Mechanosensitivity Is a Characteristic Feature of Cultured Suburothelial Interstitial Cells of the Human Bladder
Abstract
1. Introduction
2. Results
2.1. Spontaneous Ca2+ Activity in Cultured Human suICs
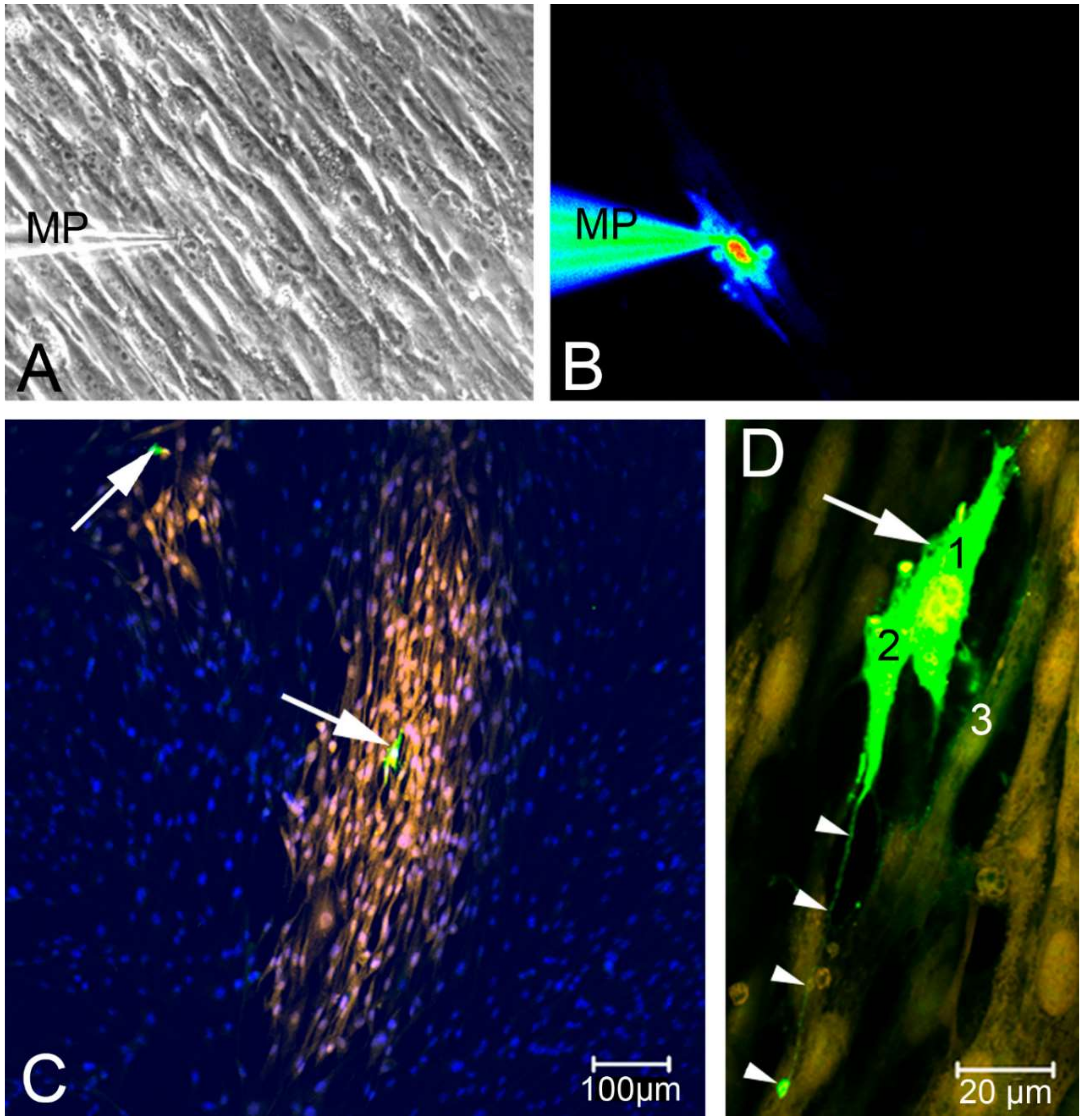
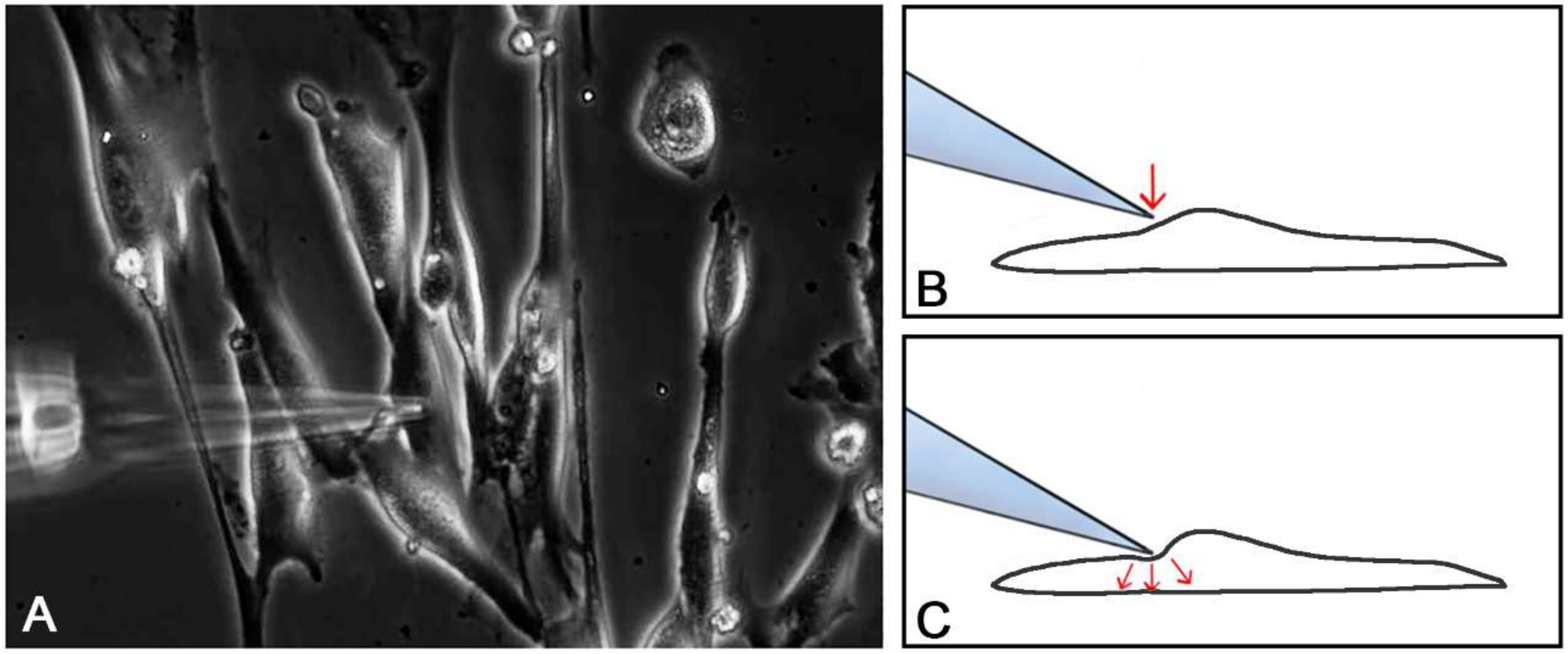
2.2. Single Cell Mechanical Stimulation by Glass Micropipette
2.3. Intercellular Propagation of the Ca2+ Signal
