Anti-Epileptic Effects of FABP3 Ligand MF1 through the Benzodiazepine Recognition Site of the GABAA Receptor
Abstract
1. Introduction
2. Results
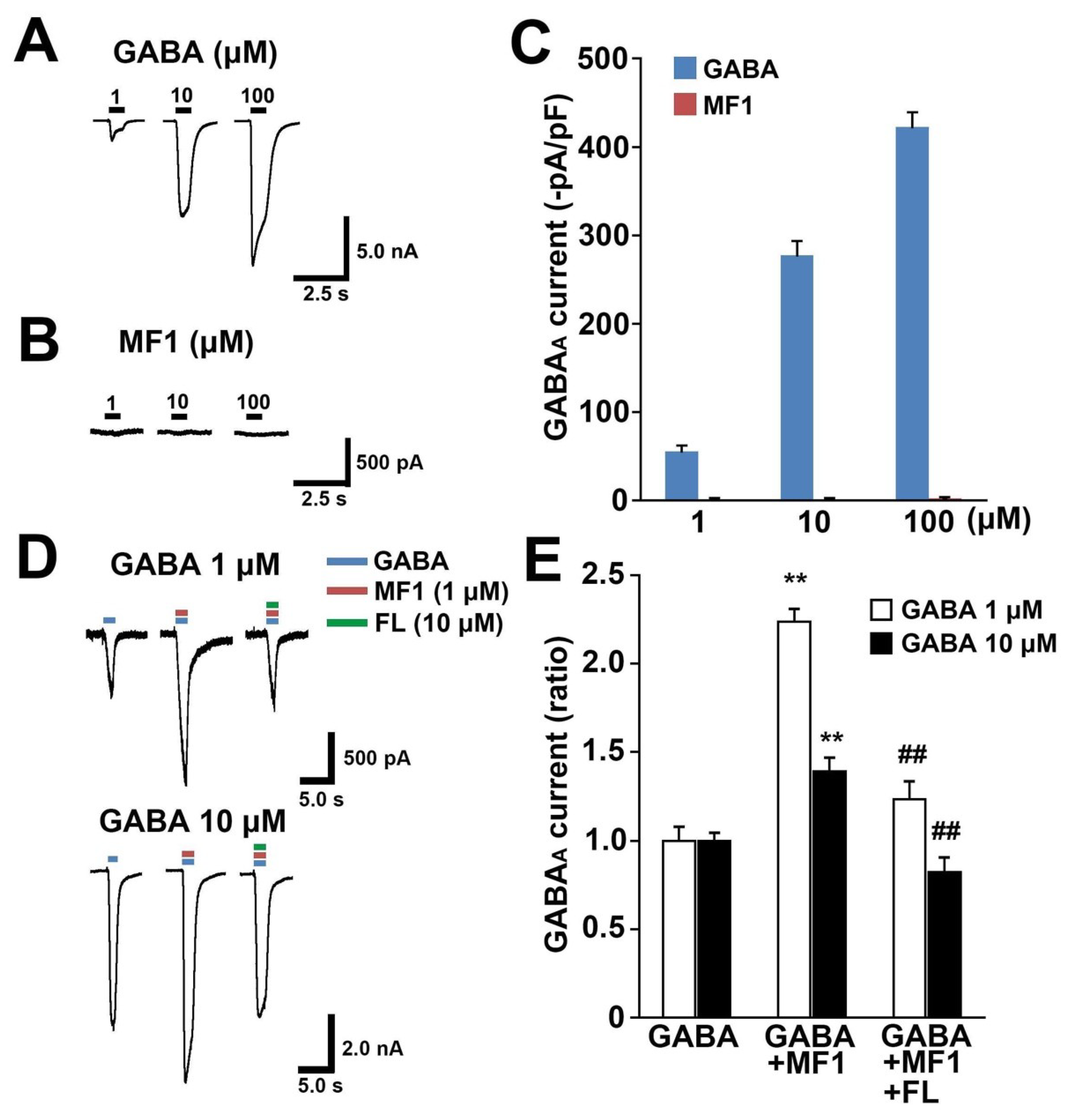
2.1. MF1 Promotes GABAA Receptor Currents through the Benzodiazepine Recognition Site
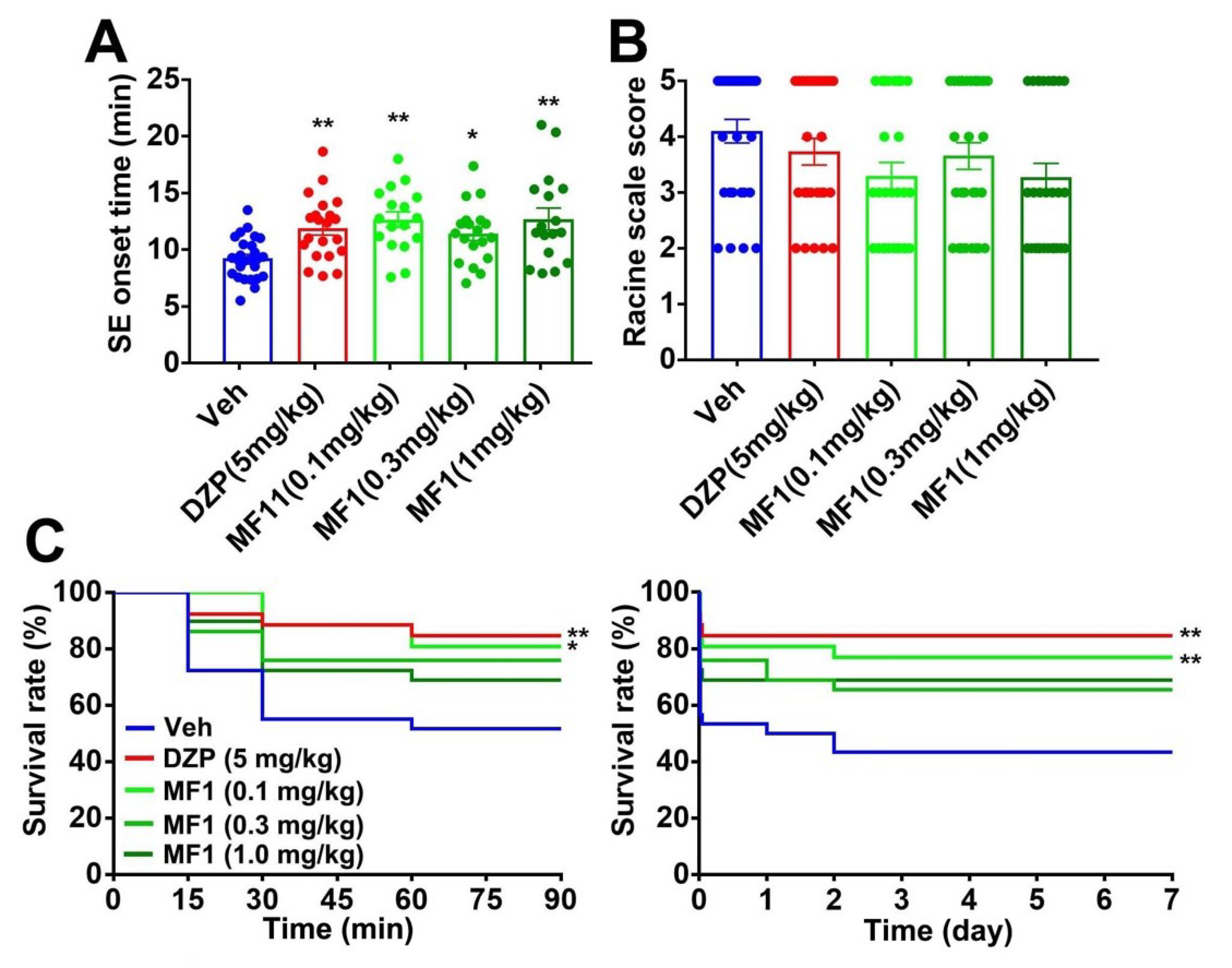
2.2. Acute MF1 Administration Attenuates SE and Mortality in PILO-Treated Mice
2.3. Acute MF1 Administration Does Not Improve Epileptic Seizures in PTZ-Treated Mice
2.4. Chronic MF1 Administration Suppresses SE and Mortality in PILO-Treated Mice
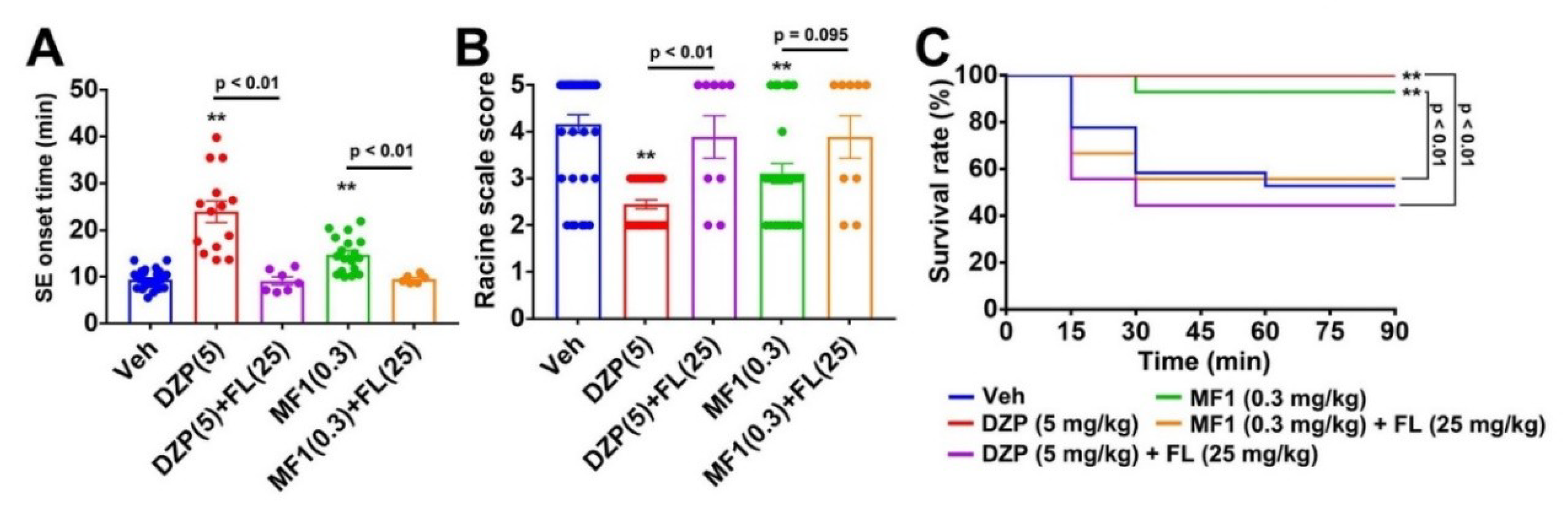
2.5. Flumazenil Blocks Anti-Epileptic Effect of MF1 in PILO-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Cell Transfection
4.4. Whole Cell Patch-Clamp Recording
4.5. Evaluation of Epileptic Behaviors
4.5.1. PILO-Induced SE Model
4.5.2. PTZ-Induced Seizure
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ANOVA | analysis of variance |
| CMC | carboxymethylcellulose |
| DMSO | dimethyl sulfoxide |
| DZP | diazepam |
| FABP | fatty acid binding protein |
| GABA | γ-aminobutyrate |
| GABAA | GABA type-A |
| GAD | glutamic acid decarboxylase |
| GFP | green fluorescent protein |
| MF1 | 4-(2-(1-(2-chlorophenyl)-5-phenyl-1H-pyrazol-3-yl)phenoxy) butanoic acid |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| PD | Parkinson’s disease |
| PFF | preformed fibril |
| PILO | pilocarpine |
| PTZ | pentylenetetrazol |
| SE | status epilepticus |
| SEM | standard error of the mean |
References
- Whiting, P.J.; Bonnert, T.P.; McKernan, R.M.; Farrar, S.; Le Bourdellès, B.; Heavens, R.P.; Smith, D.W.; Hewson, L.; Rigby, M.R.; Sirinathsinghji, D.J.; et al. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann. N. Y. Acad. Sci. 1999, 868, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Silver, R.A. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 2003, 38, 433–445. [Google Scholar] [CrossRef]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain 2020, 21, awaa072. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Löscher, W.; Rho, J.M. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harb. Perspect. Med. 2016, 6, a022780. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Wen, L.; Wu, Z.; Shen, Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimers Dement. 2020, 1–18. [Google Scholar] [CrossRef]
