Antitumor Efficacy of the Herbal Recipe Benja Amarit against Highly Invasive Cholangiocarcinoma by Inducing Apoptosis both In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. BJA-Inhibited CCA Cell Viability
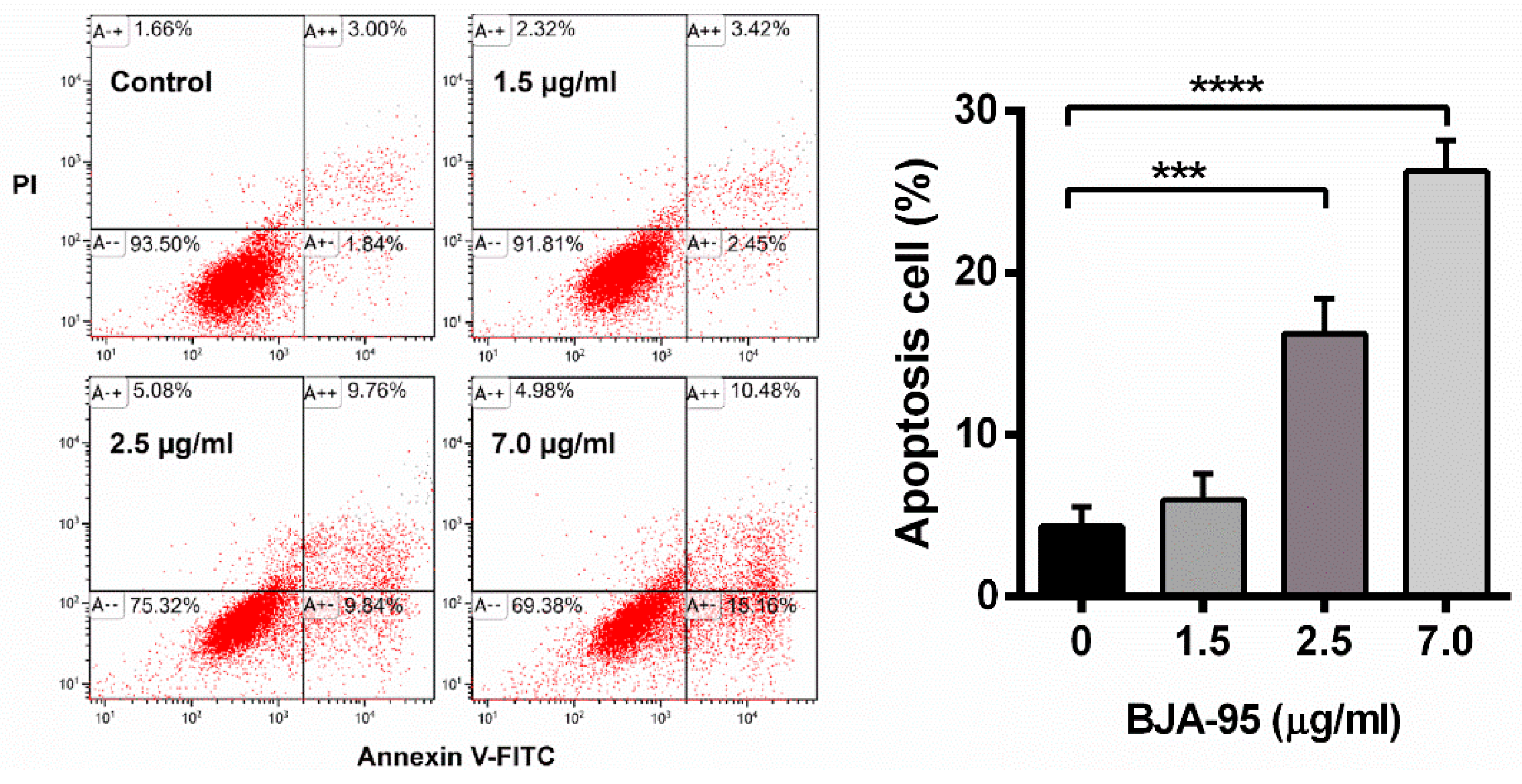
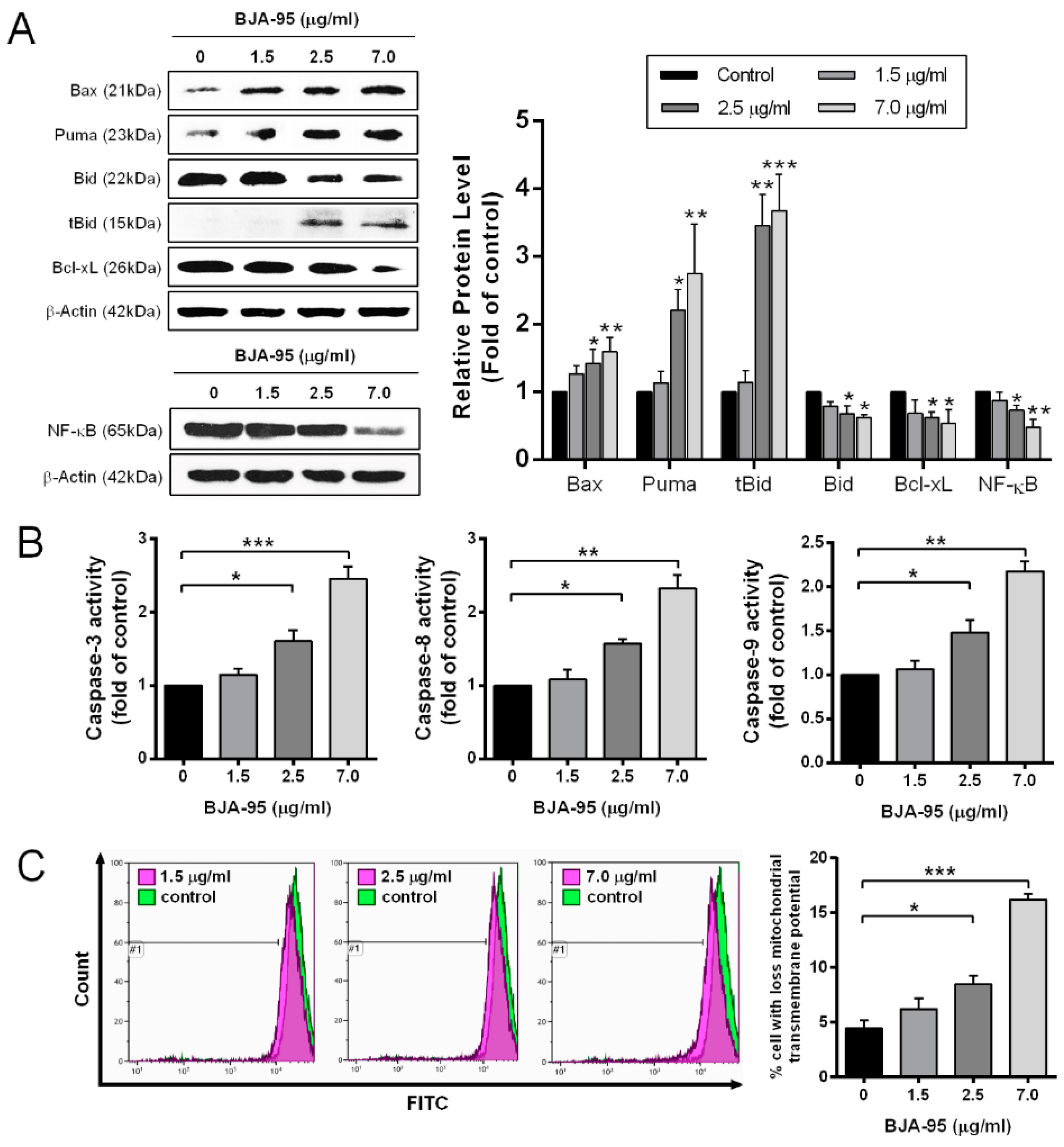
2.2. BJA-Induced Apoptosis in CCA Cells
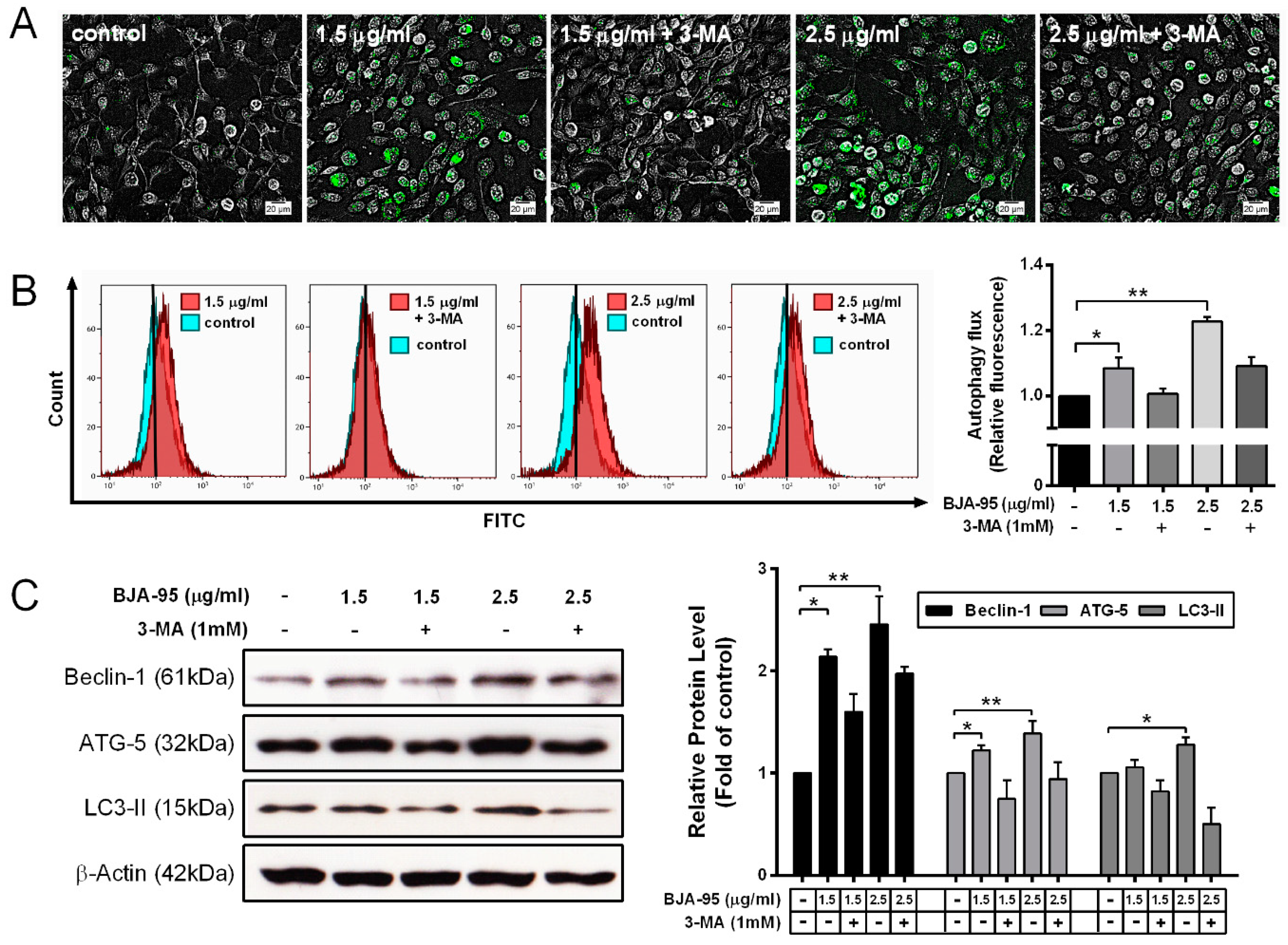
2.3. BJA-Triggered Autophagy in CCA Cells
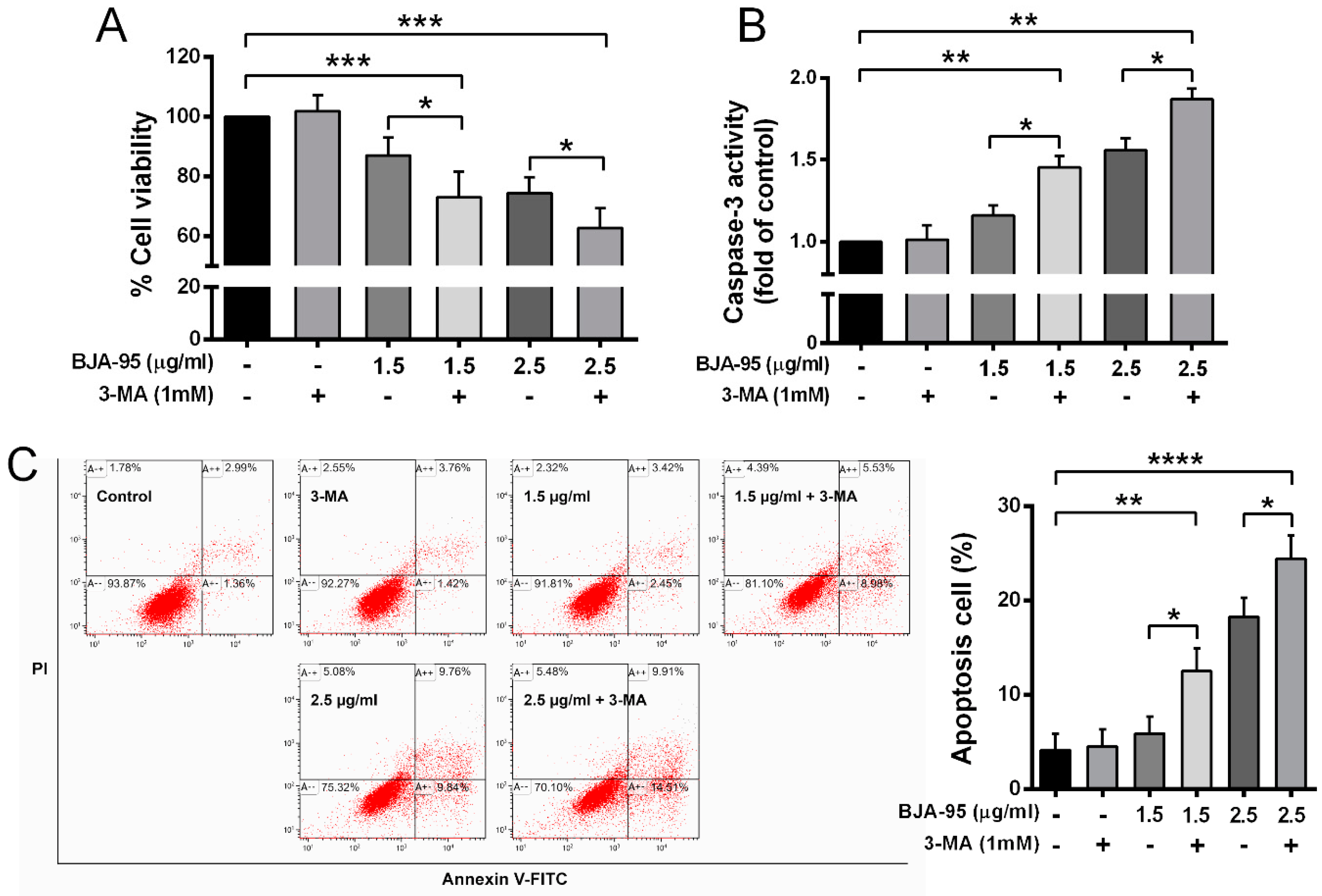
2.4. Inhibition of Autophagy-Enhanced BJA-Induced Apoptosis
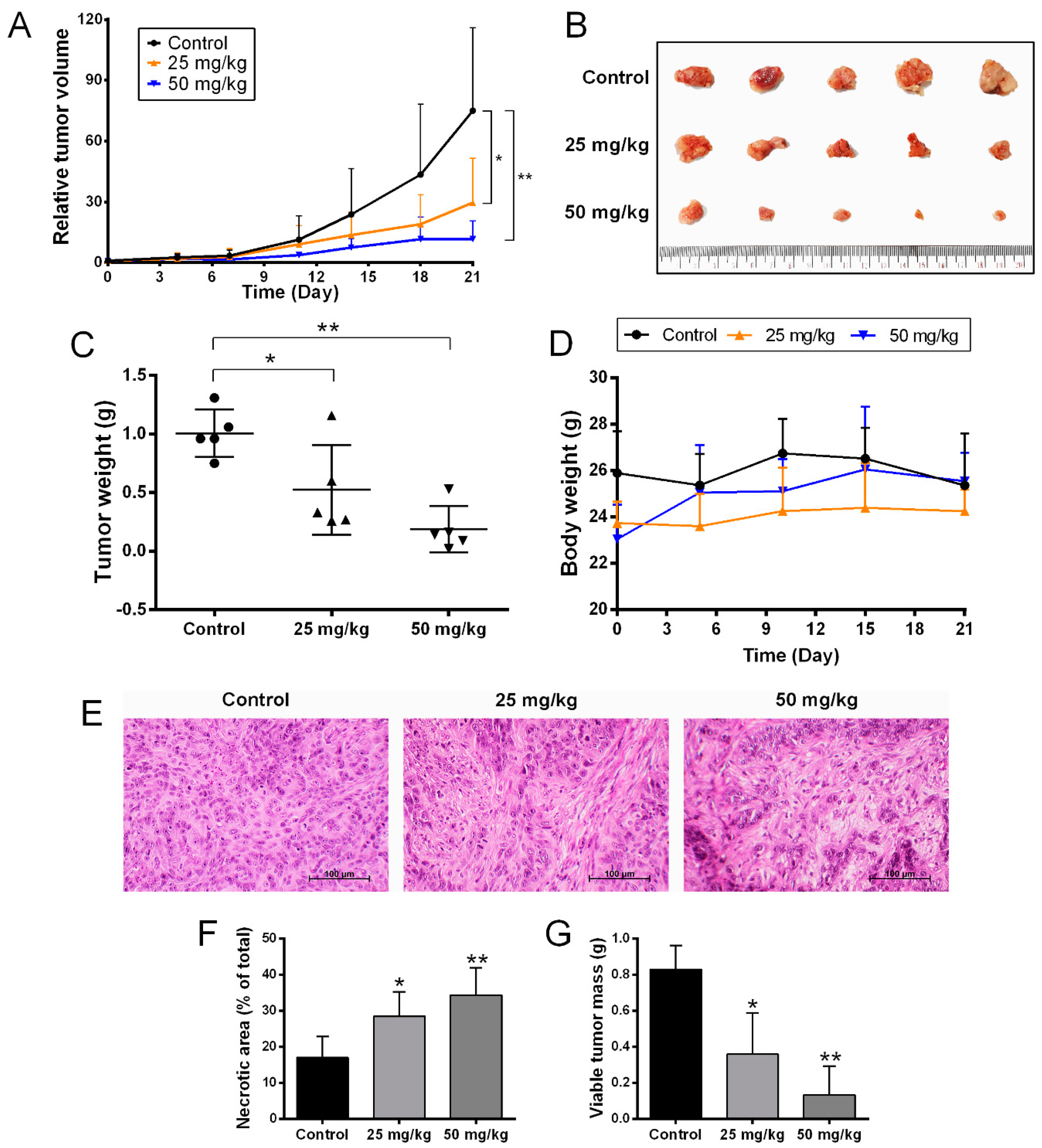
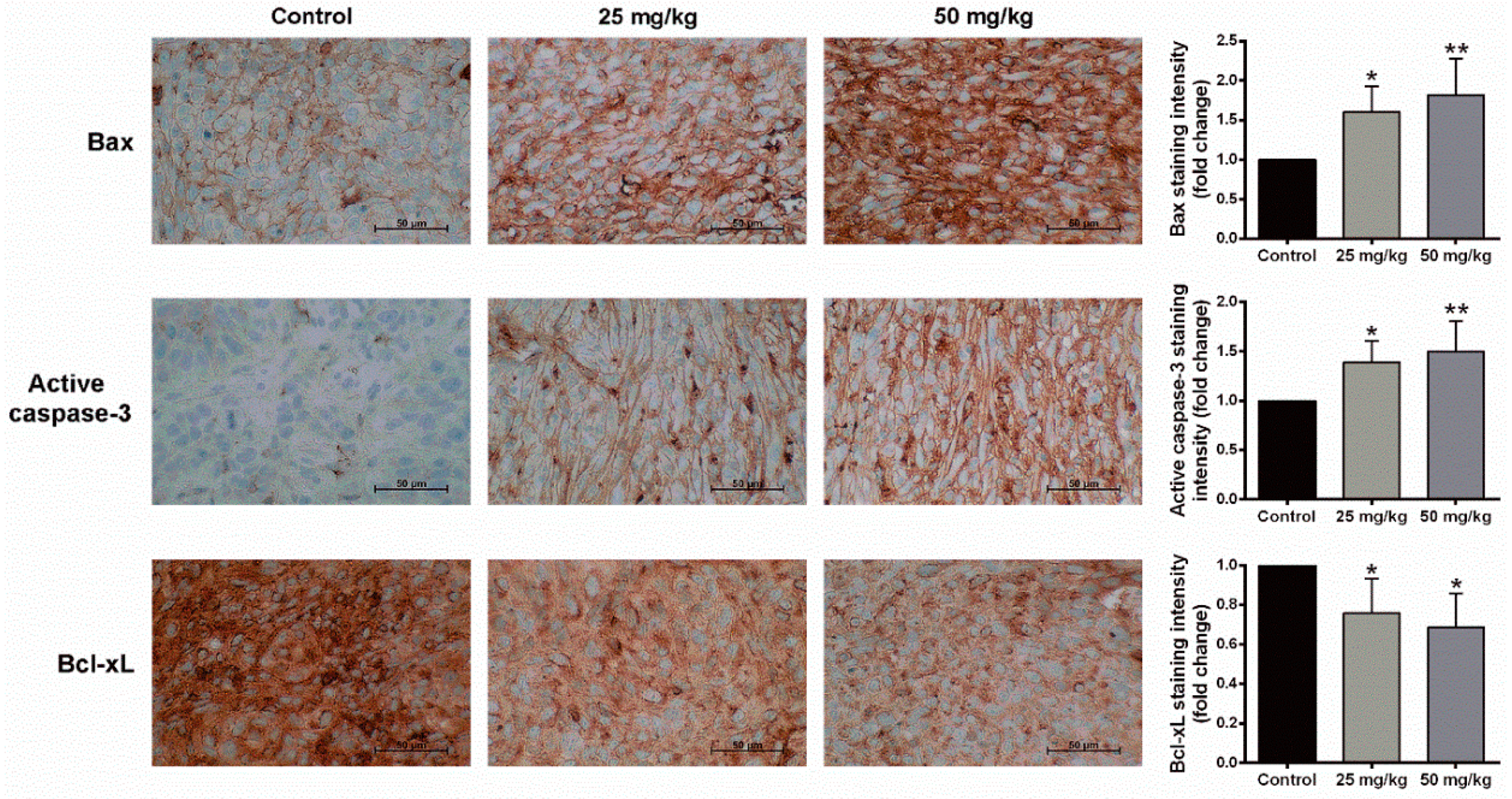
2.5. Anti-Tumor Activity of BJA in the In Vivo Model
3. Discussion
4. Materials and Methods
4.1. BJA Recipe Preparation and Extraction
4.2. Chemicals and Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Apoptosis Assay
4.6. Western Blotting Analysis
4.7. Determination of Caspase Activities
4.8. Determination of Mitochondrial Transmembrane Potential
4.9. Autophagic Vacuole Detection
4.10. Experimental Animals
4.11. Immunohistochemistry
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| Annexin V-FITC/PI | annexin V-fluorescein isothiocyanate and propidium iodide |
| AST | aspartate aminotransferase |
| BJA | Benja Amarit |
| BJA-W | water extract of BJA |
| BJA-50 | 50% ethanolic extract of BJA |
| BJA-95 | 95% ethanolic extract of BJA |
| BUN | blood urea nitrogen |
| CCA | cholangiocarcinoma |
| DEVD-p-NA | Asp-Glu-Val-Asp para-nitro aniline |
| DiOC6 | 3,3′-dihexyloxacarbocyanine iodide |
| ER | endoplasmic reticulum |
| HCC | hepatocellular carcinoma |
| H&E | hematoxylin and eosin |
| IETD-p-NA | Ile-Glu-Thr-Asp para-nitro aniline |
| LEHD-p-NA | Leu-Glu-His-Asp para-nitro aniline |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| MCV | mean corpuscular volume |
| NF-κB | nuclear factor kappa light chain enhancer of activated B cells |
| 3-MA | 3-methyladenine |
References
- Blechacz, B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349. [Google Scholar] [CrossRef] [PubMed]
