Life and Death of Fungal Transporters under the Challenge of Polarity
Abstract
:1. Introduction
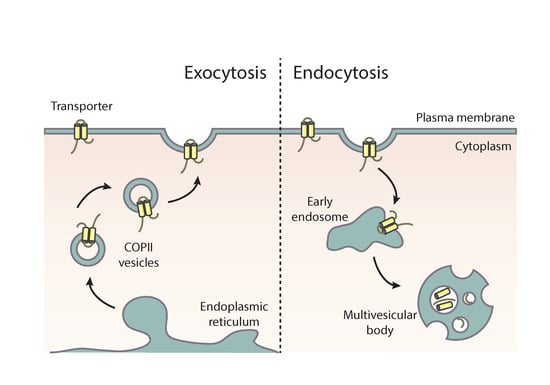
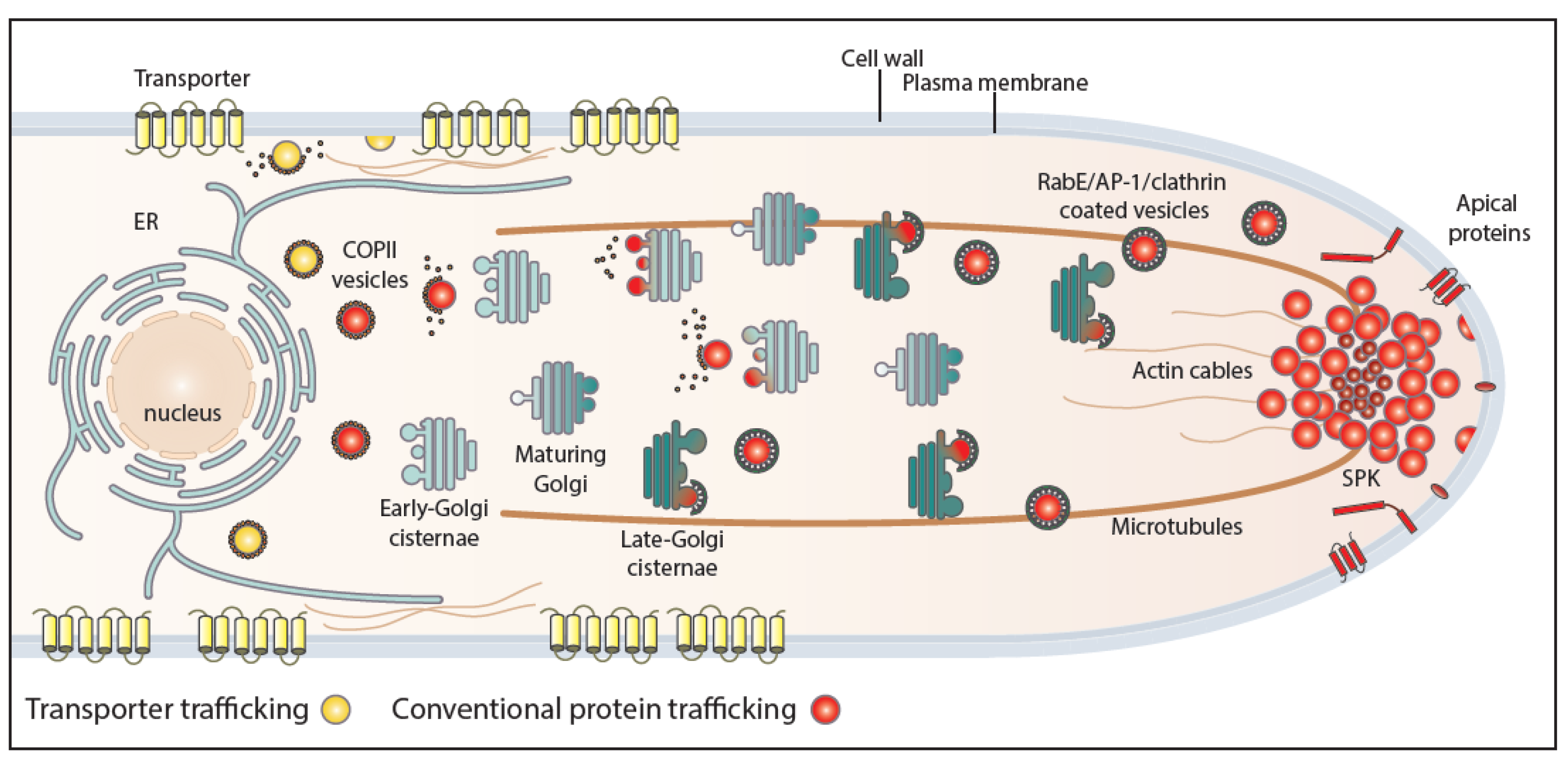
2. Brief Account on the Trafficking of Membrane Cargoes via Conventional Secretion
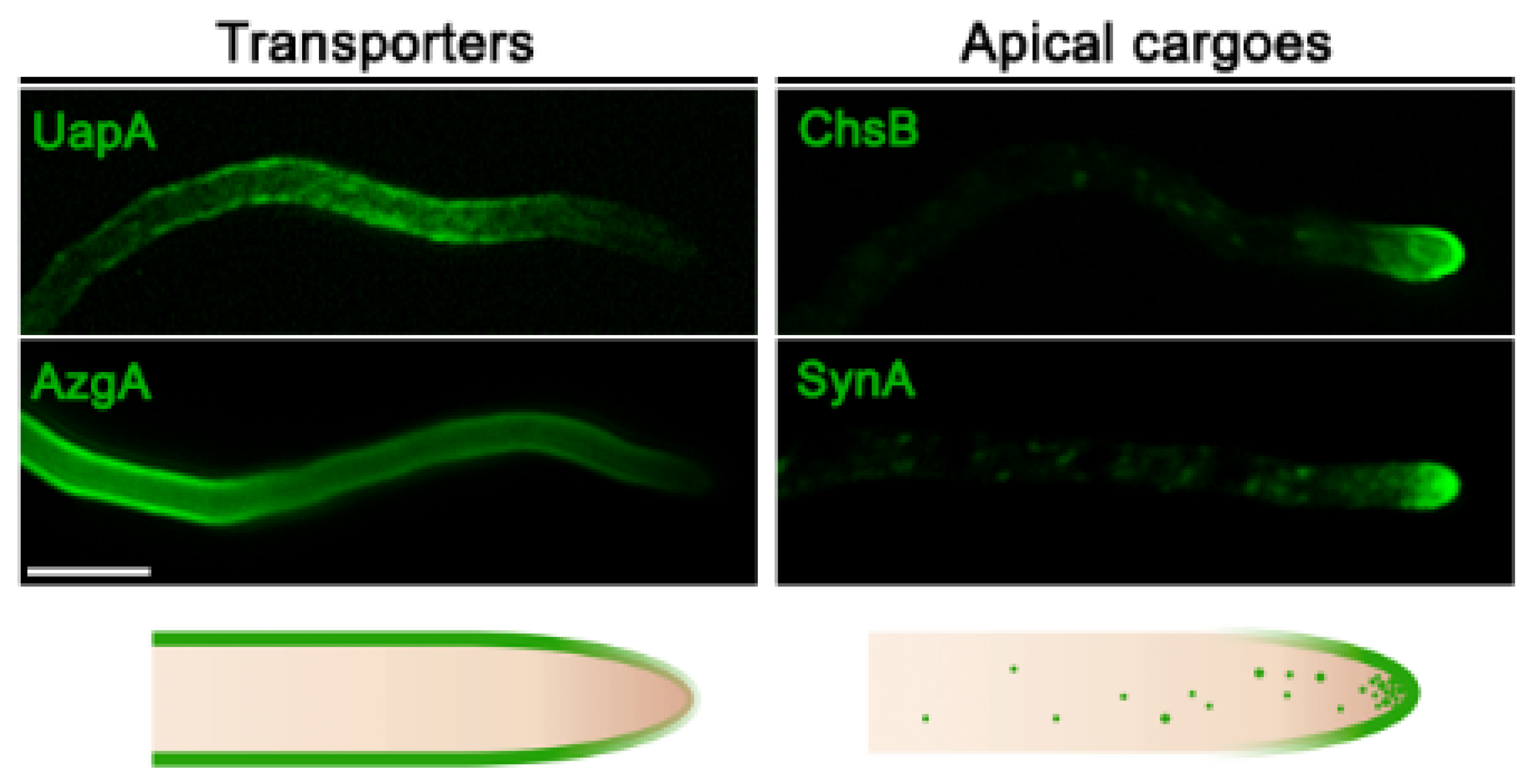
3. Do Transporters Reach the PM via Conventional Golgi-Dependent Secretion?
4. Evidence for Translocation of Aspergillus Transporters to the PM by Golgi-Independent Transfer from the ER
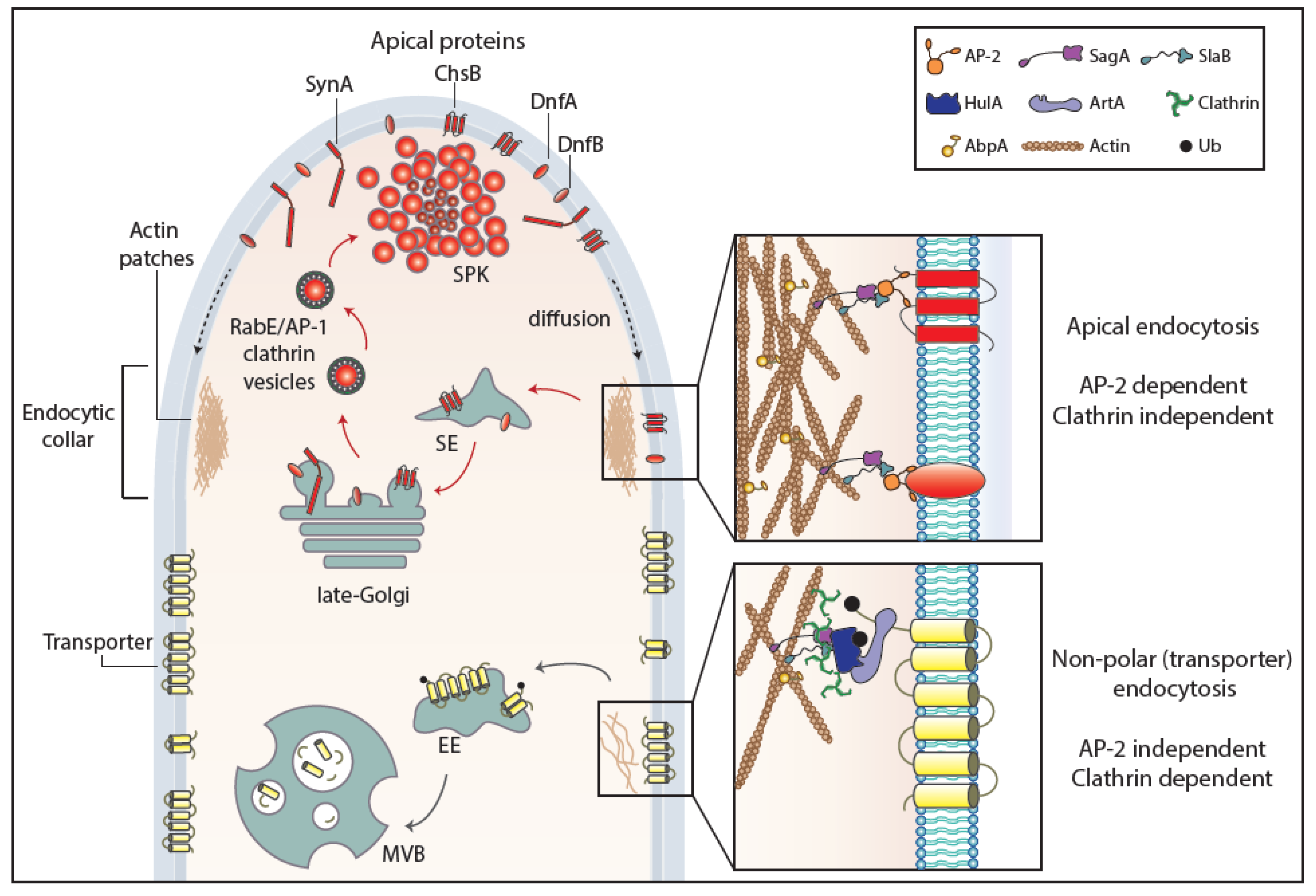
Endocytosis of Apical Cargoes and Transporters Occurs by Distinct Mechanisms that Serve Polar Growth and Nutrition, Respectively
5. Conclusions and Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, L.; Kaback, H.R. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 2006. [Google Scholar] [CrossRef] [Green Version]
- Kaback, H.R.; Smirnova, I.; Kasho, V.; Nie, Y.; Zhou, Y. The alternating access transport mechanism in LacY. J. Membr. Biol. 2011. [Google Scholar] [CrossRef] [Green Version]
- Grenson, M.; Hennaut, C. Mutation affecting activity of several distinct amino acid transport systems in Saccharomyces cerevisiae. J. Bacteriol. 1971. [Google Scholar] [CrossRef] [Green Version]
- Grenson, M. Study of the Positive Control of the General Amino-Acid Permease and Other Ammonia Sensitive Uptake Systems by the Product of the NPR1 Gene in the Yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1983. [Google Scholar] [CrossRef]
- Jauniaux, J.-C.; Grenson, M. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur. J. Biochem. 1990. [Google Scholar] [CrossRef]
- André, B. Tribute to Marcelle Grenson (1925–1996), a pioneer in the study of amino acid transport in yeast. Int. J. Mol. Sci. 2018, 19, 1207. [Google Scholar] [CrossRef] [Green Version]
- Arst, H.N.; Scazzocchio, C. Initiator constitutive mutation with an “up-promoter” effect in Aspergillus nidulans. Nature 1975. [Google Scholar] [CrossRef]
- Scazzocchio, C.; Arst, H.N. The nature of an initiator constitutive mutation in Aspergillus nidulans. Nature 1978, 274, 177–179. [Google Scholar] [CrossRef]
- Diallinas, G.; Scazzocchio, C. A gene coding for the uric acid-xanthine permease of Aspergillus nidulans: Inactivational cloning, characterization, and sequence of a cis-acting mutation. Genetics 1989, 122, 341–350. [Google Scholar]
- Wiame, J.M.; Grenson, M.; Ars, H.N. Nitrogen Catabolite Repression in Yeasts and Filamentous Fungi. Adv. Microb. Physiol. 1985. [Google Scholar] [CrossRef]
- Volland, C.; Urban-Grimal, D.; Géraud, G.; Haguenauer-Tsapis, R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J. Biol. Chem. 1994, 269, 9833–9841. [Google Scholar] [PubMed]