- Fung, L.K.; Flores, R.E.; Gu, M.; Sun, K.L.; James, D.; Schuck, R.K.; Jo, B.; Park, J.H.; Lee, B.C.; Jung, J.H.; et al. Thalamic and prefrontal GABA concentrations but not GABA(A) receptor densities are altered in high-functioning adults with autism spectrum disorder. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Faulkner, M.A.; Singh, S.P. Neurogenetic disorders and treatment of associated seizures. Pharmacotherapy 2013, 33, 330–343. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Saccaro, L.F.; Busceti, C.L.; Biagioni, F.; Fornai, F. Epilepsy and Alzheimer’s Disease: Potential mechanisms for an association. Brain Res. Bull. 2020, 160, 107–120. [Google Scholar] [CrossRef]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef]
- Czapiński, P.; Blaszczyk, B.; Czuczwar, S.J. Mechanisms of action of antiepileptic drugs. Curr. Top. Med. Chem. 2005, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bormann, J. The ‘ABC’ of GABA Receptors. Trends Pharmacol. Sci. 2000, 21, 16–19. [Google Scholar] [CrossRef]
- Rudolph, U.; Möhler, H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Sassoè-Pognetto, M.; Fritschy, J.M. Mini-review: Gephyrin, a Major Postsynaptic Protein of GABAergic Synapses. Eur. J. Neurosci. 2000, 12, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Glykys, J.; Mody, I. Activation of GABAA Receptors: Views from Outside the Synaptic Cleft. Neuron 2007, 56, 763–770. [Google Scholar] [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306. [Google Scholar] [CrossRef]
- Owada, Y.; Yoshimoto, T.; Kondo, H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J. Chem. Neuroanat. 1996, 12, 113–122. [Google Scholar] [CrossRef]
- Owada, Y. Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 2008, 214, 213–220. [Google Scholar] [CrossRef]