- Kamsa-ard, S.; Kamsa-ard, S.; Luvira, V.; Suwanrungruang, K.; Vatanasapt, P.; Wiangnon, S. Risk factors for cholangiocarcinoma in Thailand: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2018, 19, 605. [Google Scholar] [PubMed]
- Varamo, C.; Peraldo-Neia, C.; Ostano, P.; Basiricò, M.; Raggi, C.; Bernabei, P.; Venesio, T.; Berrino, E.; Aglietta, M.; Leone, F. Establishment and characterization of a new intrahepatic cholangiocarcinoma cell line resistant to gemcitabine. Cancers 2019, 11, 519. [Google Scholar] [CrossRef]
- Woradet, S.; Songserm, N.; Promthet, S.; Parkin, D.M. Health-related quality of life and survival of cholangiocarcinoma patients in northeastern region of Thailand. PLoS ONE 2016, 11, e0163448. [Google Scholar] [CrossRef]
- Wattanawongdon, W.; Hahnvajanawong, C.; Namwat, N.; Kanchanawat, S.; Boonmars, T.; Jearanaikoon, P.; Leelayuwat, C.; Techasen, A.; Seubwai, W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 2015, 47, 398–410. [Google Scholar] [CrossRef]
- Fava, G. Molecular mechanisms of cholangiocarcinoma. World J. Gastrointest. Pathophysiol. 2010, 1, 12. [Google Scholar] [CrossRef]
- Wu, H.-J.; Chu, P.-Y. Role of cancer stem cells in cholangiocarcinoma and therapeutic implications. Int. J. Mol. Sci. 2019, 20, 4154. [Google Scholar] [CrossRef]
- Marin, J.J.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.A.; Efferth, T.; Macias, R.I. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Marin, J.J.; Herraez, E.; Lozano, E.; Macias, R.I.; Briz, O. Models for understanding resistance to chemotherapy in liver cancer. Cancers 2019, 11, 1677. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.; Ryan, K.M. Autophagy and cancer–issues we need to digest. J. Cell. Sci. 2012, 125, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, S. Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 2014, 65, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.; Singh, A. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef]