- Dupré, S.; Haguenauer-Tsapis, R. Deubiquitination Step in the Endocytic Pathway of Yeast Plasma Membrane Proteins: Crucial Role of Doa4p Ubiquitin Isopeptidase. Mol. Cell. Biol. 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantazopoulou, A.; Diallinas, G. Fungal nucleobase transporters. FEMS Microbiol. Rev. 2007, 31, 657–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallinas, G. Understanding transporter specificity and the discrete appearance of channel-like gating domains in transporters. Front. Pharmacol. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallinas, G. Dissection of Transporter Function: From Genetics to Structure. Trends Genet. 2016, 32, 576–590. [Google Scholar] [CrossRef]
- Mikros, E.; Diallinas, G. Tales of tails in transporters. Open Biol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Martzoukou, O.; Amillis, S.; Zervakou, A.; Christoforidis, S.; Diallinas, G. The AP-2 complex has a specialized clathrin-independent role in apical endocytosis and polar growth in fungi. Elife 2017. [Google Scholar] [CrossRef] [Green Version]
- Martzoukou, O.; Diallinas, G.; Amillis, S. Secretory vesicle polar sorting, endosome recycling and cytoskeleton organization require the AP-1 complex in Aspergillus nidulans. Genetics 2018. [Google Scholar] [CrossRef] [Green Version]
- Diallinas, G.; Martzoukou, O. Transporter membrane traffic and function: Lessons from a mould. FEBS J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dimou, S.; Martzoukou, O.; Dionysopoulou, M.; Bouris, V.; Amillis, S.; Diallinas, G. Translocation of nutrient transporters to cell membrane via Golgi bypass in Aspergillus nidulans. EMBO Rep. 2020. [Google Scholar] [CrossRef]
- Von Heijne, G. Membrane proteins: From bench to bits. Biochem. Soc. Trans. 2011, 39, 747–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cymer, F.; Von Heijne, G.; White, S.H. Mechanisms of integral membrane protein insertion and folding. J. Mol. Biol. 2015, 427, 999–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhees, R.M.; Hegde, R.S. Toward a structural understanding of co-translational protein translocation. Curr. Opin. Cell Biol. 2016, 41, 919. [Google Scholar] [CrossRef] [PubMed]
- Aviram, N.; Schuldiner, M. Targeting and translocation of proteins to the endoplasmic reticulum at a glance. J. Cell Sci. 2017. [Google Scholar] [CrossRef] [Green Version]
- Martzoukou, O.; Karachaliou, M.; Yalelis, V.; Leung, J.; Byrne, B.; Amillis, S.; Diallinas, G. Oligomerization of the UapA Purine Transporter Is Critical for ER-Exit, Plasma Membrane Localization and Turnover. J. Mol. Biol. 2015. [Google Scholar] [CrossRef]
- Wu, X.; Rapoport, T.A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 2018, 53, 22–28. [Google Scholar] [CrossRef]
- Zanetti, G.; Pahuja, K.B.; Studer, S.; Shim, S.; Schekman, R. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 2012, 14, 20–28. [Google Scholar] [CrossRef]
- D’Arcangelo, J.G.; Stahmer, K.R.; Miller, E.A. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim. Biophys. Acta Mol. Cell Res. 2013. [Google Scholar] [CrossRef] [Green Version]
