Proteinaceous Transformers: Structural and Functional Variability of Human sHsps
Abstract
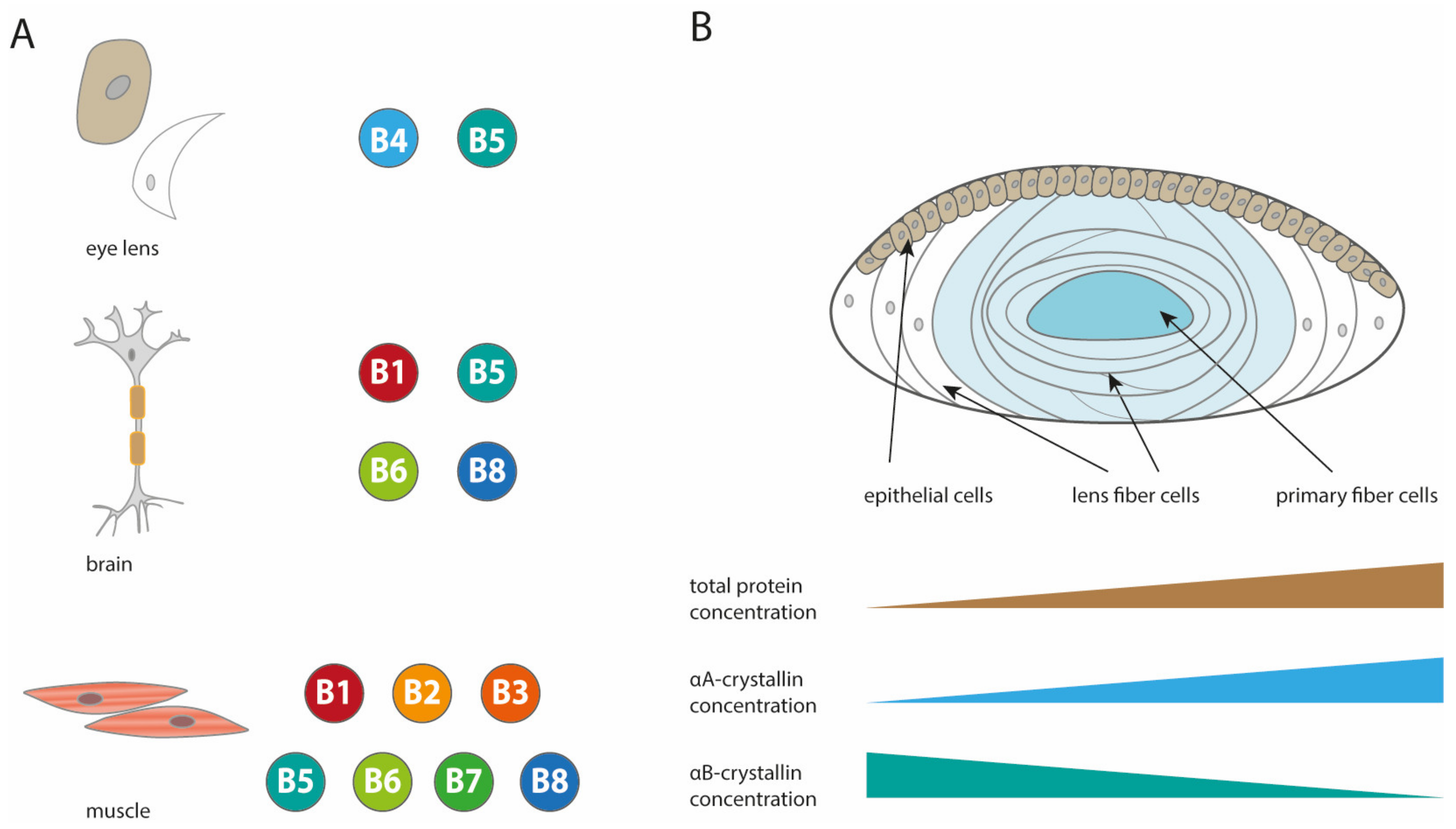
1. Introduction
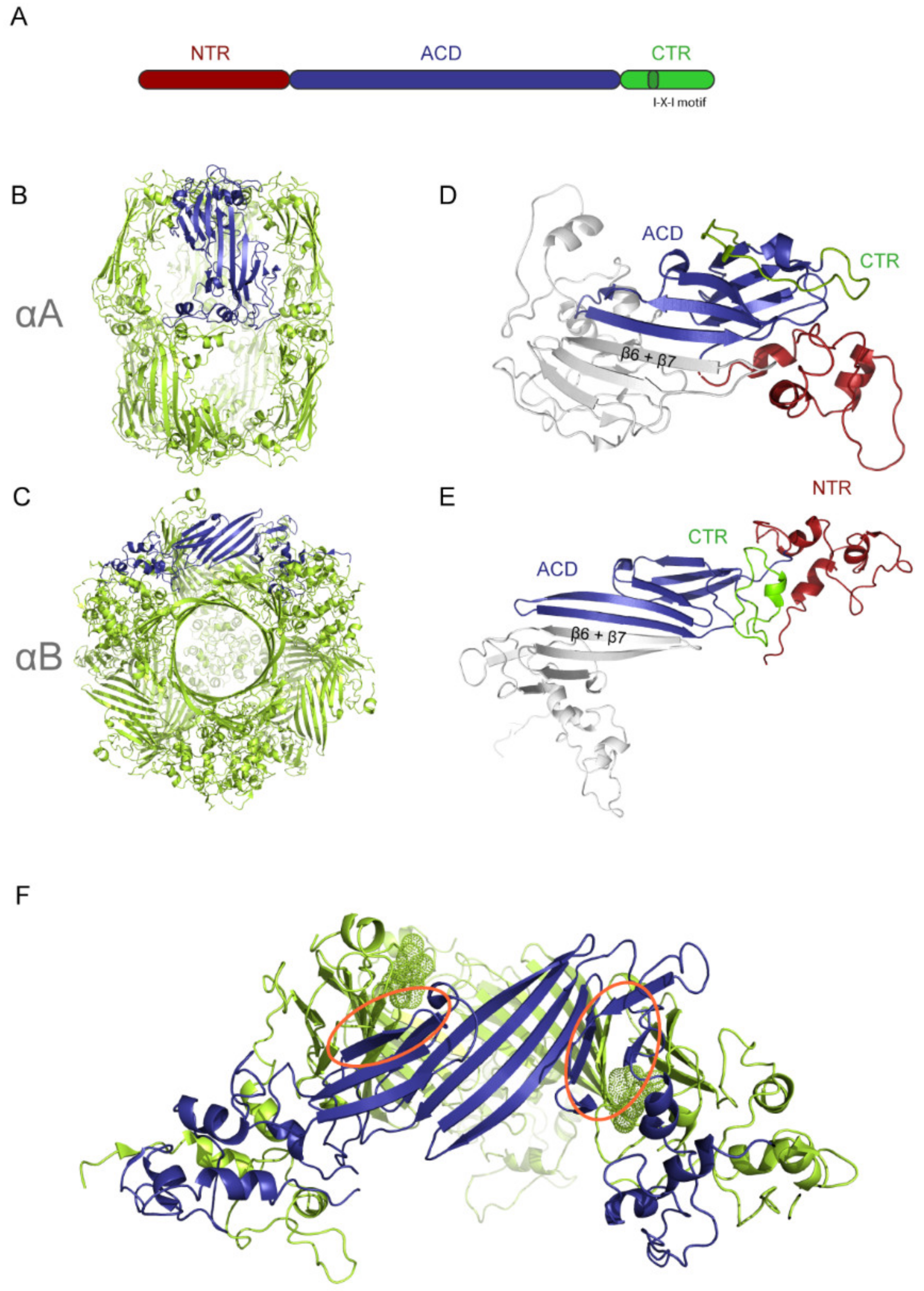
2. The Structure of Human sHsps
2.1. Structural Comparison of αA and αB
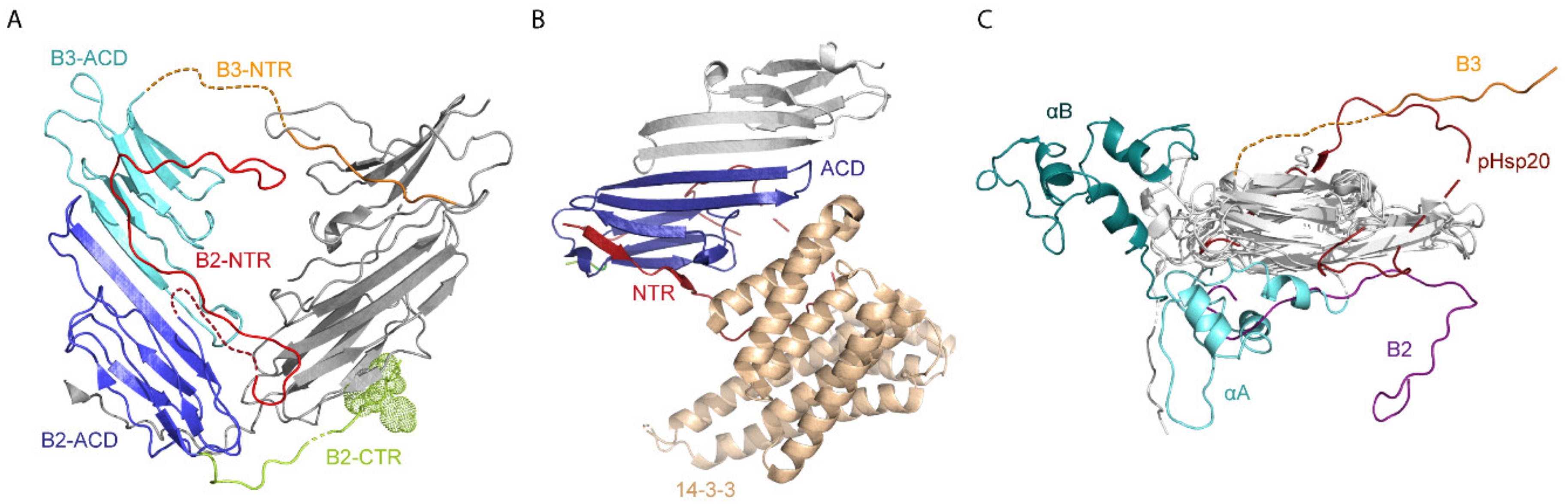
2.2. Structures of Other Human sHsps
3. Chaperone Function of Human sHsps
4. Conclusions
- sHsps are an integral component of the chaperone network and help to sustain the solubility and functionality of the proteome upon stress
- Under physiological conditions they are involved in a number of regulatory processes within the cell
- They are implicated in a variety of diseases but are usually not the causative component
- Their function is closely correlated to their structural variability
- They are commonly organized in three sequence parts, a conserved ACD flanked by divergent NTRs and CTRs
- Most sHsps form dynamic ensembles of oligomers with a variable number of subunits; some sHsps even form hetero-oligomeric species
- The assembly processes are controlled by the three sequence parts which form contacts in a hierarchical manner
- The conformational flexibility of the NTR, together with a set of binding groves on the ACD, are most likely the key to the variable oligomeric assemblies
- Changes in the composition of the dynamic ensembles of oligomers are linked to their chaperone activity and the recognition of substrates
- Changes in dynamics and composition of the ensembles lead also to modulated substrate specificities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vabulas, R.M.; Raychaudhuri, S.; Hayer-Hartl, M.; Hartl, F.U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010, 2, a004390. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Vierling, E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Ruger-Herreros, C.; Bukau, B. Cellular Functions and Mechanisms of Action of Small Heat Shock Proteins. Annu. Rev. Microbiol. 2019, 73, 89–110. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef]
- Hoffmann, J.H.; Linke, K.; Graf, P.C.; Lilie, H.; Jakob, U. Identification of a redox-regulated chaperone network. EMBO J. 2004, 23, 160–168. [Google Scholar] [CrossRef]
- Mattoo, R.U.; Goloubinoff, P. Molecular chaperones are nanomachines that catalytically unfold misfolded and alternatively folded proteins. Cell. Mol. Life Sci. 2014, 71, 3311–3325. [Google Scholar] [CrossRef]
- Zwirowski, S.; Klosowska, A.; Obuchowski, I.; Nillegoda, N.B.; Pirog, A.; Zietkiewicz, S.; Bukau, B.; Mogk, A.; Liberek, K. Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding. EMBO J. 2017, 36, 783–796. [Google Scholar] [CrossRef]
- Allen, S.P.; Polazzi, J.O.; Gierse, J.K.; Easton, A.M. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J. Bacteriol. 1992, 174, 6938–6947. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta 2014, 1854, 291–319. [Google Scholar] [CrossRef]
- Mogk, A.; Bukau, B.; Kampinga, H.H. Cellular Handling of Protein Aggregates by Disaggregation Machines. Mol. Cell 2018, 69, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Miess, A.; Stromer, T.; Walter, S.; Buchner, J. Disassembling protein aggregates in the yeast cytosol. The cooperation of Hsp26 with Ssa1 and Hsp104. J. Biol. Chem. 2005, 280, 23861–23868. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Deuerling, E.; Vorderwulbecke, S.; Vierling, E.; Bukau, B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol. Microbiol. 2003, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Zietkiewicz, S.; Liberek, K. Distinct activities of Escherichia coli small heat shock proteins IbpA and IbpB promote efficient protein disaggregation. J. Mol. Biol. 2009, 386, 178–189. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.W.; Caspers, G.J.; Leunissen, J.A. Genealogy of the alpha-crystallin--small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef]
- Treweek, T.M.; Meehan, S.; Ecroyd, H.; Carver, J.A. Small heat-shock proteins: Important players in regulating cellular proteostasis. Cell. Mol. Life Sci. 2015, 72, 429–451. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef]
- De Jong, W.W.; Leunissen, J.A.; Voorter, C.E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol. Biol. Evol. 1993, 10, 103–126. [Google Scholar]
- Kriehuber, T.; Rattei, T.; Weinmaier, T.; Bepperling, A.; Haslbeck, M.; Buchner, J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010, 24, 3633–3642. [Google Scholar] [CrossRef]
- Kappe, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.M.; De Jong, W.W. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones 2003, 8, 53–61. [Google Scholar] [CrossRef]
- Kappe, G.; Verschuure, P.; Philipsen, R.L.A.; Staalduinen, A.A.; Van de Boogaart, P.; Boelens, W.C.; De Jong, W.W. Characterization of two novel human small heat shock proteins: Protein kinase-related HspB8 and testis-specific HspB9. Biochim. Biophys. Acta 2001, 1520, 1–6. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef]