- Sepe, F.N.; Chiasserini, D.; Parnetti, L. Role of FABP3 as biomarker in Alzheimer’s disease and synucleinopathies. Future Neurol. 2018, 13, 199–207. [Google Scholar] [CrossRef]
- Chiasserini, D.; Biscetti, L.; Eusebi, P.; Salvadori, N.; Frattini, G.; Simoni, S.; De Roeck, N.; Tambasco, N.; Stoops, E.; Vanderstichele, H.; et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res. Ther. 2017, 9, 52. [Google Scholar] [CrossRef]
- Bjerke, M.; Kern, S.; Blennow, K.; Zetterberg, H.; Waern, M.; Börjesson-Hanson, A.; Östling, S.; Kern, J.; Skoog, I. Cerebrospinal Fluid Fatty Acid-Binding Protein 3 is Related to Dementia Development in a Population-Based Sample of Older Adult Women Followed for 8 Years. J. Alzheimers Dis. 2016, 49, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yabuki, Y.; Kobayashi, Y.; Onozato, M.; Owada, Y.; Fukunaga, K. FABP3 protein promotes α-synuclein oligomerization associated with 1-methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J. Biol. Chem. 2014, 289, 18957–18965. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Matsuo, K.; Kawahata, I.; Fukui, N.; Mizobata, T.; Kawata, Y.; Owada, Y.; Shioda, N.; Fukunaga, K. Fatty Acid Binding Protein 3 Enhances the Spreading and Toxicity of α-Synuclein in Mouse Brain. Int. J. Mol. Sci. 2020, 21, 2230. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Bousset, L.; Melki, R.; Fukunaga, K. Fatty Acid-Binding Protein 3 is Critical for α-Synuclein Uptake and MPP+-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons. Int. J. Mol. Sci. 2019, 20, 5358. [Google Scholar] [CrossRef]
- Cheng, A.; Shinoda, Y.; Yamamoto, T.; Miyachi, H.; Fukunaga, K. Development of FABP3 ligands that inhibit arachidonic acid-induced α-synuclein oligomerization. Brain Res. 2019, 1707, 190–197. [Google Scholar] [CrossRef]
- Matsuo, K.; Cheng, A.; Yabuki, Y.; Takahata, I.; Miyachi, H.; Fukunaga, K. Inhibition of MPTP-induced α-synuclein oligomerization by fatty acid-binding protein 3 ligand in MPTP-treated mice. Neuropharmacology 2019, 150, 164–174. [Google Scholar] [CrossRef]
- Liu, Y.M.; Fan, H.R.; Ding, J.; Huang, C.; Deng, S.; Zhu, T.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci. Rep. 2017, 7, 46654. [Google Scholar] [CrossRef]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef]