- Borgese, N. Getting membrane proteins on and off the shuttle bus between the endoplasmic reticulum and the Golgi complex. J. Cell Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kota, J.; Ljungdahl, P.O. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J. Cell Biol. 2005. [Google Scholar] [CrossRef] [Green Version]
- Kota, J.; Gilstring, C.F.; Ljungdahl, P.O. Membrane chaperone Shr3 assists in folding amino acid permeases preventing precocious ERAD. J. Cell Biol. 2007. [Google Scholar] [CrossRef]
- Martinez, P.; Ljungdahl, P.O. The SHR3 homologue from S. pombe demonstrates a conserved function of ER packaging chaperones. J. Cell Sci. 2000, 113, 4351–4362. [Google Scholar]
- Erpapazoglou, Z.; Kafasla, P.; Sophianopoulou, V. The product of the SHR3 orthologue of Aspergillus nidulans has restricted range of amino acid transporter targets. Fungal Genet. Biol. 2006. [Google Scholar] [CrossRef]
- Powers, J.; Barlowe, C. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol. Biol. Cell 2002. [Google Scholar] [CrossRef] [Green Version]
- Herzig, Y.; Sharpe, H.J.; Elbaz, Y.; Munro, S.; Schuldiner, M. A systematic approach to pair secretory cargo receptors with their cargo suggests a mechanism for cargo selection by erv14. PLoS Biol. 2012. [Google Scholar] [CrossRef]
- Pagant, S.; Wu, A.; Edwards, S.; Diehl, F.; Miller, E.A. Sec24 is a coincidence detector that simultaneously binds two signals to drive ER export. Curr. Biol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Zimmermannová, O.; Felcmanová, K.; Rosas-Santiago, P.; Papoušková, K.; Pantoja, O.; Sychrová, H. Erv14 cargo receptor participates in regulation of plasma-membrane potential, intracellular pH and potassium homeostasis via its interaction with K+-specific transporters Trk1 and Tok1. Biochim. Biophys. Acta Mol. Cell Res. 2019. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Long, K.R.; Shome, K.; Watkins, S.C.; Aridor, M. A cascade of ER exit site assembly that is regulated by p125A and lipid signals. J. Cell Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Peotter, J.; Kasberg, W.; Pustova, I.; Audhya, A. COPII-mediated trafficking at the ER/ERGIC interface. Traffic 2019, 20, 491–503. [Google Scholar] [CrossRef]
- Pantazopoulou, A.; Pinar, M.; Xiang, X.; Peñalva, M.A. Maturation of late Golgi cisternae into RabERAB11 exocytic post-Golgi carriers visualized in vivo. Mol. Biol. Cell 2014. [Google Scholar] [CrossRef]
- Feyder, S.; De Craene, J.O.; Bär, S.; Bertazzi, D.L.; Friant, S. Membrane trafficking in the yeast Saccharomyces cerevisiae model. Int. J. Mol. Sci. 2015, 16, 1509–1525. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Navarro, N.; Miller, E. Protein sorting at the ER-Golgi interface. J. Cell Biol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.S. Forty Years of Clathrin-coated Vesicles. Traffic 2015, 16, 1210–1238. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Feng, S.; Wu, B.; Guo, W. Polarized exocytosis. Cold Spring Harb. Perspect. Biol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Peñalva, M.; Galindo, A.; Abenza, J.; Pinar, M.; Calcagno-Pizarelli, A.; Arst, H.; Pantazopoulou, A. Searching for gold beyond mitosis: Mining intracellular membrane traffic in Aspergillus nidulans. Cell. Logist. 2012. [Google Scholar] [CrossRef] [Green Version]