- Thorburn, A.; Thamm, D.H.; Gustafson, D.L. Autophagy and cancer therapy. Mol. Pharmacol. 2014, 85, 830–838. [Google Scholar] [CrossRef]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726. [Google Scholar] [CrossRef]
- Yun, C.; Lee, S. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Yapasert, R.; Sripanidkulchai, B.; Teerachaisakul, M.; Banchuen, K.; Banjerdpongchai, R. Anticancer effects of a traditional Thai herbal recipe Benja Amarit extracts against human hepatocellular carcinoma and colon cancer cell by targeting apoptosis pathways. J. Ethnopharmacol. 2020, 3, 112732. [Google Scholar] [CrossRef]
- Kaewnoonual, N.; Itharat, A.; Pongsawat, S.; Nilbu-Nga, C.; Kerdput, V.; Pradidarcheep, W. Anti-angiogenic and anti-proliferative effects of Benja-ummarit extract in rats with hepatocellular carcinoma. Biomed. Rep. 2020, 12, 109–120. [Google Scholar] [CrossRef]
- Yossathera, K.; Worakunphanich, W.; Teerachaisakul, M.; Stienrut, P. Traditional Thai medicine formula“ Benja Amarit“ in liver cancer patiens: Safety and Quality of Life. J. Thai. Tradit. Altern. Med. 2017, 15, 301–311. [Google Scholar]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψ m) in apoptosis: An update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.; Harris, M.; Thompson, C. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Huang, H.Y.; Lin, H.P.; Fang, C.Y. Piperlongumine induces autophagy in biliary cancer cells via reactive oxygen species-activated Erk signaling pathway. Int. J. Mol. Med. 2019, 44, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-J.; Dong, L.-W.; Tan, Y.-X.; Yang, G.-Z.; Pan, Y.-F.; Li, Z.; Tang, L.; Wang, M.; Wang, Q.; Wang, H.-Y. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab. Investig. 2011, 91, 1146–1157. [Google Scholar] [CrossRef]
- Perez-Montoyo, H. Therapeutic Potential of Autophagy Modulation in Cholangiocarcinoma. Cells 2020, 9, 614. [Google Scholar] [CrossRef]
