Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus
Abstract
1. Introduction
2. Results
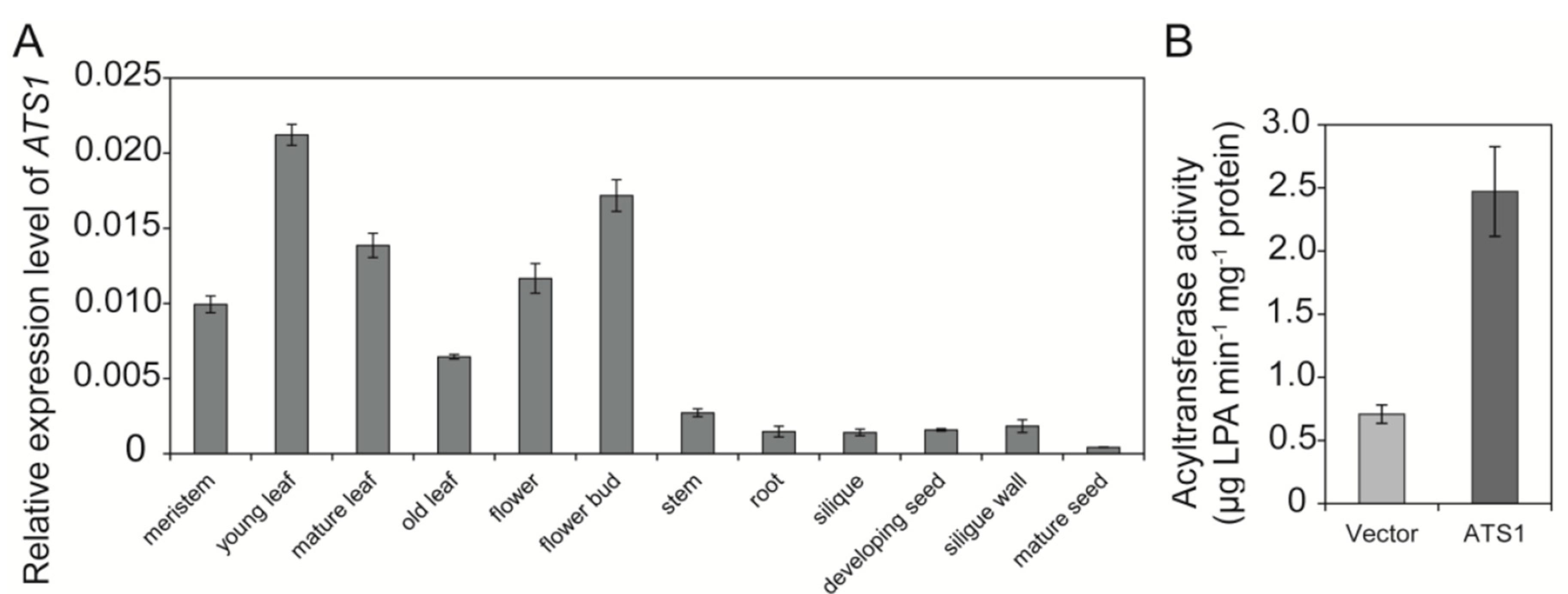
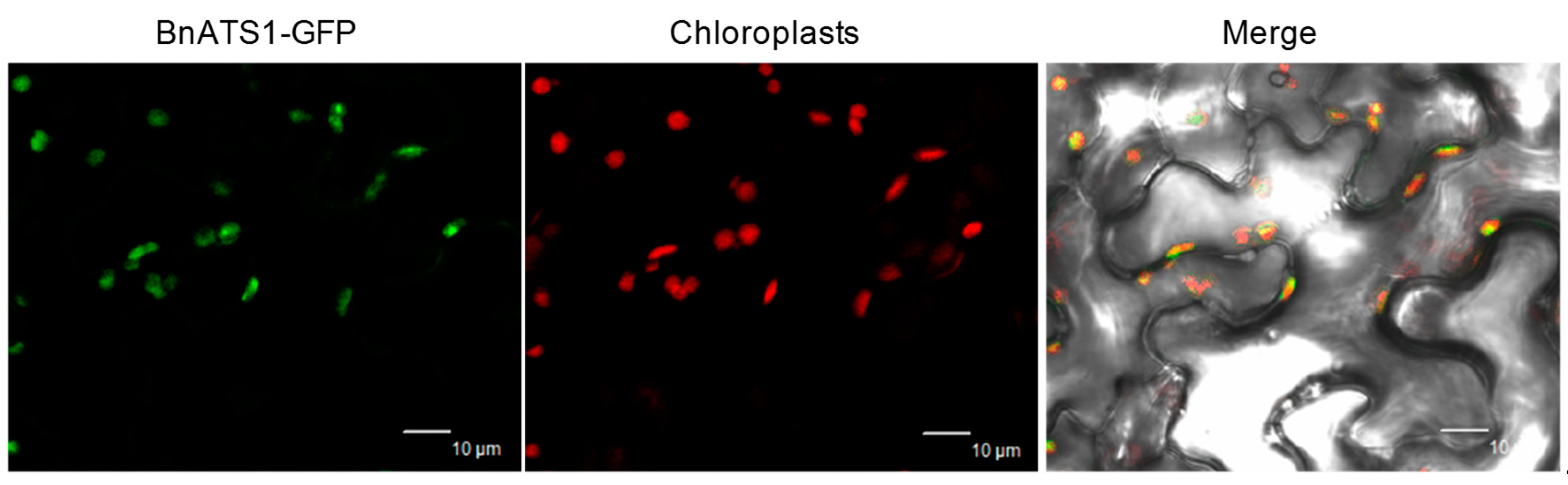
2.1. BnATS1 Is Expressed in Various Tissues and BnATS1 Is Localized in Chloroplasts
2.2. Overexpression of BnATS1 Enhanced Plant Growth during Vegetative Stage
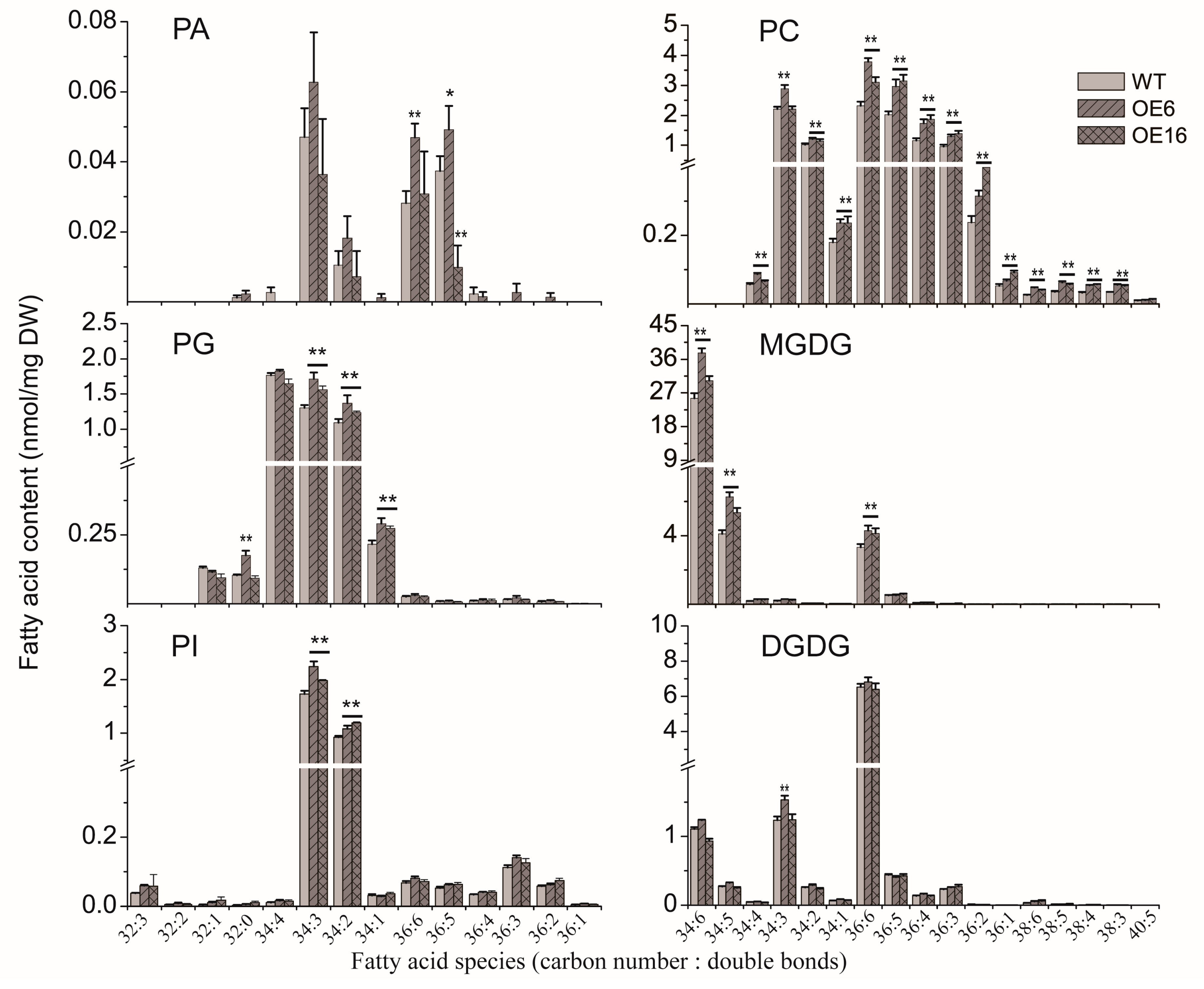
2.3. The Effect of BnATS1 on Phospholipid Metabolism
2.4. Overexpression of BnATS1 Enhanced Prokaryotic Galactolipids
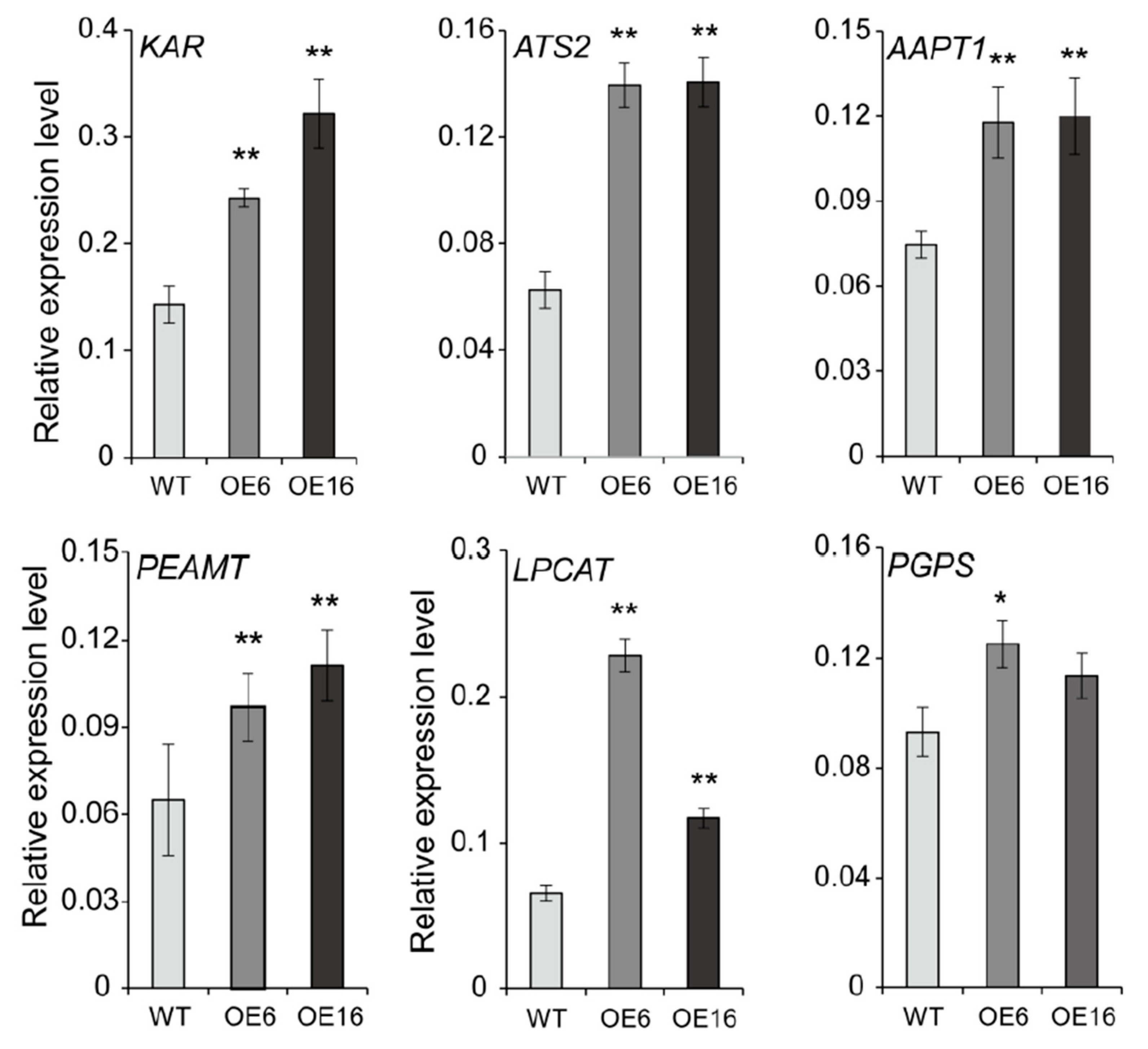
2.5. Overexpression of BnATS1 Up-Regulated the Expression of Genes Related to Lipid Anabolism
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning, Vector Construction and Plant Transformation
4.3. RNA Extraction and Real-Time PCR
4.4. BnATS1 Protein Expression and Activity Assay
4.5. Subcellular Localization
4.6. Lipid Extraction and Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li-Beisson, Y.H.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P.; et al. Arabidopsis Book; Robt, L., Ed.; The American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 11, pp. 1–70. [Google Scholar]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [PubMed]
- Kunst, L.; Browse, J.; Somerville, C. Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc. Natl. Acad. Sci. USA 1988, 85, 4143–4147. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, B.; Cornish, A.J.; Froehlich, J.E.; Benning, C. Phosphatidylglycerol biosynthesis in chloroplasts of Arabidopsis mutants deficient in acyl-ACP glycerol-3-phosphate acyltransferase. Plant J. 2006, 47, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.W.; Hosaka, K.; Plesch, G.; Mueller-Roeber, B. Cloning of Arabidopsis thaliana phosphatidylinositol synthase and functional expression in the yeast pis mutant. Plant Mol. Biol. 2000, 42, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.; Muller, F.; Eubel, H.; Braun, H.P.; Frentzen, M.; Kushnir, S. Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J. 2003, 33, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Lofke, C.; Ischebeck, T.; Konig, S.; Freitag, S.; Heiliviann, I. Alternative metabolic fates of phosphatidylinositol produced by phosphatidylinositol synthase isoforms in Arabidopsis thaliana. Biochem. J. 2008, 413, 115–124. [Google Scholar] [CrossRef]
- Nakamura, Y.; Awai, K.; Masuda, T.; Yoshioka, Y.; Takamiya, K.-i.; Ohta, H. A novel phosphatidylcholine-hydrolyzing phospholipase C induced by phosphate starvation in Arabidopsis. J. Biol. Chem. 2005, 280, 7469–7476. [Google Scholar] [CrossRef]
- Craddock, C.P.; Adams, N.; Bryant, F.M.; Kurup, S.; Eastmond, P.J. PHOSPHATIDIC ACID PHOSPHOHYDROLASE regulates phosphatidylcholine biosynthesis in Arabidopsis by phosphatidic acid-mediated activation of CTP: PHOSPHOCHOLINE CYTIDYLYLTRANSFERASE activity. Plant Cell 2015, 27, 1251–1264. [Google Scholar] [CrossRef]
- Bates, P.D.; Fatihi, A.; Snapp, A.R.; Carlsson, A.S.; Browse, J.; Lu, C.F. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012, 160, 1530–1539. [Google Scholar] [CrossRef]
- Bates, P.D.; Ohlrogge, J.B.; Pollard, M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem. 2007, 282, 31206–31216. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kondo, M.; Fukuda, H.; Nishimura, M.; Ohta, H. Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 17216–17221. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl coa:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Koizumi, R.; Shui, G.; Shimojima, M.; Wenk, M.R.; Ito, T.; Ohta, H. Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc. Natl. Acad. Sci. USA 2009, 106, 20978–20983. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, J.; Riekhof, W.; Froehlich, J.E.; Benning, C. A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J. 2003, 22, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, J.; Froehlich, J.E.; Awai, K.; Benning, C. Mutation of the tgd1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell 2005, 17, 3094–3110. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramírez, A.; Oropeza-Aburto, A.; Razo-Hernandez, F.; Ramirez-Chavez, E.; Herrera-Estrella, L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2006, 103, 6765–6770. [Google Scholar] [CrossRef]
- Li, M.; Welti, R.; Wang, X. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol. 2006, 142, 750–761. [Google Scholar] [CrossRef]
- Gaude, N.; Nakamura, Y.; Scheible, W.; Ohta, H.; Dormann, P. Phospholipase c5 (npc5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J. 2008, 56, 28–39. [Google Scholar] [CrossRef]
- Heinz, E.; Roughan, P.G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983, 72, 273–279. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Zhao, H.; Zhou, R.; Ji, B.H.; Dan, X.Y. Substrate selectivity of glycerol-3-phosphate acyltransferase in rice. J. Integr. Plant Biol. 2009, 51, 1040–1049. [Google Scholar] [CrossRef]
- Zheng, Z.F.; Xia, Q.; Dauk, M.; Shen, W.Y.; Selvaraj, G.; Zou, J.T. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 2003, 15, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Beisson, F.; Li, Y.H.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef]
- Li, Y.; Beisson, F.; Koo, A.J.K.; Molina, I.; Pollard, M.; Ohlrogge, J. Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA 2007, 104, 18339–18344. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.H.; Pollard, M.; Sauveplane, V.; Pinot, F.; Ohlrogge, J.; Beisson, F. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. USA 2009, 106, 22008–22013. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pollard, M.; Li-Beisson, Y.H.; Beisson, F.; Feig, M.; Ohlrogge, J. A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA 2009, 107, 12040–12045. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Simpson, J.P.; Li-Beisson, Y.H.; Beisson, F.; Pollard, M.; Ohlrogge, J.B. A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: Substrate specificity, sn-2 preference, and evolution. Plant Physiol. 2012, 160, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Shi, J.X.; Liang, W.Q.; Zhang, Q.F.; Lian, G.B.; Quan, S.; Zhu, L.; Lou, Z.J.; Chen, M.J.; Zhang, D.B. Glycerol-3-Phosphate Acyltransferase 3 (OsGPAT3) is required for anther development and male fertility in rice. J. Exp. Bot. 2017, 68, 513–526. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Yang, J.; Zhang, G.R.; Xing, W.F.; Zhang, S.; Yang, Z.N. Glycerol-3-phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol. Plant 2012, 5, 131–142. [Google Scholar] [CrossRef]
- Petit, J.; Bres, C.; Mauxion, J.; Tai, F.; Martin, L.; Fich, E.A.; Joubes, J.; Rose, J.; Domergue, F.; Rothan, C. The glycerol-3-phosphate acyltransferase GPAT6 from tomato plays a central role in fruit cutin biosynthesis. Plant Physiol. 2016, 171, 894–913. [Google Scholar] [CrossRef]
- Shockey, J.; Regmi, A.; Cotton, K.; Adhikari, N.D.; Browse, J.; Bates, P.D. Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis. Plant Physiol. 2016, 170, 163–179. [Google Scholar] [CrossRef]
- Singer, S.D.; Chen, G.Q.; Mietkiewska, E.; Tomasi, P.; Jayawardhane, K.; Dyer, J.M.; Weselake, R.J. Arabidopsis GPAT9 contributes to synthesis of intracellular glycerolipids but not surface lipids. J. Exp. Bot. 2016, 67, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Huang, A.H.C. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol. 2004, 134, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Payá-Milans, M.; Venegas-Calerón, M.; Salas, J.J.; Garces, R.; Martinez-Force, E. Cloning, heterologous expression and biochemical characterization of plastidial sn-glycerol-3-phosphate acyltransferase from Helianthus annuus. Phytochemistry 2015, 111, 27–36. [Google Scholar] [CrossRef]
- Mou, Z.L.; He, Y.K.; Dai, Y.; Liu, X.F.; Li, J.Y. Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 2000, 12, 405–417. [Google Scholar] [CrossRef]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ’16:3′ plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dörmann, P.; Hoffmann-Benning, S.; Balbo, I.; Benning, C. Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 1995, 7, 1801–1810. [Google Scholar]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Tjellstrom, H.; Yang, Z.; Allen, D.K.; Ohlrogge, J.B. Rapid kinetic labeling of Arabidopsis cell suspension cultures: Implications for models of lipid export from plastids. Plant Physiol. 2012, 158, 601–611. [Google Scholar] [CrossRef]
- Chen, M.; Thelen, J.J. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 1430–1444. [Google Scholar] [CrossRef]
- Mou, Z.L.; Wang, X.Q.; Fu, Z.M.; Dai, Y.; Han, C.; Jian, O.Y.; Bao, F.; Hu, Y.X.; Li, J.Y. Silencing of phosphoethanolamine N-methyltransferase results in temperature-sensitive male sterility and salt hypersensitivity in Arabidopsis. Plant Cell 2002, 14, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Huang, Y.F.; Cutler, A.J.; Abrams, S.R.; Taylor, D.C. Molecular and biochemical characterization of an aminoalcoholphosphotransferase (AAPT1) from Brassica napus: Effects of low temperature and abscisic acid treatments on AAPT expression in Arabidopsis plants and effects of over-expression of BnAAPT1 in transgenic Arabidopsis. Planta 2003, 217, 547–558. [Google Scholar] [PubMed]