- Mei, K.; Guo, W. The exocyst complex. Curr. Biol. 2018, 28, R922–R925. [Google Scholar] [CrossRef] [Green Version]
- Taheri-Talesh, N.; Horio, T.; Araujo-Bazán, L.; Dou, X.; Espeso, E.A.; Peñalva, M.A.; Osmani, S.A.; Oakley, B.R. The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 2008. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, M.; Martínez-Nñez, L. Hyphal ontogeny in Neurospora crassa: A model organism for all seasons. F1000Research 2016, 5, 2801. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.; Peñalva, M.A.; Riquelme, M.; Wösten, H.A.; Harris, S.D. Cell Biology of Hyphal Growth. In The Fungal Kingdom; ASM Press: Washington, DC, USA, 2017; Volume 5. [Google Scholar] [CrossRef] [Green Version]
- Hesse, D.; Hommel, A.; Jaschke, A.; Moser, M.; Bernhardt, U.; Zahn, C.; Kluge, R.; Wittschen, P.; Gruber, A.D.; Al-Hasani, H.; et al. Altered GLUT4 trafficking in adipocytes in the absence of the GTPase Arfrp1. Biochem. Biophys. Res. Commun. 2010. [Google Scholar] [CrossRef]
- Takazawa, K.; Noguchi, T.; Hosooka, T.; Yoshioka, T.; Tobimatsu, K.; Kasuga, M. Insulin-induced GLUT4 movements in C2C12 myoblasts: Evidence against a role of conventional kinesin motor proteins. Kobe J. Med. Sci. 2008, 54, E14–E22. [Google Scholar]
- Dawicki-McKenna, J.M.; Goldman, Y.E.; Ostap, E.M. Sites of glucose transporter-4 vesicle fusion with the plasma membrane correlate spatially with microtubules. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camus, S.M.; Camus, M.D.; Figueras-Novoa, C.; Boncompain, G.; Sadacca, L.A.; Esk, C.; Bigot, A.; Gould, G.W.; Kioumourtzoglou, D.; Perez, F.; et al. CHC22 clathrin mediates traffic from early secretory compartments for human GLUT4 pathway biogenesis. J. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stampe Jensen, C.; Watanabe, S.; Stas, J.I.; Klaphaak, J.; Yamane, A.; Schmitt, N.; Olesen, S.P.; Trimmer, J.S.; Rasmussen, H.B.; Misonou, H. Trafficking of Kv2.1 channels to the axon initial segment by a novel nonconventional secretory pathway. J. Neurosci. 2017. [Google Scholar] [CrossRef]
- Hanus, C.; Geptin, H.; Tushev, G.; Garg, S.; Alvarez-Castelao, B.; Sambandan, S.; Kochen, L.; Hafner, A.-S.; Langer, J.D.; Schuman, E.M. Unconventional secretory processing diversifies neuronal ion channel properties. Elife 2016. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.Y.; Kim, J.; Lee, M.G. Unconventional secretion of transmembrane proteins. Semin. Cell Dev. Biol. 2018, 83, 59–66. [Google Scholar] [CrossRef]
- Athanasopoulos, A.; André, B.; Sophianopoulou, V.; Gournas, C. Fungal plasma membrane domains. FEMS Microbiol. Rev. 2019, 43, 642–673. [Google Scholar] [CrossRef]
