Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their “pH” Enotype?
Abstract
1. Introduction
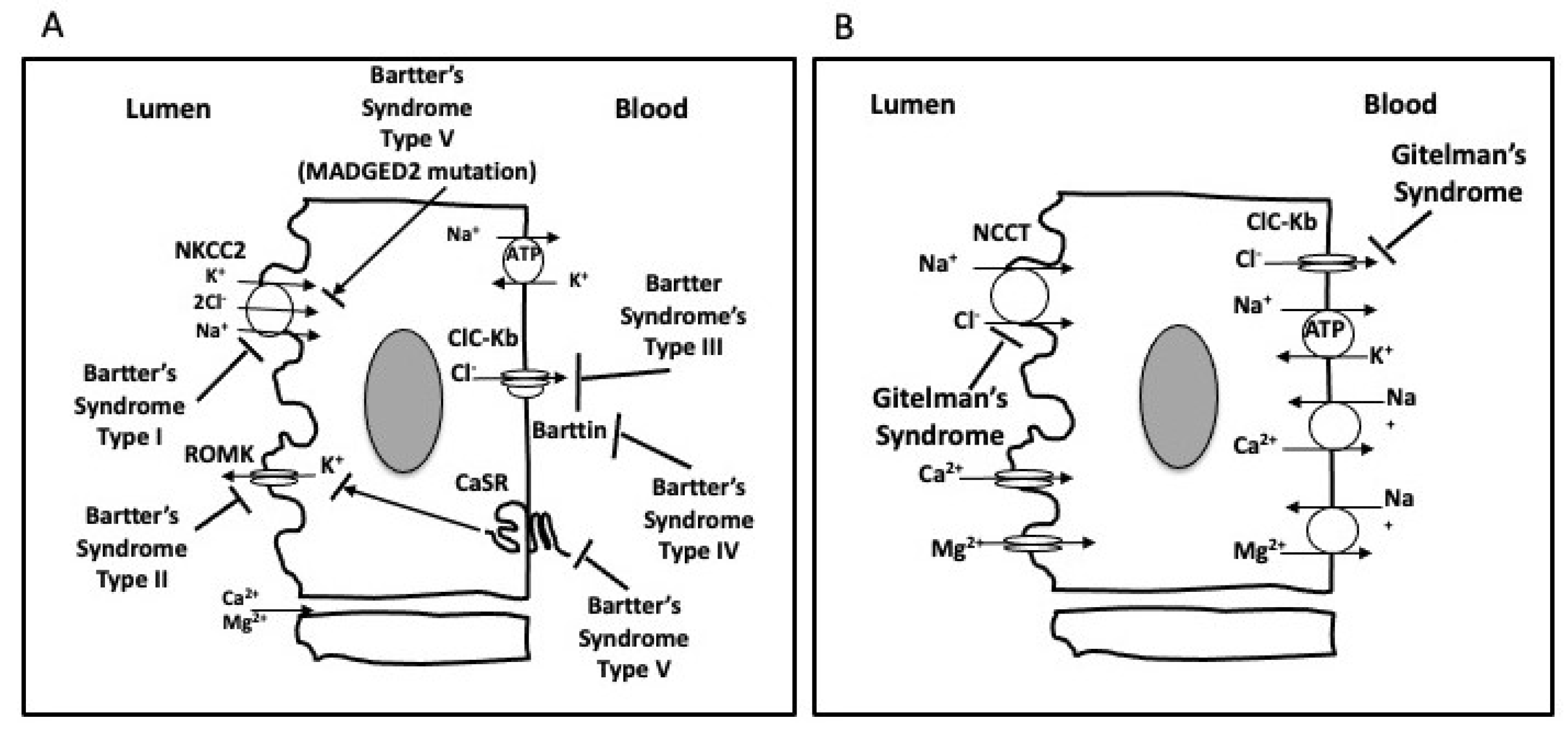
1.1. Gitelman’s Syndrome
1.2. Bartter’s Syndrome
2. The GS and BS Phenotypic Overlapping
3. RAS in Gitelman’s and Bartter’s Syndromes
4. ACE2 and Chloroquine Effect
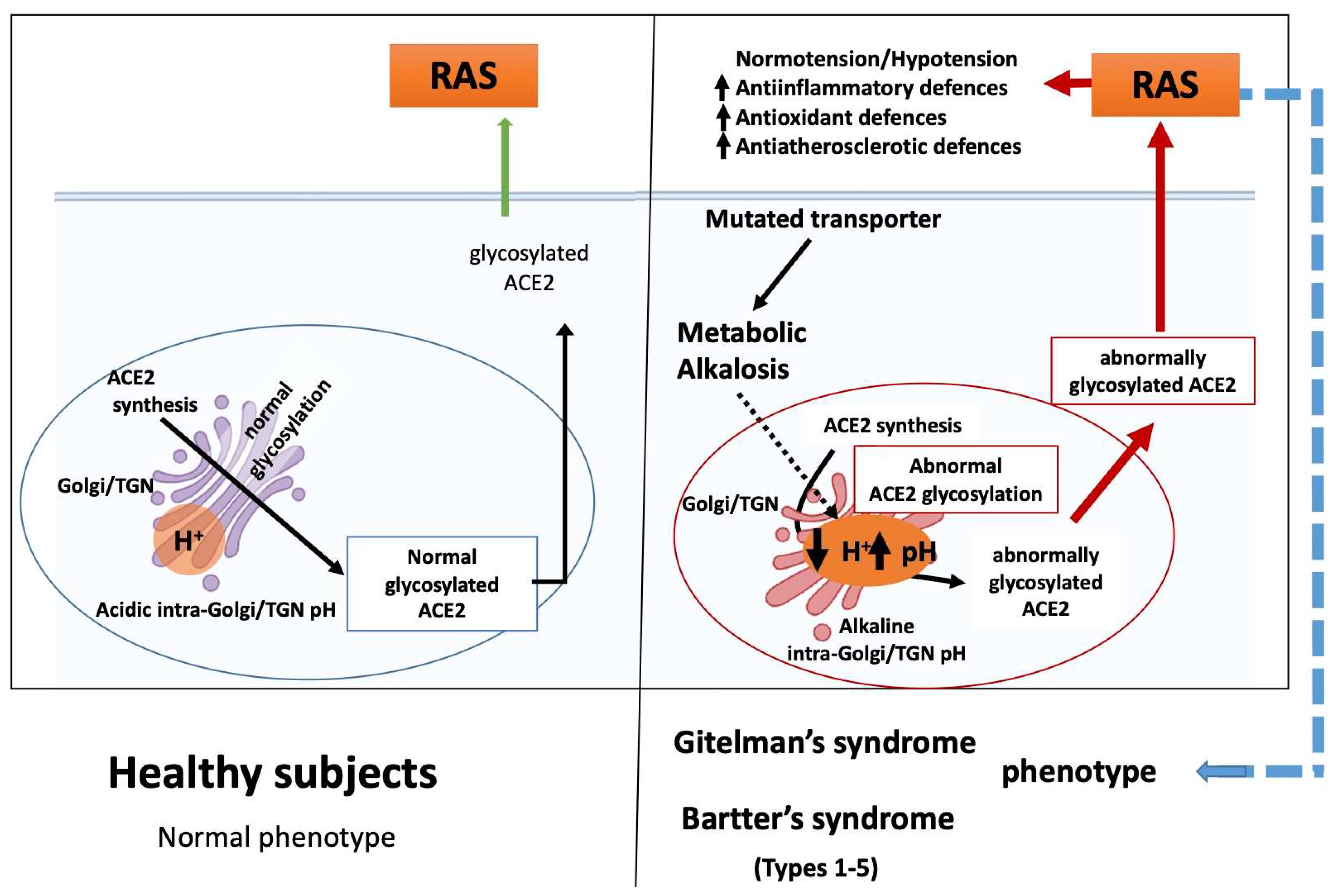
5. Golgi-localized Glycosylation: The Importance of Endosomal pH
6. The Basis for Gitelman’s and Bartter’s Syndrome Phenotypes
7. Conclusions
Funding
Conflicts of Interest
References
- Calò, L.A.; Davis, P.A.; Rossi, G.P. Understanding the mechanisms of angiotensin II signaling involved in hypertension and its long-term sequelae: Insights from Bartter’s and Gitelman’s syndromes, human models of endogenous angiotensin II signaling antagonism. J. Hypertens. 2014, 32, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Frankl, F.E.K.; Knoers, N.V.; Konrad, M.; et al. Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Ravarotto, V. Bartter and Gitelman syndromes. In Encyclopedia of Endocrine Diseases; Ilpo, H., Luciano, M., Eds.; Oxford Academic Press: Oxford, UK, 2019; Volume 3, pp. 713–721. [Google Scholar] [CrossRef]
- Zelikovic, I.; Szargel, R.; Hawash, A.; Labay, V.; Hatib, I.; Cohen, N.; Nakhoul, F. A novel mutation in the chloride channel gene, CLCNKB, as a cause of Gitelman and Bartter syndromes. Kidney Int. 2003, 63, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.; Heo, N.J.; Cheong, H.I.; Han, J.S. Mutations in SLC12A3 and CLCNKB and their correlation with clinical phenotype in patients with gitelman and gitelman-like syndrome. J. Korean Med. Sci. 2016, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Lo, Y.F.; Chen, J.C.; Huang, C.L.; Lin, S.H. Functional severity of CLCNKB mutations correlates with phenotypes in patients with classic Bartter’s syndrome. J. Physiol. 2017, 595, 5573–5586. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, D.; Kovalchuk, E.; Yu, L.; Tan, H.; Fahlke, C.; Stölting, G.; Alekov, A.K. Barttin regulates the subcellular localization and posttranslational modification of human Cl(-)/H(+) antiporter ClC-5. Front. Physiol. 2018, 9, 1490. [Google Scholar] [CrossRef]
- De Jong, J.C.; Van Der Vliet, W.A.; Willems, P.H.; Knoers, N.V.; Bindels, R.J. Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman’s syndrome. J. Am. Soc. Nephrol. 2002, 13, 1442–1448. [Google Scholar] [CrossRef]