- Slingsby, C.; Wistow, G.J.; Clark, A.R. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013, 22, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Garrido, C. HSPBs: Small proteins with big implications in human disease. Int. J. Biochem. Cell Biol. 2012, 44, 1706–1710. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Riedl, M.; Peters, C.; Weinkauf, S.; Haslbeck, M.; Buchner, J. Regulation of small heat-shock proteins by hetero-oligomer formation. J. Biol. Chem. 2020, 295, 158–169. [Google Scholar] [CrossRef]
- Bukach, O.V.; Glukhova, A.E.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim. Biophys. Acta 2009, 1794, 486–495. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 2012, 17, 157–169. [Google Scholar] [CrossRef]
- Kato, K.; Hasegawa, K.; Goto, S.; Inaguma, Y. Dissociation as a result of phosphorylation of an aggregated form of the small stress protein, hsp27. J. Biol. Chem. 1994, 269, 11274–11278. [Google Scholar]
- Siezen, R.J.; Berger, H. The quaternary structure of bovine alpha-crystallin. Size and shape studies by sedimentation, small-angle X-ray scattering and quasi-elastic light scattering. Eur. J. Biochem. 1978, 91, 397–405. [Google Scholar] [CrossRef]
- Hanson, S.R.; Smith, D.L.; Smith, J.B. Deamidation and disulfide bonding in human lens gamma-crystallins. Exp. Eye Res. 1998, 67, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Peschek, J.; Buchner, J.; Weinkauf, S. Structure and function of alpha-crystallins: Traversing from in vitro to in vivo. Biochim. Biophys. Acta 2016, 1860, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Graw, J. Genetics of crystallins: Cataract and beyond. Exp. Eye Res. 2009, 88, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.K.; Xie, L.; David, L.L.; Robinson, M.L.; Taube, J.R.; Cui, W.; Reneker, L.W. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3589–3598. [Google Scholar] [CrossRef]
- Bloemendal, H.; De Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Sharma, K.K.; Santhoshkumar, P. Lens aging: Effects of crystallins. Biochim. Biophys. Acta 2009, 1790, 1095–1108. [Google Scholar] [CrossRef]
- Clark, A.R.; Lubsen, N.H.; Slingsby, C. sHSP in the eye lens: Crystallin mutations, cataract and proteostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1687–1697. [Google Scholar] [CrossRef]
- Jaenicke, R.; Slingsby, C. Lens crystallins and their microbial homologs: Structure, stability, and function. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 435–499. [Google Scholar] [CrossRef]
- Delaye, M.; Tardieu, A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature 1983, 302, 415–417. [Google Scholar] [CrossRef]
- Tardieu, A. alpha-Crystallin quaternary structure and interactive properties control eye lens transparency. Int. J. Biol. Macromol. 1998, 22, 211–217. [Google Scholar] [CrossRef]
- Tardieu, A. Eye lens proteins and transparency: From light transmission theory to solution X-ray structural analysis. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Nahomi, R.B.; Rankenberg, J.; Glomb, M.A.; Nagaraj, R.H. Glycation-mediated inter-protein cross-linking is promoted by chaperone-client complexes of alpha-crystallin: Implications for lens aging and presbyopia. J. Biol. Chem. 2020, 295, 5701–5716. [Google Scholar] [CrossRef] [PubMed]
- Vicart, P.; Caron, A.; Guicheney, P.; Li, Z.; Prevost, M.C.; Faure, A.; Chateau, D.; Chapon, F.; Tome, F.; Dupret, J.M.; et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 1998, 20, 92–95. [Google Scholar] [CrossRef]
- Litt, M.; Kramer, P.; LaMorticella, D.M.; Murphey, W.; Lovrien, E.W.; Weleber, R.G. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum. Mol. Genet. 1998, 7, 471–474. [Google Scholar] [CrossRef]
- Klemenz, R.; Frohli, E.; Steiger, R.H.; Schafer, R.; Aoyama, A. Alpha B-crystallin is a small heat shock protein. Proc. Natl. Acad. Sci. USA 1991, 88, 3652–3656. [Google Scholar] [CrossRef]
- Horwitz, J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA 1992, 89, 10449–10453. [Google Scholar] [CrossRef]
- Sun, Y.; MacRae, T.H. The small heat shock proteins and their role in human disease. FEBS J. 2005, 272, 2613–2627. [Google Scholar] [CrossRef]
- Webster, J.M.; Darling, A.L.; Uversky, V.N.; Blair, L.J. Small Heat Shock Proteins, Big Impact on Protein Aggregation in Neurodegenerative Disease. Front. Pharmacol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Muranova, L.K.; Ryzhavskaya, A.S.; Sudnitsyna, M.V.; Shatov, V.M.; Gusev, N.B. Small Heat Shock Proteins and Human Neurodegenerative Diseases. Biochemistry 2019, 84, 1256–1267. [Google Scholar]
- Bova, M.P.; Yaron, O.; Huang, Q.; Ding, L.; Haley, D.A.; Stewart, P.L.; Horwitz, J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. USA 1999, 96, 6137–6142. [Google Scholar] [CrossRef] [PubMed]