- Grácio, D.; Magro, F.; Lima, R.T.; Máximo, V. An overview on the role of autophagy in cancer therapy. Hematol. Med. Oncol. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Fitzwalter, B.E.; Towers, C.G.; Sullivan, K.D.; Andrysik, Z.; Hoh, M.; Ludwig, M.; O’Prey, J.; Ryan, K.M.; Espinosa, J.M.; Morgan, M.J. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev. Cell 2018, 44, 555–565. [Google Scholar] [CrossRef]
- Tsai, T.-F.; Thomas, I.; Hwang, S.; Lin, J.-F.; Chen, H.-E.; Yang, S.-C.; Lin, Y.-C.; Chou, K.-Y. Suppression of quercetin-induced autophagy enhances cytotoxicity through elevating apoptotic cell death in human bladder cancer cells. Urol. Sci. 2019, 30, 58. [Google Scholar]
- Liu, D.; Gao, M.; Yang, Y.; Qi, Y.; Wu, K.; Zhao, S. Inhibition of autophagy promotes cell apoptosis induced by the proteasome inhibitor MG-132 in human esophageal squamous cell carcinoma EC9706 cells. Oncol. Lett. 2015, 9, 2278–2282. [Google Scholar] [CrossRef][Green Version]
- JG Marin, J.; Lozano, E.; Briz, O.; Al-Abdulla, R.; Serrano, M.A.; IR Macias, R. Molecular bases of chemoresistance in cholangiocarcinoma. Curr. Drug Targets 2017, 18, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Macias, R.I. Cholangiocarcinoma: Biology, clinical management, and pharmacological perspectives. ISRN Hepatol. 2014, 2014, 828074. [Google Scholar] [CrossRef]
- Baig, S.; Seevasant, I.; Mohamad, J.; Mukheem, A.; Huri, H.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, e2058. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Shabnam, B.; Girisa, S.; Harsha, C.; Banik, K.; Devi, T.B.; Choudhury, R.; Sahu, H.; Parama, D.; Sailo, B.L. Inflammation, NF-κB, and chronic diseases: How are they linked? Crit. Rev. Immunol. 2020, 40, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Ma, J.; Yu, H. Sirtuin-7 knockdown inhibits the growth of endometrial cancer cells by inducing apoptosis via the NF-κB signaling pathway. Oncol. Lett. 2019, 17, 937–943. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, P.; Xie, X.; Yu, H.; Wang, Y.; Chen, G. The role of tumour microenvironment: A new vision for cholangiocarcinoma. J. Cell. Mol. Med. 2019, 23, 59–69. [Google Scholar] [CrossRef]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-κB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.-J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634. [Google Scholar] [CrossRef]
- Thongchot, S.; Thanee, M.; Loilome, W.; Techasen, A.; Boonmars, T.; Sa-Ngiamwibool, P.; Titapun, A.; Yongvanit, P.; Isidoro, C.; Namwat, N. Curative effect of xanthohumol supplementation during liver fluke-associated cholangiocarcinogenesis: Potential involvement of autophagy. J. Tradit. Complement. Med. 2019, 10, 230–235. [Google Scholar] [CrossRef]
- Su, M.; Mei, Y.; Sinha, S. Role of the crosstalk between autophagy and apoptosis in cancer. J. Oncol. 2013, 2013, 102735. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.-Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.-L. The dual role of autophagy in cancer. Curr. Opin. Pharmacol. 2011, 11, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.D.; Thorburn, A. Focus: Death: Regulation of apoptosis by autophagy to enhance cancer therapy. Yale J. Biol. Med. 2019, 92, 707. [Google Scholar]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Wang, W.; Piao, S.; Zhou, J.; Saiyin, W.; Zheng, C.; Sun, H.; Li, Y. Inhibition of autophagy by 3-MA enhances IL-24-induced apoptosis in human oral squamous cell carcinoma cells. J. Exp. Clin. Cancer Res. 2015, 34, 97. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, M.; Arias, A.; Martínez-García, D.; Pérez-Tomás, R.; Quesada, R.; Soto-Cerrato, V. Targeting autophagy for cancer treatment and tumor chemosensitization. Cancers 2019, 11, 1599. [Google Scholar] [CrossRef]
- Liu, Z.; He, K.; Ma, Q.; Yu, Q.; Liu, C.; Ndege, I.; Wang, X.; Yu, Z. Autophagy inhibitor facilitates gefitinib sensitivity in vitro and in vivo by activating mitochondrial apoptosis in triple negative breast cancer. PLoS ONE 2017, 12, e0177694. [Google Scholar] [CrossRef]