- Khayat, W.; Hackett, A.; Shaw, M.; Ilie, A.; Dudding-Byth, T.; Kalscheuer, V.M.; Christie, L.; Corbett, M.A.; Juusola, J.; Friend, K.L.; et al. A recurrent missense variant in SLC9A7 causes nonsyndromic X-linked intellectual disability with alteration of Golgi acidification and aberrant glycosylation. Hum. Mol. Genet. 2019, 28, 598–614. [Google Scholar] [CrossRef]
- Bastug, F.; Nalcacioglu, H.; Ozaltin, F.; Korkmaz, E.; Yel, S. Nephropathic cystinosis mimicking bartter syndrome: A novel mutation. Iran. J. Kidney Dis. 2018, 12, 61–63. [Google Scholar]
- Berio, A. Nephropathy caused by cystinosis with secondary Bartter’s syndrome. Personal experience in a case treated with prolonged diet therapy and acetylsalicylic acid therapy. Minerva Pediatr. 1978, 30, 1825–1831. [Google Scholar]
- Taranta, A.; Bellomo, F.; Petrini, S.; Polishchuk, E.; De Leo, E.; Rega, L.R.; Pastore, A.; Polishchuk, R.; De Matteis, M.A.; Emma, F. Cystinosin-LKG rescues cystine accumulation and decreases apoptosis rate in cystinotic proximal tubular epithelial cells. Pediatr. Res. 2017, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Poglitsch, M.; Yogasundaram, H.; Thomas, J.; Rowe, B.H.; Oudit, G.Y. Roles of angiotensin peptides and recombinant human ace2 in heart failure. J. Am. Coll. Cardiol. 2017, 69, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Marsault, E.; Llorens-Cortes, C.; Iturrioz, X.; Chun, H.J.; Lesur, O.; Oudit, G.Y.; Auger-Messier, M. The apelinergic system: A perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 2019, 1455, 12–33. [Google Scholar] [CrossRef]
- Wang, W.; Shen, M.; Fischer, C.; Basu, R.; Hazra, S.; Couvineau, P.; Paul, M.; Wang, F.; Toth, S.; Mix, D.S.; et al. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc. Natl. Acad. Sci. USA 2019, 116, 13006–13015. [Google Scholar] [CrossRef]
- Barnes, G.D.; Alam, S.; Carter, G.; Pedersen, C.M.; Lee, K.M.; Hubbard, T.J.; Veitch, S.; Jeong, H.; White, A.; Cruden, N.L.; et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 2013, 6, 482–491. [Google Scholar] [CrossRef]
- Sato, T.; Suzuki, T.; Watanabe, H.; Kadowaki, A.; Fukamizu, A.; Liu, P.P.; Kimura, A.; Ito, H.; Penninger, J.M.; Imai, Y.; et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Investig. 2013, 123, 5203–5211. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Wang, W.; Jin, H.Y.; Chen, X.; Cheng, Y.W.; Xu, Y.L.; Song, B.; Penninger, J.M.; Oudit, G.Y.; Zhong, J.C. Apelin is a negative regulator of angiotensin ii-mediated adverse myocardial remodeling and dysfunction. Hypertension 2017, 70, 1165–1175. [Google Scholar] [CrossRef]