- Khoshnoud, M.J.; Tanideh, N.; Namdarian, S. Anticonvulsant activity of atorvastatin against seizure induced by pentylenetetrazole and maximal electroshock in mice. Trends Pharm. Sci. 2015, 1, 44–47. [Google Scholar]
- Vonderlin, N.; Fischer, F.; Zitron, E.; Seyler, C.; Scherer, D.; Thomas, D.; Katus, H.A.; Scholz, E.P. Inhibition of cardiac Kv1.5 potassium current by the anesthetic midazolam: Mode of action. Drug Des. Dev. Ther. 2014, 8, 2263–2271. [Google Scholar] [CrossRef]
- So, E.C.; Wu, K.C.; Kao, F.C.; Wu, S.N. Effects of midazolam on ion currents and membrane potential in differentiated motor neuron-like NSC-34 and NG108-15 cells. Eur. J. Pharmacol. 2014, 724, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Smolders, I.; Khan, G.M.; Manil, J.; Ebinger, G.; Michotte, Y. NMDA receptor-mediated pilocarpine-induced seizures: Characterization in freely moving rats by microdialysis. Br. J. Pharmacol. 1997, 121, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.H.; Chapman, A.G.; Meldrum, B.S. Extracellular amino acid levels in hippocampus during pilocarpine-induced seizures. Epilepsy Res. 1993, 14, 139–148. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Kapur, J. Acute cellular alterations in the hippocampus after status epilepticus. Epilepsia 1999, 40, S9–S20. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneyulu, R.; Ticku, M.K. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur. J. Pharmacol. 1984, 98, 337–345. [Google Scholar] [CrossRef]
- Hansen, S.L.; Sperling, B.B.; Sánchez, C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Pharmacology 1984, 98, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Oliveira, F.; Eleuterio Rodrigues, K.; Hamoy, M.; Sarquis, Í.R.; Otake Hamoy, A.; Crespo Lopez, M.E.; Maciel Ferreira, I.; Macchi, B.M.; Luiz Martins do Nascimento, J. Fatty Acid Amides Synthesized from Andiroba Oil (Carapa guianensis Aublet.) Exhibit Anticonvulsant Action with Modulation on GABA-A Receptor in Mice: A Putative Therapeutic Option. Pharmaceuticals 2020, 13, 43. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kida, H.; Kagawa, Y.; Yasumoto, Y.; Miyazaki, H.; Islam, A.; Ogata, M.; Yanagawa, Y.; Mitsushima, D.; Fukunaga, K.; et al. FABP3 in the Anterior Cingulate Cortex Modulates the Methylation Status of the Glutamic Acid Decarboxylase67 Promoter Region. J. Neurosci. 2018, 38, 10411–10423. [Google Scholar] [CrossRef]
- Yabuki, Y.; Takahata, I.; Matsuo, K.; Owada, Y.; Fukunaga, K. Ramelteon Improves Post-traumatic Stress Disorder-Like Behaviors Exhibited by Fatty Acid-Binding Protein 3 Null Mice. Mol. Neurobiol. 2018, 55, 3577–3591. [Google Scholar] [CrossRef]
- Riss, J.; Cloyd, J.; Gates, J.; Collins, S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008, 118, 69–86. [Google Scholar] [CrossRef]
- Parsonage, M.J.; Norris, J.W. Use of diazepam in the treatment of severe convulsive status epilepticus. Br. Med. J. 1967, 3, 85–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beagle, A.J.; Darwish, S.M.; Ranasinghe, K.G.; La, A.L.; Karageorgiou, E.; Vossel, K.A. Relative Incidence of Seizures and Myoclonus in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Dementia. J. Alzheimers Dis. 2017, 60, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Horiuchi, E.; Kanazawa, I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci. Res. 2001, 41, 397–399. [Google Scholar] [CrossRef]
- Beniyama, Y.; Matsuno, K.; Miyachi, H. Structure-guided design, synthesis and in vitro evaluation of a series of pyrazole-based fatty acid binding protein (FABP) 3 ligands. Bioorg. Med. Chem. Lett. 2013, 23, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Yamada, J.; Nikaido, Y.; Shi, X.; Kaneko, S.; Hirose, S.; Ueno, S. Properties of a novel GABAA receptor γ2 subunit mutation associated with seizures. J. Pharmacol. Sci. 2013, 121, 84–87. [Google Scholar] [CrossRef]
- Yabuki, Y.; Matsuo, K.; Izumi, H.; Haga, H.; Yoshida, T.; Wakamori, M.; Kakei, A.; Sakimura, K.; Fukuda, T.; Fukunaga, K. Pharmacological properties of SAK3, a novel T-type voltage-gated Ca2+ channel enhancer. Neuropharmacology 2017, 117, 1–13. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of Seizure Activity by Electrical Stimulation. II. Motor Seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Lowenstein, D.H.; Alldredge, B.K. Status Epilepticus. N. Engl. J. Med. 1998, 338, 970–976. [Google Scholar] [CrossRef]