- Vlanti, A.; Diallinas, G. The Aspergillus nidulans FcyB cytosine-purine scavenger is highly expressed during germination and in reproductive compartments and is downregulated by endocytosis. Mol. Microbiol. 2008. [Google Scholar] [CrossRef]
- Krypotou, E.; Kosti, V.; Amillis, S.; Myrianthopoulos, V.; Mikros, E.; Diallinas, G. Modeling, substrate docking, and mutational analysis identify residues essential for the function and specificity of a eukaryotic purine-cytosine NCS1 transporter. J. Biol. Chem. 2012. [Google Scholar] [CrossRef] [Green Version]
- Krypotou, E.; Evangelidis, T.; Bobonis, J.; Pittis, A.A.; Gabaldón, T.; Scazzocchio, C.; Mikros, E.; Diallinas, G. Origin, diversification and substrate specificity in the family of NCS1/FUR transporters. Mol. Microbiol. 2015. [Google Scholar] [CrossRef]
- Sioupouli, G.; Lambrinidis, G.; Mikros, E.; Amillis, S.; Diallinas, G. Cryptic purine transporters in Aspergillus nidulans reveal the role of specific residues in the evolution of specificity in the NCS1 family. Mol. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krypotou, E.; Lambrinidis, G.; Evangelidis, T.; Mikros, E.; Diallinas, G. Modelling, substrate docking and mutational analysis identify residues essential for function and specificity of the major fungal purine transporter AzgA. Mol. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, A.; Peñalva, M.A. Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis. Mol. Biol. Cell 2009. [Google Scholar] [CrossRef] [Green Version]
- Schultzhaus, Z.; Yan, H.; Shaw, B.D. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Schultzhaus, Z.; Zheng, W.; Wang, Z.; Mouriño-Pérez, R.; Shaw, B. Phospholipid flippases DnfA and DnfB exhibit differential dynamics within the A. nidulans Spitzenkörper. Fungal Genet. Biol. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-González, M.; Bravo-Plaza, I.; Pinar, M.; de Los Ríos, V.; Arst, H.N.; Peñalva, M.A. Endocytic recycling via the TGN underlies the polarized hyphal mode of life. PLoS Genet. 2018. [Google Scholar] [CrossRef] [Green Version]
- Apodaca, G.; Gallo, L.I.; Bryant, D.M. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat. Cell Biol. 2012, 14, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Riga, A.; Castiglioni, V.G.; Boxem, M. New insights into apical-basal polarization in epithelia. Curr. Opin. Cell Biol. 2020, 62, 1–8. [Google Scholar] [CrossRef]
- Gournas, C.; Amillis, S.; Vlanti, A.; Diallinas, G. Transport-dependent endocytosis and turnover of a uric acid-xanthine permease. Mol. Microbiol. 2010. [Google Scholar] [CrossRef]
- Karachaliou, M.; Amillis, S.; Evangelinos, M.; Kokotos, A.C.; Yalelis, V.; Diallinas, G. The arrestin-like protein ArtA is essential for ubiquitination and endocytosis of the UapA transporter in response to both broad-range and specific signals. Mol. Microbiol. 2013. [Google Scholar] [CrossRef]