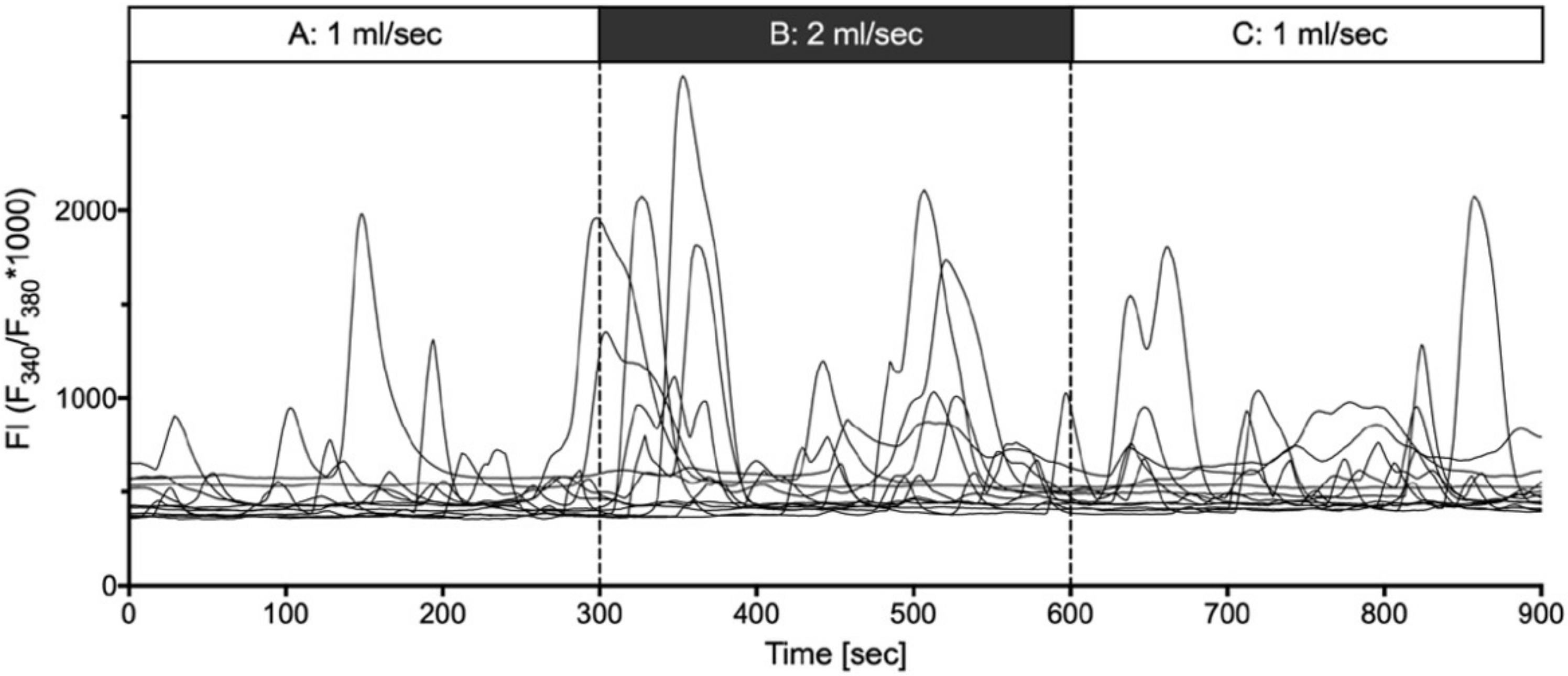
2.4. Shear Stress Evoked Calcium Transients
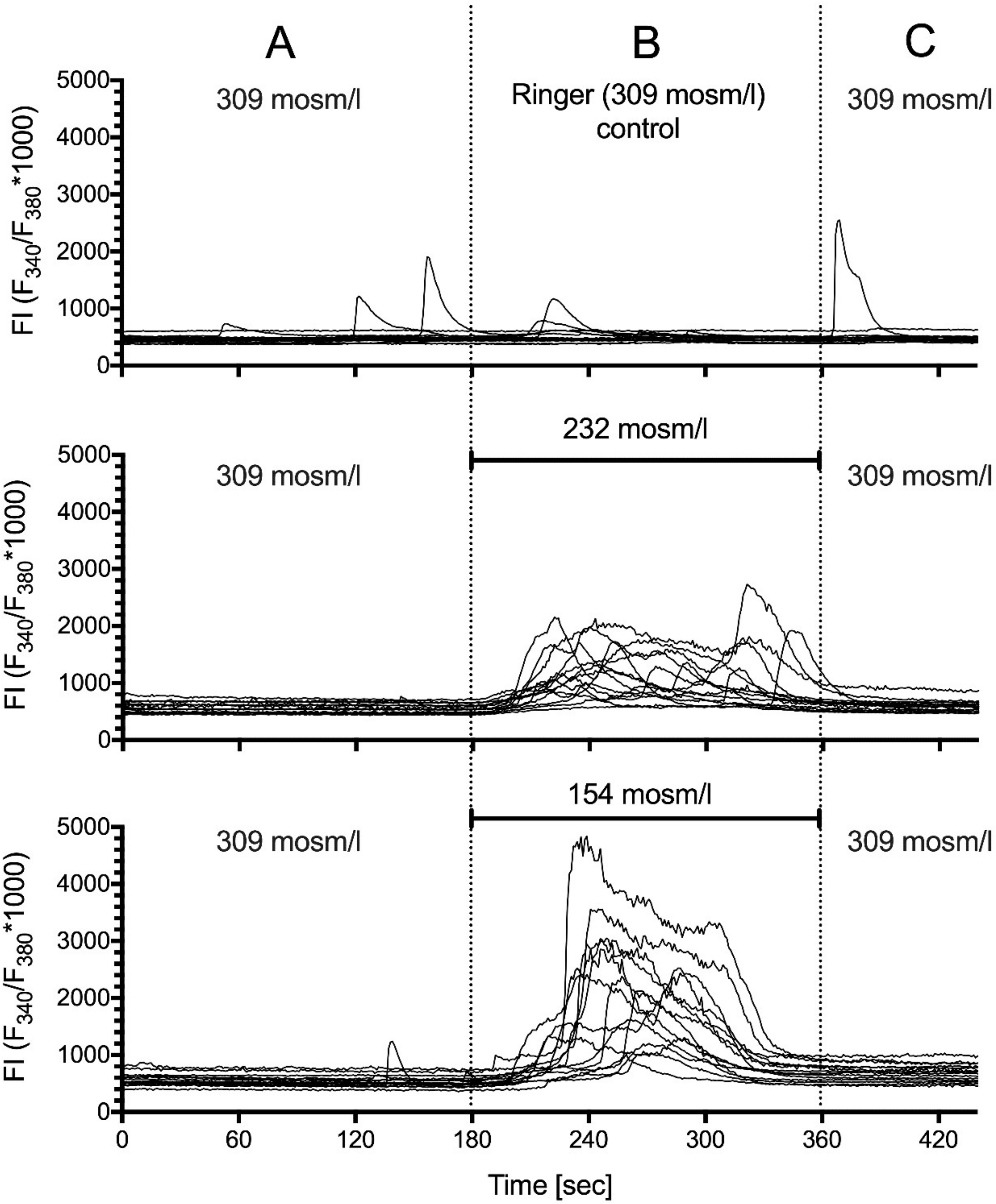
2.5. Hypotonic Stimulation
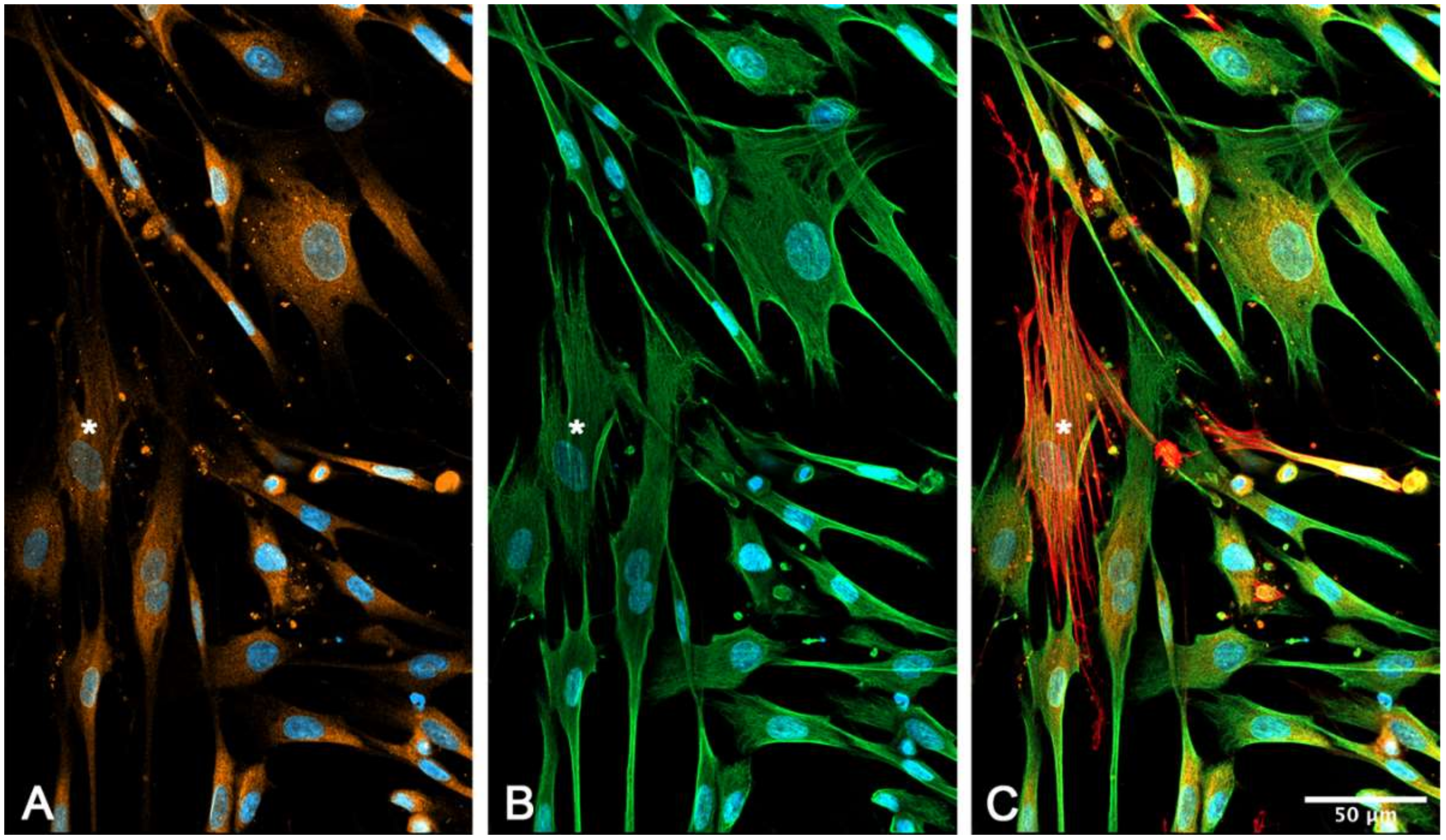
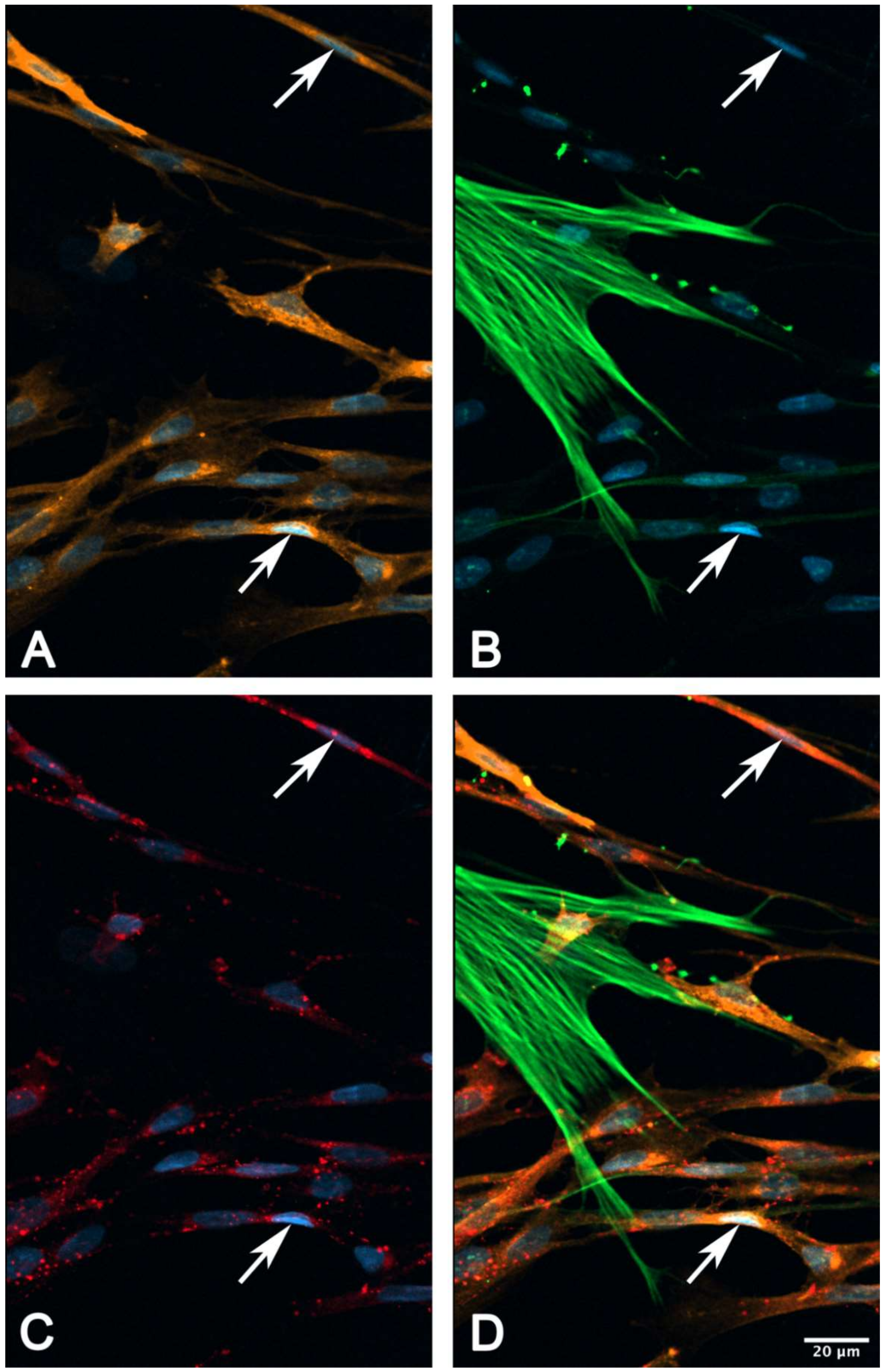
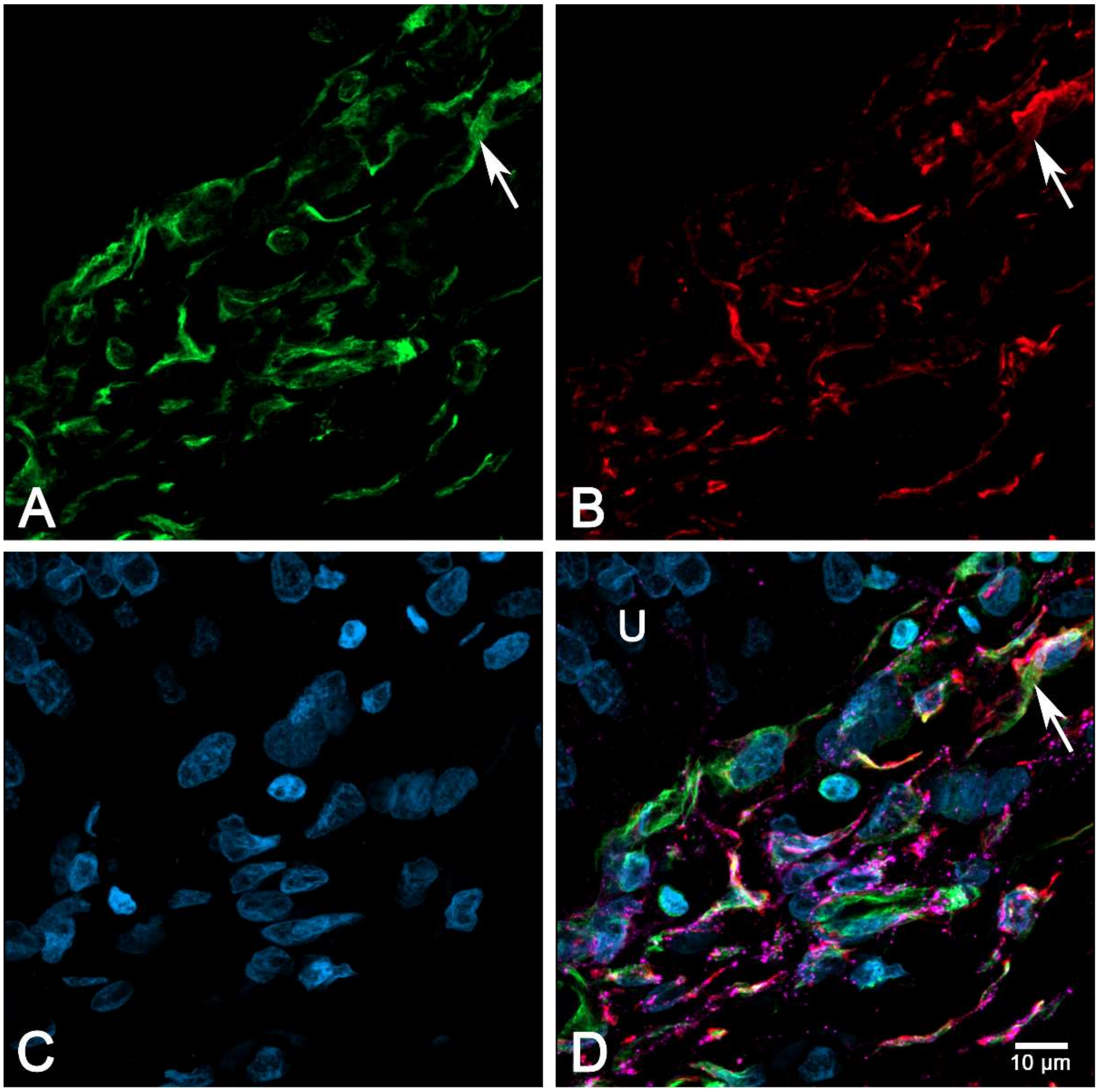
2.6. Immunocytochemical Characterization of Cultured suICs
3. Discussion
- (ii) Mechanical stimulation of human suICs either by direct indentation of the plasma membrane, swelling of the cells by hypotonic Ringer solution or membrane deformation by sheer stress induced calcium transients. Interestingly, calcium transients were also elicited when releasing the sheer stress by reduction of the flow rate to normal flow. This indicates that the cells are also capable of sensing the changing of the pressure in addition to the force of the pressure.