- Shibley, H.; Smith, B.N. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002, 49, 109–120. [Google Scholar] [CrossRef]

| Group | GTCs Onset Time (Min) | Mortality (%) | Seizure Scale |
|---|---|---|---|
| Vehicle | 1.7 ± 0.53 | 50 (5/10) | 4 ± 0 |
| DZP (5 mg/kg, i.p.) | 3.3 ± 0.70 ** | 0 (0/10) | 3.7 ± 0.15 |
| MF1 (0.1 mg/kg, p.o.) | 0.92 ± 0.15 | 40 (4/10) | 3.8 ± 0.20 |
| MF1 (0.3 mg/kg, p.o.) | 1.2 ± 0.22 | 50 (5/10) | 3.8 ± 0.20 |
| MF1 (1.0 mg/kg, p.o.) | 1.2 ± 0.28 | 70 (7/10) | 3.8 ± 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabuki, Y.; Liu, J.; Kawahata, I.; Izumi, H.; Shinoda, Y.; Koga, K.; Ueno, S.; Shioda, N.; Fukunaga, K. Anti-Epileptic Effects of FABP3 Ligand MF1 through the Benzodiazepine Recognition Site of the GABAA Receptor. Int. J. Mol. Sci. 2020, 21, 5525. https://doi.org/10.3390/ijms21155525
Yabuki Y, Liu J, Kawahata I, Izumi H, Shinoda Y, Koga K, Ueno S, Shioda N, Fukunaga K. Anti-Epileptic Effects of FABP3 Ligand MF1 through the Benzodiazepine Recognition Site of the GABAA Receptor. International Journal of Molecular Sciences. 2020; 21(15):5525. https://doi.org/10.3390/ijms21155525
Chicago/Turabian StyleYabuki, Yasushi, Jiaqi Liu, Ichiro Kawahata, Hisanao Izumi, Yasuharu Shinoda, Kohei Koga, Shinya Ueno, Norifumi Shioda, and Kohji Fukunaga. 2020. "Anti-Epileptic Effects of FABP3 Ligand MF1 through the Benzodiazepine Recognition Site of the GABAA Receptor" International Journal of Molecular Sciences 21, no. 15: 5525. https://doi.org/10.3390/ijms21155525
APA StyleYabuki, Y., Liu, J., Kawahata, I., Izumi, H., Shinoda, Y., Koga, K., Ueno, S., Shioda, N., & Fukunaga, K. (2020). Anti-Epileptic Effects of FABP3 Ligand MF1 through the Benzodiazepine Recognition Site of the GABAA Receptor. International Journal of Molecular Sciences, 21(15), 5525. https://doi.org/10.3390/ijms21155525