- Makley, L.N.; McMenimen, K.A.; DeVree, B.T.; Goldman, J.W.; McGlasson, B.N.; Rajagopal, P.; Dunyak, B.M.; McQuade, T.J.; Thompson, A.D.; Sunahara, R.; et al. Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models. Science 2015, 350, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Haslbeck, M. Medical implications of understanding the functions of human small heat shock proteins. Expert Rev. Proteom. 2015, 12, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Paul, C.; Seigneuric, R.; Kampinga, H.H. The small heat shock proteins family: the long forgotten chaperones. Int. J. Biochem. Cell. Biol. 2012, 44(10), 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Barton, K.; De Maria, A.; Petrash, J.M.; Shiels, A.; Bassnett, S. The stratified syncytium of the vertebrate lens. J. Cell Sci. 2009, 122, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.; Papaconstantinou, J. A change in the stoichiometry of assembly of bovine lens alpha-crystallin subunits in relation to cellular differentiation. Biochem. Biophys. Res. Commun. 1974, 57, 134–141. [Google Scholar] [CrossRef]
- Delbecq, S.P.; Klevit, R.E. One size does not fit all: The oligomeric states of alphaB crystallin. FEBS Lett. 2013, 587, 1073–1080. [Google Scholar] [CrossRef]
- Bagneris, C.; Bateman, O.A.; Naylor, C.E.; Cronin, N.; Boelens, W.C.; Keep, N.H.; Slingsby, C. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J. Mol. Biol. 2009, 392, 1242–1252. [Google Scholar] [CrossRef]
- Laganowsky, A.; Benesch, J.L.; Landau, M.; Ding, L.; Sawaya, M.R.; Cascio, D.; Huang, Q.; Robinson, C.V.; Horwitz, J.; Eisenberg, D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010, 19, 1031–1043. [Google Scholar] [CrossRef]
- Baranova, E.V.; Weeks, S.D.; Beelen, S.; Bukach, O.V.; Gusev, N.B.; Strelkov, S.V. Three-Dimensional Structure of alpha-Crystallin Domain Dimers of Human Small Heat Shock Proteins HSPB1 and HSPB6. J. Mol. Biol. 2011, 411, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, T.; Kastenmuller, A.; Stein, M.L.; Peters, C.; Daake, M.; Krause, M.; Weinfurtner, D.; Haslbeck, M.; Weinkauf, S.; Groll, M.; et al. The Chaperone Activity of the Developmental Small Heat Shock Protein Sip1 Is Regulated by pH-Dependent Conformational Changes. Mol. Cell 2015, 58, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Sluchanko, N.N.; Beelen, S.; Kulikova, A.A.; Weeks, S.D.; Antson, A.A.; Gusev, N.B.; Strelkov, S.V. Structural Basis for the Interaction of a Human Small Heat Shock Protein with the 14-3-3 Universal Signaling Regulator. Structure 2017, 25, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Egberts, W.V.; Kondrat, F.D.L.; Hilton, G.R.; Ray, N.J.; Cole, A.R.; Carver, J.A.; Benesch, J.L.P.; Keep, N.H.; Boelens, W.C.; et al. Terminal Regions Confer Plasticity to the Tetrameric Assembly of Human HspB2 and HspB3. J. Mol. Biol. 2018, 430, 3297–3310. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, P.; Liu, Y.; Shi, L.; Clouser, A.F.; Klevit, R.E. Structure of the alpha-crystallin domain from the redox-sensitive chaperone, HSPB1. J. Biomol. NMR 2015, 63, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Alderson, T.R.; Ying, J.; Bax, A.; Benesch, J.L.P.; Baldwin, A.J. Conditional Disorder in Small Heat-shock Proteins. J. Mol. Biol. 2020, 432, 3033–3049. [Google Scholar] [CrossRef]
- Hilton, G.R.; Hochberg, G.K.; Laganowsky, A.; McGinnigle, S.I.; Baldwin, A.J.; Benesch, J.L. C-terminal interactions mediate the quaternary dynamics of alphaB-crystallin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110405. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Benesch, J.L.; Ding, L.L.; Yaron, O.; Horwitz, J.; Robinson, C.V. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem. 2004, 279, 28675–28680. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Benesch, J.L.; Bateman, O.A.; Slingsby, C.; Robinson, C.V. Polydispersity of a mammalian chaperone: Mass spectrometry reveals the population of oligomers in alphaB-crystallin. Proc. Natl. Acad. Sci. USA 2003, 100, 10611–10616. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Shrestha, S.; Morris, A.M.; Ecroyd, H. Structural and functional aspects of hetero-oligomers formed by the small heat shock proteins alphaB-crystallin and HSP27. J. Biol. Chem. 2013, 288, 13602–13609. [Google Scholar] [CrossRef]
- Baldwin, A.J.; Lioe, H.; Robinson, C.V.; Kay, L.E.; Benesch, J.L. alphaB-Crystallin Polydispersity Is a Consequence of Unbiased Quaternary Dynamics. J. Mol. Biol. 2011, 413, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, G.K.; Benesch, J.L. Dynamical structure of alphaB-crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Kim, R.; Kim, S.H. Crystal structure of a small heat-shock protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Van Montfort, R.L.; Basha, E.; Friedrich, K.L.; Slingsby, C.; Vierling, E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001, 8, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Hanazono, Y.; Takeda, K.; Oka, T.; Abe, T.; Tomonari, T.; Akiyama, N.; Aikawa, Y.; Yohda, M.; Miki, K. Nonequivalence observed for the 16-meric structure of a small heat shock protein, SpHsp16.0, from Schizosaccharomyces pombe. Structure 2013, 21, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hilario, E.; Martin, F.J.; Bertolini, M.C.; Fan, L. Crystal structures of Xanthomonas small heat shock protein provide a structural basis for an active molecular chaperone oligomer. J. Mol. Biol. 2011, 408, 74–86. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.Y.; Yang, B.; Wang, F.H.; Wang, Y.H.; Yun, C.H. Active-State Structures of a Small Heat-Shock Protein Revealed a Molecular Switch for Chaperone Function. Structure 2015, 23, 2066–2075. [Google Scholar] [CrossRef]