- Calo, L.A.; Schiavo, S.; Davis, P.A.; Pagnin, E.; Mormino, P.; D’Angelo, A.; Pessina, A.C. ACE2 and angiotensin 1-7 are increased in a human model of cardiovascular hyporeactivity: Pathophysiological implications. J. Nephrol. 2010, 23, 472–477. [Google Scholar]
- Fang, Y.; Gao, F.; Liu, Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-kappaB and Nrf2 pathways. QJM 2019, 112, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Basque, J.; Martel, M.; Leduc, R.; Cantin, M.M. Lysosomotropic drugs inhibit maturation of transforming growth factor-beta. Can. J. Physiol. Pharmacol. 2008, 86, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskiene, S.; Dyve Lingelem, A.B.; Skotland, T.; Sandvig, K. Protection against Shiga Toxins. Toxins 2017, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Nguyen, D.; Pfeffer, S.; Förster, F.; Helms, V.; Zimmermann, R. Functions and mechanisms of the human ribosome-translocon complex. Subcell Biochem. 2019, 93, 83–141. [Google Scholar] [PubMed]
- McGill, J.B.; Johnson, M.; Hurst, S.; Cade, W.T.; Yarasheski, K.E.; Ostlund, R.E.; Schechtman, K.B.; Razani, B.; Kastan, M.B.; McClain, D.A.; et al. Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima-media thickness. Diabetol. Metab. Syndr. 2019, 11, 61. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Z.; Li, Y.; Huang, W.; Zhou, M.; Zhang, X.; Jiang, W. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogen-activated protein kinase activation. Shock 2015, 43, 395–404. [Google Scholar] [CrossRef]
- Petersen, O.H.; Gerasimenko, O.V.; Gerasimenko, J.V. Endocytic uptake of SARS-CoV-2: The critical roles of pH, Ca2+ and NAADP. Function 2020, 1, zqaa003. [Google Scholar] [CrossRef]
- Kellokumpu, S. Golgi pH, ion and redox homeostasis: How much do they really matter? Front. Cell Dev. Biol. 2019, 7, 93. [Google Scholar] [CrossRef]
- Axelsson, M.A.; Karlsson, N.G.; Steel, D.M.; Ouwendijk, J.; Nilsson, T.; Hansson, G.C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 2001, 11, 633–644. [Google Scholar] [CrossRef]
- Rivinoja, A.; Kokkonen, N.; Kellokumpu, I.; Kellokumpu, S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell Physiol. 2006, 208, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Available online: https://www.fda.gov/media/137250/download (accessed on 24 April 2020).