- Onoe-Takahashi, A.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Autophagy inhibitors regulate TRAIL sensitivity in human malignant cells by targeting the mitochondrial network and calcium dynamics. Int. J. Oncol. 2019, 54, 1734–1746. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á.J.A.H. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Chanwikruy, Y.; Rattanapanone, V.; Sripanidkulchai, B. Induction of apoptosis in the human Leukemic U937 cell line by Kaempferia parviflora Wall. ex. Baker extract and effects of paclitaxel and camptothecin. Asian Pac. J. Cancer Prev. 2009, 10, 1137–1140. [Google Scholar]
- Khaw-on, P.; Pompimon, W.; Banjerdpongchai, R. Apoptosis induction via ATM phosphorylation, cell cycle arrest, and ER stress by goniothalamin and chemodrugs combined effects on breast cancer-derived MDA-MB-231 Cells. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Khaw-On, P.; Banjerdpongchai, R. Induction of intrinsic and extrinsic apoptosis pathways in the human leukemic MOLT-4 cell line by terpinen-4-ol. Asian Pac. J. Cancer Prev. 2012, 13, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Oller, G.; Ordoñez, R.; Dotor, J. A new background subtraction method for Western blot densitometry band quantification through image analysis software. J. Immunol. Methods 2018, 457, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Na, J.; Lee, M.S.; Cha, E.Y.; Sul, J.Y.; Park, J.B.; Lee, J.S. Combination of pristimerin and paclitaxel additively induces autophagy in human breast cancer cells via ERK1/2 regulation. Mol. Med. Rep. 2018, 18, 4281–4288. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Eble, M.; Prescher, K.; Hoß, M.; Kontny, U. Chloroquine sensitizes nasopharyngeal carcinoma cells but not nasoepithelial cells to irradiation by blocking autophagy. PLoS ONE 2016, 11, e0166766. [Google Scholar] [CrossRef]