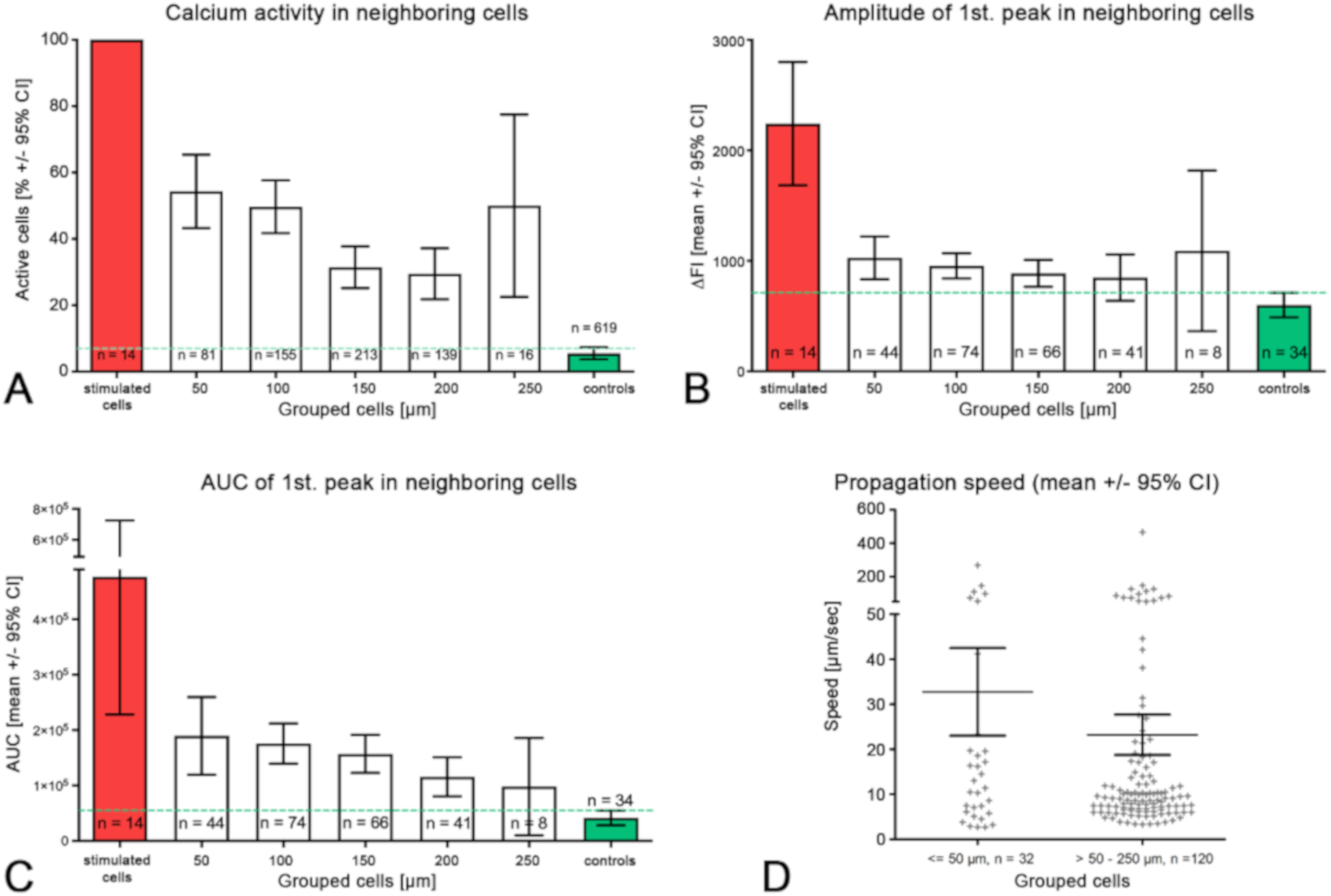
- (iii) Calcium signals propagated not only intracellularly but were transmitted to neighboring cells. This indicates that the suICs formed a functional syncytium, as previously shown [21,22]. Interestingly, we observed two populations of neighboring cells, with approximately 87% of the cells showing a delay of calcium activation after single cell stimulation, resulting in an intercellular signal propagation velocity of about 20 µm/s, while in 20 of 152 cells (13%), the delay was much shorter, with a calculated propagation speed of ≥ 50 µm/s. These cells were similarly found in close proximity to the stimulated cell (<50 µm radius), as well as within a distance of 50 to 250 µm (Figure 7D). We hypothesized that the latter cells were directly connected to the stimulated cell via long thin cell processes, thus resembling telocytes [36], which also have been proposed to be present in the upper lamina propria (ULP) of the human bladder [26,37]. This idea was directly supported by dye coupling experiments, regularly demonstrating cells with long cytoplasmic processes in cultured suICs (Figure 12), and our immunocytochemical experiments indicating the presence of typical type 1 telocytes (PDGFRa+/CALR+/aSMA−) and of PDGFRa+/CALR+/aSMA+ hybrid (type 2) telocytes (Figure 10, Figure 11, Figure S2). We found type 1 telocytes, which were characterized by very small nuclei and small cell bodies showing long thin bipolar cell protrusions spanning up to 200 µm. These cells were observed at a rate of 12.5% in early cell cultures and decreased to around 7% with increasing confluency. Since we used the cell cultures at ≥ 80% confluency (e.g., Figure 4), it is most probable that we did not directly stimulate these cells. However, the small fraction of typical telocytes would well correspond to the observed 13% of cells with short Ca2+ signal delay in up to 250 µm distance (Figure 7D), thus promoting a fast calcium wave distribution over the cellular syncytium. Interestingly, we found no Cx43-IR in typical myofibroblasts accounting for less than 1% of the cells. These very large cells, therefore, would not be considered to contribute to the propagation of the calcium signal.