- Peñalva, M.Á. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr. Opin. Microbiol. 2010, 13, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, A. The Golgi apparatus: Insights from filamentous fungi. Mycologia 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxfield, F.R.; McGraw, T.E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, C.; Piper, R.C. Cell surface recycling in yeast: Mechanisms and machineries. Biochem. Soc. Trans. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Burd, C.G. Retrograde trafficking and plasma membrane recycling pathways of the budding yeast Saccharomyces cerevisiae. Traffic 2020, 21, 45–59. [Google Scholar] [CrossRef]
- Stöckli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124, 4147–4159. [Google Scholar] [CrossRef] [Green Version]
- Sadler, J.B.A.; Bryant, N.J.; Gould, G.W.; Welburn, C.R. Posttranslational modifications of GLUT4 affect its subcellular localization and translocation. Int. J. Mol. Sci. 2013, 14, 9963–9978. [Google Scholar] [CrossRef] [Green Version]
- Bryant, N.J.; Gould, G.W. Insulin stimulated GLUT4 translocation—Size is not everything! Curr. Opin. Cell Biol. 2020, 65, 28–34. [Google Scholar] [CrossRef]
- Becuwe, M.; Léon, S. Integrated control of transporter endocytosis and recycling by the arrestin-related protein Rod1 and the ubiquitin ligase Rsp5. Elife 2014. [Google Scholar] [CrossRef]
- Gournas, C.; Saliba, E.; Krammer, E.M.; Barthelemy, C.; Prévost, M.; André, B. Transition of yeast Can1 transporter to the inward-facing state unveils an α-arrestin target sequence promoting its ubiquitylation and endocytosis. Mol. Biol. Cell 2017. [Google Scholar] [CrossRef]
- Eising, S.; Thiele, L.; Fröhlich, F. A systematic approach to identify recycling endocytic cargo depending on the GARP complex. Elife 2019. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Erpapazoglou, Z.; Haguenauer-Tsapis, R.; André, B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010, 20, 196–204. [Google Scholar] [CrossRef] [PubMed]
- MacGurn, J.A.; Hsu, P.C.; Emr, S.D. Ubiquitin and membrane protein turnover: From cradle to grave. Annu. Rev. Biochem. 2012, 81, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; MacGurn, J.A.; Chu, T.; Stefan, C.J.; Emr, S.D. Arrestin-Related Ubiquitin-Ligase Adaptors Regulate Endocytosis and Protein Turnover at the Cell Surface. Cell 2008. [Google Scholar] [CrossRef] [Green Version]
- Nikko, E.; Pelham, H.R.B. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic 2009. [Google Scholar] [CrossRef] [Green Version]
- Guiney, E.L.; Klecker, T.; Emr, S.D. Identification of the endocytic sorting signal recognized by the Art1-Rsp5 ubiquitin ligase complex. Mol. Biol. Cell 2016. [Google Scholar] [CrossRef]
- Mund, T.; Pelham, H.R. Substrate clustering potently regulates the activity of WW-HECT domain–containing ubiquitin ligases. J. Biol. Chem. 2018. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Hanson, L.; Lou, H.Y.; Akamatsu, M.; Chowdary, P.D.; Santoro, F.; Marks, J.R.; Grassart, A.; Drubin, D.G.; Cui, Y.; et al. Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat. Nanotechnol. 2017. [Google Scholar] [CrossRef]