- Mani, N.; Bhandari, S.; Moreno, R.; Hu, L.; Prasad, B.V.V.; Suguna, K. Multiple oligomeric structures of a bacterial small heat shock protein. Sci. Rep. 2016, 6, 24019. [Google Scholar] [CrossRef]
- Kaiser, C.J.O.; Peters, C.; Schmid, P.W.N.; Stavropoulou, M.; Zou, J.; Dahiya, V.; Mymrikov, E.V.; Rockel, B.; Asami, S.; Haslbeck, M.; et al. The structure and oxidation of the eye lens chaperone alphaA-crystallin. Nat. Struct. Mol. Biol. 2019, 26, 1141–1150. [Google Scholar] [CrossRef]
- Braun, N.; Zacharias, M.; Peschek, J.; Kastenmuller, A.; Zou, J.; Hanzlik, M.; Haslbeck, M.; Rappsilber, J.; Buchner, J.; Weinkauf, S. Multiple molecular architectures of the eye lens chaperone alphaB-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA 2011, 108, 20491–20496. [Google Scholar] [CrossRef]
- Pasta, S.Y.; Raman, B.; Ramakrishna, T.; Rao, C. The IXI/V motif in the C-terminal extension of alpha-crystallins: Alternative interactions and oligomeric assemblies. Mol. Vis. 2004, 10, 655–662. [Google Scholar] [PubMed]
- Clouser, A.F.; Baughman, H.E.; Basanta, B.; Guttman, M.; Nath, A.; Klevit, R.E. Interplay of disordered and ordered regions of a human small heat shock protein yields an ensemble of ‘quasi-ordered’ states. eLife 2019, 8, e50259. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S.P.; Jehle, S.; Klevit, R. Binding determinants of the small heat shock protein, alphaB-crystallin: Recognition of the ‘IxI’ motif. EMBO J. 2012, 31, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Treweek, T.M.; Rekas, A.; Walker, M.J.; Carver, J.A. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, alphaA- and alphaB-crystallin. Exp. Eye Res. 2010, 91, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.J.; Lioe, H.; Hilton, G.R.; Baker, L.A.; Rubinstein, J.L.; Kay, L.E.; Benesch, J.L. The polydispersity of alphaB-crystallin is rationalized by an interconverting polyhedral architecture. Structure 2011, 19, 1855–1863. [Google Scholar] [CrossRef]
- Horwitz, J.; Huang, Q.L.; Ding, L.; Bova, M.P. Lens alpha-crystallin: Chaperone-like properties. Methods Enzymol. 1998, 290, 365–383. [Google Scholar]
- Kelley, P.B.; Abraham, E.C. Thermally induced disintegration of the oligomeric structure of alphaB-crystallin mutant F28S is associated with diminished chaperone activity. Mol. Cell. Biochem. 2003, 252, 273–278. [Google Scholar] [CrossRef]
- Shah, D.; Li, J.; Shaikh, A.R.; Rajagopalan, R. Arginine-aromatic interactions and their effects on arginine-induced solubilization of aromatic solutes and suppression of protein aggregation. Biotechnol. Prog. 2012, 28, 223–231. [Google Scholar] [CrossRef]
- Arakawa, T.; Ejima, D.; Tsumoto, K.; Obeyama, N.; Tanaka, Y.; Kita, Y.; Timasheff, S.N. Suppression of protein interactions by arginine: A proposed mechanism of the arginine effects. Biophys. Chem. 2007, 127, 1–8. [Google Scholar] [CrossRef]
- Shatov, V.M.; Weeks, S.D.; Strelkov, S.V.; Gusev, N.B. The Role of the Arginine in the Conserved N-Terminal Domain RLFDQxFG Motif of Human Small Heat Shock Proteins HspB1, HspB4, HspB5, HspB6, and HspB8. Int. J. Mol. Sci. 2018, 19, 2112. [Google Scholar] [CrossRef]
- Cheng, G.; Basha, E.; Wysocki, V.H.; Vierling, E. Insights into small heat shock protein and substrate structure during chaperone action derived from hydrogen/deuterium exchange and mass spectrometry. J. Biol. Chem. 2008, 283, 26634–26642. [Google Scholar] [CrossRef] [PubMed]
- Lentze, N.; Narberhaus, F. Detection of oligomerisation and substrate recognition sites of small heat shock proteins by peptide arrays. Biochem. Biophys. Res. Commun. 2004, 325, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Basha, E.; Friedrich, K.L.; Vierling, E. The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J. Biol. Chem. 2006, 281, 39943–39952. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.G.; Shenoy, A.K., Jr.; Clark, J.I. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 2007, 46, 6308–6317. [Google Scholar] [CrossRef]
- Ahrman, E.; Lambert, W.; Aquilina, J.A.; Robinson, C.V.; Emanuelsson, C.S. Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase. Protein Sci. 2007, 16, 1464–1478. [Google Scholar] [CrossRef]
- Jovcevski, B.; Aquilina, J.A.; Benesch, J.L.P.; Ecroyd, H. The influence of the N-terminal region proximal to the core domain on the assembly and chaperone activity of alphaB-crystallin. Cell Stress Chaperones 2018, 23, 827–836. [Google Scholar] [CrossRef]
- Cox, D.; Selig, E.; Griffin, M.D.; Carver, J.A.; Ecroyd, H. Small Heat-shock Proteins Prevent alpha-Synuclein Aggregation via Transient Interactions and Their Efficacy Is Affected by the Rate of Aggregation. J. Biol. Chem. 2016, 291, 22618–22629. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Daake, M.; Richter, B.; Haslbeck, M.; Buchner, J. The Chaperone Activity and Substrate Spectrum of Human Small Heat Shock Proteins. J. Biol. Chem. 2017, 292, 672–684. [Google Scholar] [CrossRef]