4. Materials and Methods
4.1. Ethical Statement
4.2. Cell Cultures
4.3. Chemicals and Solutions
4.4. Calcium Imaging
4.5. Data Analysis and Statistics
4.6. Stimulation Experiments
4.6.1. Single Cell Mechanical Stimulation
4.6.2. Mechanical Stimulation by Shear Stress
4.6.3. Hypotonic Stimulation
4.7. Dye-Coupling Experiments
4.8. Confocal Imaging
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| aSMA | alpha-smooth muscle actin |
| BOO | bladder outlet obstruction |
| CALR | calreticulin |
| Cx43 | connexin 43 |
| DAPI | 4′,6-diamidin-2-phenylindol |
| FITC | fluorescein isothiocyanate |
| fps | frames per second |
| fura-2AM | fura-2-acetoxymethylester |
| IC/BPS | interstitial cystitis/bladder pain syndrome |
| OAB | overactive bladder |
| PDGFRa | platelet derived growth factor receptor-alpha |
| SD | standard deviation |
| SEM | standard error of the mean |
| suIC | suburothelial interstitial cells |
| TRP | transient receptor potential |
| VIM | vimentin |
References
- Dorschner, W.; Stolzenburg, J.U.; Neuhaus, J. Structure and function of the bladder neck. Adv. Anat. Embryol. Cell. Biol. 2001, 159, 1–113. [Google Scholar]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [PubMed]
- Birder, L.A.; Ruan, H.Z.; Chopra, B.; Xiang, Z.; Barrick, S.; Buffington, C.A.; Roppolo, J.R.; Ford, A.P.; de Groat, W.C.; Burnstock, G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. Renal. Physiol. 2004, 287, F1084–F1091. [Google Scholar] [CrossRef] [PubMed]
- Merrill, L.; Gonzalez, E.J.; Girard, B.M.; Vizzard, M.A. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 2016, 13, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; McCloskey, K.D. Lamina propria: The functional center of the bladder. Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef]
- Everaerts, W.; Gevaert, T.; Nilius, B.; De Ridder, D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol. Urodyn. 2008, 27, 264–273. [Google Scholar] [CrossRef]
- Steiner, C.; Gevaert, T.; Ganzer, R.; De Ridder, D.; Neuhaus, J. Comparative immunohistochemical characterization of interstitial cells in the urinary bladder of human, guinea pig and pig. Histochem. Cell Biol. 2018, 149, 491–501. [Google Scholar] [CrossRef]
- Gevaert, T.; Vanstreels, E.; Daelemans, D.; Franken, J.; Van Der Aa, F.; Roskams, T.; De Ridder, D. Identification of Different Phenotypes of Interstitial Cells in the Upper and Deep Lamina Propria of the Human Bladder Dome. J. Urol. 2014, 192, 1555–1563. [Google Scholar] [CrossRef]
- Gevaert, T.; Neuhaus, J.; Vanstreels, E.; Daelemans, D.; Everaerts, W.; Der Aa, F.V.; Timmermans, J.-P.; Roskams, T.; Steiner, C.; Pintelon, I.; et al. Comparative study of the organisation and phenotypes of bladder interstitial cells in human, mouse and rat. Cell Tissue Res. 2017, 370, 403–416. [Google Scholar] [CrossRef]
- Gevaert, T.; Ridder, D.D.; Vanstreels, E.; Daelemans, D.; Everaerts, W.; Aa, F.V.D.; Pintelon, I.; Timmermans, J.-P.; Roskams, T.; Steiner, C.; et al. The stem cell growth factor receptor KIT is not expressed on interstitial cells in bladder. J. Cell. Mol. Med. 2017, 21, 1206–1216. [Google Scholar] [CrossRef]
- Zhou, F.; Li, H.; Zhou, C.; Lv, H.; Ma, Y.; Wang, Y.; Song, B. Structural and functional changes in gap junctional intercellular communication in a rat model of overactive bladder syndrome induced by partial bladder outlet obstruction. Exp. Ther. Med. 2016, 11, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Negoro, H.; Kanematsu, A.; Yamamoto, S.; Kimura, Y.; Nagane, K.; Yamasaki, T.; Kanatani, I.; Ito, N.; Tabata, Y.; et al. Basic fibroblast growth factor causes urinary bladder overactivity through gap junction generation in the smooth muscle. Am. J. Physiol. Renal. Physiol. 2009, 297, F46–F54. [Google Scholar] [CrossRef]
- John, H.; Walch, M.; Lehmann, T.; Maake, C. Connexin45 expression in the human obstructed detrusor muscle. World J. Urol. 2009, 27, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, E.Y.; Hwang, T.K.; Kim, J.C. Therapeutic effects of connexin inhibitors on detrusor overactivity induced by bladder outlet obstruction in rats. Urology 2011, 78, 475.e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Kadekawa, K.; Machida, N.; Oshiro, Y.; Saito, S. Changes of bladder activity and connexin 43-derived gap junctions after partial bladder-outlet obstruction in rats. Int. Urol. Nephrol. 2009, 41, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Noguchi, M.; Matsuo, M.; Nomata, K.; Suematsu, T.; Kanetake, H. Decreased cellular membrane expression of gap junctional protein, connexin 43, in rat detrusor muscle with chronic partial bladder outlet obstruction. Urology 2005, 65, 1254–1258. [Google Scholar] [CrossRef]