- Yothaisong, S.; Dokduang, H.; Anzai, N.; Hayashi, K.; Namwat, N.; Yongvanit, P.; Sangkhamanon, S.; Jutabha, P.; Endou, H.; Loilome, W. Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Buvall, L.; Hedman, H.; Khramova, A.; Najar, D.; Bergwall, L.; Ebefors, K.; Sihlbom, C.; Lundstam, S.; Herrmann, A.; Wallentin, H. Orellanine specifically targets renal clear cell carcinoma. Oncotarget 2017, 8, 91085. [Google Scholar] [CrossRef]
- Paik, J.H.; Choe, G.; Kim, H.; Choe, J.-Y.; Lee, H.J.; Lee, C.-T.; Lee, J.S.; Jheon, S.; Chung, J.-H. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: Correlation with fluorescence in situ hybridization. J. Thorac. Oncol. 2011, 6, 466–472. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar]
- Crowe, A.R.; Yue, W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: An integrated protocol. Bio Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef]
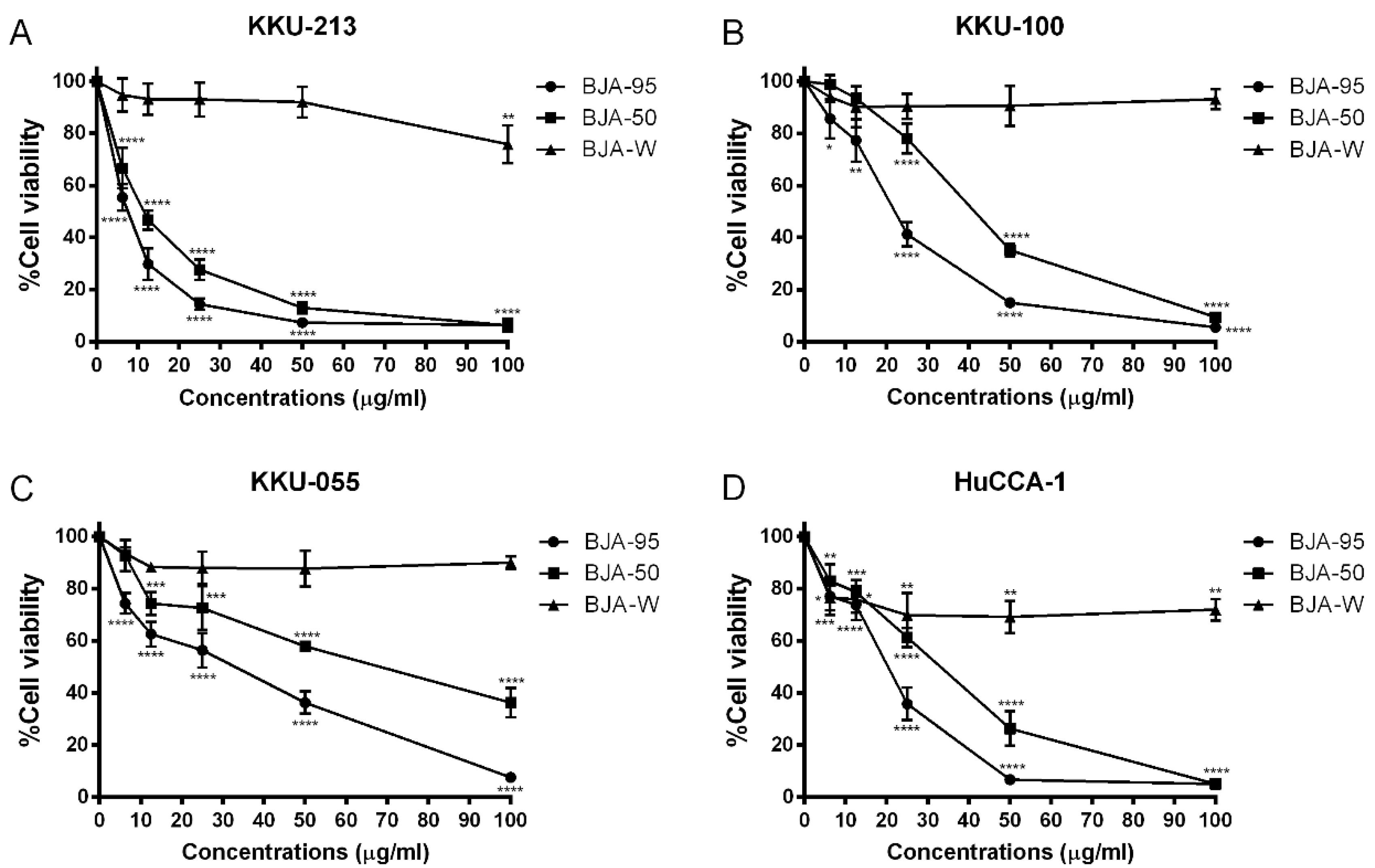
| CCA Cell Lines | IC50 (µg/mL) | ||
|---|---|---|---|
| BJA-95 | BJA-50 | BJA-W | |
| KKU-213 | 7.1 ± 1.3 | 11.1 ± 1.5 # | >100 |
| HuCCA-1 | 17.9 ± 1.2 ** | 28.2 ± 3.5 ## | >100 |
| KKU-100 | 21.0 ± 2.8 ** | 40.0 ± 2.5 ### | >100 |
| KKU-055 | 23.3 ± 1.8 *** | 61.7 ± 8.9 ## | >100 |
| Parameters | Control | BJA-95 | |
|---|---|---|---|
| 25 mg/kg | 50 mg/kg | ||
| Complete blood count (CBC) | |||
| Red blood cells (×106/µL) | 8.8 ± 0.6 | 9.4 ± 0.6 | 9.2 ± 0.9 |
| Hemoglobin (g/dL) | 13.9 ± 1.0 | 15.1 ± 1.1 | 14.6 ± 1.8 |
| Hematocrit (%) | 43.8 ± 3.4 | 47.3 ± 3.2 | 46.1 ± 5.6 |
| Mean corpuscular volume (MCV) (fL) | 49.8 ± 0.6 | 50.2 ± 0.4 | 50.2 ± 1.4 |
| Mean corpuscular hemoglobin (MCH) (pg) | 15.4 ± 0.2 | 16.0 ± 0.3 | 15.9 ± 0.5 |
| Mean corpuscular hemoglobin concentration (MCHC) (g/dL) | 31.8 ± 0.6 | 31.9 ± 0.4 | 31.7 ± 0.4 |
| White blood cells (×103/µL) | 11.9 ± 4.8 | 9.3 ± 3.8 | 12.1 ± 10.1 |
| Neutrophil (%) | 56.8 ± 11.7 | 37.3 ± 14.1 | 36.1 ± 21.0 |
| Lymphocyte (%) | 21.1 ± 7.4 | 34.8 ± 17.3 | 33.1 ± 16.1 |
| Monocyte (%) | 15.5 ± 5.9 | 25.0 ± 12.6 | 27.2 ± 12.4 |
| Eosinophil (%) | 4.9 ± 1.5 | 3.8 ± 0.8 | 3.4 ± 1.6 |