- Jiao, W.; Qian, M.; Li, P.; Zhao, L.; Chang, Z. The essential role of the flexible termini in the temperature-responsiveness of the oligomeric state and chaperone-like activity for the polydisperse small heat shock protein IbpB from Escherichia coli. J. Mol. Biol. 2005, 347, 871–884. [Google Scholar] [CrossRef]
- Peschek, J.; Braun, N.; Rohrberg, J.; Back, K.C.; Kriehuber, T.; Kastenmuller, A.; Weinkauf, S.; Buchner, J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proc. Natl. Acad. Sci. USA 2013, 110, E3780–E3789. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Watt, S.J. The N-terminal domain of alphaB-crystallin is protected from proteolysis by bound substrate. Biochem. Biophys. Res. Commun. 2007, 353, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Wintrode, P.L.; Friedrich, K.L.; Vierling, E.; Smith, J.B.; Smith, D.L. Solution structure and dynamics of a heat shock protein assembly probed by hydrogen exchange and mass spectrometry. Biochemistry 2003, 42, 10667–10673. [Google Scholar] [CrossRef] [PubMed]
- Preis, W.; Bestehorn, A.; Buchner, J.; Haslbeck, M. An alternative splice variant of human alphaA-crystallin modulates the oligomer ensemble and the chaperone activity of alpha-crystallins. Cell Stress Chaperones 2017, 22, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Vollmar, B.S.; Bardiaux, B.; Dove, K.K.; Rajagopal, P.; Gonen, T.; Oschkinat, H.; Klevit, R.E. N-terminal domain of {alpha}B-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. USA 2011, 108, 6409–6414. [Google Scholar] [CrossRef] [PubMed]
- Stamler, R.; Kappe, G.; Boelens, W.; Slingsby, C. Wrapping the alpha-crystallin domain fold in a chaperone assembly. J. Mol. Biol. 2005, 353, 68–79. [Google Scholar] [CrossRef]
- Shatov, V.M.; Strelkov, S.V.; Gusev, N.B. The Heterooligomerization of Human Small Heat Shock Proteins Is Controlled by Conserved Motif Located in the N-Terminal Domain. Int. J. Mol. Sci. 2020, 21, 4248. [Google Scholar] [CrossRef]
- Collier, M.P.; Alderson, T.R.; De Villiers, C.P.; Nicholls, D.; Gastall, H.Y.; Allison, T.M.; Degiacomi, M.T.; Jiang, H.; Mlynek, G.; Furst, D.O.; et al. HspB1 phosphorylation regulates its intramolecular dynamics and mechanosensitive molecular chaperone interaction with filamin C. Sci. Adv. 2019, 5, eaav8421. [Google Scholar] [CrossRef]
- Ito, H.; Kamei, K.; Iwamoto, I.; Inaguma, Y.; Nohara, D.; Kato, K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J. Biol. Chem. 2001, 276, 5346–5352. [Google Scholar] [CrossRef]
- Van den IJssel, P.R.; Overkamp, P.; Bloemendal, H.; De Jong, W.W. Phosphorylation of alphaB-crystallin and HSP27 is induced by similar stressors in HeLa cells. Biochem. Biophys. Res. Commun. 1998, 247, 518–523. [Google Scholar] [CrossRef]
- Gaestel, M. sHsp-phosphorylation: Enzymes, signaling pathways and functional implications. Prog. Mol. Subcell. Biol. 2002, 28, 151–169. [Google Scholar]
- Arrigo, A.P. Human small heat shock proteins: Protein interactomes of homo- and hetero-oligomeric complexes: An update. FEBS Lett. 2013, 587, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Preville, X.; Schultz, H.; Knauf, U.; Gaestel, M.; Arrigo, A.P. Analysis of the role of Hsp25 phosphorylation reveals the importance of the oligomerization state of this small heat shock protein in its protective function against TNFalpha- and hydrogen peroxide-induced cell death. J. Cell. Biochem. 1998, 69, 436–452. [Google Scholar] [CrossRef]
- Thornell, E.; Aquilina, A. Regulation of alphaA- and alphaB-crystallins via phosphorylation in cellular homeostasis. Cell Mol. Life Sci. 2015, 72, 4127–4137. [Google Scholar] [CrossRef]
- Jovcevski, B.; Kelly, M.A.; Aquilina, J.A.; Benesch, J.L.P.; Ecroyd, H. Evaluating the Effect of Phosphorylation on the Structure and Dynamics of Hsp27 Dimers by Means of Ion Mobility Mass Spectrometry. Anal. Chem. 2017, 89, 13275–13282. [Google Scholar] [CrossRef] [PubMed]
- Peschek, J.; Braun, N.; Franzmann, T.M.; Georgalis, Y.; Haslbeck, M.; Weinkauf, S.; Buchner, J. The eye lens chaperone alpha-crystallin forms defined globular assemblies. Proc. Natl. Acad. Sci. USA 2009, 106, 13272–13277. [Google Scholar] [CrossRef]
- Jehle, S.; Van, R.B.; Stout, J.R.; Noguchi, S.M.; Falber, K.; Rehbein, K.; Oschkinat, H.; Klevit, R.E.; Rajagopal, P. alphaB-crystallin: A hybrid solid-state/solution-state NMR investigation reveals structural aspects of the heterogeneous oligomer. J. Mol. Biol. 2009, 385, 1481–1497. [Google Scholar] [CrossRef]
- Jehle, S.; Rajagopal, P.; Bardiaux, B.; Markovic, S.; Kuhne, R.; Stout, J.R.; Higman, V.A.; Klevit, R.E.; Van Rossum, B.J.; Oschkinat, H. Solid-state NMR and SAXS studies provide a structural basis for the activation of alphaB-crystallin oligomers. Nat. Struct. Mol. Biol. 2010, 17, 1037–1042. [Google Scholar] [CrossRef]