- Imamura, M.; Sugino, Y.; Long, X.; Slivano, O.J.; Nishikawa, N.; Yoshimura, N.; Miano, J.M. Myocardin and microRNA-1 modulate bladder activity through connexin 43 expression during post-natal development. J. Cell. Physiol. 2013, 228, 1819–1826. [Google Scholar] [CrossRef]
- Roosen, A.; Datta, S.N.; Chowdhury, R.A.; Patel, P.M.; Kalsi, V.; Elneil, S.; Dasgupta, P.; Kessler, T.M.; Khan, S.; Panicker, J.; et al. Suburothelial Myofibroblasts in the Human Overactive Bladder and the Effect of Botulinum Neurotoxin Type A Treatment. Eur. Urol. 2009, 55, 1440–1448. [Google Scholar] [CrossRef]
- Neuhaus, J.; Pfeiffer, F.; Wolburg, H.; Horn, L.C.; Dorschner, W. Alterations in connexin expression in the bladder of patients with urge symptoms. BJU Int. 2005, 96, 670–676. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, M.; Chen, S.; Ji, Z.; Zheng, X. TGF-β1 and connexin-43 expression in neurogenic bladder from rats with sacral spinal cord injury. Neurourol. Urodyn. 2018, 37, 2502–2509. [Google Scholar] [CrossRef]
- Heinrich, M.; Oberbach, A.; Schlichting, N.; Stolzenburg, J.-U.; Neuhaus, J. Cytokine Effects on Gap Junction Communication and Connexin Expression in Human Bladder Smooth Muscle Cells and Suburothelial Myofibroblasts. PLoS ONE 2011, 6, e20792. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Heinrich, M.; Schwalenberg, T.; Stolzenburg, J.U. TGF-beta1 inhibits Cx43 expression and formation of functional syncytia in cultured smooth muscle cells from human detrusor. Eur. Urol. 2009, 55, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Makarenkova, H.P.; Shestopalov, V.I. The role of pannexin hemichannels in inflammation and regeneration. Front. Physiol. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Negoro, H.; Lutz, S.E.; Liou, L.S.; Kanematsu, A.; Ogawa, O.; Scemes, E.; Suadicani, S.O. Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model. Sci. Rep. 2013, 3, 2152. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 1995; pp. 1–244. [Google Scholar]
- Vannucchi, M.G.; Traini, C.; Guasti, D.; Del Popolo, G.; Faussone-Pellegrini, M.S. Telocytes subtypes in human urinary bladder. J. Cell. Mol. Med. 2014, 18, 2000–2008. [Google Scholar] [CrossRef]
- Kanai, A.; Andersson, K.E. Bladder afferent signaling: Recent findings. J. Urol. 2010, 183, 1288–1295. [Google Scholar] [CrossRef]
- Fry, C.H.; Sui, G.P.; Kanai, A.J.; Wu, C. The function of suburothelial myofibroblasts in the bladder. Neurourol. Urodyn. 2007, 26, 914–919. [Google Scholar] [CrossRef]
- Sui, G.P.; Wu, C.; Roosen, A.; Ikeda, Y.; Kanai, A.J.; Fry, C.H. Modulation of bladder myofibroblast activity: Implications for bladder function. Am. J. Physiol. Renal. Physiol. 2008, 295, 688–697. [Google Scholar] [CrossRef]
- Takezawa, K.; Kondo, M.; Nonomura, N.; Shimada, S. Urothelial ATP signaling: What is its role in bladder sensation. Neurourol. Urodyn. 2017, 36, 966–972. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef]
- Heppner, T.J.; Hennig, G.W.; Nelson, M.T.; Vizzard, M.A. Rhythmic Calcium Events in the Lamina Propria Network of the Urinary Bladder of Rat Pups. Front. Syst. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Scigalla, F.P.; Speroni di Fenizio, P.; Zhang, Z.G.; Stolzenburg, J.U.; Neuhaus, J. ATP enhances spontaneous calcium activity in cultured suburothelial myofibroblasts of the human bladder. PLoS ONE 2011, 6, e25769. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Fry, C.H.; Hayashi, F.; Stolz, D.B.; Griffiths, D.; Kanai, A.J. The Role of Gap Junctions in Spontaneous Activity of the Rat Bladder. Am. J. Physiol. Renal. Physiol. 2007, 293, F1018–F1025. [Google Scholar] [CrossRef]
- Sui, G.P.; Wu, C.; Fry, C.H. Electrical characteristics of suburothelial cells isolated from the human bladder. J. Urol. 2004, 171, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Faussone Pellegrini, M.S.; Popescu, L.M. Telocytes. Biomol. Concepts. 2011, 2, 481–489. [Google Scholar] [CrossRef]