- Bettinelli, A.; Tosetto, A.; Colussi, G.; Tommasini, G.; Edefonti, A.; Bianchetti, M.G. Electrocardiogram with prolonged QT interval in Gitelman disease. Kidney Int. 2002, 62, 580–584. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Calò, L.A.; Negut, C.; Coccato, M.; Mormino, P.; Pessina, A.C. Myocardial perfusion defects in Bartter and Gitelman syndromes. Eur. J. Clin. Investig. 2008, 38, 888–895. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Calò, L.A.; Bertoldi, G.; Davis, P.A. Rho kinase inhibitors for SARS-CoV-2 induced acute respiratory distress syndrome: Support from Bartter’s and Gitelman’s syndrome patients. Pharmacol. Res. 2020, 158, 104903. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Davis, P.A.; Rigato, M.; Sgarabotto, L. Angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers and risk of COVID 19: Information from Bartter’s and Gitelman’s syndromes patients. J. Hypertens. 2020, 38, 1386. [Google Scholar] [CrossRef]
- Signorelli, C.; Scognamiglio, T.; Odone, A. COVID-19 in Italy: Impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020, 91, 175–179. [Google Scholar] [CrossRef]
- Bertoldi, G.; Gianesello, L.; Calò, L.A. ACE2, Rho kinase inhibition and the potential role of Vitamin D against COVID-19. Aliment. Pharmacol. Ther. 2020, 52, 577–578. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, Y.; Wang, G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B. An ACE therapy for COVID-19. Sci. Transl. Med. 2020, 541, eabb5676. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calò, L.A.; Davis, P.A. Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their “pH” Enotype? Int. J. Mol. Sci. 2020, 21, 5660. https://doi.org/10.3390/ijms21165660
Calò LA, Davis PA. Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their “pH” Enotype? International Journal of Molecular Sciences. 2020; 21(16):5660. https://doi.org/10.3390/ijms21165660
Chicago/Turabian StyleCalò, Lorenzo A, and Paul A Davis. 2020. "Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their “pH” Enotype?" International Journal of Molecular Sciences 21, no. 16: 5660. https://doi.org/10.3390/ijms21165660
APA StyleCalò, L. A., & Davis, P. A. (2020). Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their “pH” Enotype? International Journal of Molecular Sciences, 21(16), 5660. https://doi.org/10.3390/ijms21165660