- Crapeau, M.; Merhi, A.; André, B. Stress conditions promote yeast Gap1 permease ubiquitylation and down-regulation via the arrestin-like bul and aly proteins. J. Biol. Chem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Gournas, C.; Prévost, M.; Krammer, E.M.; André, B. Function and regulation of fungal amino acid transporters: Insights from predicted structure. In Advances in Experimental Medicine and Biology; Springer Nature: Berlin/Heidelberg, Germany, 2016; Volume 892, pp. 69–106. [Google Scholar] [CrossRef]
- Talaia, G.; Gournas, C.; Saliba, E.; Barata-Antunes, C.; Casal, M.; André, B.; Diallinas, G.; Paiva, S. The α-Arrestin Bul1p Mediates Lactate Transporter Endocytosis in Response to Alkalinization and Distinct Physiological Signals. J. Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Gournas, C.; Papageorgiou, I.; Diallinas, G. The nucleobase-ascorbate transporter (NAT) family: Genomics, evolution, structure-function relationships and physiological role. Mol. Biosyst. 2008. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, G.F.; Amillis, S.; Diallinas, G. Substrate specificity of the furE transporter is determined by cytoplasmic terminal domain interactions. Genetics 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadaki, G.F.; Lambrinidis, G.; Zamanos, A.; Mikros, E.; Diallinas, G. Cytosolic N- and C-Termini of the Aspergillus nidulans FurE Transporter Contain Distinct Elements that Regulate by Long-Range Effects Function and Specificity. J. Mol. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Busto, J.V.; Wedlich-Söldner, R. Integration through separation—The role of lateral membrane segregation in nutrient uptake. Front. Cell Dev. Biol. 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Syga, L.; Moiset, G.; Spakman, D.; Schavemaker, P.E.; Punter, C.M.; Seinen, A.B.; Van Oijen, A.M.; Robinson, A.; Poolman, B. Steric exclusion and protein conformation determine the localization of plasma membrane transporters. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gournas, C.; Gkionis, S.; Carquin, M.; Twyffels, L.; Tyteca, D.; André, B. Conformation-dependent partitioning of yeast nutrient transporters into starvation-protective membrane domains. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef] [Green Version]
- Babst, M. Regulation of nutrient transporters by metabolic and environmental stresses. Curr. Opin. Cell Biol. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Kirchhausen, T.; Owen, D.; Harrison, S.C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Drubin, D.G.; Sun, Y. Clathrin-mediated endocytosis in budding yeast at a glance. J. Cell Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kirchhausen, T. Adaptors for Clathrin-Mediated Traffic. Annu. Rev. Cell Dev. Biol. 1999. [Google Scholar] [CrossRef] [Green Version]