- Gevaert, T.; De Vos, R.; Van Der Aa, F.; Joniau, S.; van den Oord, J.; Roskams, T.; De Ridder, D. Identification of telocytes in the upper lamina propria of the human urinary tract. J. Cell. Mol. Med. 2012, 16, 2085–2093. [Google Scholar] [CrossRef]
- Neuhaus, J.; Heinrich, M.; Schlichting, N.; Oberbach, A.; Fitzl, G.; Schwalenberg, T.; Horn, L.C.; Stolzenburg, J.U. Structure and function of suburothelial myofibroblasts in the human urinary bladder under normal and pathological conditions. Urol. A 2007, 46, 1197–1202. [Google Scholar] [CrossRef]
- Allbritton, N.L.; Meyer, T.; Stryer, L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 1992, 258, 1812–1815. [Google Scholar] [CrossRef]
- Sáez, J.C.; Connor, J.A.; Spray, D.C.; Bennett, M.V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc. Natl. Acad. Sci. USA 1989, 86, 2708–2712. [Google Scholar] [CrossRef]
- Sotkis, A.; Wang, X.G.; Yasumura, T.; Peracchia, L.L.; Persechini, A.; Rash, J.E.; Peracchia, C. Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell. Commun. Adhes. 2001, 8, 277–281. [Google Scholar] [CrossRef]
- Peracchia, C. Chemical gating of gap junction channels. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1662, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Kanai, A.; Roppolo, J.; Ikeda, Y.; Zabbarova, I.; Tai, C.; Birder, L.; Griffiths, D.; de Groat, W.; Fry, C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am. J. Physiol. Renal. Physiol. 2007, 292, F1065–F1072. [Google Scholar] [CrossRef]
- Oguri, G.; Nakajima, T.; Yamamoto, Y.; Takano, N.; Tanaka, T.; Kikuchi, H.; Morita, T.; Nakamura, F.; Yamasoba, T.; Komuro, I. Effects of methylglyoxal on human cardiac fibroblast: Roles of transient receptor potential ankyrin 1 (TRPA1) channels. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1339–J1352. [Google Scholar] [CrossRef]
- Hill-Eubanks, D.C.; Werner, M.E.; Heppner, T.J.; Nelson, M.T. Calcium signaling in smooth muscle. Cold Spring Harb. Perspect Biol. 2011, 3, a004549. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Bibby, K.J.; McCulloch, C.A. Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J. Cell. Physiol. 1994, 161, 187–200. [Google Scholar] [CrossRef]
- Williams, A.J.; Thomas, N.L.; George, C.H. The ryanodine receptor: Advances in structure and organization. Curr. Opin. Physiol. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2190–H2210. [Google Scholar] [CrossRef]
- Cheng, Y.; Mansfield, K.J.; Sandow, S.L.; Sadananda, P.; Burcher, E.; Moore, K.H. Porcine Bladder Urothelial, Myofibroblast, and Detrusor Muscle Cells: Characterization and ATP Release. Front. Pharmacol. 2011, 2, 27. [Google Scholar] [CrossRef]
- WMA. World medical association declaration of helsinki Ethical Principles for Medical Research Involving Human Subjects. Bull. World Health Organ. 2008, 79, 373. [Google Scholar]
- Klöckner, U.; Isenberg, G. Action potential and net membrane currents of isolated smooth muscle cells(urinary bladder of the GP). Pflügers Arch. 1985, 405, 329–339. [Google Scholar] [CrossRef]
- Neuhaus, J.; Wolburg, H.; Hermsdorf, T.; Stolzenburg, J.U.; Dorschner, W. Detrusor smooth muscle cells of the guinea-pig are functionally coupled via gap junctions in situ and in cell culture. Cell. Tissue Res. 2002, 309, 301–311. [Google Scholar] [PubMed]
- Rasband, W.S.; ImageJ. US National Institutes of Health, Bethesda, Maryland, USA, 1997–2016. Available online: http://rsb.info.nih.gov/ij/ (accessed on 15 June 2020).

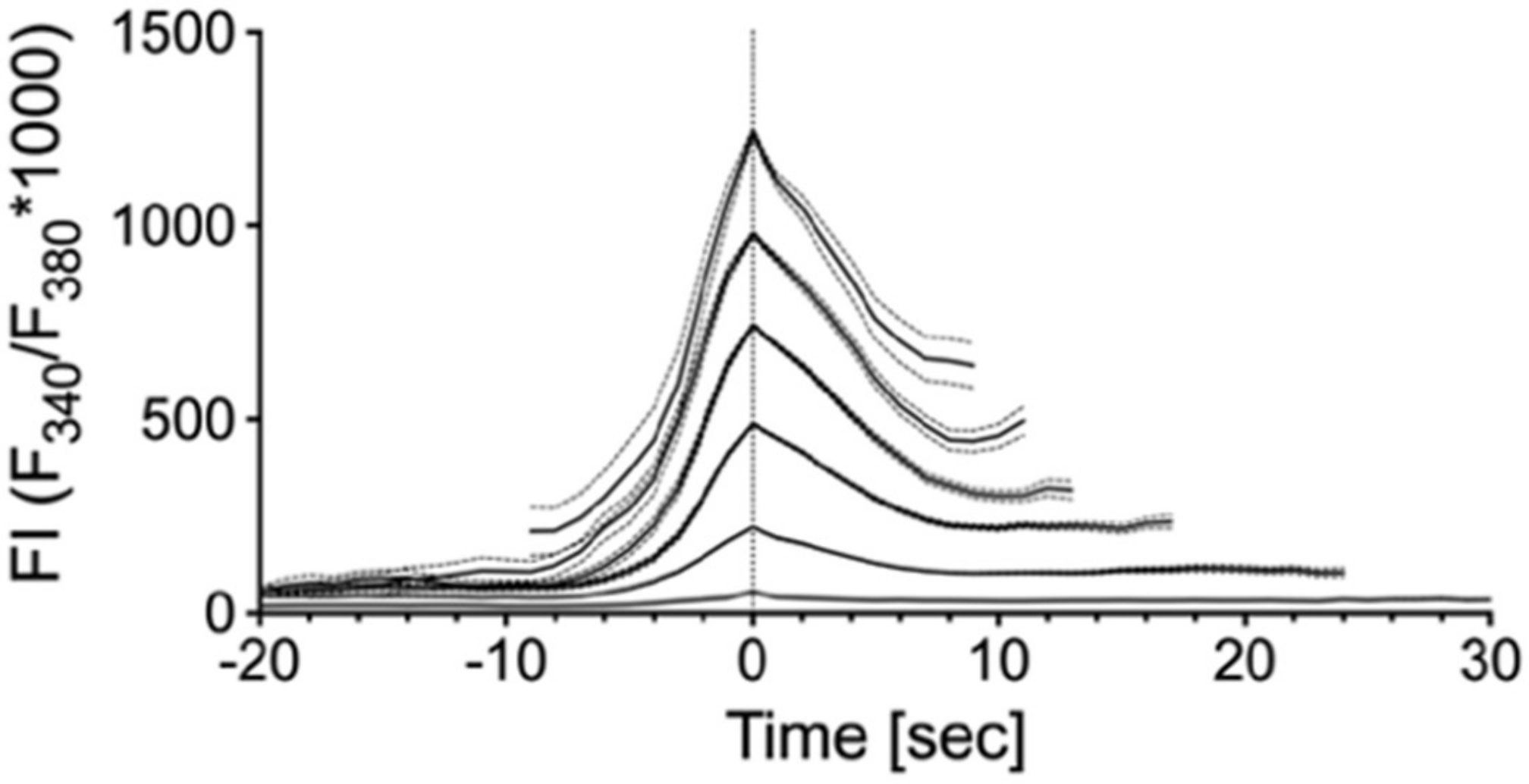
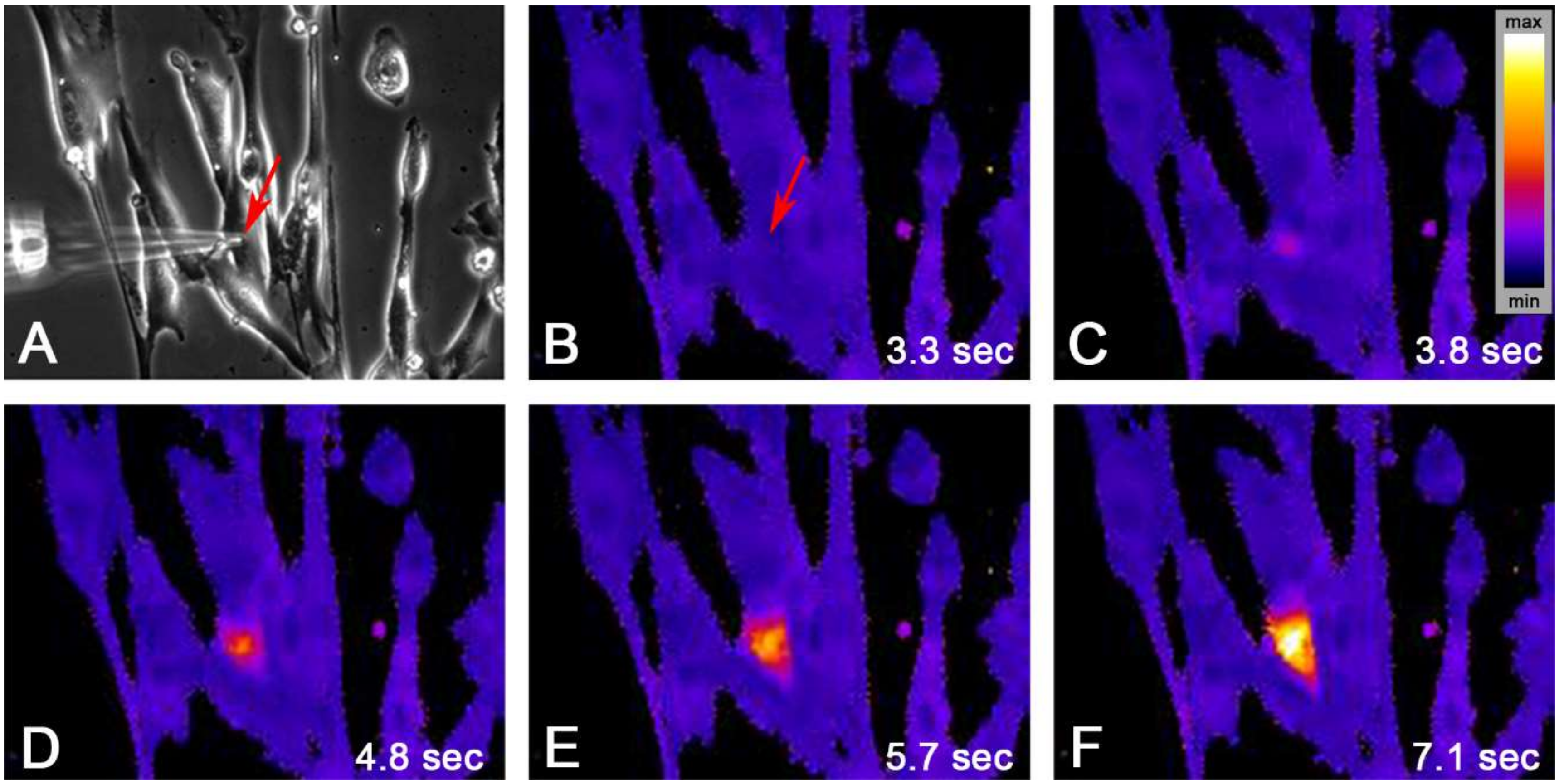

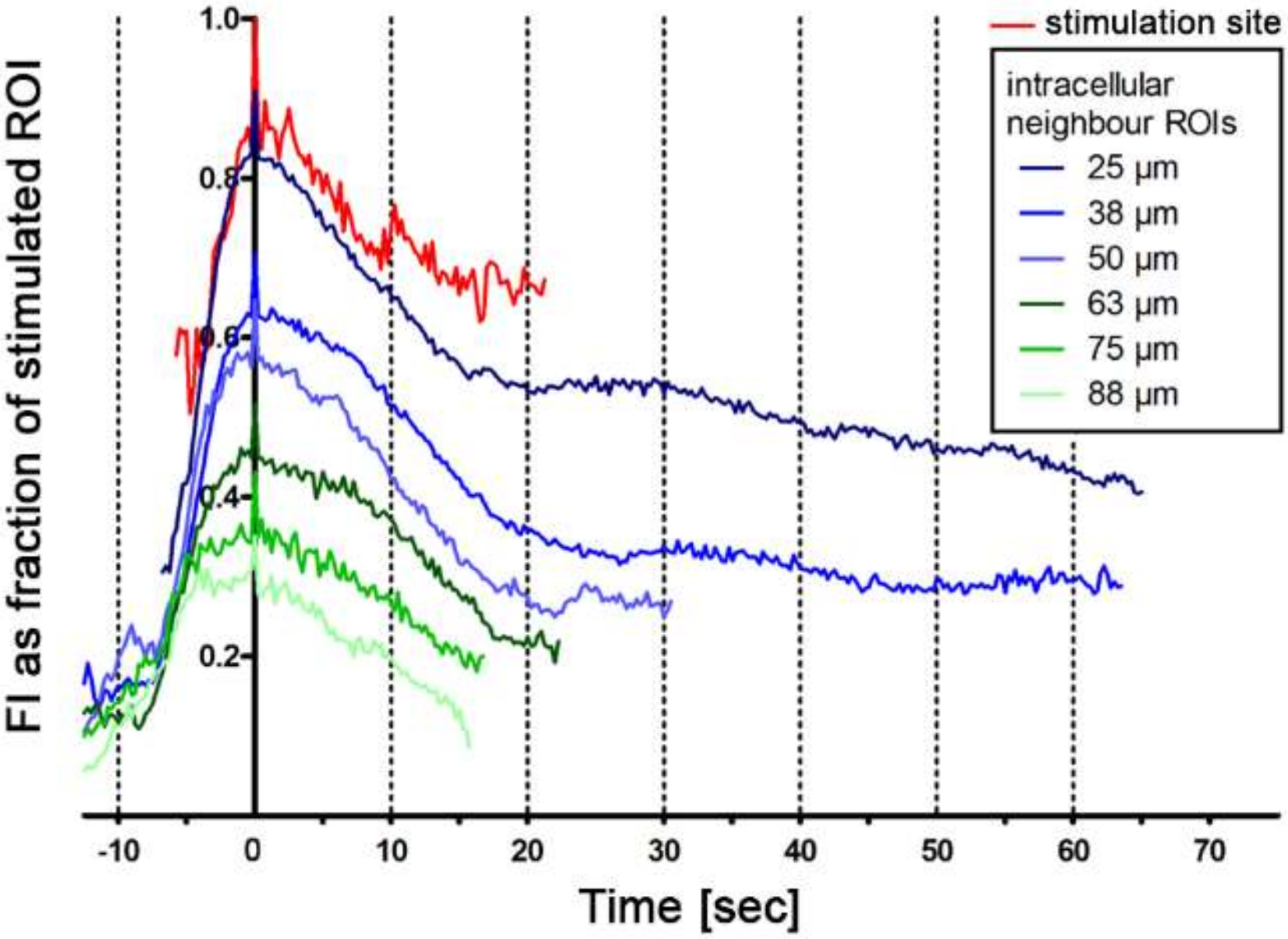
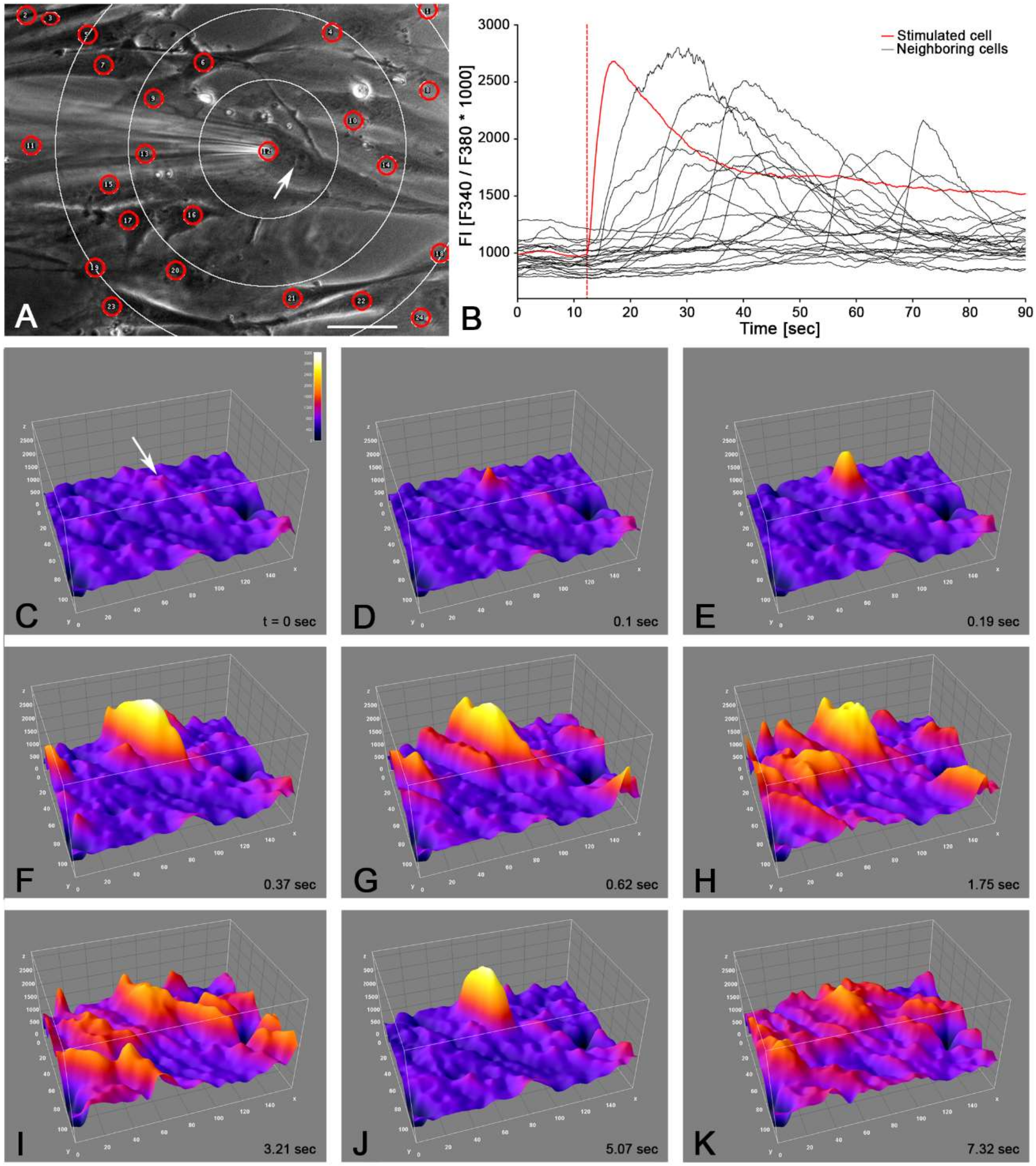
| Group | Mean | SD | N |
|---|---|---|---|
| 1 | 56.5466 | 27.46916 | 1913 |
| 2 | 221.3514 | 70.75953 | 582 |
| 3 | 488.9613 | 72.17626 | 245 |
| 4 | 743.6639 | 69.71185 | 134 |
| 5 | 978.3084 | 66.73132 | 70 |
| 6 | 1239.981 | 66.15836 | 29 |
| Cell Group | 0 | 50 | 100 | 150 | 200 | 250 | Control |
|---|---|---|---|---|---|---|---|
| Distance (µm) | Stimulated | <= 50 | >50 ≤ 100 | >100 ≤150 | >150 ≤ 200 | >200≤ 250 | NA |
| Number of cells | 14 | 81 | 155 | 213 | 139 | 16 | 619 |
| Active cells (n) | 14 | 44 | 77 | 67 | 41 | 8 | 34 |