- McDonald, E.T.; Bortolus, M.; Koteiche, H.A.; McHaourab, H.S. Sequence, structure, and dynamic determinants of Hsp27 (HspB1) equilibrium dissociation are encoded by the N-terminal domain. Biochemistry 2012, 51, 1257–1268. [Google Scholar] [CrossRef]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef]
- Clouser, A.F.; Klevit, R.E. pH-dependent structural modulation is conserved in the human small heat shock protein HSBP1. Cell Stress Chaperones 2017, 22, 569–575. [Google Scholar] [CrossRef]
- Hanson, S.R.; Hasan, A.; Smith, D.L.; Smith, J.B. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp. Eye Res. 2000, 71, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chamorro, M.; Smith, D.L.; Smith, J.B. Identification of the major components of the high molecular weight crystallins from old human lenses. Curr. Eye Res. 1994, 13, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.L.; Smith, J.B.; Smith, D.L. Modifications of the water-insoluble human lens alpha-crystallins. Exp. Eye Res. 1996, 63, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Cherian-Shaw, M.; Smith, J.B.; Jiang, X.Y.; Abraham, E.C. Intrapolypeptide disulfides in human alphaA-crystallin and their effect on chaperone-like function. Mol. Cell. Biochem. 1999, 199, 163–167. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, S.; Wang, B.; Hom, G.; Guo, M.; Li, B.; Yang, J.; Vaysburg, D.; Monnier, V.M. Evidence of Highly Conserved beta-Crystallin Disulfidome that Can be Mimicked by In Vitro Oxidation in Age-Related Human Cataract and Glutathione Depleted Mouse Lens. Mol. Cell. Proteom. 2015, 14, 3211–3223. [Google Scholar] [CrossRef]
- Hains, P.G.; Truscott, R.J. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim. Biophys. Acta 2008, 1784, 1959–1964. [Google Scholar] [CrossRef]
- Takemoto, L.J. Oxidation of cysteine residues from alpha-A crystallin during cataractogenesis of the human lens. Biochem. Biophys. Res. Commun. 1996, 223, 216–220. [Google Scholar] [CrossRef]
- Shroff, N.P.; Cherian-Shaw, M.; Bera, S.; Abraham, E.C. Mutation of R116C results in highly oligomerized alpha A-crystallin with modified structure and defective chaperone-like function. Biochemistry 2000, 39, 1420–1426. [Google Scholar] [CrossRef]
- Chiesa, R.; Spector, A. The dephosphorylation of lens alpha-crystallin A chain. Biochem. Biophys. Res. Commun. 1989, 162, 1494–1501. [Google Scholar] [CrossRef]
- Vermorken, A.J.; Bloemendal, H. alpha-Crystallin polypeptides as markers of lens cell differentiation. Nature 1978, 271, 779–781. [Google Scholar] [CrossRef]
- Spector, A.; Li, L.K.; Meretsky, D.; Augusteyn, R. What is alpha crystallin? Am. J. Ophthalmol. 1971, 71, 386–390. [Google Scholar] [CrossRef]
- Spector, A.; Li, L.-K.; Augusteyn, R.C.; Schneider, A.; Freund, T. α-Crystallin. The isolation and characterization of distinct macromolecular fractions. Biochem. J. 1971, 124, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.L.; Overbeek, P.A. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2276–2284. [Google Scholar]
- Peterson, J.J.; Young, M.M.; Takemoto, L.J. Probing alpha-crystallin structure using chemical cross-linkers and mass spectrometry. Mol. Vis. 2004, 10, 857–866. [Google Scholar]
- Santhoshkumar, P.; Murugesan, R.; Sharma, K.K. Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin. Biochemistry 2009, 48, 5066–5073. [Google Scholar] [CrossRef]
- Ryazantsev, S.N.; Poliansky, N.B.; Chebotareva, N.A.; Muranov, K.O. 3D structure of the native alpha-crystallin from bovine eye lens. Int. J. Biol. Macromol. 2018, 117, 1289–1298. [Google Scholar] [CrossRef]
- Li, L.K.; Spector, A. The reaggregation of purified subunits of alpha-crystallin. Exp. Eye Res. 1973, 15, 179–183. [Google Scholar] [CrossRef]
- Datta, S.A.; Rao, C.M. Packing-induced conformational and functional changes in the subunits of alpha -crystallin. J. Biol. Chem. 2000, 275, 41004–41010. [Google Scholar] [CrossRef]
- Sun, T.X.; Liang, J.J. Intermolecular exchange and stabilization of recombinant human alphaA- and alphaB-crystallin. J. Biol. Chem. 1998, 273, 286–290. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Minton, A.P. Macromolecular crowding: Biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 27–65. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef]
- Chebotareva, N.A.; Eronina, T.B.; Roman, S.G.; Mikhaylova, V.V.; Sluchanko, N.N.; Gusev, N.B.; Kurganov, B.I. Oligomeric state of alphaB-crystallin under crowded conditions. Biochem. Biophys. Res. Commun. 2019, 508, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, J.; Abgar, S.; Aerts, T.; Clauwaert, J. A small-angle X-ray solution scattering study of bovine alpha-crystallin. Eur. J. Biochem. 2000, 267, 3848–3858. [Google Scholar] [CrossRef] [PubMed]
- Foffi, G.; Savin, G.; Bucciarelli, S.; Dorsaz, N.; Thurston, G.M.; Stradner, A.; Schurtenberger, P. Hard sphere-like glass transition in eye lens alpha-crystallin solutions. Proc. Natl. Acad. Sci. USA 2014, 111, 16748–16753. [Google Scholar] [CrossRef] [PubMed]
- Klevit, R.E. Peeking from behind the veil of enigma: Emerging insights on small heat shock protein structure and function. Cell Stress Chaperones 2020. [Google Scholar] [CrossRef] [PubMed]
