin J. Stillman. 2020. "Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation" International Journal of Molecular Sciences 21, no. 16: 5697. https://doi.org/10.3390/ijms21165697
APA StyleWong, D. L., Yuan, A. T., Korkola, N. C., & Stillman, M. J. (2020). Interplay between Carbonic Anhydrases and Metallothioneins: Structural Control of Metalation. International Journal of Molecular Sciences, 21(16), 5697. https://doi.org/10.3390/ijms21165697