- Lu, S.P.; Bahn, S.C.; Qu, G.; Qin, H.Y.; Hong, Y.; Xu, Q.P.; Zhou, Y.M.; Hong, Y.Y.; Wang, X.M. Increased expression of phospholipase Dα1 in guard cells decreases water loss with improved seed production under drought in Brassica napus. Plant Biotechnol. J. 2013, 11, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yuan, S.; Sun, L.X.; Wang, X.M.; Hong, Y.Y. Cytidinediphosphate-diacylglycerol synthase 5 is required for phospholipid homeostasis and is negatively involved in hyperosmotic stress tolerance. Plant J. 2018, 94, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Welti, R.; Li, W.Q.; Li, M.Y.; Sang, Y.M.; Biesiada, H.; Zhou, H.; Rajashekar, C.B.; Williams, T.D.; Wang, X.M. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Jia, C.; Liu, N.; Aboagla, A.A.A.; Chen, W.; Gong, W.; Tang, S.; Hong, Y. Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus. Int. J. Mol. Sci. 2020, 21, 5325. https://doi.org/10.3390/ijms21155325
Kang H, Jia C, Liu N, Aboagla AAA, Chen W, Gong W, Tang S, Hong Y. Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus. International Journal of Molecular Sciences. 2020; 21(15):5325. https://doi.org/10.3390/ijms21155325
Chicago/Turabian StyleKang, Huiling, Chenxi Jia, Nian Liu, Alfatih Alamin Alhussain Aboagla, Wenling Chen, Wei Gong, Shaohua Tang, and Yueyun Hong. 2020. "Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus" International Journal of Molecular Sciences 21, no. 15: 5325. https://doi.org/10.3390/ijms21155325
APA StyleKang, H., Jia, C., Liu, N., Aboagla, A. A. A., Chen, W., Gong, W., Tang, S., & Hong, Y. (2020). Plastid Glycerol-3-phosphate Acyltransferase Enhanced Plant Growth and Prokaryotic Glycerolipid Synthesis in Brassica napus. International Journal of Molecular Sciences, 21(15), 5325. https://doi.org/10.3390/ijms21155325