| Basophil (%) | 0.8 ± 1.6 | 0.1 ± 0.1 | 0.1 ± 0.2 |
| Platelet (×103/µL) | 691.7 ± 323.0 | 915.4 ± 258.1 | 949.8 ± 168.8 |
| Blood chemistry (kidney) | |||
| Blood urea nitrogen (BUN) (mg/dL) | 23.9 ± 2.4 | 21.0 ± 3.6 | 24.8 ± 4.3 |
| Creatinine (mg/dL) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Blood chemistry (liver) | |||
| Aspartate aminotransferase (AST) (U/L) | 102.2 ± 20.8 | 113.8 ± 49.1 | 94.0 ± 28.5 |
| Alanine aminotransferase (ALT) (U/L) | 107.2 ± 93.8 | 70.4 ± 82.6 | 67.0 ± 18.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yapasert, R.; Lertprasertsuk, N.; Subhawa, S.; Poofery, J.; Sripanidkulchai, B.; Banjerdpongchai, R. Antitumor Efficacy of the Herbal Recipe Benja Amarit against Highly Invasive Cholangiocarcinoma by Inducing Apoptosis both In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 5669. https://doi.org/10.3390/ijms21165669
Yapasert R, Lertprasertsuk N, Subhawa S, Poofery J, Sripanidkulchai B, Banjerdpongchai R. Antitumor Efficacy of the Herbal Recipe Benja Amarit against Highly Invasive Cholangiocarcinoma by Inducing Apoptosis both In Vitro and In Vivo. International Journal of Molecular Sciences. 2020; 21(16):5669. https://doi.org/10.3390/ijms21165669
Chicago/Turabian StyleYapasert, Rittibet, Nirush Lertprasertsuk, Subhawat Subhawa, Juthathip Poofery, Bungorn Sripanidkulchai, and Ratana Banjerdpongchai. 2020. "Antitumor Efficacy of the Herbal Recipe Benja Amarit against Highly Invasive Cholangiocarcinoma by Inducing Apoptosis both In Vitro and In Vivo" International Journal of Molecular Sciences 21, no. 16: 5669. https://doi.org/10.3390/ijms21165669
APA StyleYapasert, R., Lertprasertsuk, N., Subhawa, S., Poofery, J., Sripanidkulchai, B., & Banjerdpongchai, R. (2020). Antitumor Efficacy of the Herbal Recipe Benja Amarit against Highly Invasive Cholangiocarcinoma by Inducing Apoptosis both In Vitro and In Vivo. International Journal of Molecular Sciences, 21(16), 5669. https://doi.org/10.3390/ijms21165669