- Bepperling, A.; Alte, F.; Kriehuber, T.; Braun, N.; Weinkauf, S.; Groll, M.; Haslbeck, M.; Buchner, J. Alternative bacterial two-component small heat shock protein systems. Proc. Natl. Acad. Sci. USA 2012, 109, 20407–20412. [Google Scholar] [CrossRef]
- Mchaourab, H.S.; Dodson, E.K.; Koteiche, H.A. Mechanism of chaperone function in small heat shock proteins. Two-mode binding of the excited states of T4 lysozyme mutants by alphaA-crystallin. J. Biol. Chem. 2002, 277, 40557–40566. [Google Scholar] [CrossRef]
- Muhlhofer, M.; Berchtold, E.; Stratil, C.G.; Csaba, G.; Kunold, E.; Bach, N.C.; Sieber, S.A.; Haslbeck, M.; Zimmer, R.; Buchner, J. The Heat Shock Response in Yeast Maintains Protein Homeostasis by Chaperoning and Replenishing Proteins. Cell Rep. 2019, 29, 4593–4607. [Google Scholar] [CrossRef]
- Haslbeck, M. sHsps and their role in the chaperone network. Cell Mol. Life Sci. 2002, 59, 1649–1657. [Google Scholar] [CrossRef]
- Raman, B.; Rao, C.M. Chaperone-like activity and quaternary structure of alpha-crystallin. J. Biol. Chem. 1994, 269, 27264–27268. [Google Scholar]
- Jakob, U.; Gaestel, M.; Engel, K.; Buchner, J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993, 268, 1517–1520. [Google Scholar] [PubMed]
- Reddy, G.B.; Das, K.P.; Petrash, J.M.; Surewicz, W.K. Temperature-dependent chaperone activity and structural properties of human alphaA- and alphaB-crystallins. J. Biol. Chem. 2000, 275, 4565–4570. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Walke, S.; Stromer, T.; Ehrnsperger, M.; White, H.E.; Chen, S.; Saibil, H.R.; Buchner, J. Hsp26: A temperature-regulated chaperone. EMBO J. 1999, 18, 6744–6751. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, J.; Das, K.P. Alpha-crystallin does not require temperature activation for its chaperone-like activity. Biochem. Mol. Biol. Int. 1998, 46, 249–258. [Google Scholar]
- Laskowska, E.; Wawrzynow, A.; Taylor, A. IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie 1996, 78, 117–122. [Google Scholar] [CrossRef]
- Arrigo, A.P.; Suhan, J.P.; Welch, W.J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell. Biol. 1988, 8, 5059–5071. [Google Scholar] [CrossRef]
- Stege, G.J.; Li, G.C.; Li, L.; Kampinga, H.H.; Konings, A.W. On the role of hsp72 in heat-induced intranuclear protein aggregation. Int. J. Hyperth. 1994, 10, 659–674. [Google Scholar] [CrossRef]
- Mainz, A.; Peschek, J.; Stavropoulou, M.; Back, K.C.; Bardiaux, B.; Asami, S.; Prade, E.; Peters, C.; Weinkauf, S.; Buchner, J.; et al. The chaperone alphaB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat. Struct. Mol. Biol. 2015, 22, 898–905. [Google Scholar] [CrossRef]
- Mogk, A.; Bukau, B. Role of sHsps in organizing cytosolic protein aggregation and disaggregation. Cell Stress Chaperones 2017, 22, 493–502. [Google Scholar] [CrossRef]
- Specht, S.; Miller, S.B.; Mogk, A.; Bukau, B. Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J. Cell Biol. 2011, 195, 617–629. [Google Scholar] [CrossRef]
- Ungelenk, S.; Moayed, F.; Ho, C.T.; Grousl, T.; Scharf, A.; Mashaghi, A.; Tans, S.; Mayer, M.P.; Mogk, A.; Bukau, B. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grousl, T.; Ungelenk, S.; Miller, S.; Ho, C.T.; Khokhrina, M.; Mayer, M.P.; Bukau, B.; Mogk, A. A prion-like domain in Hsp42 drives chaperone-facilitated aggregation of misfolded proteins. J. Cell Biol. 2018, 217, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Brunsting, J.F.; Stege, G.J.; Konings, A.W.; Landry, J. Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem. Biophys. Res. Commun. 1994, 204, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Ehrnsperger, M.; Graber, S.; Gaestel, M.; Buchner, J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997, 16, 221–229. [Google Scholar] [CrossRef]
- Rauch, J.N.; Tse, E.; Freilich, R.; Mok, S.A.; Makley, L.N.; Southworth, D.R.; Gestwicki, J.E. BAG3 Is a Modular, Scaffolding Protein that Physically Links Heat Shock Protein 70 (Hsp70) to the Small Heat Shock Proteins. J. Mol. Biol. 2017, 429, 128–141. [Google Scholar] [CrossRef]
- Hishiya, A.; Salman, M.N.; Carra, S.; Kampinga, H.H.; Takayama, S. BAG3 Directly Interacts with Mutated alphaB-Crystallin to Suppress Its Aggregation and Toxicity. PLoS ONE 2011, 6, e16828. [Google Scholar] [CrossRef]
- Alberti, S.; Mateju, D.; Mediani, L.; Carra, S. Granulostasis: Protein Quality Control of RNP Granules. Front. Mol. Neurosci. 2017, 10, 84. [Google Scholar] [CrossRef]
- Ulbricht, A.; Gehlert, S.; Leciejewski, B.; Schiffer, T.; Bloch, W.; Hohfeld, J. Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 2015, 11, 538–546. [Google Scholar] [CrossRef]
- Carra, S.; Brunsting, J.F.; Lambert, H.; Landry, J.; Kampinga, H.H. HspB8 participates in protein quality control by a non-chaperone-like mechanism that requires eIF2{alpha} phosphorylation. J. Biol. Chem. 2009, 284, 5523–5532. [Google Scholar] [CrossRef]
- Mounier, N.; Arrigo, A.P. Actin cytoskeleton and small heat shock proteins: How do they interact? Cell Stress Chaperones. 2002, 7, 167–176. [Google Scholar] [CrossRef]