- Merrifield, C.J.; Kaksonen, M. Endocytic Accessory Factors and Regulation of Clathrin-Mediated Endocytosis. Cold Spring Harb. Perspect. Biol. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manenschijn, H.E.; Picco, A.; Mund, M.; Rivier-Cordey, A.S.; Ries, J.; Kaksonen, M. Type-I myosins promote actin polymerization to drive membrane bending in endocytosis. Elife 2019. [Google Scholar] [CrossRef] [PubMed]
- Newpher, T.M.; Lemmon, S.K. Clathrin is important for normal actin dynamics and progression of Sla2p-containing patches during endocytosis in yeast. Traffic 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, L.J.; Tuch, B.B.; Villen, J.; Johnson, A.D.; Gygi, S.P.; Morgan, D.O. Global analysis of cdk1 substrate phosphorylation sites provides insiqhts into evolution. Science (80-. ) 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, D.B.; Gallo, G. Structure meets function: Actin filaments and myosin motors in the axon. J. Neurochem. 2014, 129, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, A.B.; Bourke, A.M.; Hiester, B.G.; Hanus, C.; Kennedy, M.J. Golgi-Independent secretory trafficking through recycling endosomes in neuronal dendrites and spines. Elife 2017. [Google Scholar] [CrossRef] [PubMed]
- Gumy, L.F.; Hoogenraad, C.C. Local mechanisms regulating selective cargo entry and long-range trafficking in axons. Curr. Opin. Neurobiol. 2018, 51, 23–28. [Google Scholar] [CrossRef]
- Gu, H.H.; Wu, X.; Giros, B.; Caron, M.G.; Caplan, M.J.; Rudnick, G. The NH2-terminus of norepinephrine transporter contains a basolateral localization signal for epithelial cells. Mol. Biol. Cell 2001. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Muth, T.; Caplan, M. The COOH-terminal tail of the GAT-2 GABA transporter contains a novel motif that plays a role in basolateral targeting. Am. J. Physiol. Cell Physiol. 2004. [Google Scholar] [CrossRef]
- Varma, S.; Sobey, K.; Campbell, C.E.; Kuo, S.M. Hierarchal contribution of N- and C-terminal sequences to the differential localization of homologous sodium-dependent vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochemistry 2009. [Google Scholar] [CrossRef]
- Kuo, S.M.; Wang, L.Y.; Yu, S.; Campbell, C.E.; Valiyaparambil, S.A.; Rance, M.; Blumenthal, K.M. The N-terminal basolateral targeting signal unlikely acts alone in the differential trafficking of membrane transporters in MDCK cells. Biochemistry 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimou, S.; Diallinas, G. Life and Death of Fungal Transporters under the Challenge of Polarity. Int. J. Mol. Sci. 2020, 21, 5376. https://doi.org/10.3390/ijms21155376
Dimou S, Diallinas G. Life and Death of Fungal Transporters under the Challenge of Polarity. International Journal of Molecular Sciences. 2020; 21(15):5376. https://doi.org/10.3390/ijms21155376
Chicago/Turabian StyleDimou, Sofia, and George Diallinas. 2020. "Life and Death of Fungal Transporters under the Challenge of Polarity" International Journal of Molecular Sciences 21, no. 15: 5376. https://doi.org/10.3390/ijms21155376