| Mean (%) | 100 | 54.3 | 49.7 | 31.5 | 29.5 | 50 | 5.5 |
| 95% CI (%) | 43.2–65.4 | 40.3–50.2 | 25.2–37.7 | 21.8–37.2 | 12.9–51.6 | 3.7–7.3 | |
| Peak ampl. mean | 2242 | 1025 | 954 | 887 | 848 | 1090 | 599 |
| 95% CI | 1683–2800 | 831–1219 | 840–1068 | 766–1008 | 639–1058 | 363–1817 | 489–711 |
| Peak AUC (au) | 476737 | 189641 | 175652 | 157005 | 115,647 | 97981 | 41535 |
| 95% CI | 228,359–725,115 | 119,516–259,766 | 139,339–211,966 | 122,889–191,121 | 80,430–150,865 | 9981–185,982 | 28,387–54,684 |
| Peak duration (s) | 40.9 | 36.8 | 36.8 | 33.4 | 28.1 | 39.3 | 28.3 |
| 95% CI | 29.4–52.6 | 32.8–40.9 | 34.1–39.6 | 30.5–36.3 | 24.6–31.7 | 19.4–39.2 | 23.4–33.2 |
| Peak delay (s) | NA | 6.534 | 11.65 | 18.34 | 18.1 | 15.68 | NA |
| 95% CI | 4.1–8.9 | 9.4–13.9 | 15.4–21.3 | 12.9–23.3 | 10.1–21.3 |
| Peak | Section A | Section B | Section C | p–Value |
|---|---|---|---|---|
| Frequency (min−1) | 0.62 (522) | 0.70 (554) | 0.75 (559) | A/B: <0.0001 § |
| [0.59–0.64] | [0.68–0.73] | [0.72–0.78] | B/C: 0.0039 § | |
| Amplitude (FI) | 269.4 (1345) | 344.7 (1628) | 207.6 (1757) | A/B: <0.0001 $ |
| [245.6–293.19] | [319.4–370.0] | [192.1–223.2] | B/C: <0.0001 $ | |
| Duration (s) | 34.26 (1345) | 39.43 (1628) | 35.74 (1757) | A/B: <0.0001 $ |
| [32.99–35.54] | [38.03–40.83] | [34.59–36.90] | B/C: <0.0001 $ | |
| AUC | 5789 (1345) | 8860 (1628) | 4602 (1757) | A/B: <0.0001 § |
| [5149–6430] | [8060–9660] | [4172–5032] | B/C: <0.0001 § |
| Osmotic Conc. | 309 | 232 | 154 | p–Value |
|---|---|---|---|---|
| (mOsm/L) | Control | Hypo25 | Hypo50 | |
| frequency (min–1) | 0.02 (44) | 0.45 (36) | 0.35(58) | Hypo25/Hypo50 |
| [0.0–0.06] | [0.39–0.49] | [0.34–0.36] | <0.0001 $ | |
| amplitude (FI) | 32.63 (1) | 733.2 (47) | 2147 (59) | Hypo25/Hypo50 |
| [NA] | [579.8–886.7] | [1891–2403] | <0.0001 $ | |
| duration (s) | 21 (1) | 93 (47) | 114.6 (59) | Hypo25/Hypo50 |
| [NA] | [80.19–105.8] | [109.4–119.8] | 0.0021 $ | |
| AUC | 419.6 (1) | 37,707 (47) | 119,702 (59) | Hypo25/Hypo50 |
| [NA] | [27,896–47,517] | [102,882–136,421] | <0.0001 $ |
| Target | Host Isotype | Class | Conjugate | Dilution | Product Number | Source |
|---|---|---|---|---|---|---|
| vimentin (VIM) | mouse gG1 | Monoclonal | na | 1:200 | V6389 | a |
| alpha-smooth muscle actin (aSMA) | mouse IgG2a | Monoclonal | na | 1:1000 | A2547 | a |
| platelet-derived growth factor receptor-alpha (PDGFRa) | goat | Polyclonal | na | 1:100 | AF-307-NA | b |
| calreticulin (CALR) | rabbit | Polyclonal | na | 1:600 | ab2907 | c |
| connexin 43 (Cx43) | rabbit | Polyclonal | na | 1:500 | C6219 | a |
| mouse IgG1 | goatIgG | Polyclonal | Alexa Fluor® 488 | 1:500 | A21121 | d |
| mouse IgG2a | goat IgG | Polyclonal | Alexa Fluor® 633 | 1:500 | A21136 | d |
| rabbit IgG | goat IgG (H+L) | polyclonal | Alexa Fluor® 555 | 1:500 | A21121 | d |
| mouse IgG | donkey IgG (H+L) | monoclonal | Alexa Fluor® 488 | 1:500 | A21202 | d |
| goat IgG | donkey IgG | polyclonal | Alexa Fluor® 555 | 1:500 | A41232 | d |
| rabbit IgG | donkey IgG | polyclonal | Dylight® 649 | 1:500 | 711-495-152 | e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuhaus, J.; Gonsior, A.; Cheng, S.; Stolzenburg, J.-U.; Berger, F.P. Mechanosensitivity Is a Characteristic Feature of Cultured Suburothelial Interstitial Cells of the Human Bladder. Int. J. Mol. Sci. 2020, 21, 5474. https://doi.org/10.3390/ijms21155474
Neuhaus J, Gonsior A, Cheng S, Stolzenburg J-U, Berger FP. Mechanosensitivity Is a Characteristic Feature of Cultured Suburothelial Interstitial Cells of the Human Bladder. International Journal of Molecular Sciences. 2020; 21(15):5474. https://doi.org/10.3390/ijms21155474
Chicago/Turabian StyleNeuhaus, Jochen, Andreas Gonsior, Sheng Cheng, Jens-Uwe Stolzenburg, and Frank Peter Berger. 2020. "Mechanosensitivity Is a Characteristic Feature of Cultured Suburothelial Interstitial Cells of the Human Bladder" International Journal of Molecular Sciences 21, no. 15: 5474. https://doi.org/10.3390/ijms21155474
APA StyleNeuhaus, J., Gonsior, A., Cheng, S., Stolzenburg, J.-U., & Berger, F. P. (2020). Mechanosensitivity Is a Characteristic Feature of Cultured Suburothelial Interstitial Cells of the Human Bladder. International Journal of Molecular Sciences, 21(15), 5474. https://doi.org/10.3390/ijms21155474