- Nicholl, I.D.; Quinlan, R.A. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994, 13, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.L.; Der Perng, M.; Prescott, A.R.; Jansen, K.A.; Koenderink, G.H.; Quinlan, R.A. The specificity of the interaction between alphaB-crystallin and desmin filaments and its impact on filament aggregation and cell viability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120375. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Haslbeck, M.; Dempfle, L.; Krause, M.; Grashoff, C.; Buchner, J.; Herrmann, H.; Bausch, A.R. The small heat shock protein Hsp27 affects assembly dynamics and structure of keratin intermediate filament networks. Biophys. J. 2013, 105, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Chernik, I.S.; Seit-Nebi, A.S.; Marston, S.B.; Gusev, N.B. Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3gamma. Mol. Cell. Biochem. 2007, 295, 9–17. [Google Scholar] [CrossRef]
- Sathish, H.A.; Koteiche, H.A.; Mchaourab, H.S. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: The role of a folding intermediate. J. Biol. Chem. 2004, 279, 16425–16432. [Google Scholar] [CrossRef]
- Mishra, S.; Stein, R.A.; McHaourab, H.S. Cataract-linked gammaD-crystallin mutants have weak affinity to lens chaperones alpha-crystallins. FEBS Lett. 2012, 586, 330–336. [Google Scholar] [CrossRef]
- Serebryany, E.; King, J.A. Wild-type human gammaD-crystallin promotes aggregation of its oxidation-mimicking, misfolding-prone W42Q mutant. J. Biol. Chem. 2015, 290, 11491–11503. [Google Scholar] [CrossRef]
- Van den IJssel, P.R.; Overkamp, P.; Knauf, U.; Gaestel, M.; De Jong, W.W. Alpha A-crystallin confers cellular thermoresistance. FEBS Lett. 1994, 355, 54–56. [Google Scholar] [CrossRef]
- Christopher, K.L.; Pedler, M.G.; Shieh, B.; Ammar, D.A.; Petrash, J.M.; Mueller, N.H. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim. Biophys. Acta 2014, 1843, 309–315. [Google Scholar] [CrossRef]
- Alderson, T.R.; Roche, J.; Gastall, H.Y.; Dias, D.M.; Pritisanac, I.; Ying, J.; Bax, A.; Benesch, J.L.P.; Baldwin, A.J. Local unfolding of the HSP27 monomer regulates chaperone activity. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Regulation of the Chaperone Function of Small Hsps. In The Big Book on Small Heat Shock Proteins; Tanguay, R.M., Hightower, L.E., Eds.; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 155–176. [Google Scholar]
- Wu, D.; Vonk, J.J.; Salles, F.; Vonk, D.; Haslbeck, M.; Melki, R.; Bergink, S.; Kampinga, H.H. The N terminus of the small heat shock protein HSPB7 drives its polyQ aggregation-suppressing activity. J. Biol. Chem. 2019, 294, 9985–9994. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Chebotareva, N.; Gurvits, B. Transient transformation of oligomeric structure of alpha-crystallin during its chaperone action. Int. J. Biol. Macromol. 2013, 55, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Miesbauer, L.R.; Zhou, X.; Yang, Z.; Yang, Z.; Sun, Y.; Smith, D.L.; Smith, J.B. Post-translational modifications of water-soluble human lens crystallins from young adults. J. Biol. Chem. 1994, 269, 12494–12502. [Google Scholar] [PubMed]
- Wunderlich, M.; Glockshuber, R. Redox properties of protein disulfide isomerase (DsbA) from Escherichia coli. Protein Sci. 1993, 2, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Preville, X.; Salvemini, F.; Giraud, S.; Chaufour, S.; Paul, C.; Stepien, G.; Ursini, M.V.; Arrigo, A.P. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Exp. Cell Res. 1999, 247, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Hsp27: Novel regulator of intracellular redox state. IUBMB Life 2001, 52, 303–307. [Google Scholar] [CrossRef]
- Mehlen, P.; Kretz-Remy, C.; Preville, X.; Arrigo, A.P. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996, 15, 2695–2706. [Google Scholar] [CrossRef]
- Bova, M.P.; Ding, L.L.; Horwitz, J.; Fung, B.K. Subunit exchange of alphaA-crystallin. J. Biol. Chem. 1997, 272, 29511–29517. [Google Scholar] [CrossRef]
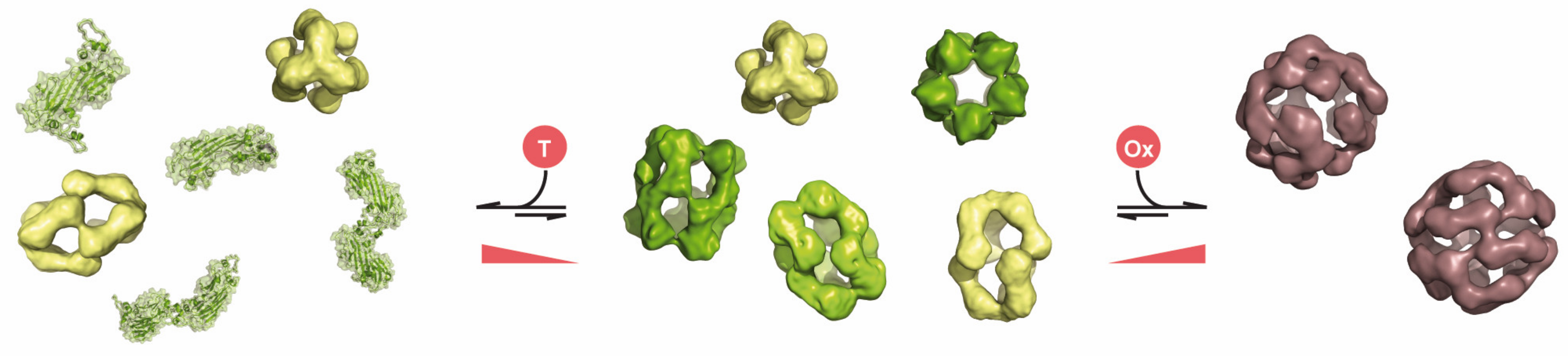
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, M.; Strauch, A.; Catici, D.A.M.; Haslbeck, M. Proteinaceous Transformers: Structural and Functional Variability of Human sHsps. Int. J. Mol. Sci. 2020, 21, 5448. https://doi.org/10.3390/ijms21155448
Riedl M, Strauch A, Catici DAM, Haslbeck M. Proteinaceous Transformers: Structural and Functional Variability of Human sHsps. International Journal of Molecular Sciences. 2020; 21(15):5448. https://doi.org/10.3390/ijms21155448
Chicago/Turabian StyleRiedl, Mareike, Annika Strauch, Dragana A.M. Catici, and Martin Haslbeck. 2020. "Proteinaceous Transformers: Structural and Functional Variability of Human sHsps" International Journal of Molecular Sciences 21, no. 15: 5448. https://doi.org/10.3390/ijms21155448
APA StyleRiedl, M., Strauch, A., Catici, D. A. M., & Haslbeck, M. (2020). Proteinaceous Transformers: Structural and Functional Variability of Human sHsps. International Journal of Molecular Sciences, 21(15), 5448. https://doi.org/10.3390/ijms21155448



